Abstract
In an implicit-solvent description of molecular solvation, the electrostatic free energy is given through the electrostatic potential. This potential solves a boundary-value problem of the Poisson–Boltzmann equation in which the dielectric coefficient changes across the solute-solvent interface—the dielectric boundary. The dielectric boundary force acting on such a boundary is the negative first variation of the electrostatic free energy with respect to the location change of the boundary. In this work, the concept of shape derivative is used to define such variations and formulas of the dielectric boundary force are derived. It is shown that such a force is always in the direction toward the charged solute molecules.
Keywords: implicit solvent, the Poisson–Boltzmann equation, dielectric boundary force, shape derivative
1 Introduction
We consider electrostatic interactions in the solvation of molecules within the framework of widely used implicit-solvent or continuum-solvent modeling [9, 19, 24, 45, 53]. In such a model, the solvent molecules and ions are treated implicitly and their effects are coarse grained. Most of the existing implicit-solvent models are based on various kinds of fixed solute-solvent interfaces, such as the van der Waals surface, solvent-excluded surface, or solvent-accessible surface [17, 18, 35, 43, 44]. Such a predefined interface is used to compute the solvation free energy as the sum of two separate parts. One is the surface energy, proportional to the area of interface. The other is the electrostatic contribution determined by the Poisson–Boltzmann (PB) [1, 7, 20, 25–27, 30, 31, 36, 37, 39, 49, 58] or generalized Born (GB) [2, 3, 52] approach in which the solute-solvent interface is used as the dielectric boundary.
In recent years, a new class of implicit-solvent models, termed variational implicit-solvent models (VISM), have emerged [22, 23]. Coupled with the robust level-set numerical method [41, 42, 46], such models allow an efficient and quantitative description of molecular solvation [12, 13, 15]. Central in the VISM is a free-energy functional of all possible solute-solvent interfaces, or dielectric boundaries, that separate the continuum solvent from all solute atoms. In a simple setting, such a free-energy functional consists of surface energy of solute molecules, solute-solvent van der Waals interaction energy, and continuum electrostatic free energy, all coupled together and depending solely on a given solute-solvent interface. Minimizing the functional determines the solvation free energy and stable equilibrium solute-solvent interfaces. Initial applications of the level-set VISM to nonpolar molecular systems have demonstrated its success in capturing the hydrophobic interaction, multiple equilibrium states of hydration, and fluctuation between such states [12, 14, 15, 47, 57], all of which are difficult to be captured by a fixed-surface implicit-solvent model. See [4, 9, 10, 24, 56] for other related models and methods.
In this work, we study the dielectric boundary force—the normal component of such a force, to be more precisely—acting on a dielectric boundary or solute-solvent interface. Such a force is the negative variation of the electrostatic free energy with respect to the location change of the dielectric boundary. It is the electrostatic part of the total boundary force associated with the VISM free-energy functional, determining the conformation and dynamics of an underlying molecular system with an implicit solvent. Practically, it is also the electrostatic part of the “normal velocity” in the level-set relaxation of the free-energy functional.
For a given solvation system with a fixed charge density and dielectric coefficient, any given, possible dielectric boundary Γ determines the electrostatic potential ψ = ψΓ as the unique solution to the nonlinear PB equation, which in turn determines the electrostatic free energy G[Γ]. Appealing the notion and method of shape derivatives, we give a precise definition of the dielectric boundary force and derive formulas for such a force. We notice that in [57] the dielectric boundary force with the Coulomb-field approximation of electrostatic free energy is derived and implemented in the level-set VISM for charged molecules. In this approach, there is no need to estimate the GB radii as usually done in a GB model which is also based on the Coulomb-field approximation in a simple setting. In [11], the Yukawa-field approximation of the electrostatic free energy is proposed and the formula of the corresponding dielectric boundary force is derived. These approaches require no solutions to any partial differential equations. In comparison, our current approach is more accurate analytically but can be less efficient computationally. Related work on electrostatic forces in molecular systems can be found in [29, 32, 38].
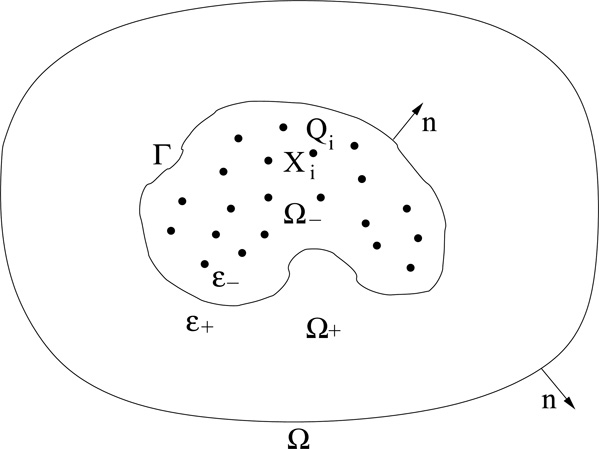
We assume that the entire solvation system occupies a bounded region Ω ⊂ ℝ3. It is divided into three disjoint parts: the region of solute Ω− (e.g., charged biomolecules such as proteins), the region of solvent Ω+ (e.g., salted water), and the solute-solvent interface or the dielectric boundary Γ that separates Ω− and Ω+. See Figure 1, where n denotes the unit normal to the boundary Γ pointing from Ω− to Ω+ and also the exterior unit normal to ∂Ω, the boundary of Ω. The solute region Ω− is completely contained in the entire system region Ω, i.e., , where an over-line denotes the closure. The solute region Ω− contains all the solute atoms located at X1, …, XN, carrying charges Q1, …, QN, respectively.
Figure 1.
The geometry of a solvation system with an implicit solvent. Dots represent solute atoms at Xi carrying charge Qi (i = 1, …, N). The solute-solvent interface Γ separates the solute region Ω− and the solvent region Ω+. The corresponding dielectric coefficients are denoted by ε− and ε+, respectively. The unit normal at the interface Γ pointing from Ω− to Ω+ and the exterior unit normal at the boundary of the entire solvation region Ω are both denoted by n.
In the PB theory, the electrostatic part of the solvation free energy—the electrostatic free energy—is given by
| (1.1) |
through the electrostatic potential ψ : Ω → ℝ [8, 20, 29, 37, 49]. Here, εΓ : Ω → ℝ is the dielectric coefficient defined by
| (1.2) |
where ε− and ε+ are two positive constants. We have and , respectively, where ε0 is the vacuum permittivity, and and are the temperature-dependent relative permittivities. Typically, the value of is in between 1 and 10 for proteins and that of is close to 80 for water at normal conditions. The function f : Ω → ℝ is the fixed charge density of charged solute molecules. It is usually the sum of the point charges Qi located at Xi (i = 1, …, N). Here we assume that f is an integrable function that approximates these point charges. The function χ+ is the characteristic function of Ω+ defined by χ+(X) = 1 if X ∈ Ω+ and χ+(X) = 0 otherwise. The parameter β > 0 is the inverse thermal energy, M ≥ 2 is the number of ionic species in the solvent, and qj ∈ ℝ and are the charge and bulk concentration, respectively, of the jth ionic species with j = 1, …, M. Note that all ε+, ε−, f, β, and qj and are input data. We use the SI units of electrostatics. The electrostatic potential ψ is the unique solution of a boundary-value problem of the nonlinear PB equation [1, 20, 25, 37, 48]
| (1.3) |
See also [5, 34, 36, 37, 54, 55, 58] for generalized PB equations to include ionic excluded-volume effects. Eq. (1.3) is the Euler–Lagrange equation of the right-hand side of (1.1) viewed as a functional of ψ. Note that this functional is concave in ψ.
Let V : ℝ3 → ℝ3 be a smooth map vanishing outside a small neighborhood of the dielectric boundary Γ. Let x = x(t, X) be the solution map of the dynamical system [6, 21, 33, 51]
The shape derivative of the electrostatic free energy G[Γ] in the direction of V : ℝ3 → ℝ3 is defined to be
where Γt(V) = {x(t, X) : X ∈ Γ} and G[Γt(V)] is defined similarly using Γt(V) instead of Γ. It will be shown that δΓ,V G[Γ] is an integral of the product of V · n and some function on Γ that is independent of V, where n is the unit normal along Γ (cf. Theorem 4.1). We identify that function on Γ as the shape derivative of G[Γ] and denote it by δΓG[Γ]. We define the dielectric boundary force, or more precisely the normal component of the dielectric boundary force, to be −δΓG[Γ] and denote it by Fn. Notice that it is only the normal component, not the tangential components of the boundary force, that determines the motion of the boundary.
Our main result is the following formula of the shape derivative δΓG[Γ] : Γ → ℝ of the electrostatic free energy G[Γ] with respect to the dielectric boundary Γ:
| (1.4) |
where ψ is the electrostatic potential, a superscript + or − denotes the restriction onto Ω+ or Ω−, respectively, and
| (1.5) |
Note that ε+∇ψ+ · n = ε−∇ψ− · n on Γ and this common value is denoted by εΓ∇ψ · n. The dependence on the direction of n is in V · n in the integral over Γ. See Theorem 4.1 for the details. A different but useful form of the shape derivative δΓG[Γ] : Γ → ℝ is
| (1.6) |
where I is the identity matrix. See Corollary 4.1. The vector (I − n ⊗ n)∇ψ is the tangential component of ∇ψ. It is continuous across the boundary Γ. The corresponding term, the middle term, in the boundary force (1.6), may not be small compared with the first term in (1.6), since a solute-solvent interface can be rough.
We remark that our results hold true for the function B : ℝ → ℝ that is more general than that defined in (1.5). Our formula (1.4) corrects that in [8] (cf. (3.13) in [8]). If we define E = −∇ψ and
then it is easy to verify by the formula (1.4) that
The quantity T is the Maxwell stress tensor of our underlying charged molecular system [29, 38, 40].
A direct consequence of our result (1.6) is that, under the assumption ε− < ε+ which is true in general, we always have
| (1.7) |
See Corollary 4.1. This gives a quantitative interpretation, in the framework of implicit solvent, of the following phenomenon described by Debye in 1960s [16]: “Under the combined influence of electric field generated by solute charges and their polarization in the surrounding medium which is electrostatic neutral, an additional potential energy emerges and drives the surrounding molecules to the solutes.” A significant consequence of our mathematical statement (1.7) is as follows: A neutral cavity close to a charged solute (e.g., a protein) will move away from the solute due to the dielectric boundary force. This important charge effect to the hydrophobic interaction in biomolecules has been observed in the recent level-set variational implicit-solvent modeling of BphC, a two-domain protein [57].
In the proof of our main results, we use some uniform bounds of the potential ψ. Such bounds are obtained in the proof of the existence and uniqueness of the solution to the boundary-value problem of the PB equation. We also use the variational principle that the electrostatic free energy G[Γ] maximizes a corresponding functional of ψ for a fixed boundary Γ. In deriving the formula of boundary force, we do not use an abstract lemma as usually done [21,51]. Rather we give a direct and simpler derivation using the definition of shape derivatives. Notice that we assume in this work that the fixed charge density f is a compactly supported smooth function that approximates point charges. Numerically such approximation can be made by that of the Dirac delta function [50]. Our analysis does not directly extend to the case of point charges which can be in general more difficult to treat. In fact, with point charges in the PB model, a rigorous definition of the electrostatic free energy must be given with caution. This will be our future work.
The rest of the paper is organized as follows: In Section 2, we recall the boundary-value problem of the PB equation and the related electrostatic free energy. In Section 3, we define the dielectric boundary force using the notion of shape derivative. Finally, in Section 4, we derive our main formula of the dielectric boundary force and show that the force is always attractive to solutes.
2 The Poisson–Boltzmann Equation and Electrostatic Free Energy
We make the following assumptions throughout the rest of the paper:
A1. All Ω, Ω−, and Ω+ are non-empty, bounded, and open subsets of ℝ3 with and . The boundary . Both ∂ Ω and Γ are of C2. The unit normal at Γ pointing from Ω− to Ω+ is denoted by n. The unit exterior normal at the boundary of Ω is also denoted by n. See Figure 1.
A2. M ≥ 2 is an integer. All β > 0, qj ∈ ℝ and , ε− > 0, and ε+ > 0 are constants. The function εΓ ∈ L∞(Ω) is defined in (1.2). The parameters qj and satisfy the condition of charge neutrality: .
- A3. The fixed charge density f : Ω → ℝ satisfies that f ∈ L2(Ω) and supp (f) ⊂ Ω−. The boundary data in the Dirichlet boundary condition that we use is given by a function g ∈ W2,∞(Ω). Moreover, we use the notation
Here and below we use the standard notation for the Sobolev space Wk,p(Ω) which, for any fixed integer k ≥ 1 and any extended real number p : 1 ≤ p ≤ ∞, consists of all k-times (weakly) differentiable functions u : Ω → ℝ with the pth power of u or any of its derivatives of order ≤ k integral over Ω if 1 ≤ p < ∞ or with u and all its derivatives of order ≤ k essentially bounded in Ω. A4. There exists a smooth function B : ℝ → ℝ that satisfies B(s) > B(0) = 0 for all s ≠ 0, B′(0) = 0, infℝ B″ > 0 and hence B is strictly convex, B(±∞) = ∞, and B′(∞) = ∞ and B′(−∞) = −∞.
In the usual PB theory, the function B : ℝ → ℝ is given by (1.5). One easily verifies using the charge neutrality and Jensen’s inequality that for this specific form of B, all the properties listed in A4 are satisfied. A general function B : ℝ → ℝ as we assumed in A4 above covers other cases such as those including ionic size effects [5, 34, 36, 37, 54, 55].
We define G[Γ, ·] : H1(Ω) → ℝ ∪ {±∞} by
| (2.1) |
We consider the maximization of the functional G[Γ, ·] and the boundary-value problem of the Poisson–Boltzmann (PB) equation
| (2.2) |
| (2.3) |
Theorem 2.1. (1) The functional has a unique maximizer , defined by
Moreover, this maximum value is finite and
for some constant C1 > 0 depending on ε−, ε+, f, g, B and Ω but not on Γ.
(2) The maximizer ψ0 is the unique solution to the boundary-value problem of the PB equation (2.2) and (2.3).
We define the electrostatic free energy corresponding to the dielectric boundary Γ to be
| (2.4) |
Proof of Theorem 2.1. Let be such that
| (2.5) |
Standard regularity theory implies that u0 ∈ L∞(Ω) and there exists a constant Ĉ1 > 0, depending possibly on ε−, ε+, f, g, and Ω but not on Γ, such that
| (2.6) |
cf. [28] (Chapter 8). Let and . By (2.1), we have
where
Hence the maximization of G[Γ, ϕ] over all is equivalent to the minimization of I[Γ, u] over all .
By the Poincaré inequality and the fact that B : ℝ → ℝ is non-negative, there exists a constant C > 0 such that
Thus I[Γ, u] is finite. Let (k = 1, 2…) be such that
Then {uk} is bounded in H1(Ω) and hence it has a subsequence, not relabeled, that weakly converges to some . Since the embedding H1(Ω) ↪ L2(Ω) is compact, up to a further subsequence, again not relabeled, uk → u∞ a. e. in Ω. Therefore, since B : ℝ → ℝ is continuous and non-negative, Fatou’s lemma implies
Since
is convex and H1(Ω)-continuous, it is sequentially weakly lower semi-continuous. Consequently,
Thus u∞ is a minimizer of and the minimum value is clearly finite. The uniqueness of such a minimizer follows from the strict convexity of I[Γ, ·]. Therefore is the unique maximizer of G[Γ, ·] over and the maximum value is finite.
We show now the boundedness of the minimizer u∞ of I[Γ, ·] over and hence that of the maximizer ψ0 = u0 + u∞ of G[Γ, ·] over . By (2.6) and the assumption that B′(∞) = ∞ and B′(−∞) = −∞, there exists λ > 0 depending on Ĉ1 and B such that
Define uλ : Ω → ℝ by
Clearly and hence I[Γ, u∞] ≤ I[Γ, uλ]. This and the fact that |∇uλ| ≤ |∇u∞| a.e. in Ω imply
Consequently, we have by the convexity of B : ℝ → ℝ imply that
Hence the last integral is 0. This implies that the Lebesgue measure of the set {X ∈ Ω+ : |u∞(X)| > λ} is 0. Therefore |u∞| ≤ λ a.e. Ω+.
Since is a minimizer of , it is a weak solution of the corresponding Euler–Lagrange equation, which can be rewritten as
The right-hand side of this equation is bounded in Ω by a constant depending only on Ĉ1 and B but not on Γ. Thus, by the regularity theory, both the H1(Ω) and L∞(Ω) norms of u∞ and hence those of ψ0 = u∞ + u0 are bounded by a constant that only depends on Ĉ1 and B but not on Γ. This proves the desired boundedness of ψ0.
Now routine calculations lead to
Hence ψ0 is a weak solution to the boundary-value problem of PB equation (2.2) and (2.3).
If is another weak solution to the boundary-value problem of PB equation (2.2) and (2.3), then
for any . Choosing and using the convexity of B : ℝ → ℝ, we obtain ψ0 = ϕ0, proving the uniqueness.
We remark that the boundary-value problem of the PB equation (2.2) and (2.3) is equivalent to the elliptic interface problem
| (2.7) |
where 〚u〛 = u|Ω+−u|Ω− denotes the jump across Γ of a function u from Ω+ to Ω−. See [37] for a proof. In particular, this equivalence implies the continuity of εΓ∂ψ/∂n across the boundary Γ.
3 Dielectric boundary forces as shape derivatives
Let V ∈ C∞(ℝ3,ℝ3). Assume that V (x) = 0 if dist (x, Γ) > d for some d > 0 such that
| (3.1) |
Let X ∈ ℝ3 and consider the dynamical system for x = x(t):
| (3.2) |
The solution of this dynamical system defines a smooth map from ℝ3 to ℝ3 at each t ≥ 0. We shall denote this map by x = x(t, X) = Tt(X) for all t ≥ 0 and X ∈ ℝ3. Each Tt : ℝ3 → ℝ3 is a homeomorphism with both Tt and being smooth. Clearly T0 is the identity map.
Notice that for t > 0 small we have by Taylor’s expansion that
This means that the perturbation of identity defined by X ↦ X + tV (X) and the map Tt(X) = x(t, X) agree with each other up to the leading order term in t. Notice also that we only consider V = V (x) instead of a more general V = V (t, x), since the shape derivative defined via V = V (t, x) only depends on V (0, ·) by the Structure Theorem [21, 51].
For each t ≥ 0, we denote
Clearly, all Ωt, Ωt−, and Ωt+ are open sets, and . To indicate the dependence on V, we also write Γt = Γt(V). Note that each Tt perturbs Γ locally: Tt(X) = X if dist (X, Γ) > d. Since d > 0 satisfies (3.1), we have Tt(X) = X if f(X) ≠ 0 or X ∈ ∂Ω. In particular, Ωt = Tt(Ω) = Ω and Tt(∂Ω) = ∂Tt(Ω) = ∂Ω.
We recall that the electrostatic free energy G[Γ] is given by (2.4), where the functional G[Γ, ·] is given in (2.1) and ψ0 is the weak solution to (2.2) and (2.3). For t > 0, we define
| (3.3) |
where εΓt : Ω → ℝ is defined by
| (3.4) |
and χt+ is the characteristic function of Ωt+. By Theorem 2.1 there exists a unique that maximizes G[Γt, ·] over . The maximum is finite and is given by
| (3.5) |
This is the electrostatic free energy corresponding to Γt. In addition, ψt is the unique weak solution to the corresponding boundary-value problem of the Poisson–Boltzmann equation
| (3.6) |
| (3.7) |
Finally,
| (3.8) |
where C1 is the same constant in Theorem 4.1. In particular, C1 does not depend on Γt.
Definition 3.1. Let V and Γt(V) (t ≥ 0) be given as above. The shape derivative of G[Γ] in the direction of V is
if the limit exists.
In general, there exists w : Γ → ℝ such that
where n is the unit normal to Γ. We shall define
and call it the shape derivative of G[Γ].
We recall some properties of the transformation Tt(X) defined by V = V (X). These properties can be proved by direct calculations, cf. [21] (Chapter 8 and Chapter 9).
- Let X ∈ ℝ3 and t ≥ 0. Let ∇Tt(X) be the Jacobian matrix of Tt at X defined by , where is the ith component of Tt (i = 1, 2, 3). Let
Then for each X the function t → Jt(X) is in C∞ and at X
where ◦ denotes the composition of functions or maps. Clearly, ∇T0 is the identity matrix and J0 = 1. The continuity of Jt at t = 0 then implies that Jt > 0 for t > 0 small enough.(3.9) - Define A(t) : Ω → ℝ for t ≥ 0 small enough by
where a superscript T denotes the matrix transpose. We have at each point in Ω that(3.10) (3.11) - For any u ∈ L2(Ω) and t ≥ 0, u ◦ Tt ∈ L2(Ω) and . Moreover,
(3.12) - Let t ≥ 0 and u ∈ H1(Ω). Then both u ↦ u◦Tt and are one-to-one and onto maps from H1(Ω) (resp. ) to H1(Ωt) = H1(Ω) (resp. ). Moreover, for any u ∈ H1(Ω),
(3.13) - For any u ∈ H1(Ω) and t ≥ 0,
(3.14)
4 Formulas of the dielectric boundary force
Let V ∈ C∞(ℝ3,ℝ3) be such that V (X) = 0 if dist (X, Γ) > d for some d > 0 that satisfies (3.1). Let the transformations Tt (t ≥ 0) be defined by (3.2). For t > 0, the electrostatic free energy G[Γt] is given by (3.5), where the functional G[Γt, ·] is given in (3.3) and ψt is the weak solution to (3.6) and (3.7), respectively. For t = 0, the electrostatic free energy G[Γ] is given by (2.4), where the functional G[Γ, ·] is given in (2.1) and ψ0 is the weak solution to (2.2) and (2.3).
Theorem 4.1. Assume f ∈ H1(Ω). Then the shape derivative of the electrostatic free energy G[Γ] in the direction of V is given by
| (4.1) |
Proof. We divide our proof into four steps.
Step 1. Let t ≥ 0. Since each corresponds uniquely to , we have by (3.5) that
Let ϕ ∈ H1(Ω) ∩ L∞(Ω) and t ≥ 0, and denote
| (4.2) |
We prove that ∂tz(t, ϕ) exists for t ≥ 0 small and derive a formula for this derivative.
Recall that Jt(X) = det∇Tt(X). By the continuity of t ↦ Jt(X) and the fact that J0(X) = 1 at each X ∈ Ω, there exists τ > 0 such that Jt(X) > 0 for all t ∈ [0, τ] and all X ∈ Ω. Now let t ∈ [0, τ] and ϕ ∈ H1(Ω) ∩ L∞(Ω). By the definition of G[Γt, ϕ] (cf. (3.3)), the change of variable x = Tt(X), and (3.13), we obtain
| (4.3) |
where A(t) is given in (3.10). By the properties of the transformations Tt (t ≥ 0), cf. (3.9), (3.11), and (3.14), each term in the above integral is differentiable with respect to t, and
| (4.4) |
Step 2. Let t ∈ (0, τ]. Since and maximize G[Γt, ·] and G[Γ, ·], respectively, over (cf. (3.5) and (2.4)), we have
Hence
Consequently, we obtain by (4.2) that
From Step 1, there exist ξ(t), η(t) ∈ [0, t] for each t ∈ (0, τ] such that
| (4.5) |
Step 3. We prove
| (4.6) |
| (4.7) |
We only prove (4.7). The proof of (4.6) is similar and in fact simpler.
Since V ∈ C∞(ℝ3,ℝ3) is compactly supported in Ω, it is easy to see that
uniformly on Ω as t → 0 which implies η(t) → 0 as t → 0. By (3.12), we also have as t → 0 that
We shall prove at the end of this step
| (4.8) |
Notice by Theorem 2.1 that ψt is uniformly bounded in L∞(Ω) with respect to t ∈ [0, τ], cf. (3.8). Thus
as t → 0, where λ(t) : Ω → ℝ is in between ψt ◦Tt and ψ0 at each point in Ω and hence λ(t) is uniformly bounded in L∞(Ω) with respect to t ∈ [0, τ]. Applying all these convergence results, we obtain by (4.4) that
proving (4.7).
We now prove (4.8). Fix t > 0. Since maximizes G[Γt, ·] over , ψt ◦Tt maximizes over all . Since ψt is bounded in Ω by (3.8), we obtain from (4.3) and
that
Since maximizes G[Γ, ·] over ,
Subtracting one of these two equations from the other and choosing , we deduce by rearranging the terms that
Notice by the convexity of B that
Therefore, applying the Poincaré inequality to and using the Cauchy–Schwarz inequality, we obtain that
Here and below we denote by C > 0 a generic constant that can depend on ε−, ε+, Ω, B but not on t or ψ0. Note that
Consequently, the uniform boundedness of ψt in L∞(Ω) and in H1(Ω) for small t > 0 (cf. (3.8)), the uniform convergence A(t) → A(0) = I and Jt → 1 as t → 0, and the convergence f ◦ Tt → f in L2(Ω) (cf. (3.12)) imply that
This is (4.8).
Step 4. It follows from (4.5)–(4.7) that
We now show that ∂tz(0, ψ0) is the right-hand side of (4.1).
It follows from (4.4) with t = 0 and ϕ = ψ0, and (3.11) with t = 0 that
Denote by Vi and ni the ith components of V and n, respectively. Recall that the unit normal n to Γ points from Ω+ to Ω−. By integration by parts and the fact that V vanishes in a neighborhood of ∂ Ω, by (2.7) with ψ = ψ0, and using the summation convention, we continue to have
| (4.9) |
Since along Γ,
By (2.7),
| (4.10) |
We thus have
This and (4.9) imply that ∂tz(0, ψ0) is the right-hand side of (4.1).
Corollary 4.1. Under the assumption of Theorem 4.1 we have
| (4.11) |
In particular, if ε− < ε+ then the dielectric boundary force in the normal direction from Ω− to Ω+ is always negative: Fn = −δΓG[Γ] < 0 on Γ.
Proof. We have the orthogonal decomposition
Moreover, since ψ0 is continuous, its tangential derivatives along Γ are continuous. Hence
We denote this common value by (I − n ⊗ n)∇ψ0. Notice that
We thus have by (4.10) that
This and (4.1) imply (4.11). If ε− < ε+ then (4.11) implies Fn = −δΓG[Γ] < 0.
Acknowledgment
B. L. was supported by the US National Science Foundation (NSF) through the grant DMS-0811259, by the NSF Center for Theoretical Biological Physics (CTBP) through the NSF grant PHY-0822283, and by the National Institutes of Health through the grant R01GM096188. Z. Z. was supported by a fellowship from the Lu Graduate Education International Exchange Fund, Zhejiang University, China. Part of this work was completed during Li’s visit to the Department of Mathematics, Zhejiang University, in the summer of 2010, and during Zhang’s visit to the Department of Mathematics, UC San Diego, in Fall 2010 and Winter 2011. The hospitality of these departments is greatly appreciated. The authors thank Dr. Ray Luo for helpful discussions and for sharing with them his unpublished notes on the Maxwell stress tensor.
References
- 1.Andelman D. Electrostatic properties of membranes: The Poisson–Boltzmann theory. In: Lipowsky R, Sackmann E, editors. Handbook of Biological Physics. ume 1. Elsevier; 1995. pp. 603–642. [Google Scholar]
- 2.Baker NA. Improving implicit solvent simulations: a Poisson-centric view. Curr. Opin. Struct. Biol. 2005;15:137–143. doi: 10.1016/j.sbi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bashford D, Case DA. Generalized Born models of macromolecular solvation effects. Ann. Rev. Phys. Chem. 2000;51:129–152. doi: 10.1146/annurev.physchem.51.1.129. [DOI] [PubMed] [Google Scholar]
- 4.Bates PW, Chen Z, Sun YH, Wei GW, Zhao S. Geometric and potential driving formation and evolution of biomolecular surfaces. J. Math. Biology. 2009;59:193–231. doi: 10.1007/s00285-008-0226-7. [DOI] [PubMed] [Google Scholar]
- 5.Borukhov I, Andelman D, Orland H. Steric effects in electrolytes: a modified Poisson–Boltzmann equation. Phys. Rev. Lett. 1997;79:435–438. [Google Scholar]
- 6.Bucur D, Buttazzo G. Progress in Nonlinear Differential Equations and Their Applications. Boston: Birkhäuser; 2005. Variational Methods in Shape Optimization Problems. [Google Scholar]
- 7.Chapman DL. A contribution to the theory of electrocapillarity. Phil. Mag. 1913;25:475–481. [Google Scholar]
- 8.Che J, Dzubiella J, Li B, McCammon JA. Electrostatic free energy and its variations in implicit solvent models. J. Phys. Chem. B. 2008;112:3058–3069. doi: 10.1021/jp7101012. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Brooks CL, III, Khandogin J. Recent advances in implicit solvent based methods for biomolecular simulations. Curr. Opin. Struct. Biol. 2008;18:140–148. doi: 10.1016/j.sbi.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Baker NA, Wei GW. Differential geometry based solvation model I: Eulerian formulation. J. Comput. Phys. 2010;229:8231–8258. doi: 10.1016/j.jcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng HB, Cheng L-T, Li B. Yukawa-field approximation of electrostatic free energy and dielectric boundary force. Nonlinearity. 2011 doi: 10.1088/0951-7715/24/11/011. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng L-T, Dzubiella J, McCammon JA, Li B. Application of the level-set method to the implicit solvation of nonpolar molecules. J. Chem. Phys. 2007;127:084503. doi: 10.1063/1.2757169. [DOI] [PubMed] [Google Scholar]
- 13.Cheng L-T, Li B, Wang Z. Level-set minimization of potential controlled Hadwiger valuations for molecular solvation. J. Comput. Phys. 2010;229:8497–8510. doi: 10.1016/j.jcp.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng L-T, Wang Z, Setny P, Dzubiella J, Li B, McCammon JA. Interfaces and hydrophobic interactions in receptor-ligand systems: A level-set variational implicit solvent approach. J. Chem. Phys. 2009;131:144102. doi: 10.1063/1.3242274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng L-T, Xie Y, Dzubiella J, McCammon JA, Che J, Li B. Coupling the level-set method with molecular mechanics for variational implicit solvation of nonpolar molecules. J. Chem. Theory Comput. 2009;5:257–266. doi: 10.1021/ct800297d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu B. Interscience. John Wiley & Sons; 1967. Molecular Forces, Based on the Lecture of Peter J. W. Debye. [Google Scholar]
- 17.Connolly ML. Analytical molecular surface calculation. J. Appl. Cryst. 1983;16:548–558. [Google Scholar]
- 18.Connolly ML. The molecular surface package. J. Mol. Graphics. 1992;11:139–141. doi: 10.1016/0263-7855(93)87010-3. [DOI] [PubMed] [Google Scholar]
- 19.Cramer CJ, Truhlar DG. Implicit solvation models: Equilibria, structure, spectra, and dynamics. Chem. Rev. 1999;99:2161–2200. doi: 10.1021/cr960149m. [DOI] [PubMed] [Google Scholar]
- 20.Davis ME, McCammon JA. Electrostatics in biomolecular structure and dynamics. Chem. Rev. 1990;90:509–521. [Google Scholar]
- 21.Delfour MC, Zolésio J-P. Shapes and Geometries: Analysis, Differential Calculus, and Optimization. SIAM. 1987 [Google Scholar]
- 22.Dzubiella J, Swanson JMJ, McCammon JA. Coupling hydrophobicity, dispersion, and electrostatics in continuum solvent models. Phys. Rev. Lett. 2006;96:087802. doi: 10.1103/PhysRevLett.96.087802. [DOI] [PubMed] [Google Scholar]
- 23.Dzubiella J, Swanson JMJ, McCammon JA. Coupling nonpolar and polar solvation free energies in implicit solvent models. J. Chem. Phys. 2006;124:084905. doi: 10.1063/1.2171192. [DOI] [PubMed] [Google Scholar]
- 24.Feig M, Brooks CL., III Recent advances in the development and applications of implicit solvent models in biomolecule simulations. Curr. Opin. Struct. Biol. 2004;14:217–224. doi: 10.1016/j.sbi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Fixman F. The Poisson–Boltzmann equation and its application to polyelecrolytes. J. Chem. Phys. 1979;70:4995–5005. [Google Scholar]
- 26.Fogolari F, Briggs JM. On the variational approach to Poisson–Boltzmann free energies. Chem. Phys. Lett. 1997;281:135–139. [Google Scholar]
- 27.Fogolari F, Brigo A, Molinari H. The Poisson–Boltzmann equation for biomolecular electrostatics: a tool for structural biology. J. Mol. Recognit. 2002;15:377–392. doi: 10.1002/jmr.577. [DOI] [PubMed] [Google Scholar]
- 28.Gilbarg D, Trudinger NS. Elliptic Partial Differential Equations of Second Order. 2nd edition. Springer-Verlag; 1998. [Google Scholar]
- 29.Gilson MK, Davis ME, Luty BA, McCammon JA. Computation of electrostatic forces on solvated molecules using the Poisson–Boltzmann equation. J. Phys. Chem. 1993;97:3591–3600. doi: 10.1063/1.1924448. [DOI] [PubMed] [Google Scholar]
- 30.Gouy M. Sur la constitution de la charge électrique a la surface d’un électrolyte. J. de Phys. 1910;9:457–468. [Google Scholar]
- 31.Grochowski P, Trylska J. Continuum molecular electrostatics, salt effects and counterion binding—A review of the Poisson–Boltzmann model and its modifications. Biopolymers. 2008;89:93–113. doi: 10.1002/bip.20877. [DOI] [PubMed] [Google Scholar]
- 32.Im W, Beglov D, Roux B. Continuum Solvation Model: computation of electrostatic forces from numerical solutions to the Poisson–Boltzmann equation. Comput. Phys. Commun. 1998;111:59–75. [Google Scholar]
- 33.Kawohl B, Pironneau O, Tartar L, Zolésio J-P. Optimal Shape Design, volume 1740 of Lecture Notes in Mathematics. Springer; 2000. [Google Scholar]
- 34.Kralj-Iglic V, Iglic A. A simple statistical mechanical approach to the free energy of the electric double layer including the excluded volume effect. J. Phys. II (France) 1996;6:477–491. [Google Scholar]
- 35.Lee B, Richards FM. The interpretation of protein structures: Estimation of static accessibility. J. Mol. Biol. 1971;55:379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- 36.Li B. Continuum electrostatics for ionic solutions with nonuniform ionic sizes. Non-linearity. 2009;22:811–833. [Google Scholar]
- 37.Li B. Minimization of electrostatic free energy and the Poisson–Boltzmann equation for molecular solvation with implicit solvent. SIAM J. Math. Anal. 2009;40:2536–2566. [Google Scholar]
- 38.Lu B, Cheng X, Hou T, McCammon JA. Calculation of the Maxwell stress tensor and the Poisson–Boltzmann force on a solvated molecular surface using hypersingular boundary integrals. J. Chem. Phys. 2005;123:084904. doi: 10.1063/1.2008252. [DOI] [PubMed] [Google Scholar]
- 39.Lu BZ, Zhou YC, Holst MJ, McCammon JA. Recent progress in numerical methods for the Poisson–Boltzmann equation in biophysical applications. Commun. Comput. Phys. 2008;3:973–1009. [Google Scholar]
- 40.Luo R. Personal communications. 2010 [Google Scholar]
- 41.Osher S, Fedkiw R. Level Set Methods and Dynamic Implicit Surfaces. New York: Springer; 2002. [Google Scholar]
- 42.Osher S, Sethian JA. Fronts propagating with curvature-dependent speed: Algorithms based on Hamilton-Jacobi formulations. J. Comput. Phys. 1988;79:12–49. [Google Scholar]
- 43.Richards FM. Areas, volumes, packing, and protein structure. Annu. Rev. Biophys. Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- 44.Richmond TJ. Solvent accessible surface area and excluded volume in proteins. Analytical equations for overlapping spheres and implications for the hydrophobic effect. J. Mol. Biol. 1984;178:63–89. doi: 10.1016/0022-2836(84)90231-6. [DOI] [PubMed] [Google Scholar]
- 45.Roux B, Simonson T. Implicit solvent models. Biophys. Chem. 1999;78:1–20. doi: 10.1016/s0301-4622(98)00226-9. [DOI] [PubMed] [Google Scholar]
- 46.Sethian JA. Level Set Methods and Fast Marching Methods: Evolving Interfaces in Geometry, Fluid Mechanics, Computer Vision, and Materials Science. 2nd edition. Cambridge University Press; 1999. [Google Scholar]
- 47.Setny P, Wang Z, Cheng L-T, Li B, McCammon JA, Dzubliella J. Dewetting-controlled binding of ligands to hydrophobic pockets. Phys. Rev. Lett. 2009;103:187801. doi: 10.1103/PhysRevLett.103.187801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharp KA, Honig B. Calculating total electrostatic energies with the nonlinear Poisson–Boltzmann equation. J. Phys. Chem. 1990;94:7684–7692. [Google Scholar]
- 49.Sharp KA, Honig B. Electrostatic interactions in macromolecules: Theory and applications. Annu. Rev. Biophys. Biophys. Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]
- 50.Smereka P. The numerical approximation of a delta function with application to level set methods. J. Comput. Phys. 2006;211:77–90. [Google Scholar]
- 51.Sokolowski J, Zolésio J-P. Springer Series in Computational Mathematics. Springer; 1992. Introduction to Shape Optimization: Shape Sensitivity Analysis. [Google Scholar]
- 52.Still WC, Tempczyk A, Hawley RC, Hendrickson T. Semianalytical treatment of solvation for molecular mechanics and dynamics. J. Amer. Chem. Soc. 1990;112:6127–6129. [Google Scholar]
- 53.Tomasi J, Persico M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994;94:2027–2094. [Google Scholar]
- 54.Treset G. Generalized Poisson–Fermi formalism for investigating size correlation effects with multiple ions. Phys. Rev. E. 2008;78:061506. doi: 10.1103/PhysRevE.78.061506. [DOI] [PubMed] [Google Scholar]
- 55.Trizac E, Raimbault J-L. Long-range electrostatic interactions between like-charged colloids: Steric and confinement effects. Phys. Rev. E. 1999;60:6530–6533. doi: 10.1103/physreve.60.6530. [DOI] [PubMed] [Google Scholar]
- 56.Wagoner JA, Baker NA. Assessing implicit models for nonpolar mean salvation forces: The importance of dispersion and volume changes. Proc. Natl. Acad. Sci., USA. 2006;103:8331–8336. doi: 10.1073/pnas.0600118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Che J, Cheng L-T, Dzubiella J, Li B, McCammon JA. Level-set variational implicit solvation with the Coulomb-field approximation. J. Chem. Theory Comput. 2011 doi: 10.1021/ct200647j. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou S, Wang Z, Li B. Mean-field description of ionic size effects with non-uniform ionic sizes: A numerical approach. Phys. Rev. E. 2011;84:021901. doi: 10.1103/PhysRevE.84.021901. [DOI] [PMC free article] [PubMed] [Google Scholar]