The main causes of visual impairment and sight loss in developed countries are retinal diseases. Ocular diseases are generally categorized on basis of the ocular region, that is, anterior and posterior segment. Common posterior eye diseases which usually cause visual impairment include diabetic retinopathy, age-related macular degeneration, uveitis, macular edema secondary to retinal vein occlusion, retinitis, retinitis pigmentosa, and cytomegalovirus. Posterior diseases including diabetic retinopathy, glaucoma, and retinitis pigmentosa causes irreversible blindness. A total of 1.7 million Americans (age >60 years) suffer from age-related macular degeneration and this number will grow to almost 3 million by 2020. Treating such disease requires availability of therapeutic concentration in posterior segment. Recent researches in drug delivery area promises to improve ocular retention and contact time of drug via in situ gels, suspension, collagen matrix gels, poly(lactic/glycolic) acid (PLGA) film, microparticles, nanoparticles, episcleral implants, polyorthoesters gels, implants, iontophoresis, liposomes, micelles, microcannulation, microneedle, osmotic pumps, and vectosomes. There are four main routes used for posterior delivery, that is, topical, systemic, intravitreal, and periocular. Topical delivery to eye region is the preferred method of ocular drug delivery, but it is not capable enough to provide effective therapeutic concentration in posterior segment of eye due to ocular physiological barriers. In systemic delivery, the blood-retinal barrier hinders the availability of drugs to the posterior segment. Frequent delivery of drug from topical/systemic route to meet the therapeutic level may cause drug-related toxicities and side effects. In intravitreal route; formulation (i.e., solution, suspensions, nanosuspensions, and implants) are injected directly in to the posterior section of eye. It helps in attaining appropriate therapeutic level in posterior section, but repeated injection may lead to retinal detachment. To avoid such situation, newer biodegradable implants have been developed to provide prolonged delivery and to prohibit frequent intravitreal injections. The main barriers to deliver drug from vitreous to retina are the inner limiting membrane and the blood retinal barrier. The periocular route of drug delivery was considered as efficient in terms of less pain and better bioavailability. It minimizes the risk of intravitreal injection by enabling the deposition of molecules against the external surface of the sclera (retrobulbar, peribulbar, sub-Tenon, and subconjunctival routes). There are many research and clinical trials which are going on developing new drug delivery systems for posterior segment. A recent discovery in gene and protein therapy including upcoming new drugs requires advance delivery systems with proper bioavailability and fewer side effects. Here an update of current clinical trials [Table 1] and marketed products [Table 2] are provided. We will try to update professionals with more updates in this era from time to time.
Table 1.
List of some current clinical trials for posterior ocular delivery
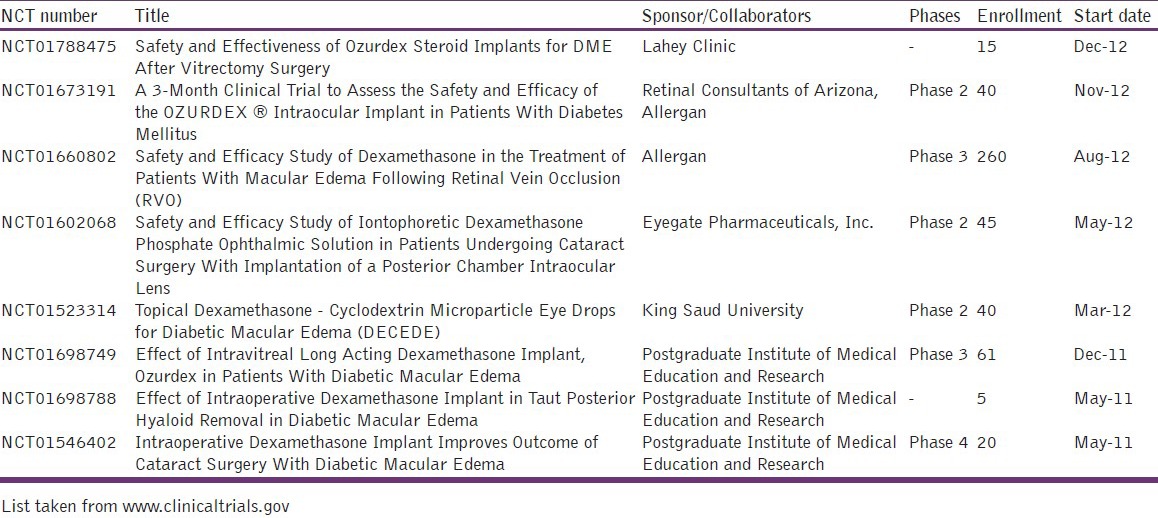
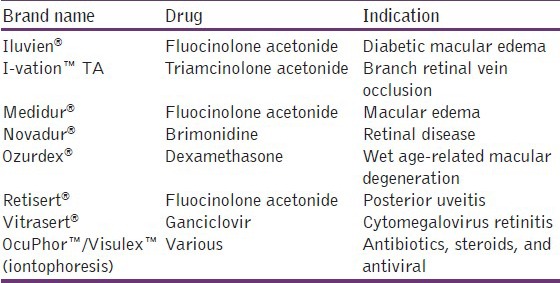
Table 2.
List of available intravitreal implants and posterior delivery systems