Abstract
BACKGROUND:
Experimental unilateral cryptorchidism (ULC) and bilateral cryptorchidism (BLC) are excellent methods to study undescended testis in relation to spermatogenesis against a temperature gradient.
OBJECTIVES:
In case of ULC, it is possible to compare the testicular functions between normal condition and cryptorchidism in the same animal, whereas BLC shows the necessity of testicular androgens for proper maintenance of reproductive structures and functions.
MATERIALS AND METHODS:
In the present study, experimental ULC and BLC was done on same-aged adult mature male mice and kept for 15 days and 30 days, respectively, to observe the changes due to the induced cryptorchidism on the different reproductive organs, viz., the testis and accessory sex organs along with epididymal sperm count. Reproductive tissues were collected from individual animals and histopathological studies of testis were done to investigate different cytological changes.
RESULTS:
The size of the testes and accessory sex organs were found to be significantly reduced in BLC mice, whereas only testicular weight reduction was observed in ULC mice. Histopathological studies showed degenerative changes throughout the seminiferous tubules.
CONCLUSION:
Thus, the present investigation showed compensatory androgen production in ULC mice, whereas absence of androgen mediated reproductive functions in BLC animals.
KEY WORDS: Cryptorchidism, infertility, male reproduction, sperm count, testis
INTRODUCTION
Male infertility is both a private and a social problem.[1] Cryptorchidism is a well-described clinical condition associated with male infertility.[2] Cryptorchidism is a defect involving maldescent of the testicle. The objectives of therapy for cryptorchidism include preservation of fertility, reduction of the risk of malignancy, and alleviation of physiological stress in human male. The incidence of cryptorchidism in the newborn is 2-6%; however, with spontaneous descent, this incidence drops to 1% by the age of 3 months.[3] Both the testes and ovaries originate from a common bipotential precursor. In the embryonic level, several factors such as SRY genes (sex-determining region on Y chromosome), SRY proteins, anti-mullerian hormone (AMH), and Mullerian-inhibiting substance (MIS) helps in the differentiation of the male gonad from their bipotential precursor. The male gonads undergo SRY directed changes in an organization. At that time, male gonad, i.e. the testis remains situates into the abdominal cavity because the genital ridge develops in the upper lumber region at the 8th week of the embryonic life.[4] Therefore, a phenomenon called testicular descendence occurs in different animals at a particular time in their life. In case of humans, the testis starts to descend from its retroperitoneal, abdominal position to the pelvic position between 10th and 15th week of gestation. Within 35th to 40th week of human pregnancy, it descends into the scrotum and remains permanently in that position. After the descendence of the testis, the inguinal canal through which it comes to the scrota closes.[4] Soon after, sertoli cells develop and secrete MIS, the level of which remains high throughout gestation and causes regression of Mullerian ducts. At 9th week of gestation, Leydig cells develop and secrete testosterone. Prenatal ultrasonography shows no testicular descent before 28 weeks’ gestation other than transabdominal movement to the internal inguinal ring. Trans-inguinal migration, thought to be under hormonal control, occurs at 28-40 weeks’ gestation, usually resulting in a scrotal testis by the end of a full-term of gestation.[5]
Although most mammals have notable scrota, rhinoceros, seal, elephant and whale are notable exceptions. In those vertebrates who do not have the scrota, the testis remains in the abdominal cavity throughout the life. In rodents, the testis remains in the abdominal cavity, but due to their opened inguinal canal, it comes into the scrotal sac during their breeding season. However, the chiropteran does not have any scrotal sac and their testis descend in the caudal abdominal wall only during their breeding season.[6]
There are several factors that affects this transabdominal descend of the testis, such as MIS that acts on a fibrous structure gubernaculums, which attaches the testis in the posterior abdominal wall; as the body increases in size, the gubernaculums does not elongate, i.e. it becomes shorter in males. Thus, in males, the relative positions of the testis become increasingly caudal. The passage of the testis through the inguinal canal into the scrotum is probably controlled by the action of androgens.[4]
In man and domesticated mammals, it is not uncommon for the testis to be retained in the abdominal cavity rather than to descend normally into the scrotum; this condition is called cryptorchidism or cryptorchism.[6] The word cryptorchidism is obtained from the Greek word “Kryptos” meaning “hidden” and “Orchid” meaning “testicles.”[7] This pathophysiological condition may occur in different animals due to different reasons. Thus, there are several types of cryptorchidism found according to its reasons, such as natural cryptorchidism, which may occur due to the obstruction of the inguinal canal or by the inguinal canal dysfunction in human beings; evolutionary cryptorchidism, which is found in some animals due to the evolutionary effects (e.g. monotrems, elephants).[4]
Conversely, for experimental purpose, cryptorchidism may also be induced in animals such as bilateral cryptorchidism (BLC), in which both the testis remains within the abdominal cavity. It ultimately results in complete sterility and unilateral cryptorchidism (ULC), in which one of the testes remains within the abdominal cavity and the other descends into the scrotal sac and remains normal. The one that remains normal can continue both the spermatogenesis and steroidogenesis in normal manner, but at a reduced rate. However, in the cryptorchid testes, both these two functions of the gonad become degenerated.
Overall, 32-79% of undescended testes are associated with some type of epididymal abnormality. However, abnormalities that inhibit sperm transport (e.g. complete caput separation, artesia, agenesis) have been reported in only 8% of patients with cryptorchidism. In addition, when the processus vaginalis is patent, the epididymis is more likely to be abnormal.[8]
Cryptorchidism is a pre-existing factor in 3-8% of infertile men and in 20% of men with azoospermia.[9] Animal studies also supports the effect of cryptorchidism on fertility. It is generally accepted that a relatively low temperature is preferable in spermatogenesis in mammalian species.[10] Actually, several studies indicate that the temperature in the scrotum is 4°C to 5°C lower than that in the abdomen in most mammals.[10] Waites and Moule[11] measured the temperature of the abdominal aorta and testicular artery at various points and found that the temperature of the testicular artery was significantly smaller. A major deleterious effect of high intratesticular temperature on spermatogenesis has been demonstrated.[12] It is well-known that the function of the cryptorchid testis is impaired by the rise in temperature.[2,3] The effect of ULC on ipsilateral testicular function has been well studied in humans and experimental animals.[13,14] However, the effect of ULC on contralateral testicular function has not been extensively studied. Ono and Sofikitis[15] have held that the endocrine and exocrine functions of the contralateral testis are impaired by a rise in temperature. The present study focuses on the effects of left cryptorchidism on the right testicular and epididymal function and the overall sperm fertilizing capacity.
MATERIALS AND METHODS
Animals
Male mice (Parkes strain) weighing 25-30 g were used in this study. The animals were randomly distributed into cages and allowed to acclimatize for 10 days in a well-ventilated room at a room temperature of 25.0 ± 2.0°C under natural lighting condition, 12 h light/dark cycles were maintained in the animal house. The animals were fed with standard laboratory diet and allowed free access to water daily. All animals used in this study were handled in accordance with the Institutional guidelines for Care and Use of Laboratory Animals.
Experimental protocol
Animals were divided into three main groups: ULC, BLC, and control. Each group was further subdivided into 2 groups (n = 8), for 15-days study and for 30-days study. To create ULC (groups B and C), left inguinal incision was performed. The left testis was sutured at the posterior abdominal wall with nylon sutures under ether anesthesia. The sham operation (group A) included a left inguinal incision, placement of the left testis into the abdomen and replacement of the testis into its normal position. Group of ULC mice were weighed and killed by cervical dislocation after 15th and 30th day. Weight of the cryptorchid testis, normal testis, epididymis, and seminal vesicle were measured. In addition, the control and cryptorchid testes were fixed by immersion in Bouin's solution and processed into paraffin. Sections (5-μm thick) were stained with Hematoxylin and Eosin.[16]
Sperm count
Sperm samples were collected from the cauda epididymis and counted by a hemocytometer chamber under light microscope.[12] To minimize error, count was repeated at least five times for each rat.
Statistical analysis
Results were expressed as mean ± SD. One-way analysis of variance (ANOVA) test was first carried out to test for any differences between the mean values of all groups. If differences between groups were established, the values of the treated groups were compared with those of the control group by a modified t-test. P > 0.05 was interpreted as statistically significant.[17]
RESULTS
As the testes of the mature male mice were surgically confined into the abdominal cavity, a significant decrease in the weight [Table 1], size, and turgidity of these organs were found in comparison between the unilaterally cryptorchid and control mice [Figure 1]. In addition, the accessory sex organs such as the epididymis, seminal vesicle, and vas difference also became significantly reduced in their size, weight, and function as compared to their corresponding control tissues [Table 1]. The experimental animals were also found to have significantly reduced sperm count than the sham-operated mice [Figure 2].
Table 1.
Weight of testis and accessory sex organs in control and treated rats
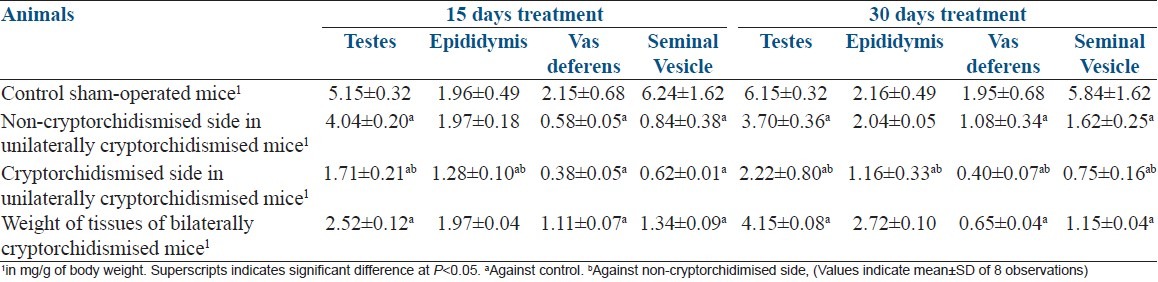
Figure 1.
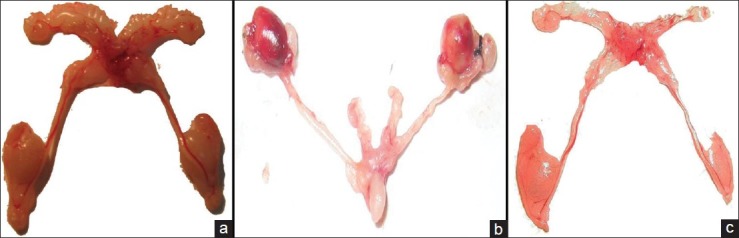
Reproductive organs of (a) Sham-operated, (b) Unilateral cryptochid mice and, (c) Bilateral cryptorchid mice
Figure 2.
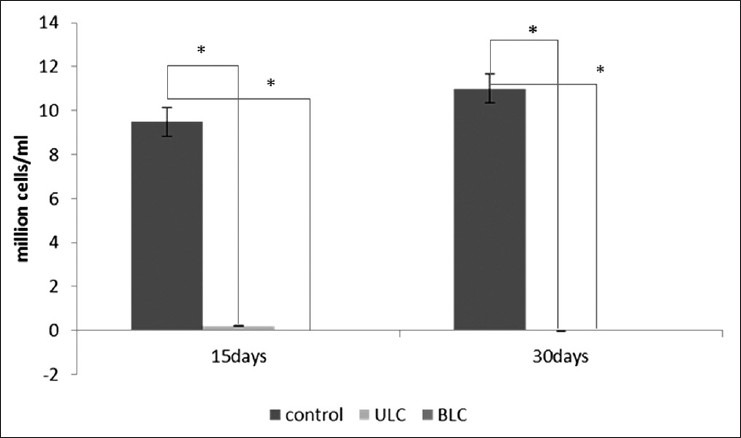
Comparison of sperm count between 15 days and 30 days of ULC and BLC
Histologically severe disorganization of the seminiferous tubules was observed time-dependently. Histopathological examinations showed deterioration in germinal epithelium and seminiferous tubules [Figure 3]. In case of the 30-days BLC testis, it was found that all germinal elements of the tubules were lost and little remained except a single layer of sertoli cell-like cells next to the basement membrane of the tubules. This has been referred to as sertoli cell-only syndrome in the ULC and BLC animals within 15-days period. Most highly differentiated cells have disappeared first in order of degeneration were spermatozoa, spermatids, spermatocytes, and spermatogonia.
Figure 3.
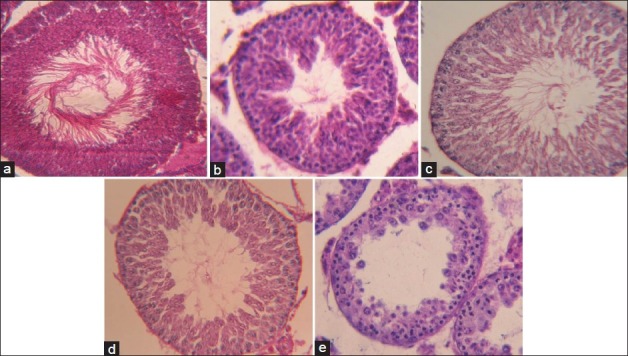
Histological sections of testis (×400) showing, (a) The changes in spermatogenic cell count in control, (b) Unilaterally cryptorchid mice (15 days), (c) Unilaterally cryptorchid mice (30 days), (d) Bilaterally cryptorchid mice (15 days), (e) Bilaterally cryptorchid mice (30 days)
DISCUSSION
In the present experiment, almost no sperm was observed in the caudal part of the epididymis of abdominal testis in ULC and BLC mice for both 15- and 30-days study. However, in the caudal part of the normal non-cryptorchidimized testis (right) in the ULC mice, normal sperm was found, though quite lower than that in the control mice [Figure 2]. According to the sperm count of that cryptorchid testis, it can be assumed that the spermatogenesis and other testicular functions of experimental animals were totally disrupted, because the temperature of the abdominal cavity is approximately 4°C higher than that of the scrotum. It has been reported that higher temperature causes impairment of the testicular functions. In the scrotum, pampiniform plexus, a network of veins from the testes and epididymis, keeps the temperature of the testes lower than that of the body cavity. This plexus is supplied by spermatic artery and the venous blood empties into the spermatic vein. The plexus functions to cool the blood from the body before it enter the testes and also to warm the blood from the testes before it is returned to the systemic circulation. Thus, the pampiniform plexus controls the temperature of the testes and the scrota and keeps these two in a lower temperature of about 36°C. In that low temperature of the scrotum, different testicular functions and the spermatogenesis can occur in their normal manner. As the testis was removed from its scrotal position, it could not regulate the temperature through the pampiniform plexus. Thus, at higher temperature, spermatogenesis becomes totally disrupted.[6] Decrease in sperm count, testicular, and accessory sex organ weights are relatively higher in BLC mice because of impairment of androgen biosynthesis by Leydig cells. A time-dependant decrease was also noticed in both cases [Table 1]. On the contrary, it was found that the weights of accessory sex glands in ULC mice were not affected, as the remaining testis was capable of producing adequate amount of testosterone to maintain structural integrity of these androgen-dependent glands. It was also reported that cryptorchid testis is equally capable of producing testosterone in vitro as in the control testis.[18] Cellular degeneration was observed in histological sections of testis, which is also an indicator of low testosterone level. However, some reports demonstrated a reduced serum testosterone level after 7 days of cryptorchidism in rat, but it came to normal in subsequent days up to day 28.[19,20] Measurement of serum testosterone and luteinizing hormone (LH) levels demonstrated that the cryptorchid mouse testes are capable of secreting significant level of testosterone to cause a normal regulating function of the hypertrophy. Some investigations reported that follicle stimulating hormone (FSH) increases on day 7 of cryptorchidism, which progressively rises with increased duration of cryptorchidism in the mouse.[20] Another report demonstrated extensive damage along with lower level of FSH in comparison to castrated condition, which suggests that BLC can exert suppressive feedback activity on FSH reduced activity, possibly due to continued secretion of testosterone by the Leydig cells.[4]
Particularly in the case of ULC, it has been reported that spermatogenesis does not proceed within the clinically unilateral undecended testis during puberty.[19,20,21,22,23,24,25,26,27,28,29,30,31] In the present study, the arrest of spermatogenesis was also observed in both 15- and 30-days ULC, although some androgens were produced from the remaining testis in the ULC mice. However, the secretion rates of those androgens are considerably lower than that in normal mice.[19] While correlating this ULC with the unilateral ovariectomy (ULO), it can observed that there is no compensatory stimulation by the increased levels of LH in case of the testis that remains normal in the ULC.[7] Thus, it is reduced in size, weight, and sperm count in a negligible manner than in the control.
CONCLUSION
The present investigation showed compensatory androgen production in ULC mice, whereas absence of androgen mediated reproductive functions in experimental animals after BLC. Thus, the current findings suggested that ULC and BLC may cause detrimental effects on male reproductive physiology.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sengupta P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem Toxicol. 2013;36:353–68. doi: 10.3109/01480545.2012.710631. [DOI] [PubMed] [Google Scholar]
- 2.Diamond DA, Flores C, Kumar S, Malhotra R, Seethalakshmi L. The effects of an LHRH agonist on testicular function in the cryptorchid rat. J Urol. 1992;147:264–9. doi: 10.1016/s0022-5347(17)37210-5. [DOI] [PubMed] [Google Scholar]
- 3.Taskinen S, Hovatta O, Wikström S. Early treatment of cryptorchidism, semen quality and testicular endocrinology. J Urol. 1996;156:82–4. [PubMed] [Google Scholar]
- 4.Johnson HM, Everitt JB. Essential Reproduction. 5th ed. pp. 11–4. [Google Scholar]
- 5.Chandra AK, Goswami H, Sengupta P. Dietary calcium induced cytological and biochemical changes in thyroid. Environ Toxicol Pharmacol. 2012;34:454–65. doi: 10.1016/j.etap.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Turner CD, Bagnara JT. General Endocrinology. 6th ed. pp. 432–4. [Google Scholar]
- 7.Knobil E, Neill DJ. The Physiology of Reproduction. vol 1. pp. 727–53. [Google Scholar]
- 8.Docimo SG. The results of surgical therapy for cryptorchidism: A literature review and analysis. J Urol. 1995;154:1148–52. [PubMed] [Google Scholar]
- 9.Sengupta P. Health impacts of yoga and pranayama: A state-of-the-art review. Int J Prev Med. 2012;3:444–58. [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison RG, Weiner JS. Vascular patterns of the mammalian testis and their functional significance. J Exp Biol. 1949;26:304–16. doi: 10.1242/jeb.26.3.304. [DOI] [PubMed] [Google Scholar]
- 11.Waites GM, Moule GR. Relation of vascular heat exchange to temperature regulation in the testis of the ram. J Reprod Fertil. 1961;2:213–24. doi: 10.1530/jrf.0.0020213. [DOI] [PubMed] [Google Scholar]
- 12.Chandra AK, Sengupta P, Goswami H, Sarkar M. Excessive dietary calcium in the disruption of structural and functional status of adult male reproductive system in rat with possible mechanism. Mol Cell Biochem. 2012;364:181–91. doi: 10.1007/s11010-011-1217-3. [DOI] [PubMed] [Google Scholar]
- 13.Keel BA, Abney TO. Influence of bilateral cryptorchidism in the mature rat: Alterations in testicular function and serum hormone levels. Endocrinology. 1980;107:1226–33. doi: 10.1210/endo-107-4-1226. [DOI] [PubMed] [Google Scholar]
- 14.Rager K, Arnold E, Hauschild A, Gupta D. Effect of bilateral cryptorchidism on the in vitro transformation of progesterone by testicular tissue at different stages of sexual maturation. J Steroid Biochem. 1975;6:1537–41. doi: 10.1016/0022-4731(75)90211-3. [DOI] [PubMed] [Google Scholar]
- 15.Ono K, Sofikitis N. A novel mechanism to explain the detrimental effects of left cryptorchidism on right testicular function. Yonago Acta Med. 1997;40:79–89. [Google Scholar]
- 16.Chandra AK, Sengupta P, Goswami H, Sarkar M. Effects of dietary magnesium on testicular histology, steroidogenesis, spermatogenesis and oxidative stress markers in adult rats. Indian J Exp Biol. 2013;51:37–47. [PubMed] [Google Scholar]
- 17.Rosner B. Fundamentals of Biostatistics. 5th ed. Duxbury: 2000. pp. 80–240. [Google Scholar]
- 18.Mendis-Handagama SM, Kerr JB, de Kretser DM. Experimental cryptorchidism in the adult mouse: II. A hormonal study. J Androl. 1990;11:548–54. [PubMed] [Google Scholar]
- 19.Sengupta P. The laboratory rat: Relating its age with human's. Int J Prev Med. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- 20.Risbridger GP, Kerr JB, de Kretser DM. Evaluation of Leydig cell function and gonadotropin binding in unilateral and bilateral cryptorchidism; evidence for local control of Leydig cell function by the seminiferous tubule. Biol Reprod. 1981;24:534–40. doi: 10.1095/biolreprod24.3.534. [DOI] [PubMed] [Google Scholar]
- 21.Sengupta P, Chaudhuri P, Bhattacharya K. Male reproductive health and yoga. Int J Yoga. 2013;6(2):87–95. doi: 10.4103/0973-6131.113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sengupta P, Chaudhuri P, Bhattacharya K. A small-scale cross-sectional study for the assessment of cardiorespiratory fitness in relation to body composition and morphometric characters in fishermen of Araku valley, Andhra Pradesh, India. Int J Prev Med. 2013 [PMC free article] [PubMed] [Google Scholar]
- 23.Sengupta P, Sahoo S. A Cross Sectional Study to Evaluate the Fitness Pattern among the Young Fishermen of Coastal Orissa. Indian J Pub Health Res Dev. 2013;4:171–5. [Google Scholar]
- 24.Sengupta P, Chaudhuri P, Bhattacharya K. Screening obesity by direct and derived anthropometric indices with evaluation of physical efficiency among female college students of Kolkata. Ann Med Health Sci Res. 2013 doi: 10.4103/2141-9248.122066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sengupta P. Chemosterilization: Spermatogenesis, Steroidogenesis, Reproductive Functions, and Behavior from Historical Perspective to Contemporary Practice. J Basic Clin Repro Sci. 2013;2(1):1–2. [Google Scholar]
- 26.Sengupta P, Sahoo S. Evaluation of Health Status of Fishers: Prediction of Cardiovascular Fitness and Anaerobic Power. World J Life Sci Med Res. 2011;1(2):25–30. [Google Scholar]
- 27.Sengupta P, Goswami H, Chandra AK. Environmental threat to male fertility by hard water metals: How protective are the citrus foods? 100th Indian Science Congress. 2013:201–202. [Google Scholar]
- 28.Sengupta P. A Scientific Review of Age Determination for a Laboratory Rat: How Old is it in Comparison with Human Age? Biomed Int. 2011;2(2):81–89. [Google Scholar]
- 29.Sengupta P, Goswami H, Chandra AK. Which one is more potent in ameliorating the impacts of chronic hard water induced male infertility, Citric Acid or EDTA? 19th West Bengal State Science & Technology Congress. 2013:247. [Google Scholar]
- 30.Sengupta P. Challenge of infertility: How protective the yoga therapy is? Ancient Sci Life. 2012;32(1):61–62. doi: 10.4103/0257-7941.113796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta P. Potential Health Impacts of Hard Water. Int J Prev Med. 2013 [PMC free article] [PubMed] [Google Scholar]


