Abstract
Gingival enlargement comprises any clinical condition in which an increase in the size of the gingiva is observed. Among the drugs that induce gingival enlargement, the antiepileptic agent phenytoin has been widely related to this condition. The Cytochrome P450(CYP) superfamily is the most commonly involved enzymes in metabolism of drugs. Common coding region CYP variants that affects drug elimination and response has been studied in great detail. Pharmacogenetic influences on drug metabolism have been widely reviewed and gene polymorphism of cytochrome P450 2C9 appeared to be responsible for much of the interindividual variability on drug elimination. Genetic variation in the CYP2C9 gene can affect metabolism, leading to altered phenotypes. Individuals with poor metaboliser alleles of CYP2C9 gene were shown to have a reduced metabolism of phenytoin compared with wild-type alleles. Thus identification of patients genotype prior to anti-epileptic drug administration could potentially prevent higher serum drug concentrations leading to adverse side effects such as gingival enlargement. This case report addresses the influence of CYP2C9 genetic polymorphism on Phenytoin drug metabolism thereby causing gingival enlargement.
KEY WORDS: Cytochrome2C9 gene, gene polymorphism, gingival enlargement
Epilepsy is the most common chronic neurological disorder in humans. The first report of gingival enlargement associated with the chronic use of phenytoin was made in 1939. This agent remains as one of the most commonly prescribed medications to treat epilepsy and it may also be used in cases of neuralgias and cardiac arrhythmias. It is estimated that about 30-50% of patients taking phenytoin develop significant gingival alterations.[1] Not all patients taking phenytoin develop phenytoin induced gingival enlargement. Evidence suggests that genetic factors also might have a significant role in the pathogenesis of phenytoin induced gingival enlargement and in the patient's susceptibility to this unwanted effect. A genetic predisposition could influence a variety of factors in the drug plaque-induced inflammation. These include gingival fibroblast functional heterogeneity, collagenolytic activity, drug metabolism, and collagen synthesis.[2]
Regarding drug metabolism, the cytochrome P450 2C9 (CYP2C9) is responsible for phenytoin conversion on its hydroxylated form in the liver. Pharmacogenetic influences on drug metabolism have been widely reviewed and gene polymorphism of CYP2C9 appeared to be responsible for much of the interindividual variability on drug elimination.[3] Genetic variation in the CYP2C9 gene can affect metabolism, leading to altered phenotypes.[4] Individuals with poor metaboliser alleles of CYP2C9 gene were shown to have a reduced metabolism of phenytoin compared with wild-type alleles. Thus, identification of patient's genotype prior to anti-epileptic drug administration could potentially prevent higher serum drug concentrations leading to adverse side effects such as gingival enlargement.
Case Report
A 18-year-old male patient came to Department of Periodontics, Mahatma Gandhi Post Graduate Institute of Dental Sciences, Pondicherry, with a complain of swelling of upper and lower teeth gums for the past 10 months. Patient's medical history revealed that he had been on long-term phenytoin therapy (10 years) for primary generalized epilepsy at a daily dose of 300-mg/day. On intraoral clinical examination, there was generalized gingival enlargement involving marginal, attached, interdental papilla of upper and lower anterior teeth both labial and lingual/palatal aspects [Figures 1-3] and on the buccal aspects of upper and lower premolars and first and second molars. The enlargement was pale pink in color, firm, resilient with minutely lobulated surface and no tendency to bleed on probing. There was also presence of supra and subgingival deposits. There were no any other significant dental findings. Based on these clinical findings a provisional diagnosis of Phenytoin induced gingival enlargement with superimposed inflammatory component was made.
Figure 1.
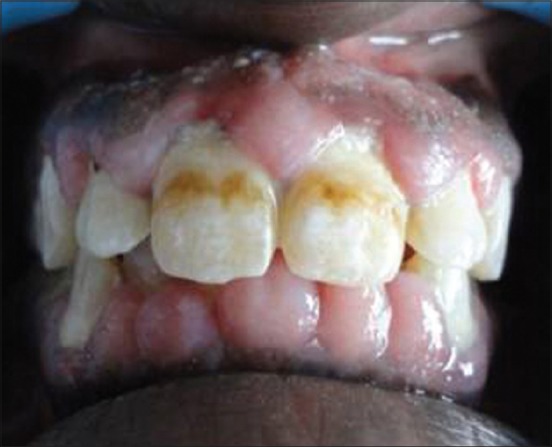
Gingival enlargement involving the labial surfaces of upper and lower anterior teeth
Figure 3.
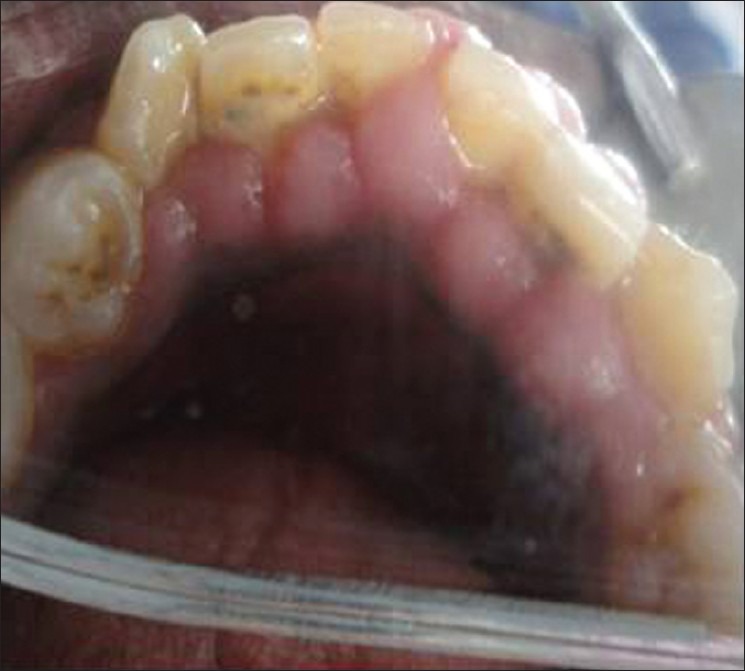
Gingival enlargement involving the lingual surfaces of lower anterior teeth
Figure 2.
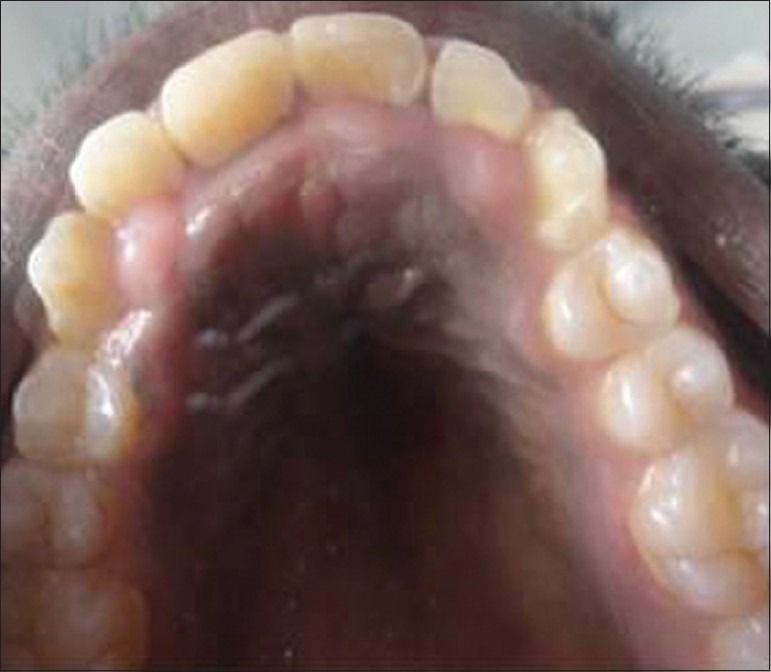
Gingival enlargement involving the palatal surfaces of upper anterior teeth
Routine blood investigations were done which were found to be within normal limits. Gingival enlargement was due to phenytoin toxicity. This phenytoin toxicity prompted appropriate pharamacogenomics studies. Patient was informed verbally and written informed consent was obtained. 5 ml of venous blood was collected from the antecubital vein into ethylenediaminetetraacetic acid tubes. Plasma was separated by centrifugation and stored at −20°C until the assay. After separation of plasma, the cellular fraction was subjected to DNA extraction from leukocytes by the standard phenol: Chloroform procedure and genotyping of CYP2C9 was performed by a polymerase chain reaction-restriction fragment length polymorphism method. The CYP2C9* (Arg144 Cys) was detected using the forward and reverse primers 5´ TACAAATACAATGAAAATATCATG 3´ and 5´ CTAACAACCAGA CTCATAATG 3´, respectively and the genotype result was found to be homozygous CYP2C9*3*3.
Discussion
Phenytoin is one of the most-widely prescribed anti-epileptic agents in the Indian population. This, combined with the fact that the CYP2C9 enzyme which metabolizes phenytoin is polymorphically expressed and that phenytoin has a narrow safety margin makes it important to study the effect of the CYP2C9 polymorphism with regard to phenytoin metabolism. Phenytoin has been found to be safe and suitable for use as a probe to study the pharmacogenetics of CYP2C9.[5] Phenytoin follows non-linear pharmacokinetics. At a concentration of <10 μg/ml, it follows first order (linear) kinetics, and beyond this, zero-order kinetics. Since the influence of enzymes can only be studied on the drugs that follow first-order kinetics, only a single dose of phenytoin was administered to the subjects, although epileptic patients receive long-term therapy in the clinical setting.
This 18-year-old male patient came with the complain of swelling in the gums, but prior to the treatment we planned for the pharmacogenomics study which proved to be homozygous mutant of CYP2C9 in our patient, but still larger samples should be taken to confirm the study. After the pharmacogenomic study, immediately drug substitution was done to sodium valproate under the patient's physician opinion. Oral prophylaxis was done for the patient and was educated about the oral hygiene maintenance. After 6 months of drug substitution and oral hygiene maintenance the gingival enlargement was persisting in relation to upper and lower anteriors only and hence Gingivectomy was planned for the patient and the excised tissue was sent for biopsy. The histopathological examination [Figure 4] revealed epithelial acanthosis, and elongated rete ridges. Hyperplasia of connective tissue, along with dense fibrous connective tissue was seen and there was a mild to moderate inflammatory cell infiltrate within connective tissue. Patient was recalled after 3 months, which revealed no evidence of recurrence [Figure 5].
Figure 4.
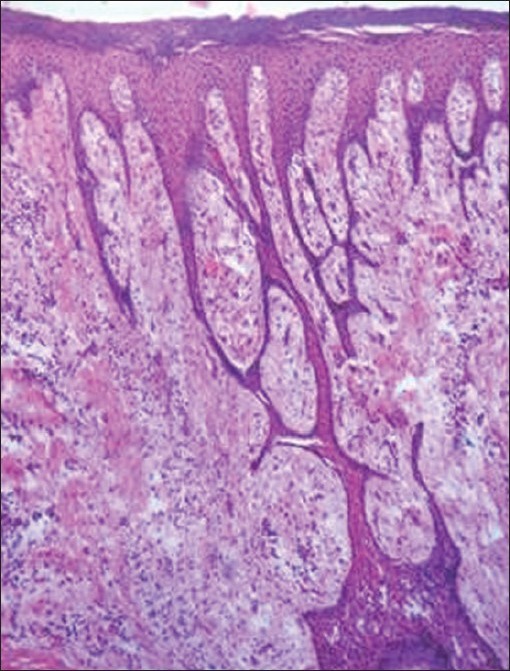
Photomicrograph showing connective tissue hyperplasia, acanthosis of overlying epithelium, and elongated rete ridges (×10)
Figure 5.
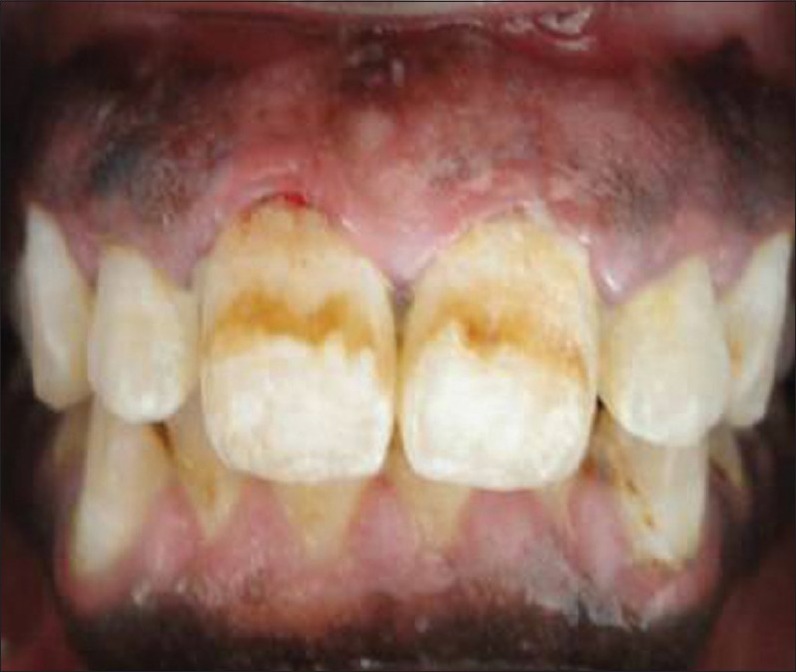
After drug substitution and oral prophylaxis with reduction in gingival enlargement
Conclusion
Pharmacogenomics studies before initiating antiepileptic therapy could have easily identified the unfavorable genotype of CYP2C9, a major phenytoin metabolizing enzyme in this case, and prompted the physician to choose the correct dosage or to convince the patient on the need for an alternate antiepileptic drug such as sodium valproate. This would have avoided a number of iatrogenic complications of antiepileptic drug therapy. We recommend that wherever possible, the clinicians may do CYP2C9 genotyping of the epileptic patients before prescribing Phenytoin, with advancement in the molecular technology; the clinicians may do the genotypic analysis which enables them to have personalized prescription and to avoid the adverse drug reaction.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: A comprehensive review of the in vitro and human data. Pharmacogenetics. 2002;12:251–63. doi: 10.1097/00008571-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Soga Y, Nishimura F, Ohtsuka Y, Araki H, Iwamoto Y, Naruishi H, et al. CYP2C polymorphisms, phenytoin metabolism and gingival overgrowth in epileptic subjects. Life Sci. 2004;74:827–34. doi: 10.1016/j.lfs.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Odani A, Hashimoto Y, Otsuki Y, Uwai Y, Hattori H, Furusho K, et al. Genetic polymorphism of the CYP2C subfamily and its effect on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Clin Pharmacol Ther. 1997;62:287–92. doi: 10.1016/S0009-9236(97)90031-X. [DOI] [PubMed] [Google Scholar]
- 4.Ramasamy K, Narayan SK, Chanolean S, Chandrasekaran A. Severe phenytoin toxicity in a CYP2C9 *3 *3 homozygous mutant from India. Neurol India. 2007;55:408–9. doi: 10.4103/0028-3886.33300. [DOI] [PubMed] [Google Scholar]
- 5.Adithan C, Gerard N, Vasu S, Balakrishnan R, Shashindran CH, Krishnamoorthy R. Allele and genotype frequency of CYP2C9 in Tamilnadu population. Eur J Clin Pharmacol. 2003;59:707–9. doi: 10.1007/s00228-003-0666-3. [DOI] [PubMed] [Google Scholar]


