Abstract
Although 36% of US men are normal weight (BMI <25 kg/m2), the health benefits of greater leanness in normal-weight individuals are seldom acknowledged. To assess the optimal body weight with respect to minimizing coronary heart disease (CHD) risk, we applied Cox proportional hazard analyses of 20,525 nonsmoking, nondiabetic, normal-weight men followed prospectively for 7.7 years, including 20,301 who provided follow-up questionnaires. Two-hundred and forty two men reported coronary artery bypass graph (CABG) or percutaneous transluminal coronary angioplasty (PTCA) and 82 reported physician-diagnosed incident myocardial infarction (267 total). The National Death Index identified 40 additional ischemic heart disease deaths. In these normal-weight men, each kg/m2 decrement in baseline BMI was associated with 11.2% lower risk for total CHD (P = 0.005), 13.2% lower risk for nonfatal CHD (P = 0.002), 19.0% lower risk for nonfatal myocardial infarction (P = 0.01), and 12.2% lower risk for PTCA or CABG (P = 0.007). Compared to men with BMI between 22.5 and 25 kg/m2, those <22.5 kg/m2 had 24.1% lower total CHD risk (P = 0.01), 27.9% lower nonfatal CHD risk (P = 0.01), 37.8% lower nonfatal myocardial infarction risk (P = 0.05), and 27.8% lower PTCA or CABG risk (P = 0.02). In nonabdominally obese men (waist circumference <102 cm), CHD risk declined linearly with declining waist circumference. CHD risk was unrelated to change in waist circumference between 18 years old and baseline except as it contributed to baseline circumference. These results suggest that the optimal BMI for minimizing CHD risk lies somewhere <22.5 kg/m2, as suggested from our previous analyses of incident diabetes, hypertension, and hypercholesterolemia in these men.
Introduction
Current public health guidelines classify adult BMI between 18.5 and 24.9 kg/m2 as normal weight, between 25.0 and 29.9 kg/m2 as overweight, and 30 kg/m2 and above as obese [1]. In addition, waist circumference provides a convenient measure of abdominal obesity, with men’s circumferences ≥102 cm classified as abdominally obese [1].
The health implications of greater leanness in ostensibly normal- weight individuals receive little attention [1]. Most studies contrast disease risk for overweight and obese vis- a-vis normal weight without regard to variations in risk within the normal range [1–4]. Although the increase in coronary heart disease (CHD) risk with body weight is generally acknowledged to begin at the low end of the normal-weight range [1], this conclusion is primarily deduced from trends in risk rather than statistically significant differences within the normal range [5]. Other studies show no greater CHD risk with increasing BMI within the normal-weight range [6–11], or with BMI overall [12,13]. Many relevant studies use BMI intervals that overlap the normal and overweight categories [14–17]. Those studies showing significant differences within the normal-weight range for women [18,19] or Asians [20] are unlikely to be applicable to white males because at the same level of body fat, Asians have lower BMIs than whites [21], and women have lower BMIs than men [22].
The Third National Health and Nutrition Examination Survey estimates that 36% of US men are normal weight [4]. Attaining and preserving health should be personally important to the ~90 million US adults who are of normal weight. Emphasizing the health benefits of the optimal body weight in currently lean men and women could be more effective in preventing obesity than achieving sustained weight loss in those who are already overweight or obese [1,23,24]. Moreover, inevitable advancements in pharmacological or behavioral weight control will require, at some point, the definition of optimal body weight.
This report examines prospectively the incidence of CHD in relation to body weight, body circumferences, and weight history in normal-weight men who are participants of the National Runners’ Health Study [23]. We have previously shown that less body weight within the normal-weight range is associated with a lower incidence of diabetes, hypertension, and hypercholesterolemia in these men [23]. The current analyses show that despite an active lifestyle, lower body weight and waist circumference are associated with significantly lower CHD risk within the normal-weight range, hence the importance of avoiding weight gain even among lean, ostensibly normal- weight, physically active individuals.
Methods And Procedures
The design and methods of the National Runners’ Health Study are described elsewhere [23]. Briefly, recruitment of this cohort occurred between 1991 and 1994 by nationwide distribution of a two-page questionnaire to runners identified through running magazine subscription lists and participation in foot race events. The questionnaire solicited information on demographics, running history, weight history, smoking habit, prior history of heart attacks and cancer, and medications for blood pressure, thyroid, cholesterol, and diabetes. We estimate that ~15% of recipients returned questionnaires among the total number contacted (the percentage is approximate due to the uncertainty in the number of questionnaires distributed at footraces and the number of persons who received multiple questionnaires). To be eligible for the study, participants were required to provide signed informed consent, be at least 18 years old, and provide, at a minimum, their name, contact information, sex, birth date, weekly running distance, body weight, and height. Follow-up questionnaires were mailed between 1998 and 2001. The University of California Berkeley Committee for the Protection of Human Subjects approved the study protocol and all participants provided written informed consent.
BMI was calculated as weight in kilograms divided by the square of height in meters. Self-reported waist and chest circumferences were elicited by the question, “Please provide, to the best of your ability, your body circumference in inches: waist___, hip___, and chest___,” without further instruction. Elsewhere, we have reported the strong correlations between self-reported and clinically measured heights (r = 0.96) and weights (r = 0.96) (ref. 24), and the significant association of self-reported BMIs and waist circumferences vs. running distance cross-sectionally [25] and diabetes, hypertension, and hypercholesterolemia prospectively [23,26,27]. Self-reported waist circumferences are somewhat less precise, as indicated by their correlations with reported circumferences on a second questionnaire (r = 0.84) and with their clinical measurements (r = 0.68) (ref. 24). Participants were also asked to report their body circumferences at age 18. Running distances were reported in usual miles run per week at baseline. Previously, we reported strong correlations between repeated questionnaires for self-reported running distance (r = 0.89) (ref. 24). Intakes of meat, fish and fruit were based on the questions “During an average week, how many servings of beef, lamb, or pork do you eat”, “…servings of fish do you eat”, and “…pieces of fruit do you eat”. Alcohol intake was estimated from the corresponding questions for 4-oz. glasses of wine, 12-oz. bottles of beer, and mixed drinks and liqueurs.
In their follow-up questionnaires, participants reported whether they had percutaneous transluminal coronary angioplasty (PTCA) and coronary artery bypass graph (CABG), and whether they had been diagnosed by a physician as having had a heart attack since baseline, and if so, they provided the year of the procedure or diagnosis. Persons reporting any of these procedures or conditions in the year of their baseline survey or before were excluded from these analyses. Nonfatal CHD was defined as the earliest myocardial infarction, CABG, or PTCA. Fatal CHD was defined as an ICD-9 diagnosis of 410–414 or 429.2, or an ICD-10 diagnosis of I20–I25. The endpoint “total CHD” includes both fatal and nonfatal CHD as described above. Fatal CHD were too few for their separate analyses.
Regression analyses (JMP software version 5.0; SAS Institute, Cary, NC) were used to test for significant trends in exercise levels, pack-years of prior cigarette use, age, education, body circumferences, and weekly intakes of meat, fish, fruit, alcohol, and aspirin by baseline BMI. Cox proportional hazard model (JMP software) was used with weekly intakes of alcohol, meat, fish, fruit, and aspirin, pack-years of prior cigarette use, and years of education, along with age (age and age2) and usual distance run (km/day and km2/day2) as covariates. Results are presented for BMI as a continuous measure of weight standardized for height and as a categorical variable for the data partitioned by evenly spaced cut-points at 22.5, 25, and 27.5 kg/m2. The analyses of nonfatal events include only those participants with follow-up questionnaires, and the analyses of all fatal and nonfatal CHD include those subjects identified through the National Death Index in addition to the follow-up survey respondents.
Results
There were 36,537 men who had complete data on age, education, diet, aspirin use, BMI, smoking history, and running distance, of whom we excluded 527 for baseline cigarette use, 214 for baseline diabetes, and 230 for pre-existing heart disease. Of the remaining 35,566 men, follow-up questionnaires were obtained for 28,462 men (80.0%) and causes of death were obtained from the National Death Index on an additional 314 men with complete baseline data who were not current smokers, nondiabetic, and without pre-existing heart disease at baseline (115 men had both follow-up questionnaires and subsequently died, thus 28,776 or 80.1% were known dead or provided follow- up questionnaires). Those responding to the follow-up survey were significantly older (mean ± s.e. for responder vs. nonresponder: 44.81 ± 0.06 vs. 41.95 ± 0.12 years, P < 0.0001), more educated (16.54 ± 0.01 vs. 15.89 ± 0.03 years, P < 0.0001), leaner as indicated by their lower BMI (23.86 ± 0.02 vs. 24.24 ± 0.03 kg/m2, P < 0.0001) and circumferences of the waist (84.44 ± 0.04 vs. 84.62 ± 0.08, P = 0.03) and chest (101.75 ± 0.05 vs. 102.73 ± 0.10 cm, P < 0.0001), took fewer aspirin (2.44 ± 0.03 vs. 2.61 ± 0.06 tablets/ week, P = 0.01), and ate more meat (2.84 ± 0.02 vs. 2.67 ± 0.03 serving/week, P < 0.0001), but did not differ in reported weekly intakes of fish (1.54 ± 0.01 vs. 1.53 ± 0.02 serving, P = 0.59), fruit (11.0 ± 0.05 vs. 10.84 ± 0.11 pieces, P = 0.09), or alcohol (80.92 ± 0.6 vs. 80.50 ± 1.41 ml, P = 0.78).
Men reported physician diagnoses of 464 CHD (262 PTCA, 183 CABG, and 152 myocardial infarctions, including 133 men with multiple events). The National Death Index search identified an additional 61 deaths due to ischemic heart disease. Women were not analyzed because there were only 20 women who reported physician diagnosis of CHD and only 3 female deaths due to ischemic heart disease.
Table 1 presents the baseline characteristics of the sample by BMI. Leaner men were significantly more likely to be younger, more-highly educated, use less aspirin, have smoked less in the past and consume less meat, fish, and alcohol and more fruit. There was a strong inverse association of running distance and BMI, as has been reported previously for this cohort [25]. The sample was self-identified as 94.8% white, 2.3% Hispanic, 0.8% black, 0.3% Native American, 1.3% Asian, and 0.5% other racial groups.
Table 1.
Baseline characteristics (mean±SD) of nondiabetic, currently nonsmoking runners without a history of coronary heart disease.
| Body mass index (kg/m2) | ||||
|---|---|---|---|---|
| <22.5 | 22.5–25 | 25–27.5 | ≥27.5 | |
| Sample size | 9,044 | 11,481 | 5,971 | 2,280 |
| BMIbaseline (kg/m2) | 21.25 ± 1.01 | 23.67 ± 0.68 | 26.02 ± 0.72 | 29.51 ± 2.03 |
| Waist circumference (cm) | 80.11 ± 4.31 | 84.09 ± 4.36 | 88.16 ± 4.93 | 93.86 ± 7.16 |
| Chest circumference (cm) | 96.96 ± 6.03 | 101.56 ± 5.86 | 105.80 ± 5.83 | 110.77 ± 7.12 |
| Age (years) | 43.66 ± 11.72 | 45.40 ± 10.13 | 45.76 ± 9.38 | 44.27 ± 8.85 |
| Education (years) | 16.57 ± 2.45 | 16.61 ± 2.43 | 16.40 ± 2.44 | 16.32 ± 2.52 |
| Meat intake (servings/wk) | 2.60 ± 2.63 | 2.77 ± 2.56 | 3.08 ± 2.75 | 3.48 ± 2.98 |
| Fish intake (servings/wk) | 1.47 ± 1.37 | 1.57 ± 1.46 | 1.58 ± 1.48 | 1.60 ± 1.48 |
| Fruit intake (pieces/wk) | 11.91 ± 9.31 | 11.09 ± 8.93 | 10.28 ± 8.25 | 9.48 ± 7.90 |
| Alcohol intake (ml/wk) | 72.25 ± 102.60 | 82.91 ± 112.66 | 90.78 ± 125.17 | 80.01 ± 125.19 |
| Aspirin (tablets/wk) | 2.18 ± 4.67 | 2.41 ± 4.67 | 2.75 ± 5.13 | 2.99 ± 6.29 |
| Past cigarettes use (pack years) | 4.17 ± 10.93 | 5.86 ± 13.08 | 7.57 ± 14.98 | 8.72 ± 16.82 |
| Running distance (km/day) | 6.59 ± 3.67 | 5.36 ± 2.99 | 4.44 ± 2.56 | 3.55 ± 2.59 |
All trends were significant at P < 0.0001 by simple least-squares regression analyses. Sample sizes for waist circumference were: 8,525 for <22.5 kg/m2, 10,823 for 22.5–25 kg/m2, 5,587 for 25–27.5 kg/m2, and 2,149 for ≥27.5 kg/m2; and for chest circumference were: 7,301 for <22.5 kg/m2, 9,368 for 22.5–25 kg/m2, 4,908 for 25–27.5 kg/m2, and 1,901 for ≥27.5 kg/m2.
Table 2 presents the relative risks for CHD by BMI and waist circumference. In normal-weight men, each kg/m2 decrement in BMI was associated with 11.2% lower risk for fatal plus nonfatal CHD, 13.2% lower risk for nonfatal CHD, 19% lower risk for myocardial infarction, and 12.2% lower risk for PTCA or CABG. Per kg/m2, the decreases in risk associated with lower BMI were generally greater for BMI <25 kg/m2 than for the entire sample. The decrement in risk was also generally greater for nonfatal myocardial infarction than other endpoints. Tests for nonlinearity by the inclusion of a quadratic term for BMI did not achieve statistical significance in predicting total CHD risk (P = 0.06), nonfatal CHD (P = 0.09), myocardial infarction (P = 0.32), or PTCA or CABG (P = 0.08) in normal-weight men. Adjustment for waist circumference did not eliminate the significance of decrement in risk associated with BMI, whereas adjustment for baseline BMI diminished the relative risk for waist circumference sufficiently to marginalize its statistical significance. In normal weight men, each centimeter decrement in waist circumference was associated with a 3.8% lower risk for total CHD, 4.1% lower risk for nonfatal CHD, 5.3% lower risk for myocardial infarction, and 4.3% lower risk for PTCA or CABG.
Table 2.
Men’s relative risk for CHD by decreasing BMI (per kg/m2) and decreasing waist circumference (per cm).
| Model | Men with BMI<25 kg/m2 | All men | ||
|---|---|---|---|---|
| Total CHD | BMI (per kg/m2 decrease) |
Waist circumference (per cm decrease) |
BMI (per kg/m2 decrease) |
Waist circumference (per cm decrease) |
| BMI only | 0.888 (0.815, 0.966) P=0.005 |
0.909 (0.882, 0.939) P<0.0001 |
||
| Waist circumference only | 0.962 (0.938, 0.987) P=0.003 |
0.963 (0.950, 0.976) P<0.0001 |
||
| BMI & Waist circumference | 0.906 (0.819, 1.000) P=0.05 |
0.975 (0.947, 1.003) P=0.08 |
0.926 (0.887, 0.968) P=0.0008 |
0.983 (0.965, 1.001) P=0.07 |
| Nonfatal CHD | ||||
| BMI only | 0.868 (0.791, 0.951) P=0.002 |
0.904 (0.876, 0.935) P<0.0001 |
||
| Waist circumference only | 0.959 (0.934, 0.985) P=0.003 |
0.962 (0.949, 0.977) P<0.0001 |
||
| BMI & Waist circumference | 0.903 (0.811, 1.003) P=0.06 |
0.972 (0.943, 1.002) P=0.07 |
0.921 (0.880, 0.965) P=0.0007 |
0.984 (0.965, 1.004) P=0.12 |
| Myocardial infarction | ||||
| BMI only | 0.810 (0.678, 0.959) P=0.01 |
0.881 (0.835, 0.932) P<0.0001 |
||
| Waist circumference only | 0.947 (0.904, 0.995) P=0.03 |
0.948 (0.927, 0.971) P<0.0001 |
||
| BMI & Waist circumference | 0.853 (0.695, 1.037) P=0.11 |
0.966 (0.916, 1.022) P=0.23 |
0.917 (0.851, 0.994) P=0.03 |
0.970 (0.940, 1.002) P=0.07 |
| PTCA & CABG | ||||
| BMI only | 0.878 (0.796, 0.966) P=0.007 |
0.910 (0.879, 0.943) P<0.0001 |
||
| Waist circumference only | 0.957 (0.930, 0.984) P=0.003 |
0.966 (0.951, 0.981) P<0.0001 |
||
| BMI & Waist circumference | 0.914 (0.816, 1.020) P=0.11 |
0.968 (0.938, 0.999) P=0.05 |
0.923 (0.879, 0.970) P=0.002 |
0.987 (0.967, 1.008) P=0.24 |
95% confidence intervals given in parentheses. All relative risks were adjusted for age (age and age2), education, pack-years of previous cigarette use, running distance (km/week and km2/week2), and weekly intake of meat, fish, fruit, alcohol, and aspirin. Sample sizes for analyses of all men involving BMI only were N = 28,776 for total CHD and N = 28,462 for other outcomes, and involving waist circumference were N = 27,084 for total CHD and N = 26,799 for other outcomes. Sample sizes for analyses of men with BMI <25 kg/m2 involving BMI only were N = 20,525 for total CHD and N = 20,301 for other outcomes, and involving waist circumference were N = 19,348 for total CHD and N = 19,145 for other outcomes.
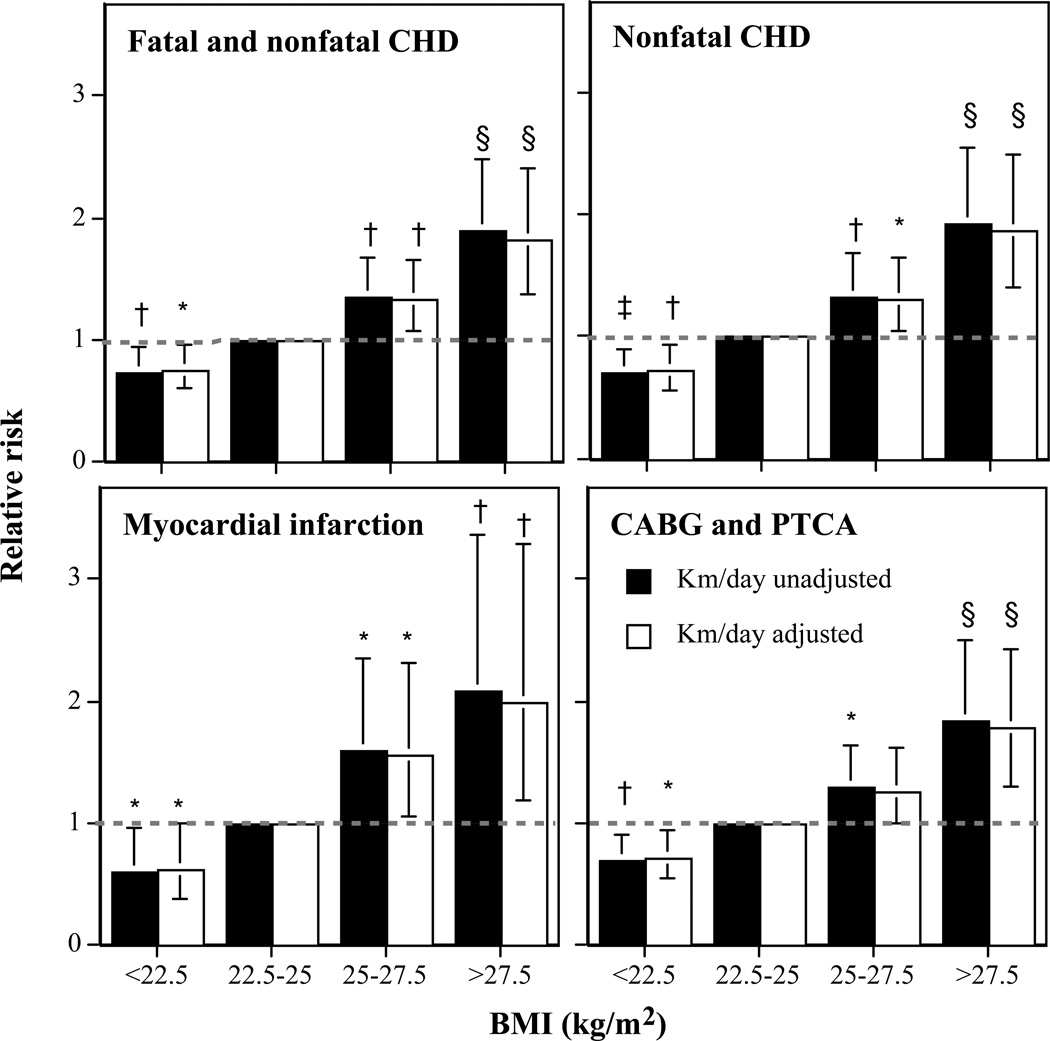
Figure 1 displays the risk for incident CHD by BMI. The relative risks are computed relative to the incident CHD for BMI between 22.5 and 25 kg/m2. As expected from Table 2, higher baseline BMI was associated with significantly greater CHD risk. For each CHD endpoint, however, a BMI <22.5 kg/m2 was associated with significantly lower risk than for BMI between 22.5 and 25 kg/m2. Relative to a BMI between 22.5 and 25 kg/m2, the decline in risk for BMI <22.5 kg/m2 was comparable to the increase in risk for BMI between 25 and 27.5 kg/m2. Adjustment for distance run per week had little affect on the relationship between BMI and CHD.
Figure 1.
Relative risk for incident total CHD vs. BMI in 35,566 men followed prospectively for 7.7 years. Results are presented with and without adjustments for physical activity (km/day) to demonstrate that the relationships with BMI are largely unaffected by exercise level. Significance levels for the relative risk relative to men 22.5 < BMI < 25 kg/m2 are coded: *P < 0.05, †P < 0.01, ‡P < 0.005, and §P < 0.001 by Cox proportional hazards analyses. Brackets designate 95% confidence intervals. All analyses were adjusted for age, education, pack-years of prior cigarette use, and reported weekly intakes of meat, fish, fruit, alcohol, and aspirin.
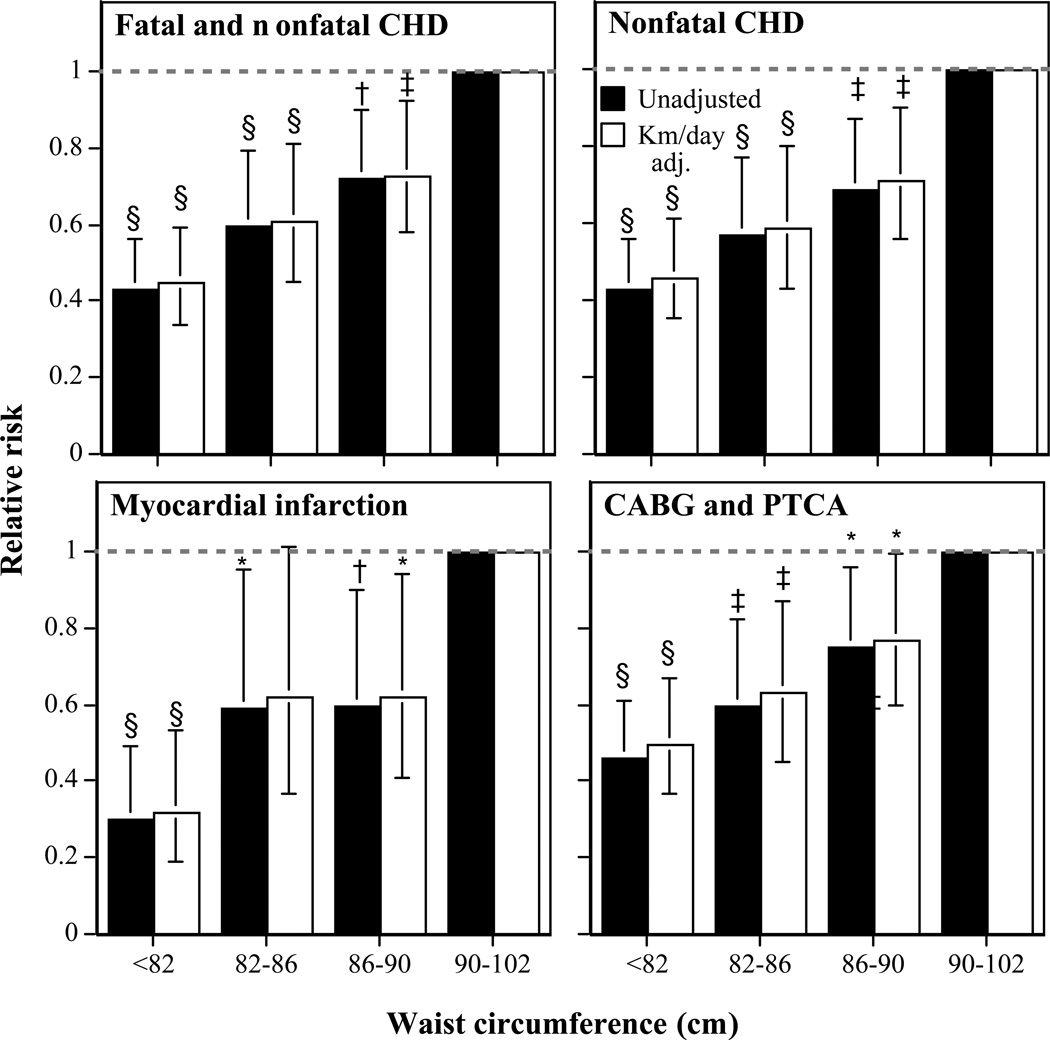
Figure 2 presents the corresponding analyses for waist circumference in men lacking abdominal obesity as defined by a waist circumference <102 cm. There was a linear decline in risk with decreasing waist circumference, including significantly lower risk for 86–90 cm vis-à-vis 90–102 cm. Adjustment for distance run had little affect on the decline in risk associated with waist circumference.
Figure 2.
Relative risk for incident total CHD vs. waist circumference in 33,298 men followed prospectively for 7.7 years. Results are presented with and without adjustments for physical activity (km/day) to demonstrate that the relationships with BMI are largely unaffected by exercise level. Significance levels for the relative risk relative to men 90–102 cm are coded: *P < 0.05, †P < 0.01, ‡P < 0.005, and §P < 0.001 by Cox proportional hazards analyses. Brackets designate 95% confidence intervals. All analyses were adjusted for age, education, and reported weekly intakes of meat, fish, fruit, alcohol, and aspirin.
Other circumference measures
Smaller baseline chest circumference predicted lower incident total CHD (relative risk = 0.979 per cm, P = 0.003), nonfatal CHD (relative risk = 0.980 per cm, P = 0.007), nonfatal myocardial infarction (relative risk = 0.970 per cm, P = 0.01), and revascularization procedures (PTCA and CABG: relative risk = 0.981 per cm, P = 0.02), but not when adjusted for either waist circumference or BMI (P > 0.05). Similarly, smaller hip circumference was weakly predictive of decreasing risk for total CHD (relative risk = 0.985 per cm, P = 0.09), nonfatal CHD (relative risk = 0.981 per cm, P = 0.05), nonfatal myocardial infarction (relative risk = 0.976 per cm, P = 0.13), and revascularization procedures (relative risk = 0.978 per cm, P = 0.03) but not when adjusted for either BMI or waist circumference. Waist-to-hip ratio was not significantly related to any CHD outcome except total combined fatal and nonfatal CHD (P = 0.04).
Excluding early events and other sensitivity analyses
Because only 44 of the 525 total events occurred during the first 2 years of follow-up, excluding these early events had little effect on the relative risks of Table 2. For the total sample, when these 44 early events were excluded, each kg/m2 decrement in BMI was associated with an 8.9% lower risk for fatal plus nonfatal CHD, 9.7% lower risk for nonfatal CHD, 12.9% lower risk for myocardial infarction, and 9.2% lower risk for PTCA or CABG. Other sensitivity analyses showed that the relative risk for total CHD-risk per kg/m2 decrease in BMI in all men (relative risk = 0.909, Table 2) remained essentially unchanged if the analyses: (i) statistically adjusted for current smokers, pre-existing diabetes, and prior myocardial infarction (relative risk = 0.902) rather than excluding them from the analyses; (ii) excluded men with pre-existing hypertension, cancer or stroke (relative risk = 0.910); or (iii) was restricted to white males (relative risk = 0.912).
Weight history
Table 3 examines the relationship of waist circumference at age 18 and at baseline to total CHD risk (qualitatively similar results reported for other endpoints). Larger waist circumference at age 18 was marginally associated with increasing CHD risk during follow-up but not when adjusted for baseline circumference, whereas baseline circumference was strongly related to CHD risk with or without adjustment for waist circumference at 18. Changes in waist circumference (Δwaist circumference) between 18 years old and baseline predicted CHD risk but not when adjusted for baseline circumference. However, it is possible to create a strongly significant association between Δwaist circumference and CHD risk by adjusting for waist circumference at 18 years (see Discussion).
Table 3.
Relationship of total (fatal plus nonfatal) CHD versus increasing current waist circumference, waist circumference at age 18, and Δwaist circumference since age 18.
| Relative risk per cm increase (95% confidence interval) | |||
|---|---|---|---|
| Model components | waist circumference at 18 years old |
Current waist circumference |
Δwaist circumference |
| Waist circumference at age 18 only | 1.015 (1.000, 1.029) P=0.06 |
||
| Current waist circumference only | 1.045 (1.029, 1.060) P<0.0001 |
||
| Both current waist circumference and at 18 years old | 0.996 (0.980, 1.012) P=0.61 |
1.047 (1.029, 1.064) P<0.0001 |
|
| Δwaist circumference only | 1.024 (1.009, 1.039) P=0.002 |
||
| Δwaist circumference and waist circumference at 18 years old | 1.042 (1.024, 1.060) P<0.0001 |
1.047 (1.029, 1.064) P<0.0001 |
|
| Δwaist circumference and current waist circumference | 1.042 (1.024, 1.060) P<0.0001 |
1.004 (0.988, 1.021) P=0.61 |
|
95% confidence intervals given in parentheses. All analyses restricted to men who reported their waist circumferences at baseline and for age 18 (N= 23,970) including 418 incident fatal or nonfatal CHD. All relative risks adjusted for (age (age and age2), education, pack-years of previous cigarette use, running distance (km/wk and km2/wk2), and weekly intake of meat, fish, fruit, alcohol, and aspirin.
Discussion
Even among active, ostensibly normal-weight men, greater leanness predicts significantly lower CHD risk. The apparent risk reduction between <22.5 and 22.5–25 kg/m2 was comparable to the risk increase between 22.5–25 kg/m2 and 25–27.5 kg/m2 (Figure 1). The distinction between normal and overweight may have pragmatic utility for health promotion in the general population, however, for these men, the distinction appears arbitrary and ignores clinically important CHD risk reductions from greater leanness.
The reductions in CHD risk shown in this report are consistent with our previous findings of decreased incidence of hypertension, hypercholesterolemia, and diabetes with greater leanness within the normal-weight range [23]. Having a desirable BMI between 22.5 and 25 kg/m2 meant having 55% greater odds of becoming hypertensive relative to men between 20 and 22.5 kg/m2, and over twice the odds of those <20 kg/m2. Normal-weight men with a BMI between 22.5 and 25 kg/m2 had a 37% increase in their odds of acquiring high cholesterol relative to men with BMIs between 20 and 22.5 kg/m2, and over twice the odds of those with BMIs <20 kg/m2. Men with BMIs between 22.5 and 25.0 kg/m2 were over twice as likely to become diabetic as were leaner men.
Although it is generally acknowledged that the increase in CHD risk begins at the lower end of the normal-weight range [1], this supposition derives more from apparent trends rather than statistically significant differences within the normal-weight range [15,28,29]. Many relevant studies use BMI intervals that overlap the normal and overweight categories, making it difficult to assess differences within the normal range [14,16,17]. There are, in fact, a notable number of studies that report no decline in risk within the normal-weight range [12,13,30,31]. Variations in BMI were not predictive of men’s CHD risk in the Physicians’ Health Study <25.7 kg/m2 [6], in the Health Professionals Follow-up Study <25 kg/m2 [11], in male Parisians <28.5 kg/m2 [7], in northwestern Germans <25.3 kg/m2 [8], in men residing in Buffalo New York <25.1 kg/m2 [9], and in Chicago Western Electric Company employees <24.8 kg/m2 [10]. Jonsson et al. did report that men with BMI <20 had significantly fewer coronary events than men with BMI between 20 and 24.9 kg/m2 [32].
In the current report, adjustment for BMI largely eliminated the relationship between waist circumference and CHD risk (Table 2). Although our waist circumferences were less reproducible over duplicate questionnaires and more weakly correlated with direct standardized measurements than those used by the Physician’s Health Study and the Health Professionals Follow-up Study (they report r ≥ 0.95, ref. 33), our findings are nevertheless generally consistent with theirs [6,11]. The heights and weights reported to us showed the same high correlation with clinic measurements as cited by others (r = 0.96) (ref. 18).
Weight gain during young adulthood has become an established risk factor for CHD [19,20,32]. In each case, the analyses demonstrating the significance of the weight gain included adjustment for early-adulthood weight (i.e., inclusion as a covariate).
Table 3 shows that waist circumference at age 18 and Δwaist circumference (i.e., the difference between baseline and age 18) were marginally associated with CHD risk but not when adjusted for baseline waist circumference (the analyses are not presented for BMI because weight at 18 years old was not solicited). Table 3 also demonstrates that statistical artifact can produce a strongly significant effect of Δwaist circumference on CHD risk by including circumference at age 18 as a covariate. Specifically, if the disease risk is related to baseline circumference only, and if another variable that has no relationship to the disease (e.g., circumference at age 18 years old) is subtracted from baseline circumference, then in order to achieve the best fit the regression analyses will resurrect the baseline value by adding circumference at age 18 to the difference, i.e.,
Waistbaseline = Waist18 + Waistbaseline − Waist18 = Waist18 + Δwaist
Thus, ΔWaist is the baseline adiposity contaminated with noise, and the adjustment for Waist18 is the correction for this noise. In creating the best prediction equation for the disease, the regression analyses will assign statistical significance to both ΔWaist and Waist18, which is often misinterpreted as showing that “weight gain since early adulthood affects the disease outcome, independent of the weight at age 18”. Most studies do not simply test whether early-adult weight affects disease risk when adjusted for baseline weight, as done in Table 3.
The demonstration of significant associations between multiple adiposity measures (BMI, waist circumference, chest circumference) and multiple CHD endpoints (total CHD, myocardial infarctions, revascularization procedures) strongly supports the validity of the observed associations. Nevertheless, the primary limitation of this study is the absence of clinical validation and medical record verification of CHD endpoints. The Nurses’ Health Study reported that although 32% of participant- reported myocardial infarctions could not be confirmed using strict World Health Organization criteria (symptoms and either typical electrocardiogram changes or elevations of serum cardiac enzyme levels), most were nevertheless hospitalizations for cardiac disease [34]. In other studies the inclusion of revascularization procedures with myocardial infarction is acknowledged as a possible bias due to their occurrence at an earlier age with less disease progression than the infarcts. It is therefore noteworthy that our data show significant risk reductions separately for myocardial infarction and revascularization procedures. We acknowledge that 20% of the cohort did not respond to our follow-up questionnaires, and differences between the responders and nonresponders may affect the generalizability of the results. We also caution that men and women who are physically active may differ from others genetically, socioeconomically, psychologically, and with respect to other health behaviors, and that statistical assumptions upon which the statistical adjustment depends may not strictly apply [24]. We also did not ascertain family history of CHD and therefore cannot adjust for its effect.
The current weight standards are more liberal than the Metropolitan Life Insurance Company tables of 1959 (ref. 35). Our analyses suggest that weight gain during adulthood is only relevant to the extent that it contributes to current adiposity. For men who satisfy the public health recommendations for physical activity, the 25 kg/m2 cutoff for normal weight is arbitrary and clinically important reductions in CHD risk are likely to be achieved with a BMI <22.5 kg/m2. Others report that excess total mortality in very lean men is due primarily to cancer and respiratory diseases rather than CHD [15], and that the optimal body weight with respect to minimizing the risk for all-cause mortality is a BMI of 25.3 kg/m2 [36]. In our opinion, current public health recommendations acknowledge but underplay the importance of vigorous physical activity in preventing obesity [37–39]. A greater emphasis on maintaining vigorous physical activity while men are still young and lean may facilitate the return of public health targets that are closer to optimal body weight.
Acknowledgments
This work was supported in part by grants HL45652 and HL072110 from the National Heart Lung and Blood Institute, DK066738 from the National Institute of Diabetes and Digestive and Kidney Diseases, and AG032004 from the Institute of Aging, and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03- 76SF00098 to the University of California).
References
- 1.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report: National Institutes of Health. Obes Res. 1998;2(Suppl):51S–209S. [PubMed] [Google Scholar]
- 2.McGee DL. Diverse Populations Collaboration. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 4.Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 5.Kivimäki M, Ferrie JE, Batty GD, et al. Optimal form of operationalizing BMI in relation to all-cause and cause-specific mortality: the Original Whitehall Study. Obesity (Silver Spring) 2008;16:1926–1932. doi: 10.1038/oby.2008.322. [DOI] [PubMed] [Google Scholar]
- 6.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 7.Empana JP, Ducimetiere P, Charles MA, Jouven X. Sagittal abdominal diameter and risk of sudden death in asymptomatic middle-aged men: the Paris Prospective Study I. Circulation. 2004;110:2781–2785. doi: 10.1161/01.CIR.0000146395.64065.BA. [DOI] [PubMed] [Google Scholar]
- 8.Schulte H, Cullen P, Assmann G. Obesity, mortality and cardiovascular disease in the Münster Heart Study (PROCAM) Atherosclerosis. 1999;144:199–209. doi: 10.1016/s0021-9150(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 9.Dorn JM, Schisterman EF, Winkelstein W, Jr, Trevisan M. Body mass index and mortality in a general population sample of men and women. The Buffalo Health Study. Am J Epidemiol. 1997;146:919–931. doi: 10.1093/oxfordjournals.aje.a009218. [DOI] [PubMed] [Google Scholar]
- 10.Spataro JA, Dyer AR, Stamler J, et al. Measures of adiposity and coronary heart disease mortality in the Chicago Western Electric Company Study. J Clin Epidemiol. 1996;49:849–857. doi: 10.1016/0895-4356(96)00067-4. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141:1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 12.Larsson B, Svärdsudd K, Welin L, et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288:1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keys A. A Multivariate Analysis of Death and Coronary Heart Disease. Cambridge, MA: Harvard University Press; 1980. Seven countries. [Google Scholar]
- 14.Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chronic Dis. 1979;32:563–576. doi: 10.1016/0021-9681(79)90119-x. [DOI] [PubMed] [Google Scholar]
- 15.Shaper AG, Wannamethee SG, Walker M. Body weight: implications for the prevention of coronary heart disease, stroke, and diabetes mellitus in a cohort study of middle aged men. BMJ. 1997;314:1311–1317. doi: 10.1136/bmj.314.7090.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh PN, Lindsted KD, Fraser GE. Body weight and mortality among adults who never smoked. Am J Epidemiol. 1999;150:1152–1164. doi: 10.1093/oxfordjournals.aje.a009942. [DOI] [PubMed] [Google Scholar]
- 17.Imeson JD, Haines AP, Meade TW. Skinfold thickness, body mass index and ischaemic heart disease. J Epidemiol Community Health. 1989;43:223–227. doi: 10.1136/jech.43.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 20.Galanis DJ, Harris T, Sharp DS, Petrovitch H. Relative weight, weight change, and risk of coronary heart disease in the Honolulu Heart Program. Am J Epidemiol. 1998;147:379–386. doi: 10.1093/oxfordjournals.aje.a009460. [DOI] [PubMed] [Google Scholar]
- 21.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–1171. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 22.Jackson AS, Stanforth PR, Gagnon J, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26:789–796. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- 23.Williams PT, Hoffman K, La I. Weight-related increases in hypertension, hypercholesterolemia, and diabetes risk in normal weight male and female runners. Arterioscler Thromb Vasc Biol. 2007;27:1811–1819. doi: 10.1161/ATVBAHA.107.141853. [DOI] [PubMed] [Google Scholar]
- 24.Williams PT. Exercise and the population distribution of body weight. Int J Obes. 2004;28:120–128. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 25.Williams PT, Pate RR. Age-related weight gain, and age-specific weight loss, in 60,617 physically active men studied cross-sectionally. Med Sci Sports Exer. 2005;37:1329–1337. doi: 10.1249/01.mss.0000174894.05236.45. [DOI] [PubMed] [Google Scholar]
- 26.Williams PT. Increases in weight and body size increase the odds for hypertension during 7 years of follow-up. Obesity (Silver Spring) 2008;16:2541–2548. doi: 10.1038/oby.2008.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams PT. Changes in body weight and waist circumference affect incident hypercholesterolemia during 7 years of follow-up. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.299. e-pub ahead of print 26 June 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15-year follow-up of middle-aged men and women in eastern Finland. Circulation. 1996;93:1372–1379. doi: 10.1161/01.cir.93.7.1372. [DOI] [PubMed] [Google Scholar]
- 29.Jarrett RJ, Shipley MJ, Rose G. Weight and mortality in the Whitehall Study. Br Med J (Clin Res Ed) 1982;285:535–537. doi: 10.1136/bmj.285.6341.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcosky T, Hyde J, Anderson JJ, Bangdiwala S, Duncan B. Obesity and mortality in the Lipid Research Clinics Program Follow-up Study. J Clin Epidemiol. 1990;43:743–752. doi: 10.1016/0895-4356(90)90232-e. [DOI] [PubMed] [Google Scholar]
- 31.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 32.Jonsson S, Hedblad B, Engström G, et al. Influence of obesity on cardiovascular risk. Twenty-three-year follow-up of 22,025 men from an urban Swedish population. Int J Obes Relat Metab Disord. 2002;26:1046–1053. doi: 10.1038/sj.ijo.0802060. [DOI] [PubMed] [Google Scholar]
- 33.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Colditz G, Martin AP, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 35.Metropolitan Insurance Company. New weight standards for men and women. Stat Bull Metrop Insur Co. 1959 Nov-Dec;40:1–4. [Google Scholar]
- 36.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 37.Williams PT. Maintenance of vigorous activity and 7-year weight gain in 8,340 male and female runners. Med Sci Sports Exer. 2007;39:801–809. doi: 10.1249/mss.0b013e31803349b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams PT. Asymmetric weight gain and loss from increasing and decreasing exercise. Med Sci Sports Exerc. 2008;40:296–302. doi: 10.1249/mss.0b013e31815b6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams PT, Thompson PD. Dose-dependent effects of training and detraining on weight in 6406 runners during 7.4 years. Obesity (Silver Spring) 2006;14:1975–1984. doi: 10.1038/oby.2006.231. [DOI] [PubMed] [Google Scholar]