Abstract
CONTEXT:
Insulin-like growth factor-1 (IGF-1) has been reported to play a role in human follicular and embryonic development. However, earlier studies carried out mostly in animal models or in culture mediums supplemented with IGF-1 have been unable to directly link IGF-1 with embryo quality. Results correlating IGF-1 with pregnancy outcome have also been ambiguous so far.
AIM:
The aim of this study is to find if in situ follicular-fluid level of IGF-1 is predictive of embryo quality and implantation rates in in vitro fertilization (IVF) cycles.
SETTINGS AND DESIGN:
Prospective study involving 120 cycles of conventional IVF-embryo transfer in infertile women.
SUBJECTS AND METHODS:
IGF-1 concentrations were estimated in pooled follicular-fluid on the day of oocyte-pickup. Embryo quality was assessed daily at different developmental stages. Cycles were sorted into low and high follicular fluid insulin-like growth factor-1 (FF IGF-1) groups according to the median value of measurement. Embryo quality, clinical pregnancy and implantation rate were the main outcome measures.
STATISTICAL ANALYSIS:
Graph-pad Prism 5 statistical package.
RESULTS:
FF IGF-1 correlates with embryo quality (Pearson r = 0.3894, r2 = 0.1516, P > 0.0001) and clinical pregnancy (Pearson r = 0.5972, r2 = 0.36, P > 0.0001). High FF IGF-1 group shows significantly higher rates of fertilization, cleavage, blastocyst formation and top grade embryos compared with low FF IGF-1 group. Clinical pregnancy rates (38.33 vs. 20%, P = 0.0272) and embryo implantation rates (21.6 vs. 10.32%, P = 0.0152) are also significantly higher in the high versus low FF IGF-1 group. Threshold value of FF IGF-1 for clinical pregnancy is <58.50 ng/mg protein (receiver operating characteristics AUC : 0.85 ± 0.03, 95% CI: 0.78-0.91).
CONCLUSION:
FF IGF-1 is a plausible biochemical marker of embryo quality and implantation rate and correlates with clinical pregnancy rates in conventional IVF cycles.
KEY WORDS: Clinical pregnancy, embryo quality, FF IGF-1, implantation rate, IVF cycles
INTRODUCTION
Selection of the best quality embryos for transfer may be a key factor in influencing implantation and pregnancy rates of in vitro fertilization (IVF) procedure. Several workers have employed various embryo grading systems which may be unreliable due to discrepancies in the morphological evaluation criteria used. Recently, pre-implantation genetic diagnosis (PGD) studies and use of embryoscope have augmented the possibilities for selection of good quality embryos. However, such expensive techniques may not be an economically viable option for all infertility centers. Furthermore, in stimulated cycles, where at least 2-3 embryos are selected for transfer, it is difficult to trace the fate of individual embryos of a particular morphological quality. Therefore, appraisal of embryo quality based solely on morphological examination may be incongruous and should be accompanied by biochemical evaluation. A robust biochemical marker predictive of embryo quality and its implantation potential remains elusive and hence warrants examination.
Insulin-like growth factor-1 (IGF-1), a member of the ovarian IGF system, has been shown to serve as an intra-ovarian regulator of follicle function in rodents and exerts direct effects on human and rodent granulosa cell function.[1,2] IGF-I acts on granulosa cells in an autocrine fashion[3,4] and in conjunction with gonadotropins, appears to have a role in promoting follicle growth,[5] steroid secretion,[6,7] and as an anti-atretic hormone.[8] Few workers[9,10] have reported a major role of IGF-1 in regulation of human follicular and embryonic development through regulation of the cell cycle. It has also been implicated in mediating aromatase activity and estrogen production by the developing follicle.[11,12] Though the presence of IGF-1 has been reported in human follicular fluid (FF),[13,14,15,16,17,18] most studies on follicular fluid insulin-like growth factor-1 (FF IGF-1) have so far been carried out in animal models.[19,20,21] Human studies with IGF-1 have mostly employed in vitro cell culture techniques,[22,23,24] with exogenous supplementation instead of its estimation in in situ sources like FF.[25] A definitive role of IGF-1 in FF has not yet been established or is poorly understood with conflicting reports,[26,27,28,29,30] on its predictive values. Moreover, no approach has yet been made to directly correlate FF IGF-1 with embryo quality. This study contended that FF IGF-1 may be a plausible marker for assessment of embryo quality and hence implantation rates in IVF cycles.
This study aimed at evaluating IGF-1 in FF pooled from follicles from which oocytes had been retrieved in each IVF cycle. Per cycle pooled FF was used to get a comprehensive replicate of granulosa cell function since it has recently been reported that FF specimens collected from single dominant follicles might not truly reflect granulosa or thecal cell production.[30] The objective was to establish in situ FF IGF-1 as a plausible biochemical marker influencing embryo development; thus, affecting its quality and implantation potential. This is arguably the first ever study, which attempts to correlate FF IGF-1 with embryo quality and implantation rate in IVF cycles.
SUBJECTS AND METHODS
Subjects
Prospective study involved 146 normoovulatory women (mean age 32.22 ± 4.25 years, body mass index 23.97 ± 4.53, W/H ratio 0.88 ± 0.06, menstrual cycle length range 25-32 days) undergoing their first conventional IVF cycle. 26 cycles were abandoned due to either no oocytes retrieved (12 cycles) or fertilization failure (14 cycles). Finally, 120 cycles were considered for assessment of embryo quality, clinical pregnancy and implantation rates. Informed consent was sought from all patients for participation in this study. Study protocol was approved by the local Hospital Ethical Committee.
Exclusion criteria
Women older than 42 years of age
Women with polycystic ovary syndrome (as defined according to the Rotterdam consensus)
Oocyte pickup failure and fertilization failure cycles
Women laparoscopically diagnosed with endometriosis
Intracytoplasmic sperm injection cycles were excluded in order to remove any male factor bias.
Main outcome measure
Embryo quality
Clinical pregnancy rate: Gestational sac with positive cardiac activity observed at ultrasound at around 6th week of amenorrhea was defined as confirmation of clinical pregnancy
-
Implantation rate:
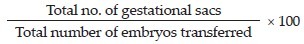
Cycle monitoring
Pituitary desensitization involving treatment with gonadotropin-releasing hormone (GnRH) agonists (500 μg/day of Luprolide acetate) was started in the mid-luteal phase of the menstrual cycle 7 days prior to the earliest expected date of menstruation. Comprehensive down-regulation was confirmed by measurement of serum follicle stimulating hormone (FSH) and estradiol (E2) levels below 1.0 mIU/mL and >20 pg/ml respectively, either on the day of onset of menstruation or 1 or 2 days at the most, after onset.
After confirmation of comprehensive down-regulation, standard long protocol followed for controlled ovarian hyperstimulation involved daily administration of recombinant FSH (Recagon 200 IU/day). Transvaginal ultrasound scan was performed on days 8 and 10 of ovarian stimulation and every 1 or 2 days thereafter as required. Final oocyte maturation and trigger for ovulation was induced by administering human chorionic gonadotropin (hCG) 5000 IU, when there was at least one leading follicle reaching a mean diameter of 18 mm and at least two-four other follicles reaching mean diameter of 16 mm.
Transvaginal ultrasound-guided oocyte retrieval under patient sedation was done between 34 h and 36 h after hCG administration. FF was aspirated from follicles (≥16 mm) using a double lumen needle and maintained at steady 37°C temperature conditions. Only the original follicular aspirate was collected in the few instances wherein oocyte was retrieved in the flush. In every cycle, FF from each follicle was collected separately and equal volume of FF from individual follicles from which an oocyte had been obtained, was pooled together. FF was then centrifuged at 3,000 g for 15 min at 4°C to eliminate cellular elements. Clear supernatant was used for estimations.
Hormonal estimations
FF obtained on the day of oocyte retrieval was estimated for IGF-1 levels by enzyme-linked immunosorbent assay technique using diagnostic kits [Diagnostic Systems Laboratories, Texas, USA (DSL-10-2800)]. Protocol was followed as per manufacturer's instructions. Theoretical sensitivity or lowest detection limit was 0.01 ηg/ml with no detectable cross reactivity. Since extraction method has been reported to involve overestimations due to interference by binding proteins,[31] we followed the non-extraction method of estimation. The intra-assay precision determined from a mean of 10 replicates each with three human FF samples (mean ± standard deviation [SD]) was 12 ± 3.6, 87 ± 9.6, 295 ± 44.8 ng/ml (coefficient of variation 3.4, 6.4 and 7.2% respectively), whereas the inter-assay precision was 14 ± 4.1, 79 ± 8.1, 347 ± 59.8 ng/ml (coefficient of variation 2.9, 7.4 and 10.8% respectively).
Levels in FF were expressed as the ratio of corresponding total protein content to remove bias due to volume variability. Protein estimation was performed by Folin-phenol reagent method described by Lowry et al. (1951).[32] The original method was scaled down to accommodate micro-quantities of sample and reagents.
Estradiol levels were measured in FF by radio-immuno-assay kits [Diagnostic Systems Laboratories, Texas, USA (DSL-4400)]. Estimations were performed as per manufacturer's protocol. Values were expressed as pg/ml with theoretical sensitivity or lowest detection limit 4.7 pg/ml.
Assessment of embryo quality
Embryos were evaluated on the basis of following parameters:
Fertilization: Assessed 16-18 h after insemination, was characterized by the presence of two pronuclei and two polar bodies.
-
Cleavage: Main morphologic characteristics assessed were:
- Evenness of blastomeres
- Lack of multinucleation
- 4-5 blastomeres on day 2
- <6 blastomeres on day 3
- Multicellular (morula) stage with compaction on day 4
-
Embryo fragmentation %.Taking into consideration above characteristics, cleavage stage embryos were graded as per Veeck's criteria[33] as grade 1 (top), grade 2 (average) and grade 3 (poor).
-
Blastocyst: Timing of blastocyst formation, expansion and hatching was evaluated alongwith following criteria:
- Day 5: Formation of blastocoel cavity, orientation of the inner cell mass and thinning of zona
- Day 6: Formation of hypoblast and point of hatching/spontaneous hatching.
Blastocyst stage embryos were graded as per Gardner grading system,[34] as:
Early blastocyst: Blastocele > half the volume of embryo
Blastocyst: Blastocele ≥ half the volume of embryo
Full blastocyst: Blastocele completely filling the embryo
Expanded blastocyst: Blastocele volume larger than that of the early embryo and thinning zona-pellucida
Hatching blastocyst: Brophectoderm has started to herniate through the zona pellucida
Hatched blastocyst: Blastocyst has completely escaped from zona.
In addition, grades A, B or C were assigned on the basis of composition of inner cell mass (ICM) and number and texture of trophectodermal (TE) cells. Depending on cumulative gradation, embryos were designated top, average or poor quality.
For example, 4AA (top/grade 1): Represents fully expanded blastocyst with distinct round or oval shape, compact inner cell mass and trophectoderm consisting of a high number of flat epithelium-like cells without dark granulation.
5BB (average/grade 2): Represents hatching blastocyst with slightly dispersed ICM and consisting of few number of TE cells.
4CC (poor/grade 3): Represents fully expanded blastocyst characterized by flat, irregularly shaped or fragmented ICM and trophectoderm formed by very few cells with poor cell-to-cell attachment.
ET
Quality of embryos influenced the decision on whether the transfer was performed at cleavage stage or at the blastocyst stage. If there were at least three morphologically good quality embryos available on day 3, culture was extended to blastocyst stage. If number of good quality embryos was >3 or if quality was average/poor, cleavage stage transfer was done. Also, if patients were unwilling for blastocyst transfer due to financial constraints, transfer was done at cleavage stage even if <3 good quality embryos were available.
Micronized progesterone 200 mg twice daily was administered to support luteal phase starting from the evening of day ET until day 14 of ET. On d14 ET, serum β-hCG <50 mIU/ml was considered as a positive indicator of pregnancy. Regular trans-vaginal ultrasound scan was done and presence of gestational sac/s with positive cardiac activity at around 6th week of amenorrhea confirmed clinical pregnancy.
Statistical analysis
Data was analyzed for statistical significance using Graph Pad Prism 5.0 statistical package. Student's t-test was used to assess the difference between means. Comparisons between continuous variables from more than two groups were performed using one-way analysis of variance (ANOVA). Receiver operating characteristics (ROC) analysis was done to obtain cutoff values. Correlation was obtained and expressed as Pearson correlation coefficient (r). All values are expressed as mean ± SD. In all cases, P > 0.05 was considered to be statistically significant.
RESULTS
Cycles were sorted into pregnant and non-pregnant groups depending on clinical pregnancy and then into low (≤59.25 ng/mg protein) and high (<59.25 ng/mg protein) groups according to FF IGF-1 concentrations. Cutoffs for defining low and high concentrations corresponded to round value of median (50th centile) of each measurement.
120 cycles of conventional IVF led to an overall clinical pregnancy rate of 29.17% (35/120) with 5 (14.29%) twin pregnancies. A total of 251 embryos were transferred giving an implantation rate of 15.94% (40 × 100/251). Table 1 depicts a comparison of FF IGF-1 levels and embryological data between pregnant and non-pregnant groups. FF IGF-1 levels, fertilization and blastocyst formation rates were significantly higher in pregnant group than in non-pregnant group. However, cleavage rates showed no statistically significant difference between the two groups. One-way ANOVA test for fertilization, cleavage and blastocyst formation rates between the two groups was highly significant (P ≤ 0.0001). Embryo grades (top, average and poor) differed significantly (one-way ANOVA P > 0.0001) between pregnant and non-pregnant groups.
Table 1.
Embryological data in pregnant versus non-pregnant groups
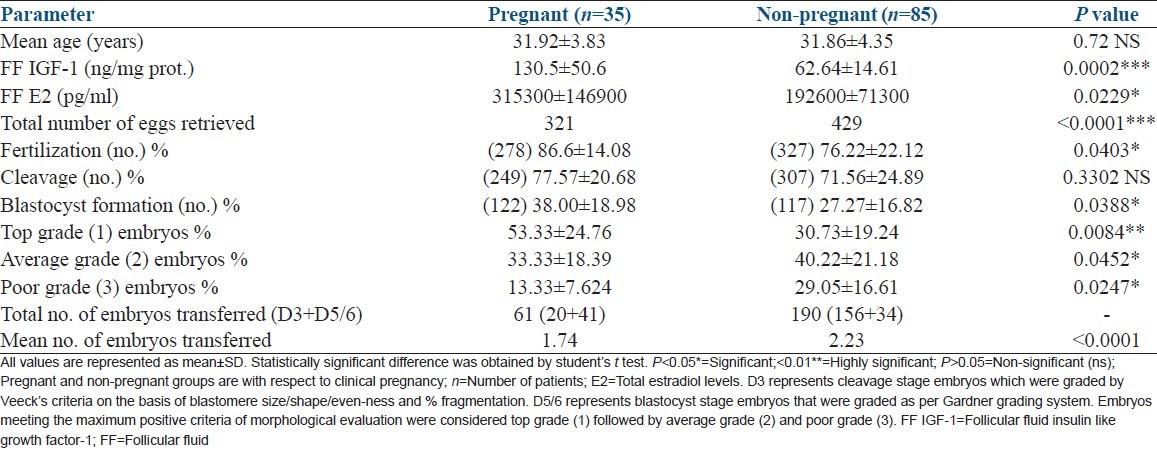
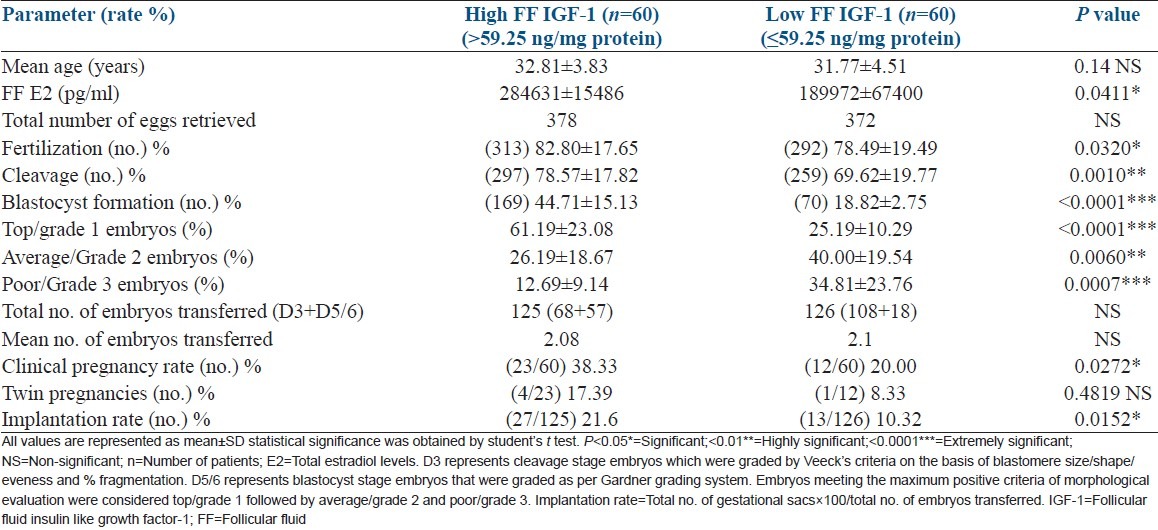
Table 2 represents a comparison of embryological data between high and low FF IGF-1 groups. Not only was the difference in fertilization and blastocyst formation rate more significantly pronounced; but cleavage rate also differed significantly between high versus low FF IGF-1 groups. Difference in embryo grades was also more significantly demarcated when cycles were divided into high and low IGF-1 groups (one-way ANOVA for embryo grades P > 0.0001). Notably, clinical pregnancy and embryo implantation rates showed considerable improvement, above the overall rates, when cycles were grouped on the basis of FF IGF-1 levels [Table 2]. Age of the women did not seem to have any impact on the FF IGF-1 levels in the selected group of patients [Tables 1 and 2].
Table 2.
Embryological data in high versus low FF IGF-1 groups
An interesting finding was the significant difference in FF IGF-1 levels between cycles involving day 5/6 blastocyst transfers (36 cycles, 75 blastocysts), day 3 ET (73 cycles, 153 embryos) and “forced” (due to few number of embryos available or due to patient's economic status) day 3 ET (11 cycles, 23 embryos). Cycles involving day 3 ET showed significantly lower FF IGF-1 compared with both: “forced” day 3 ET cycles (44.99 ± 14.69 vs. 124.0 ± 49.76, P > 0.0001) and blastocyst transfer cycles (44.99 ± 14.69 vs. 151.1 ± 71.20, P > 0.0001).
FF IGF-1 shared a very strong correlation with embryo quality (Pearson r = 0.3894) as well as clinical pregnancy (Pearson r = 0.5972) [Table 3]. Table 4 shows ROC curve data with a threshold value of FF IGF-1 for clinical pregnancy (<58.5 ng/mg protein, sensitivity 89.58%, specificity 60.24%).
Table 3.
Correlation of FF IGF-1 with embryo quality and clinical pregnancy
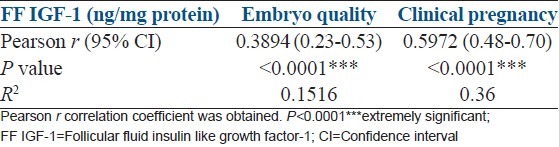
Table 4.
ROC analysis of FF IGF-1

DISCUSSION
This is the first ever study that has successfully investigated the significance of in situ FF IGF-1 in effecting embryo quality and its efficacy in influencing implantation rates. The presence of IGF-1 has been reported in FF. FF microenvironment may thus be considered to be a dynamic milieu with its rich source of growth factors and their binding proteins facilitating follicular growth and embryonic development. In recent years, several components have been assayed in monofollicular fluids (fluid obtained from each individual follicle) to study oocyte and embryo quality. However, studies in monofollicular fluids are cumbersome and have their limitations.[35] Though few studies have also focused on monodominant follicles (fluid obtained from single lead follicle), such estimations may not truly reflect granulosa or thecal cell production.[30] Therefore, in each cycle, we estimated IGF-1 levels in fluid pooled from follicles from which oocytes had been retrieved. It may also be pertinent to note that this study followed the recommended non-extraction method for estimation of IGF-1 as the extraction method has been reported to involve interference due to binding proteins.[31]
Our study was based on the hypothesis that apropos role of IGF-1 in follicular and embryonic development,[36] FF IGF-1 may be a potential biochemical marker of embryo quality and its implantation potential in IVF cycles. This contention is amply supported by our findings of significantly higher rates of fertilization, cleavage, blastocyst formation and top quality embryos in high FF IGF-1 group than in low FF IGF-1 group.
Two earlier studies,[26,27] had linked FF IGF-1 with follicular development and oocyte maturation but found no significant correlation with embryo cleavage rates. Bencomo et al. (2006)[28] reported a dose dependent decrease in the rate of apoptosis of human granulosa lutein (GL) cells and embryo fragmentation rates. However, their study was carried out with GL cells cultured in a medium exogenously supplemented by IGF-1 and could not correlate response to exogenous IGF-1 with IVF outcome. They also failed to establish a direct correlation between IGF-1 and embryo quality. Pertinently, in our study, cleavage rate was significantly much higher in high FF IGF-1 group compared with low FF IGF-1 group [Table 2] as opposed to the non-significant difference in cleavage rates observed between pregnant and non-pregnant groups [Table 1]. This finding emphasizes the role of IGF-1 as a cell cycle regulator through promotion of cell division. It is also evident from Table 1 that the fertilization and blastocyst formation rates remained significantly lower in non-pregnant group compared with pregnant group.
We also obtained a strong correlation of FF IGF-1 with embryo quality. Further, significant differences in FF IGF-1 levels observed between cycles involving day 5/6 blastocyst transfers, day 3 ET and “forced” day 3 ET indicates that the good quality embryos that were transferred on day 3 itself owing to the fewer number available or due to patient's economic considerations, also carried the potential to develop to blastocyst stage. This remarkable finding further underlines the competence of FF IGF-1 in regulating embryonic development, influencing its quality.
Another important finding of this study was the significant difference in estrogen levels observed not only between pregnant and non-pregnant groups [Table 1] but also between high and low FF IGF-1 groups [Table 2]. Our result corroborates with earlier observations implicating IGF-1 in mediating aromatase activity and estrogen production.[11,12,36]
The role of FF IGF-1 in predicting pregnancy outcome seemed ambiguous from previous studies. Though Dorn et al. (2003),[29] reported higher levels of IGF-1 in conception versus non-conception cycles in serum on the day of oocyte retrieval; no such association was observed in FF samples. Another study[30] found no significant difference in levels of FF IGF-1 or pregnancy rates in women undergoing IVF using agonist versus antagonist stimulation protocols. Yet another study,[37] indirectly indicated an IGF-1 mediated influence of embryo on the endometrial milieu during early implantation. However, our study not only reports significantly higher levels of FF IGF-1 in conception versus non-conception cycles but has also found a strong, direct correlation of FF IGF-1 with clinical pregnancy. In our study, when cycles were grouped on the basis of FF IGF-1 levels, high FF IGF-1 group showed significantly demarcated differences in embryo quality despite comparable number of eggs retrieved between low and high groups. Furthermore, a considerable improvement in clinical pregnancy and embryo implantation rates above the overall rates was obtained inspite of comparable number of embryos transferred, suggesting that FF IGF-1 is not only an add-on to morphological evaluation as an indicator of embryo quality but also influences clinical pregnancy and embryo implantation rates.
It was our conjecture that the FF micro-environmental milieu with its rich source of growth factors, cytokines and hormones may provide the trigger for embryonic development and may dictate the course of events leading to successful implantation of ensuing embryo. Therefore, evaluating levels of biochemical molecules in in situ sources like FF may be a more feasible approach than extrapolating data from animal models or cell-culture studies involving GL cells/endometrial tissue in culture exogenously supplemented by the molecule of interest. Moreover, as mentioned earlier, in each cycle, we used pooled FF obtained from a follicular cohort from which oocytes had been retrieved, to get a comprehensive replicate of granulosa cell function and assessment of embryo quality. Our stance stands vindicated by a direct correlation obtained in our study between FF IGF-1 levels and embryo quality (especially cleavage rates) as well as pregnancy outcome, both of which have not yet been established together for IGF-1 in IVF cycles by any of the earlier studies. However, supplementation of clinical findings and biochemical data with genetic studies like PGD could offer a more promising recourse in future studies on embryo quality. Another remarkable finding of higher fertilization rates in high FF IGF-1 and pregnant groups in our study may prompt future research into the significance of IGF-1 in effecting fertilization.
In conclusion, it may be said that this study establishes FF IGF-1 as a plausible biochemical marker of embryo quality and implantation rate. It also successfully correlates FF IGF-1 with clinical pregnancy rates in conventional IVF cycles.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mason HD, Martikainen H, Beard RW, Anyaoku V, Franks S. Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J Endocrinol. 1990;126:R1–4. doi: 10.1677/joe.0.126r001. [DOI] [PubMed] [Google Scholar]
- 2.Mason HD, Margara R, Winston RM, Seppala M, Koistinen R, Franks S. Insulin-like growth factor-I (IGF-I) inhibits production of IGF-binding protein-1 while stimulating estradiol secretion in granulosa cells from normal and polycystic human ovaries. J Clin Endocrinol Metab. 1993;76:1275–9. doi: 10.1210/jcem.76.5.7684393. [DOI] [PubMed] [Google Scholar]
- 3.Adashi EY, Resnick CE, D’Ercole AJ, Svoboda ME, Van Wyk JJ. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev. 1985;6:400–20. doi: 10.1210/edrv-6-3-400. [DOI] [PubMed] [Google Scholar]
- 4.Gates GS, Bayer S, Seibel M, Poretsky L, Flier JS, Moses AC. Characterization of insulin-like growth factor binding to human granulosa cells obtained during in vitro fertilization. J Recept Res. 1987;7:885–902. doi: 10.3109/10799898709054568. [DOI] [PubMed] [Google Scholar]
- 5.Di Blasio AM, Viganó P, Ferrari A. Insulin-like growth factor-II stimulates human granulosa-luteal cell proliferation in vitro. Fertil Steril. 1994;61:483–7. doi: 10.1016/s0015-0282(16)56580-7. [DOI] [PubMed] [Google Scholar]
- 6.Christman GM, Randolph JF, Jr, Peegel H, Menon KM. Differential responsiveness of luteinized human granulosa cells to gonadotropins and insulin-like growth factor I for induction of aromatase activity. Fertil Steril. 1991;55:1099–105. doi: 10.1016/s0015-0282(16)54359-3. [DOI] [PubMed] [Google Scholar]
- 7.Yong EL, Baird DT, Yates R, Reichert LE, Jr, Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab. 1992;74:842–9. doi: 10.1210/jcem.74.4.1548349. [DOI] [PubMed] [Google Scholar]
- 8.Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: Follicle-stimulating hormone as a major survival factor. Endocrinology. 1996;137:1447–56. doi: 10.1210/endo.137.4.8625923. [DOI] [PubMed] [Google Scholar]
- 9.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–82. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 10.Eden JA, Jones J, Carter GD, Alaghband-Zadeh J. A comparison of follicular fluid levels of insulin-like growth factor-1 in normal dominant and cohort follicles, polycystic and multicystic ovaries. Clin Endocrinol (Oxf) 1988;29:327–36. doi: 10.1111/j.1365-2265.1988.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 11.Devoto L, Kohen P, Castro O, Vega M, Troncoso JL, Charreau E. Multihormonal regulation of progesterone synthesis in cultured human midluteal cells. J Clin Endocrinol Metab. 1995;80:1566–70. doi: 10.1210/jcem.80.5.7745001. [DOI] [PubMed] [Google Scholar]
- 12.Devoto L, Kohen P, Vega M, Castro O, González RR, Retamales I, et al. Control of human luteal steroidogenesis. Mol Cell Endocrinol. 2002;186:137–41. doi: 10.1016/s0303-7207(01)00654-2. [DOI] [PubMed] [Google Scholar]
- 13.Owen EJ, Torresani T, West C, Mason BA, Jacobs HS. Serum and follicular fluid insulin like growth factors I and II during growth hormone co-treatment for in-vitro fertilization and embryo transfer. Clin Endocrinol (Oxf) 1991;35:327–34. doi: 10.1111/j.1365-2265.1991.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 14.Giudice LC. Insulin-like growth factors and ovarian follicular development. Endocr Rev. 1992;13:641–69. doi: 10.1210/edrv-13-4-641. [DOI] [PubMed] [Google Scholar]
- 15.Huyser C, Fourie FL, Bosmans E, Levay PF. Interleukin-1 beta, interleukin-6, and growth hormone levels in human follicular fluid. J Assist Reprod Genet. 1994;11:193–202. doi: 10.1007/BF02211808. [DOI] [PubMed] [Google Scholar]
- 16.Thierry van Dessel HJ, Chandrasekher Y, Yap OW, Lee PD, Hintz RL, Faessen GH, et al. Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab. 1996;81:1224–31. doi: 10.1210/jcem.81.3.8772603. [DOI] [PubMed] [Google Scholar]
- 17.Mendoza C, Ruiz-Requena E, Ortega E, Cremades N, Martinez F, Bernabeu R, et al. Follicular fluid markers of oocyte developmental potential. Hum Reprod. 2002;17:1017–22. doi: 10.1093/humrep/17.4.1017. [DOI] [PubMed] [Google Scholar]
- 18.Cunha-Filho JS, Lemos NA, Freitas FM, Kiefer K, Faller M, Passos EP. Insulin-like growth factor (IGF)-1 and IGF binding protein-1 and-3 in the follicular fluid of infertile patients with endometriosis. Hum Reprod. 2003;18:423–8. doi: 10.1093/humrep/deg077. [DOI] [PubMed] [Google Scholar]
- 19.Holtorf AP, Furuya K, Ivell R, McArdle CA. Oxytocin production and oxytocin messenger ribonucleic acid levels in bovine granulosa cells are regulated by insulin and insulin-like growth factor-I: Dependence on developmental status of the ovarian follicle. Endocrinology. 1989;125:2612–20. doi: 10.1210/endo-125-5-2612. [DOI] [PubMed] [Google Scholar]
- 20.Guthrie HD, Garrett WM, Cooper BS. Follicle-stimulating hormone and insulin-like growth factor-I attenuate apoptosis in cultured porcine granulosa cells. Biol Reprod. 1998;58:390–6. doi: 10.1095/biolreprod58.2.390. [DOI] [PubMed] [Google Scholar]
- 21.Rao JU, Shah KB, Puttaiah J, Rudraiah M. Gene expression profiling of preovulatory follicle in the buffalo cow: Effects of increased IGF-I concentration on periovulatory events. PLoS One. 2011;6(6):e20754. doi: 10.1371/journal.pone.0020754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergh C, Carlsson B, Olsson JH, Billig H, Hillensjö T. Effects of insulin-like growth factor I and growth hormone in cultured human granulosa cells. Ann N Y Acad Sci. 1991;626:169–76. doi: 10.1111/j.1749-6632.1991.tb37911.x. [DOI] [PubMed] [Google Scholar]
- 23.Irwin JC, de las Fuentes L, Giudice LC. Growth factors and decidualization in vitro. Ann N Y Acad Sci. 1994;734:7–18. doi: 10.1111/j.1749-6632.1994.tb21730.x. [DOI] [PubMed] [Google Scholar]
- 24.Irwin JC, Utian WH, Eckert RL. Sex steroids and growth factors differentially regulate the growth and differentiation of cultured human endometrial stromal cells. Endocrinology. 1991;129:2385–92. doi: 10.1210/endo-129-5-2385. [DOI] [PubMed] [Google Scholar]
- 25.Geisthoevel F, Moretti-Rojas IM, Rojas FJ, Asch RH. Immunoreactive insulin-like growth factor I in human follicular fluid. Hum Reprod. 1989;4:35–8. doi: 10.1093/oxfordjournals.humrep.a136841. [DOI] [PubMed] [Google Scholar]
- 26.Jimena P, Castilla JA, Peran F, Molina R, Ramirez JP, Acebal M, et al. Insulin and insulin-like growth factor I in follicular fluid after induction of ovulation in women undergoing in vitro fertilization. J Reprod Fertil. 1992;96:641–7. doi: 10.1530/jrf.0.0960641. [DOI] [PubMed] [Google Scholar]
- 27.Stadtmauer L, Vidali A, Lindheim SR, Sauer MV. Follicular fluid insulin-like growth factor-I and insulin-like growth factor-binding protein-1 and-3 vary as a function of ovarian reserve and ovarian stimulation. J Assist Reprod Genet. 1998;15:587–93. doi: 10.1023/A:1020377209952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bencomo E, Pérez R, Arteaga MF, Acosta E, Peña O, Lopez L, et al. Apoptosis of cultured granulosa-lutein cells is reduced by insulin-like growth factor I and may correlate with embryo fragmentation and pregnancy rate. Fertil Steril. 2006;85:474–80. doi: 10.1016/j.fertnstert.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Dorn C, Reinsberg J, Kupka M, van der Ven H, Schild RL. Leptin, VEGF, IGF-1, and IGFBP-3 concentrations in serum and follicular fluid of women undergoing in vitro fertilization. Arch Gynecol Obstet. 2003;268:187–93. doi: 10.1007/s00404-002-0366-8. [DOI] [PubMed] [Google Scholar]
- 30.Choi YS, Ku SY, Jee BC, Suh CS, Choi YM, Kim JG, et al. Comparison of follicular fluid IGF-I, IGF-II, IGFBP-3, IGFBP-4 and PAPP-A concentrations and their ratios between GnRH agonist and GnRH antagonist protocols for controlled ovarian stimulation in IVF-embryo transfer patients. Hum Reprod. 2006;21:2015–21. doi: 10.1093/humrep/del091. [DOI] [PubMed] [Google Scholar]
- 31.Roussi M, Royère M, Guillonueau M, Lansac J, Muh JP. Human antral fluid IGF-I and oocyte maturity: Effect of stimulation therapy. Acta Endocrinol (Copenh) 1989;121:90–4. doi: 10.1530/acta.0.1210090. [DOI] [PubMed] [Google Scholar]
- 32.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 33.Veeck LL. Atlas of the Human Oocyte and Early Conceptus. Baltimore: Williams and Wilkins; 1991. pp. 121–246. [Google Scholar]
- 34.Gardner DK, Schoolcraft WB, Wagley L, Schlenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in-vitro fertilization. Hum Reprod. 1998;13:3434–40. doi: 10.1093/humrep/13.12.3434. [DOI] [PubMed] [Google Scholar]
- 35.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erickson GF, Garzo VG, Magoffin DA. Insulin-like growth factor-I regulates aromatase activity in human granulosa and granulosa luteal cells. J Clin Endocrinol Metab. 1989;69:716–24. doi: 10.1210/jcem-69-4-716. [DOI] [PubMed] [Google Scholar]
- 37.Fluhr H, Carli S, Deperschmidt M, Zwirner M, Wallwiener D, Licht P. Different expression of IGF-1, IGF-2 and IGF-1R in human endometrial stromal cells during decidualization in vitro and under the influence of hCG. Fertil Steril. 2006;86:S278–9. [Google Scholar]


