Abstract
Objective:
The objective of the present review was to determine the effectiveness of maxillary sinus floor augmentation without bone grafts using lateral window technique.
Materials and Methods:
PubMed and Cochrane databases were searched for relevant articles. We also included articles by hand search until June 2012. The analysis included both human and animal studies which satisfied the following criteria: Minimum of 6 months follow-up, no use of bone grafts, and lateral window approach to the sinus.
Results:
We included 22 articles in the review. A descriptive analysis of the constructed evidence tables indicated that there is evidence of predictable a mount of bone formation in the maxillary sinus augmentation without the use of bone grafts.
Conclusion:
Within the limits of the articles and data available, maxillary sinus augmentation without bone graft might be considered effective inpredictable bone formation.
KEY WORDS: Augmentation, dental implant, lateral window, maxillary sinus
Endosseous implants have been frequently used for prosthetic reconstruction in the partially edentulous patient. Sufficient volume and density of the alveolar bone for implant integration and load bearing have always been considered as prerequisites for good results. Bone resorption following the extraction of posterior maxillary teeth sometimes results in severe loss of bone in vertical and/or horizontal dimensions, which may preclude the use of dental implants.
Various grafting procedures to re-establish an adequate bone volume to enable the placement of endosseous implants in the posterior maxilla have been described. The most commonly used technique is augmentation of the maxillary sinus floor, a technique introduced by Tatum[1] and modified by Boyne and James[2] and by Wood and Moore.[3] With this technique, the maxillary sinus is accessed by creating a bone window in the lateral sinus wall with a small round bur, with the aim of leaving the sinus membrane intact. The sinus membrane is then carefully elevated and the bone window is rotated medially.
Several studies have evaluated maxillary sinus augmentation using autogenous bone grafts from the iliac crest, mandibular chin,[4] mandibular ramus[5] or calvarium, as well as bone substitutes alone[6] or in combination with autogenous bone via lateral window technique or indirect technique by Summer's.[7]
Autogenous bone (AB) graft is very popular for sinus floor augmentation because it possesses osteogenic, osteoinductive, and osteoconductive properties.[8] Unfortunately, the harvest of AB required donor site surgery and potentially increases patient morbidity.[9,10,11,12]
The maxillary sinus augmentation being an elective procedure, priority should be to minimize patient morbidity. Earlier studies indicate that the mere lifting of the sinus membrane, creation of a void space and blood clot formation might result in new bone owing to the principles of guided bone regeneration.[13,14]
Therefore, the aim of the present review was to determine the effectiveness of maxillary sinus floor augmentation without bone grafts using lateral window technique. The primary and secondary variables of interest analyzed in the study are given in Table 1. Structured question: What is the effectiveness of maxillary sinus floor augmentation without bone grafts using lateral window technique?
Table 1.
Variables of interest

Materials and Methods
A systematic search strategy was used. In the initial phase of the review, a computerized literature search for human and animal studies was performed in the PubMed and Cochrane databases, till June 2012. In addition, a hand search was carried out in the following journals:
International Journal of Oral and Maxillofacial Surgery, British Journal of Oral and Maxillofacial Surgery, Journal of Oral and Maxillofacial Surgery, Journal of Cranio-Maxillofacial Surgery, Clinical Implant Dentistry and Related Research, Clinical Oral Implants Research, Journal of Oral Implantology, The International Journal of Oral and Maxillofacial Implants, International Journal of Periodontics and Restorative Dentistry, Journal of Clinical Periodontology, Journal of Periodontology, Periodontology 2000, International Journal of Prosthodontics, Journal of Prosthetic Dentistry, Oral Surgery Oral Medicine Oral Pathology. Additional publications were identified from the reference lists of the retrieved articles.
Further, we checked the Cochrane Controlled Trials Register and The Cochrane Health Group Specialized Register for publications on sinus floor augmentation. Articles were searched from 1997 (the first known study of sinus lift surgery without the use of grafts[13]) to June 2012.
Search terms
Keywords were ‘sinus Augmentation’ or ‘Maxillary Sinus Augmentation’ or ‘Maxillary Sinus Floor Augmentation’ and ‘Blood’ or ‘Venous Blood’ or ‘Peripheral Venous Blood’ or ‘Gelatin Sponge’ or ‘Gel Sponge’ or ‘Resorbable Sponge’ or ‘Platelet Rich Plasma’ or ‘Platelet Rich Fibrin’ or ‘Fibrin Rich Block’ or ‘Platelet Concentrates’ or ‘space Maintaining Device’ or ‘Resorbable Device’.
Inclusion criteria
The inclusion criteria for the articles to be included in this present systematic review were as follows:
Articles that showed evidence of atrophy of the maxilla in the premolar/molar area that required a sinus lift before implantation in case of human studies
Case series in which bone graft or bone substitutes were not used in the maxillary sinus augmentation
Clinical trials in which bone graft or bone substitutes were not used in any one of the groups
Articles having information on either animals/humans in this area
Articles with either histological or radiographic evaluation for maxillary sinus floor augmentation.
Exclusion criteria
Articles having studies not done with lateral window approach
Articles other than in English language
Articles having studies done with less than 6 months follow-up.
Selection of studies
Titles derived from this broad search were independently screened by two authors based on the inclusion criteria. Disagreements were resolved by discussion. Following this, abstracts of all titles agreed on by both authors were obtained and screened for meeting the inclusion criteria. If no abstract was available in the database, the abstract of the printed article was used. The selected articles were then obtained in full text. If the title and abstract did not provide sufficient information regarding the inclusion criteria, the full report was obtained as well. Again, disagreements were resolved by discussion. Finally, the selection based on inclusion and exclusion criteria was made for the full-text articles. For this purpose, Material and Methods and Results sections of these studies were screened. This step was again carried out independently by both the authors. Disagreements were resolved by discussion [Figure 1].
Figure 1.
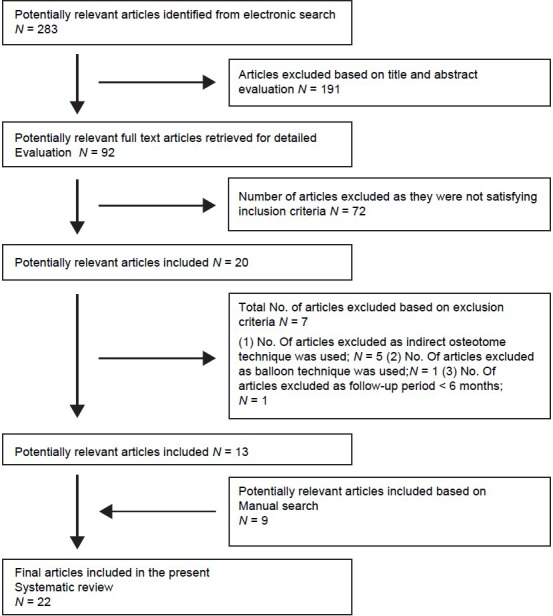
Selection of article
Reasons for exclusion of articles from the present review have been tabulated in Table 2.
Table 2.
Reason for exclusion
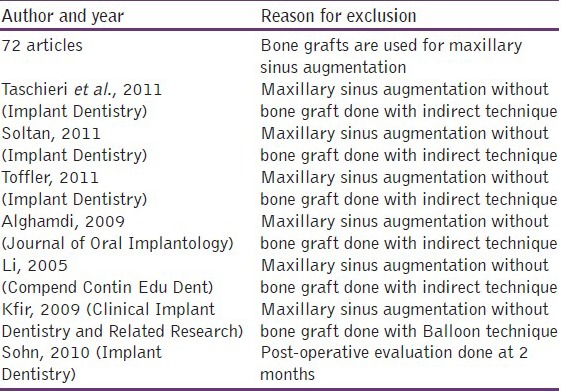
Data extraction
Data was extracted using data extraction tables from the selected papers based on author(s), year of publication, study design, human study or animal study, sample size, inclusion and exclusion criteria, follow-up period, surgical technique, height of residual alveolar crest, material used for sinus floor augmentation, bone gain, method of examination of bone gain, sinus mucosa perforation, patient morbidity and disease transmission.
The study design and basic data are presented in Tables 3 and 4, while the summary of surgical procedures and variables of interest evaluated are given in Tables 5 and 6.
Table 3.
General information of selected (non-comparative) articles
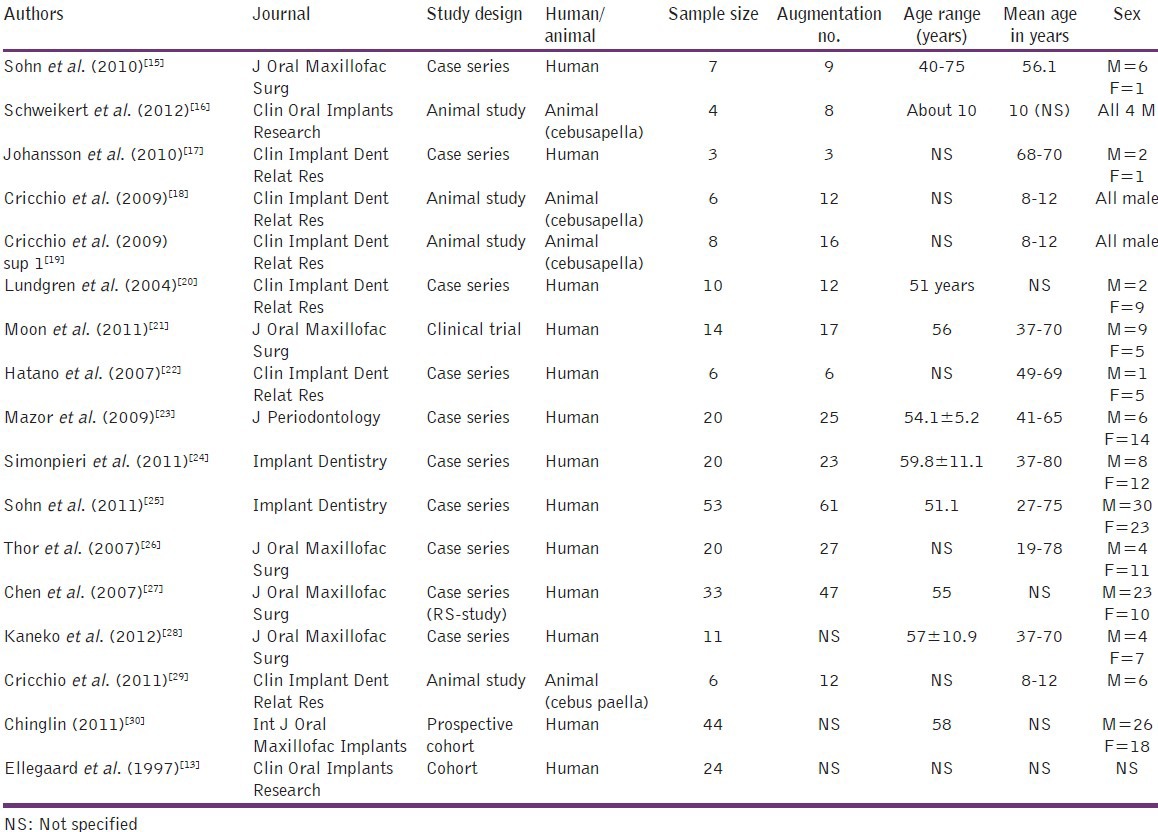
Table 4.
General information of selected (comparative) articles
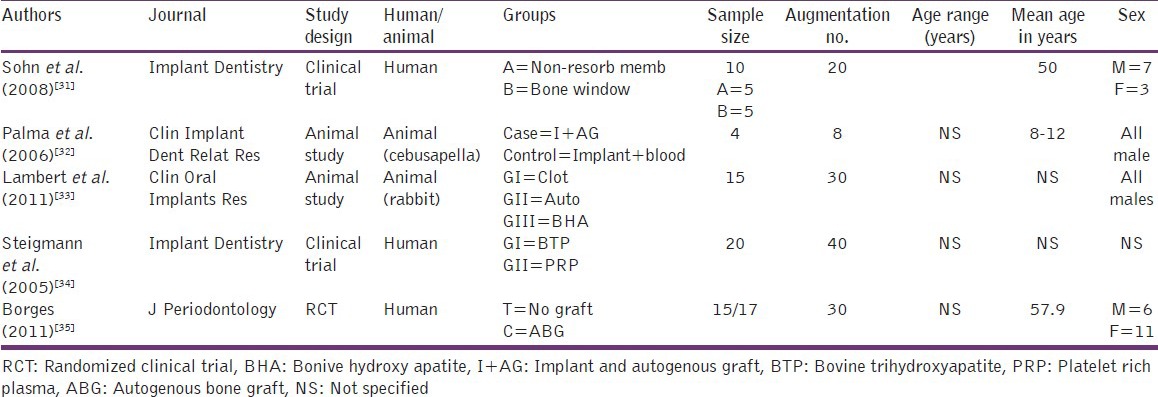
Table 5.
Summary of surgical procedure and type of grafting material for selected non-comparative studies
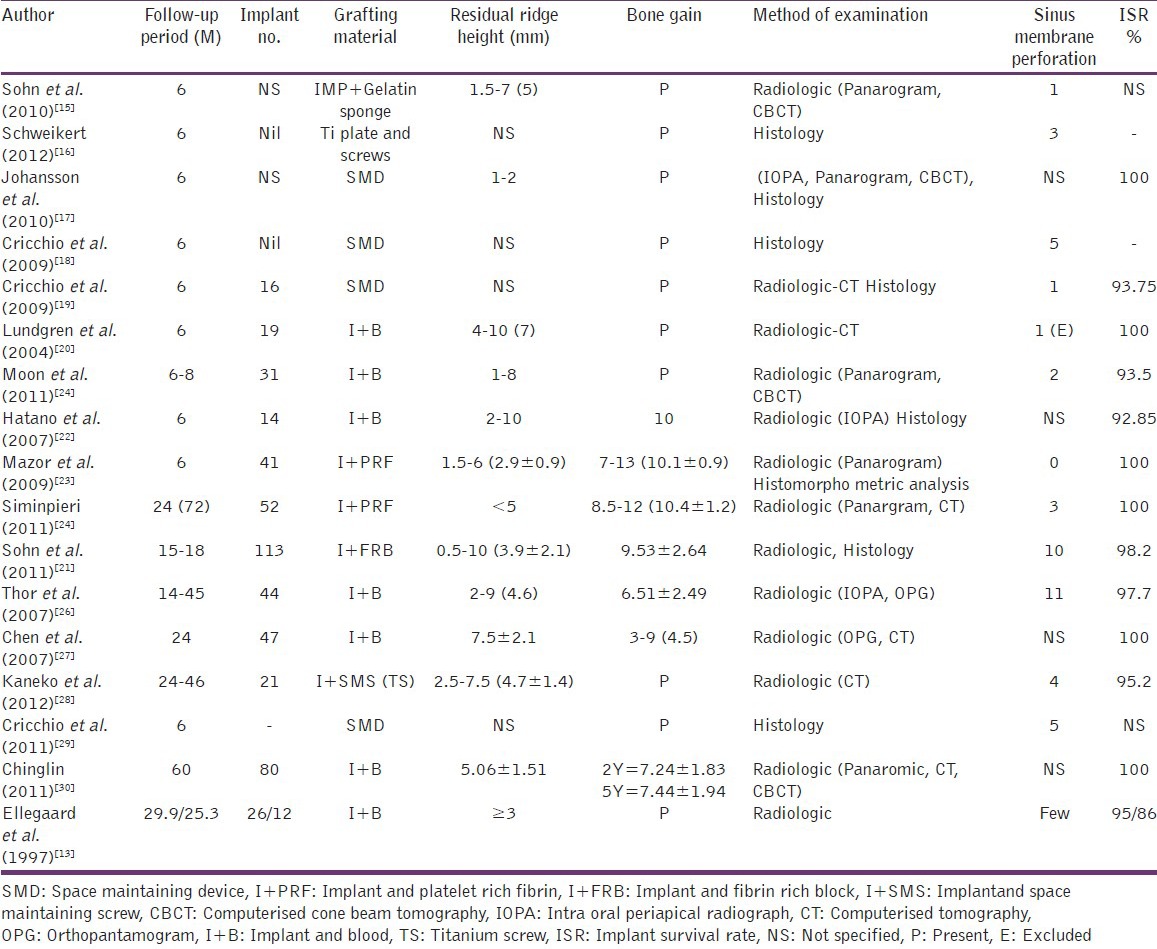
Table 6.
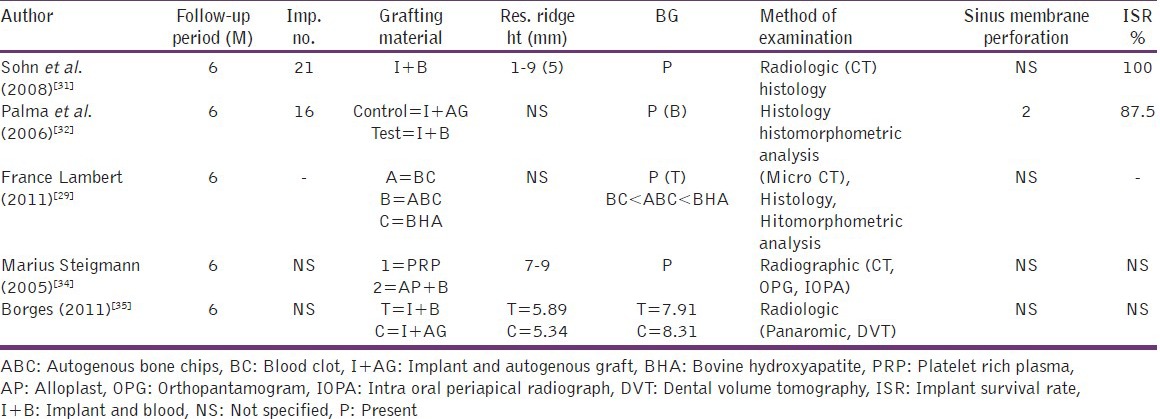
Summary of surgical procedure and type of grafting material for selected comparative studies
Results
Among the 22 articles included in the review, most of them revealed the presence of bone formation in the maxillary sinus augmentation without bone grafts. Following is a count of the number of articles based on the grafting material used: Blood - 13, platelet rich fibrin - 2, space maintaining device - 4, platelet rich plasma - 1, fibrin rich block - 1, and gel sponge - 1. Out of the 22 articles included, 16 were human studies and 6 were animal studies.
Though most of the articles reviewed showed evidence of bone formation, variation in levels of bone formation was noticed among different groups as well as among different studies. No standaradization was done regarding study design, methodology or method of evaluation.
Discussion
The primary objective of the present review was to assess the effectiveness of bone gain in the maxillary sinus floor augmentation without any bone grafts using lateral window technique. Sinus floor augmentation is one of the most reliable procedures in preprosthetic surgery.
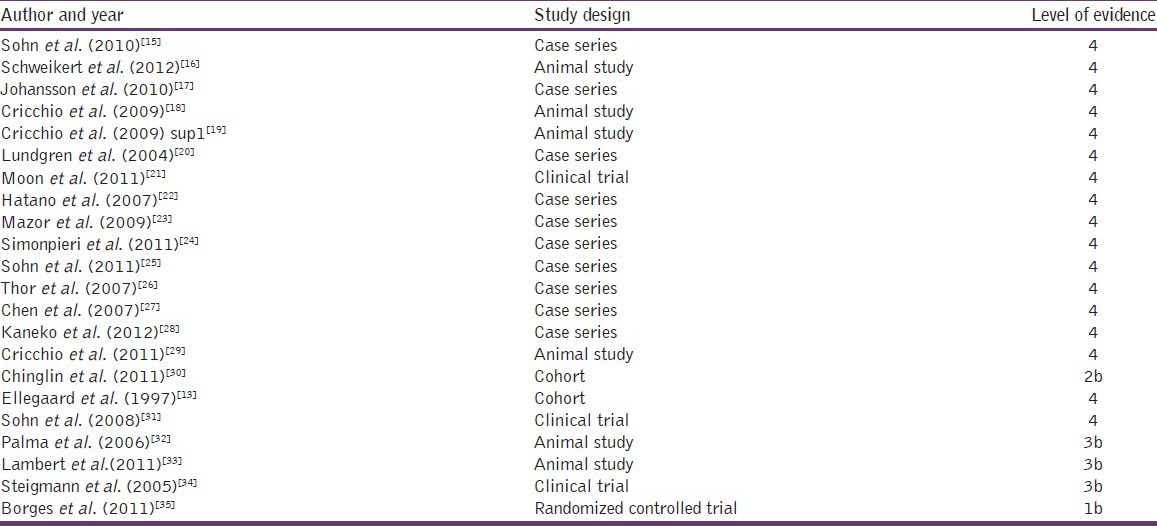
The levels of evidences of these selected articles are presented in Table 7.
Table 7.
Level of evidence of selected articles from the centre for evidence-based medicine, Oxford
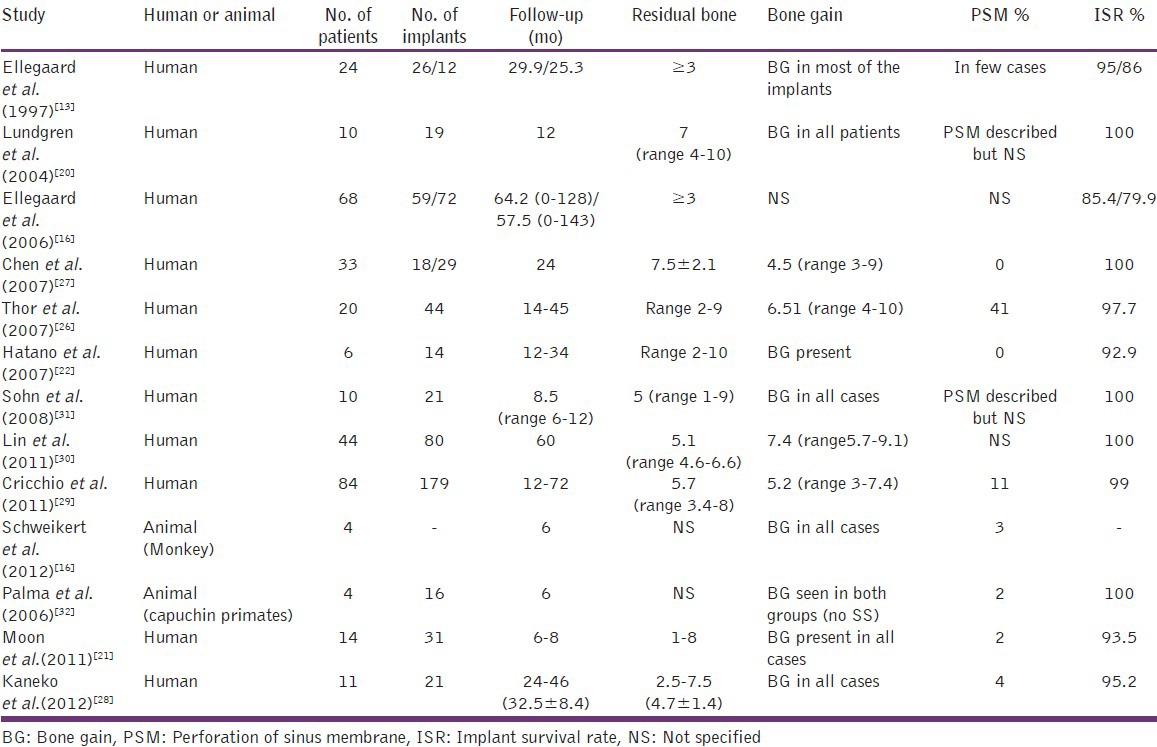
In the present review, 13 studies were included to evaluate the effect of bone gain in the maxillary sinus floor augmentation with implant and blood alone using lateral window technique. Among these, 11 were human studies and 2 were animal studies.
In an animal study conducted by Schweikert et al.[16] and Palma et al.[32] using blood as sole grafting material, presence of bone gain was shown in all cases.
Long-term human studies using blood as grafting material in sinus augmentation and with a minimum of 1 year follow-up also revealed the presence of bone gain in most of the cases. In the short-term human studies, though there was evidence of new bone formation, it required long-term follow-up to arrive at conclusions.
Maxillary sinus augmentation with blood showed no statistical difference in the amount of bone gain when compared to the autogenous grafts.[20] Thus this technique is considered to be cost-effective, less time-consuming and associated with lower morbidity since no bone harvesting is needed summary of published studies (1997-2011) of sinus lift with blood only is presented in Table 8.
Table 8.
Summary of studies of sinus lift with blood only
The maxillary sinus floor augmentation with platelet rich fibrin, platelet rich plasma, fibrin rich block, gel sponge, and space maintaining device also showed evidence of bone formation with varying levels of bone formation among different groups.
All the 22 articles included in the review showed predictable amount of bone formation in the maxillary sinus augmentation.
Septae and sinus membrane perforations
The membrane elevation technique without the use of grafting materials, as described in this paper, is not initially an easy technique as it may require adaptation from the more common technique using grafts where the bony window is prepared with a burr.[25,36] However, with results comparable to the routine sinus lift techniques, the membrane elevation technique displayed possible advantages. However, the problems encountered such as sinus septae and membrane perforations are factors that need to be taken in to consideration.
The maxillary sinus is often divided into compartments by complete or incomplete bony septae. These must be taken into account during the surgical planning of the procedure and are best visualized by preoperative computed tomography evaluation.[37] The premolar region is also the location of most septae in atrophic edentulous ridges and it has been shown that septae in dentate maxillae are of greater heights than in edentulous patients.[38] While planning the surgery, these septae are usually considered to be a problem during the procedure. However, they might prove to be helpful in achieving satisfactory primary stability for the implant when placed in these septae of the basal bone of the maxillary atrophied crest. The use of a wide bony window for access to the sinus mucosa is also important.
Sinus membrane perforation has been the most frequently reported complication. The anatomical features like septae and a fragile mucosa may develop into lacerations of the sinus mucosa during the dissection, which requires careful and delicate surgical approach. It is evident from the data available in existing systematic reviews that there are reported cases of the sinus membrane perforation and it might be one of the reasons affecting the success rate of the procedure. Piezosurgery may be advantageous when performing this stage in the procedure. Suturing of the mucosa to the superior part of the bony window after extensive dissection has also been recommended, but not yet evaluated by randomized controlled trials.[20,26]
Implant survival rate
The articles included in this review show evidence of good survival rate when implant is placed either along with or after maxillary sinus augmentation without bone grafts. Cricchio et al.[18] in his study has shown that augmenting maxillary sinus using space maintaining device without bone grafts was associated with evidence of more bone formation when placed simultaneously along with maxillary sinus augmentation.
The implant survival rate depends on various conditions such as quality of the bone, stage of implant installation, implant system, implant surface, implant length, implant diameter, prosthetic considerations, systemic health of the patient, and oral hygiene status.
Limitations
The present systematic review limited the studies included to be of English language only. This might have limited the inclusion of studies assessing similar objectives into this review. This systematic review also considered only the published data for result interpretation. The unpublished and the raw data of few studies have not been included for interpretation. Due to lack of studies available, the standardization of methodology was not considered, which might have led to difficulty in comparison of bone gain among different group of non-bone grafting materials. Further, only articles that had minimum 6 months follow-up were included, for better observation of bone formation, randomized controlled trials with long-term follow-up would be required.
Future scope
Variations have been observed in the study design adopted, methodology used, type of grafting material used, follow-up period and method of evaluation of bone gain among the different articles.
Selection and inclusion of randomized controlled trials with long-term follow-up and studies with standardization in study design, methodology, follow-up period and method of evaluation of bone gain are required to obtain a clear interpretation of the effectiveness of maxillary sinus augmentation without bone grafts.
Conclusion
Within the limits of the articles and data available, maxillary sinus augmentation without bone graft might be considered effective in predictable bone formation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tatum H., Jr Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986;30:207–29. [PubMed] [Google Scholar]
- 2.Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38:613–6. [PubMed] [Google Scholar]
- 3.Wood RM, Moore DL. Grafting of the maxillary sinus with intraorally harvested autogenous bone prior to implant placement. Int J Oral Maxillofac Implants. 1988;3:209–14. [PubMed] [Google Scholar]
- 4.Lundgren S, Moy P, Johansson C, Nilsson H. Augmentation of the maxillary sinus floor with particulated mandible: A histologic and histomorphometric study. Int J Oral Maxillofac Implants. 1996;11:760–6. [PubMed] [Google Scholar]
- 5.Tulasne JF. Commentary on maxillary pre-implant rehabilitation. A study of 55 cases using autologous bone graft augmentation] Rev Stomatol Chir Maxillofac. 1999;100:265–6. [PubMed] [Google Scholar]
- 6.Hallman M, Sennerby L, Lundgren S. A clinical and histologic evaluation of implant integration in the posterior maxilla after sinus floor augmentation with autogenous bone, bovine hydroxyapatite, or a 20:80 mixture. Int J Oral Maxillofac Implants. 2002;17:635–43. [PubMed] [Google Scholar]
- 7.Pjetursson BE, Tan WC, Zwahlen M, Lang NP. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J Clin Periodontol. 2008;35:216–40. doi: 10.1111/j.1600-051X.2008.01272.x. [DOI] [PubMed] [Google Scholar]
- 8.Galindo-Moreno P, Avila G, Fernández-Barbero JE, Mesa F, O’Valle-Ravassa F, Wang HL. Clinical and histologic comparison of two different composite grafts for sinus augmentation: A pilot clinical trial. Clin Oral Implants Res. 2008;19:755–9. doi: 10.1111/j.1600-0501.2008.01536.x. [DOI] [PubMed] [Google Scholar]
- 9.Nkenke E, Radespiel-Tröger M, Wiltfang J, Schultze-Mosgau S, Winkler G, Neukam FW. Morbidity of harvesting of retromolar bone grafts: A prospective study. Clin Oral Implants Res. 2002;13:514–21. doi: 10.1034/j.1600-0501.2002.130511.x. [DOI] [PubMed] [Google Scholar]
- 10.Nkenke E, Schultze-Mosgau S, Radespiel-Tröger M, Kloss F, Neukam FW. Morbidity of harvesting of chin grafts: A prospective study. Clin Oral Implants Res. 2001;12:495–502. doi: 10.1034/j.1600-0501.2001.120510.x. [DOI] [PubMed] [Google Scholar]
- 11.Nkenke E, Weisbach V, Winckler E, Kessler P, Schultze-Mosgau S, Wiltfang J, et al. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int J Oral Maxillofac Surg. 2004;33:157–63. doi: 10.1054/ijom.2003.0465. [DOI] [PubMed] [Google Scholar]
- 12.Kübler NR, Will C, Depprich R, Betz T, Reinhart E, Bill JS, et al. Comparative studies of sinus floor elevation with autologous or allogeneic bone tissue. Mund Kiefer Gesichtschir. 1999;3:S53–60. doi: 10.1007/PL00014517. [DOI] [PubMed] [Google Scholar]
- 13.Ellegaard B, Kølsen-Petersen J, Baelum V. Implant therapy involving maxillary sinus lift in periodontally compromised patients. Clin Oral Implants Res. 1997;8:305–15. doi: 10.1034/j.1600-0501.1997.080409.x. [DOI] [PubMed] [Google Scholar]
- 14.Lundgren S, Andersson S, Sennerby L. Spontaneous bone formation in the maxillary sinus after removal of a cyst: Coincidence or consequence? Clin Implant Dent Relat Res. 2003;5:78–81. doi: 10.1111/j.1708-8208.2003.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 15.Sohn DS, Moon JW, Moon KN, Cho SC, Kang PS. New bone formation in the maxillary sinus using only absorbable gelatin sponge. J Oral Maxillofac Surg. 2010;68:1327–33. doi: 10.1016/j.joms.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Schweikert M, Botticelli D, de Oliveira JA, Scala A, Salata LA, Lang NP. Use of a titanium device in lateral sinus floor elevation: An experimental study in monkeys. Clin Oral Implants Res. 2012;23:100–5. doi: 10.1111/j.1600-0501.2011.02200.x. [DOI] [PubMed] [Google Scholar]
- 17.Johansson LA, Isaksson S, Adolfsson E, Lindh C, Sennerby L. Bone regeneration using a hollow hydroxyapatite space-maintaining device for maxillary sinus floor augmentation-A clinical pilot study. Clin Implant Dent Relat Res. 2010 doi: 10.1111/j.1708-8208.2010.00293.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Cricchio G, Palma VC, Faria PE, de Olivera JA, Lundgren S, Sennerby L, et al. Histological outcomes on the development of new space-making devices for maxillary sinus floor augmentation. Clin Implant Dent Relat Res. 2011;13:224–30. doi: 10.1111/j.1708-8208.2009.00208.x. [DOI] [PubMed] [Google Scholar]
- 19.Cricchio G, Palma VC, Faria PE, de Oliveira JA, Lundgren S, Sennerby L, et al. Histological findings following the use of a space-making device for bone reformation and implant integration in the maxillary sinus of primates. Clin Implant Dent Relat Res. 2009;11:e14–22. doi: 10.1111/j.1708-8208.2009.00158.x. [DOI] [PubMed] [Google Scholar]
- 20.Lundgren S, Andersson S, Gualini F, Sennerby L. Bone reformation with sinus membrane elevation: A new surgical technique for maxillary sinus floor augmentation. Clin Implant Dent Relat Res. 2004;6:165–73. [PubMed] [Google Scholar]
- 21.Moon JW, Sohn DS, Heo JU, Shin HI, Jung JK. New bone formation in the maxillary sinus using peripheral venous blood alone. J Oral Maxillofac Surg. 2011;69:2357–67. doi: 10.1016/j.joms.2011.02.092. [DOI] [PubMed] [Google Scholar]
- 22.Hatano N, Sennerby L, Lundgren S. Maxillary sinus augmentation using sinus membrane elevation and peripheral venous blood for implant-supported rehabilitation of the atrophic posterior maxilla: Case series. Clin Implant Dent Relat Res. 2007;9:150–5. doi: 10.1111/j.1708-8208.2007.00043.x. [DOI] [PubMed] [Google Scholar]
- 23.Mazor Z, Horowitz RA, Del Corso M, Prasad HS, Rohrer MD, Dohan Ehrenfest DM. Sinus floor augmentation with simultaneous implant placement using Choukroun's platelet-rich fibrin as the sole grafting material: A radiologic and histologic study at 6 months. J Periodontol. 2009;80:2056–64. doi: 10.1902/jop.2009.090252. [DOI] [PubMed] [Google Scholar]
- 24.Simonpieri A, Choukroun J, Del Corso M, Sammartino G, Dohan Ehrenfest DM. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: A six-year experience. Implant Dent. 2011;20:2–12. doi: 10.1097/ID.0b013e3181faa8af. [DOI] [PubMed] [Google Scholar]
- 25.Sohn DS, Heo JU, Kwak DH, Kim DE, Kim JM, Moon JW, et al. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant Dent. 2011;20:389–95. doi: 10.1097/ID.0b013e31822f7a70. [DOI] [PubMed] [Google Scholar]
- 26.Thor A, Sennerby L, Hirsch JM, Rasmusson L. Bone formation at the maxillary sinus floor following simultaneous elevation of the mucosal lining and implant installation without graft material: An evaluation of 20 patients treated with 44 Astra Tech implants. J Oral Maxillofac Surg. 2007;65:64–72. doi: 10.1016/j.joms.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 27.Chen TW, Chang HS, Leung KW, Lai YL, Kao SY. Implant placement immediately after the lateral approach of the trap door window procedure to create a maxillary sinus lift without bone grafting: A 2-year retrospective evaluation of 47 implants in 33 patients. J Oral Maxillofac Surg. 2007;65:2324–8. doi: 10.1016/j.joms.2007.06.649. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko T, Masuda I, Horie N, Shimoyama T. New bone formation in nongrafted sinus lifting with space-maintaining management: A novel technique using a titanium bone fixation device. J Oral Maxillofac Surg. 2012;70:e217–24. doi: 10.1016/j.joms.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Cricchio G, Sennerby L, Lundgren S. Sinus bone formation and implant survival after sinus membrane elevation and implant placement: A 1- to 6-year follow-up study. Clin Oral Implants Res. 2011;22:1200–12. doi: 10.1111/j.1600-0501.2010.02096.x. [DOI] [PubMed] [Google Scholar]
- 30.Lin IC, Gonzalez AM, Chang HJ, Kao SY, Chen TW. A 5-year follow-up of 80 implants in 44 patients placed immediately after the lateral trap-door window procedure to accomplish maxillary sinus elevation without bone grafting. Int J Oral Maxillofac Implants. 2011;26:1079–86. [PubMed] [Google Scholar]
- 31.Sohn DS, Lee JS, Ahn MR, Shin HI. New bone formation in the maxillary sinus without bone grafts. Implant Dent. 2008;17:321–31. doi: 10.1097/ID.0b013e318182f01b. [DOI] [PubMed] [Google Scholar]
- 32.Palma VC, Magro-Filho O, de Oliveria JA, Lundgren S, Salata LA, Sennerby L. Bone reformation and implant integration following maxillary sinus membrane elevation: An experimental study in primates. Clin Implant Dent Relat Res. 2006;8:11–24. doi: 10.2310/j.6480.2005.00026.x. [DOI] [PubMed] [Google Scholar]
- 33.Lambert F, Léonard A, Drion P, Sourice S, Layrolle P, Rompen E. Influence of space-filling materials in subantral bone augmentation: Blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin Oral Implants Res. 2011;22:538–45. doi: 10.1111/j.1600-0501.2010.02069.x. [DOI] [PubMed] [Google Scholar]
- 34.Steigmann M, Garg AK. A comparative study of bilateral sinus lifts performed with platelet-rich plasma alone versus alloplastic graft material reconstituted with blood. Implant Dent. 2005;14:261–6. doi: 10.1097/01.id.0000177412.84225.05. [DOI] [PubMed] [Google Scholar]
- 35.Borges FL, Dias RO, Piattelli A, Onuma T, Gouveia Cardoso LA, Salomão M, et al. Simultaneous sinus membrane elevation and dental implant placement without bone graft: A 6-month follow-up study. J Periodontol. 2011;82:403–12. doi: 10.1902/jop.2010.100343. [DOI] [PubMed] [Google Scholar]
- 36.Ellegaard B, Baelum V, Kølsen-Petersen J. Non-grafted sinus implants in periodontally compromised patients: A time-to-event analysis. Clin Oral Implants Res. 2006;17:156–64. doi: 10.1111/j.1600-0501.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim MJ, Jung UW, Kim CS, Kim KD, Choi SH, Kim CK, et al. Maxillary sinus septa: Prevalence, height, location, and morphology. A reformatted computed tomography scan analysis. J Periodontol. 2006;77:903–8. doi: 10.1902/jop.2006.050247. [DOI] [PubMed] [Google Scholar]
- 38.Krennmair G, Ulm CW, Lugmayr H, Solar P. The incidence, location, and height of maxillary sinus septa in the edentulous and dentate maxilla. J Oral Maxillofac Surg. 1999;57:667–71. doi: 10.1016/s0278-2391(99)90427-5. [DOI] [PubMed] [Google Scholar]


