Abstract
BACKGROUND:
Immunotherapy with paternal lymphocytes plays an important role in preventing recurrent spontaneous abortion (RSA) and is an effective treatment for it. This kind of treatment is performed as an immunotherapy method in several centers in the world. It attributes to the production of anti-paternal cytotoxic antibodies (APCAs) in women with RSA. Production of APCA after lymphocyte immunotherapy (LIT) in RSA patients gives them a better chance for successful pregnancy. Regarding the important effect of trace elements on the function of the immune system, we tried to investigate the correlation between serum zinc level and the success of LIT in RSA.
MATERIALS AND METHODS:
Serum zinc concentration was determined in two groups of RSA patients using atomic absorption spectrophotometer systems. Group (a) that responded to the paternal lymphocytes and their cross-match test was positive, and group (b) that had no response to the paternal lymphocytes immunizations and their cross-match test was negative.
RESULTS:
Serum zinc levels in group (a) patients were 74.98 ± 11.88 μg/dl, which was significantly higher than those in group (b) with the zinc concentration of 64.22 ± 9.22 μg/dl.
CONCLUSIONS:
Zinc deficiency may be one of the substantial causes of negative results for LIT in RSA patients. Therefore, compensation of zinc defect before LIT can be a promising approach to improve the immune response in patients.
KEY WORDS: Immunotherapy, recurrent spontaneous abortion, serum zinc levels
INTRODUCTION
Recurrent spontaneous abortion (RSA) is considered as three or more clinically detectable failure of pregnancy that occurs before 20 or even 28 weeks or abortion of fetus weighting >500 g.[1,2,3,4]
RSA is the most common complication of pregnancy with the involvement of 1 in 300 pregnant women.[3] Several factors assume to have a correlation with RSA, including genetic factors,[5,6] anatomical problems,[7,8] endocrine hormones,[9] infections,[10] placental abnormalities, alcohol and tobacco, and exposure to environmental factors such as lead, ethylene oxide, ionizing radiation,[11] and immunological factors.[12,13,14,15]
Immunological relationship between mother and fetus is due to interaction of embryonic antigens and mother's immune system that recognizes and reacts with foreign antigens. There is clear evidence that shows recognition of fetus antigens is essential for maintaining pregnancy and that inadequate recognition may lead to abortion in RSA women.[3,16,17]
Many studies confirm the association between recurrent abortions and parent similarity of human leukocyte antigens (HLA). This similarity induces hyporesponsiveness and can inhibit production of humeral blocker antibodies as immunological regulators to maintain the pregnancy. Anti-paternal cytotoxic antibodies (APCAs), anti-idiopathic antibodies (Ab2), and mixed lymphocyte-reaction blocking antibodies (MLR-Bf) are a part of regulators that cover paternal HLA molecules in the surface of fetuses and make a barrier for attacking the maternal T cells and NK cells.[1,16,18,19] Production of APCA, Ab2, and MLR-Bf antibodies, inhibition of T lymphocytes by reducing the maternal IL-2 receptors, shifting of the Th1 to Th2 immune response, and decreasing NK cell function are beneficial effects of immunotherapy with paternal lymphocytes.[3,4,18,20,21,22]
On the other hand, in an effective treatment, the white blood cells (WBC) cross-match test becomes positive, which is a promising indicative of a high chance of pregnancy. Increased pregnancy outcome was observed after immunotherapy with paternal lymphocyte in RSA patients by many research groups in the world[20,21,23,24,25,26] as well as in our previous investigation at Sarem Infertility Center.[23] However, the efficacy of lymphocyte immunotheraphy (LIT) for RSA was doubted by some researchers, because no beneficial effect was found after LIT.[27,28] According to previous studies, the effectiveness of LIT in different patients is variable and the cross-match test becomes positive in some cases only.[3,23] This difference in response to immunotherapy might be associated with various factors such as the amount of trace elements. In this research study, zinc is suggested to have a critical effect on the outcome of immunotherapy.
Zinc is an essential trace element that has multifunctional activity in the body as follows:
Cell division, muscle growth, function of the hormones, blood coagulation, hair and nails growth, immune system function, menstrual disorders in women regeneration, reproduction, and fertility in men. There is a complicate relationship between zinc and the immune system.[29,30,31,32,33,34,35]
Because of the considerable impact of zinc on specific and nonspecific immune responses, its deficiency can lead to reduction of appropriate responses.[36,37,38,39,40,41,42,43]
Regarding the importance of APCA production after LIT, we tried to investigate the relationship between serum zinc level and the success of LIT in RSA patients.
MATERIALS AND METHODS
Patients
Six hundred and thirty-five age-matched patients with a history of miscarriage, who referred to Sarem women's hospital, were evaluated during a 10-month period. The exclusion criteria were abnormal karyotype of both parents, impaired glucose tolerance test, any anatomical abnormalities, intrauterine adhesions, cervical incompetence revealed during hysterosalpingography, positive toxoplasmosis serology, any thyroid dysfunction, any abnormality in luteal phase, prolactin level, and presence of anti-nuclear factor or anticardiolipin antibodies. Finally, 240 women who tested negative or normal to all the above screening tests and had a history of three or more consecutive abortions were included in the study. Also, in this study, 70 normal women age-matched with the RSA patients without any history of abortion having 1-3 children, who had referred for checking up, were studied as healthy controls.
After LIT, we divided the patients with RSA into two groups: Group (a) included those who responded to the LIT and showed a positive cross-match test (≥30%). Group (b) included those who had no response to LIT with a negative cross-match test result (>30%).
This study was approved by the ethics committee at Immunology, Asthma and Allergy Research Institute and Ethics committee at Sarem women's hospital. Also, all subjects completed consent form for this study.
Immunization procedure
This procedure was described by Orgad et al.[21] Briefly, paternal peripheral blood mononuclear cell (PBMC) were prepared from 20 mL heparinized blood in sterile conditions.
The 80-85 × 10 6 PBMC were separated by Ficoll-Hypaque density centrifugation (Cederland, Canada). Then, the PBMCs were resuspended in 4 mL of 0.9% Hartman's solution. The aliquots of 0.5 mL were injected twice subcutaneously in a 3-week interval into forearm or arm. Two weeks after the second immunization, the patients’ sera were collected and tested for APCA and serum zinc levels.
A cross-match test between maternal undiluted fresh serum and paternal fresh peripheral PBMCs was performed for the measurement of APCA. Cross-match result was considered positive when the antibodies in maternal serum could react and kill paternal PBMCs at a proportion of more than 30% in comparison to negative serum. Cell viability was assessed by counting the eosin-stained cells.[23]
Determination of serum zinc
Serum samples were evaluated in terms of zinc concentration using GBC Atomic Absorption Spectrophotometer Systems (Victoria, Australia).
All samples and standards were analyzed in duplicates. The accuracy of the procedure was evaluated by analyzing commercially available samples of lyophilized human serum trace element, seronorm™, level 1 and 2 (Seronorm, UK).
Statistical analysis
Kolmogorov-Smirnov test was carried out for assessing normal distribution. Differences in zinc concentration between groups were analyzed with the Mann-Whitney U-test. P > 0.05 was considered significant. The data were expressed as means ± SD. Statistical analysis was done using SPSS16 Inc.
RESULTS
In the present study, 240 females with RSA and the mean age of 31.76 ± 5.64 years were selected. The cross-match test was positive in 86 patients with a mean age of 31.6 ± 5.06 years (21-42 years) and negative for 154 patients with a mean age of 31.84 ± 5.96 (20-49 years).
The mean concentration of serum zinc levels in group (a) was 74.98 ± 11.88 μg/dl, which was significantly higher than those in group (b) with the concentration of 64.22 ± 9.22μg/dl (P > 0.001).
Also, serum zinc level in healthy controls with a mean age of 32.37 ± 5.76 years and without any history of abortion was 82.90 ± 12.36 μg/dl.
In Figure 1, we compared serum concentrations of zinc in group (a) (APCA positive) and group (b) (APCA negative) to those of normal subjects (normal). Our results showed that serum zinc level in group (b) was significantly lower than that in group (a) and healthy controls. As we indicated in Figure 2, there was a significant and positive correlation (r = 0.462) between serum zinc level and APCA production in patients treated with paternal PBMC. Patient with lower levels of zinc could not produce APCA as an indicative of humoral immune response to immunotherapy.
Figure 1.
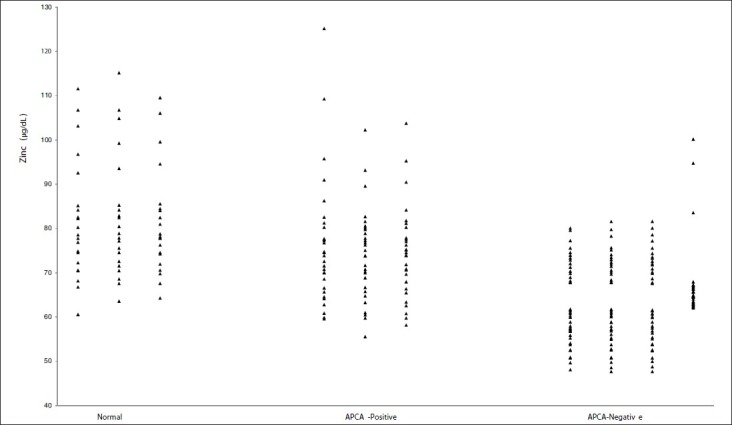
Serum zinc levels in normal subjects (normal) and two groups of RSA patients: Group (a) (APCA positive) responded to the paternal lymphocytes and their cross-match test was positive and group (b) (APCA negative) did not respond to paternal lymphocytes immunizations and their cross-match test was negative
Figure 2.
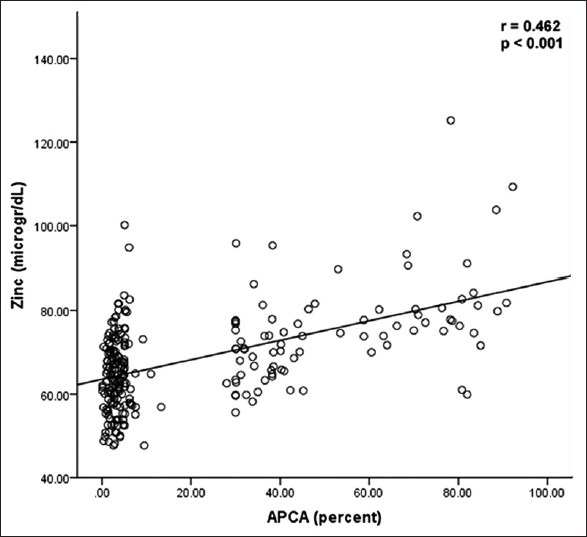
Correlation between serum zinc level and APCA production in patients treated with paternal PBMC; patient with zinc deficiency could not respond to immunotherapy
DISCUSSION
Several different studies have investigated the effect of zinc supplementation on the function of the immune system, especially production of specific antibodies after vaccination. Kreft et al., and Provinciali et al., showed that immune response to vaccination dependent on the zinc status in patients. In those patients, non responders had lower serum zinc levels and supplementation with zinc could elevate the amount of antibody after vaccination.[37,44,45] Izdebska-Szymona et al., reported the effect of zinc on humoral and cellular response in mice and found that anti-SRBC antibody levels was greater in supplemented mice than in control ones.[40] Zinc deficiency can affect the immune response and the production of antibodies. Because the aim of LIT is production of blocker antibodies such as APCA, zinc deficiency can affect it.
Chaichian et al., in Sarem Infertility Center followed-up 93 patients with positive cross-match test after LIT. In this study, pregnancy occurred in 49 of 93 patients, which was clinically successful in about 52.7% of them.[23] In the present study, among 86 patients with positive cross-match test, we could follow-up 74 patients. Of them, 51 (68.9%) patients were revealed to have documented pregnancy and, among them, successful clinical pregnancy (gestational age of at least 28 weeks) were recorded in only 38 subjects. Therefore, successful clinical pregnancy was reported in 51.3% (38/74) of those with positive APCA. In present study, was observed that different patients have variable responses to this kind of immunotherapy and the cross-match test becomes negative in most cases (154 patients). On the other hand, some studies reported that LIT for unexplained RSA had no beneficial effect,[27,28] and, until date, the use of LIT for the treatment of RSA remains a matter of controversy. Lack of response to LIT may be associated with the amount of zinc.
To the best of our knowledge, there has been no study on correlation between serum zinc level and success of LIT in RSA. Apgar et al., showed that zinc intake affects the maintenance of guinea pig pregnancy.[46] Also, Graham et al., in 2009 demonstrated that serum zinc and copper concentrations had a positive correlation with spontaneous abortion in cows.[47] In this study, patients with RSA who responded to LIT due to their positive cross-match had higher serum zinc levels than those with negative cross-match test. In other words, LIT could not be effective for induction of APCA production in zinc-deficient patients.
In this study, we also revealed that serum zinc levels were higher in normal age-matched group of women than both groups of patients (with positive and negative cross-match results).
In conclusion, supplementation of RSA patient with zinc before LIT is highly recommended and would be a promising strategy to produce more blocking antibodies after immunotherapy toward keeping the fetus in patients with RSA.
ACKNOWLEDGMENTS
This work was supported by research grants from Tehran University of Medical Sciences and Health Services, Immunology, Asthma and Allergy Research Institute and the Sarem Women's Hospital, Sarem Cell Research Center, Tehran, Iran. The authors report no conflicts of interest.
Footnotes
Source of Support: Tehran University of Medical Sciences and Health Services, Immunology, Asthma and Allergy Research Institute and the Sarem Women's Hospital, Sarem Cell Research Center, Tehran, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Salat-Baroux J. Recurrent spontaneous abortions. Reprod Nutr Dev. 1988;28:1555–68. [PubMed] [Google Scholar]
- 2.Crosignani PG, Rubin BL. Recurrent spontaneous abortion. Hum Reprod. 1991;6:609–10. doi: 10.1093/oxfordjournals.humrep.a137390. [DOI] [PubMed] [Google Scholar]
- 3.Pandey MK, Thakur S, Agrawal S. Lymphocyte immunotherapy and its probable mechanism in the maintenance of pregnancy in women with recurrent spontaneous abortion. Arch Gynecol Obstet. 2004;269:161–72. doi: 10.1007/s00404-003-0560-3. [DOI] [PubMed] [Google Scholar]
- 4.Yokoo T, Takakuwa K, Ooki I, Kikuchi A, Tamura M, Tanaka K. Alteration of TH1 and TH2 cells by intracellular cytokine detection in patients with unexplained recurrent abortion before and after immunotherapy with the husband's mononuclear cells. Fertil Steril. 2006;85:1452–8. doi: 10.1016/j.fertnstert.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez JM, Franzi L, Collia F, De Diaz SL, Panal M, Dubner M. Cytogenetic study of spontaneous abortions by transabdominal villus sampling and direct analysis of villi. Prenat Diagn. 1999;19:601–3. [PubMed] [Google Scholar]
- 6.Tunc E, Demirhan O, Demir C, Tastemir D. Cytogenetic study of recurrent miscarriages and their parents. Genetika. 2007;43:545–52. [PubMed] [Google Scholar]
- 7.Hill JA, Polgar K, Harlow BL, Anderson DJ. Evidence of embryo- and trophoblast-toxic cellular immune response(s) in women with recurrent spontaneous abortion. Am J Obstet Gynecol. 1992;166:1044–52. doi: 10.1016/s0002-9378(11)90589-4. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66:24–9. [PubMed] [Google Scholar]
- 9.Arredondo F, Noble LS. Endocrinology of recurrent pregnancy loss. Semin Reprod Med. 2006;24:33–9. doi: 10.1055/s-2006-931799. [DOI] [PubMed] [Google Scholar]
- 10.Nigro G, Mazzocco M, Mattia E, Di Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Neonatal Med. 2011;24:983–9. doi: 10.3109/14767058.2010.547963. [DOI] [PubMed] [Google Scholar]
- 11.Gardella JR, Hill JA., 3rd Environmental toxins associated with recurrent pregnancy loss. Semin Reprod Med. 2000;18:407–24. doi: 10.1055/s-2000-13731. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen HS, Witvliet MD, Steffensen R, Haasnoot GW, Goulmy E, Christiansen OB, et al. The presence of HLA-antibodies in recurrent miscarriage patients is associated with a reduced chance of a live birth. J Reprod Immunol. 2010;87:67–73. doi: 10.1016/j.jri.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Hadinedoushan H, Mirahmadian M, Aflatounian A. Increased natural killer cell cytotoxicity and IL-2 production in recurrent spontaneous abortion. Am J Reprod Immunol. 2007;58:409–14. doi: 10.1111/j.1600-0897.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 14.Saini V, Arora S, Yadav A, Bhattacharjee J. Cytokines in recurrent pregnancy loss. Clin Chim Acta. 2011;412:702–8. doi: 10.1016/j.cca.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Daher S, de Arruda Geraldes Denardi K, Blotta MH, Mamoni RL, Reck AP, Camano L, et al. Cytokines in recurrent pregnancy loss. J Reprod Immunol. 2004;62:151–7. doi: 10.1016/j.jri.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Coulam CB, Goodman C, Roussev RG, Thomason EJ, Beaman KD. Systemic CD56 + cells can predict pregnancy outcome. Am J Reprod Immunol. 1995;33:40–6. doi: 10.1111/j.1600-0897.1995.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramhorst R, Agriello E, Zittermann S, Pando M, Larriba J, Irigoyen M, et al. Is the paternal mononuclear cells’ immunization a successful treatment for recurrent spontaneous abortion? Am J Reprod Immunol. 2000;44:129–35. doi: 10.1111/j.8755-8920.2000.440301.x. [DOI] [PubMed] [Google Scholar]
- 18.Li TC, Makris M, Tomsu M, Tuckerman E, Laird S. Recurrent miscarriage: Aetiology, management and prognosis. Hum Reprod Update. 2002;8:463–81. doi: 10.1093/humupd/8.5.463. [DOI] [PubMed] [Google Scholar]
- 19.Makino T. Recurrent reproductive wastage and immunologic factors. Am J Reprod Immunol. 2002;48:266–8. doi: 10.1034/j.1600-0897.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- 20.Coulam CB. Report from the Ethics Committee for Immunotherapy. Am J Reprod Immunol. 1993;30:45–7. doi: 10.1111/j.1600-0897.1993.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 21.Orgad S, Loewenthal R, Gazit E, Sadetzki S, Novikov I, Carp H. The prognostic value of anti-paternal antibodies and leukocyte immunizations on the proportion of live births in couples with consecutive recurrent miscarriages. Hum Reprod. 1999;14:2974–9. doi: 10.1093/humrep/14.12.2974. [DOI] [PubMed] [Google Scholar]
- 22.Kaji T, Mishima A, Koyanagi E, Yamamoto C, Sakamoto M, Kozuka H. Possible mechanism for zinc protection against cadmium cytotoxicity in cultured vascular endothelial cells. Toxicology. 1992;76:257–70. doi: 10.1016/0300-483x(92)90194-j. [DOI] [PubMed] [Google Scholar]
- 23.Chaichian S, Shoaee S, Saremi A, Pedar S, Firouzi F. Factors influencing success rate of leukocyte immunization and anti-paternal antibodies in spontaneous recurrent miscarriage. Am J Reprod Immunol. 2007;57:169–76. doi: 10.1111/j.1600-0897.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 24.Khonina NA, Broitman EV, Shevela EY, Pasman NM, Chernykh ER. Mixed lymphocyte reaction blocking factors (MLR-Bf) as potential biomarker for indication and efficacy of paternal lymphocyte immunization in recurrent spontaneous abortion. Arch Gynecol Obstet. 2013 doi: 10.1007/s00404-013-2832-x. [DOI] [PubMed] [Google Scholar]
- 25.Wilczynski JR, Radwan P, Tchorzewski H, Banasik M. Immunotherapy of patients with recurrent spontaneous miscarriage and idiopathic infertility: Does the immunization-dependent Th2 cytokine overbalance really matter? Arch Immunol Ther Exp (Warsz) 2012;60:151–60. doi: 10.1007/s00005-012-0161-6. [DOI] [PubMed] [Google Scholar]
- 26.Liang P, Mo M, Li GG, Yin B, Cai J, Wu T, et al. Comprehensive analysis of peripheral blood lymphocytes in 76 women with recurrent miscarriage before and after lymphocyte immunotherapy. Am J Reprod Immunol. 2012;68:164–74. doi: 10.1111/j.1600-0897.2012.01141.x. [DOI] [PubMed] [Google Scholar]
- 27.Illeni MT, Marelli G, Parazzini F, Acaia B, Bocciolone L, Bontempelli M, et al. Immunotherapy and recurrent abortion: A randomized clinical trial. Hum Reprod. 1994;9:1247–9. doi: 10.1093/oxfordjournals.humrep.a138687. [DOI] [PubMed] [Google Scholar]
- 28.Ober C, Karrison T, Odem RR, Barnes RB, Branch DW, Stephenson MD, et al. Mononuclear-cell immunisation in prevention of recurrent miscarriages: A randomised trial. Lancet. 1999;354:365–9. doi: 10.1016/S0140-6736(98)12055-X. [DOI] [PubMed] [Google Scholar]
- 29.Uriu-Adams JY, Keen CL. Zinc and reproduction: Effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res B Dev Reprod Toxicol. 2010;89:313–25. doi: 10.1002/bdrb.20264. [DOI] [PubMed] [Google Scholar]
- 30.Haase H, Rink L. The immune system and the impact of zinc during aging. Immun Ageing. 2009;6:9. doi: 10.1186/1742-4933-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferencik M, Ebringer L. Modulatory effects of selenium and zinc on the immune system. Folia Microbiol (Praha) 2003;48:417–26. doi: 10.1007/BF02931378. [DOI] [PubMed] [Google Scholar]
- 32.Zinc and the immune system. Treatment Update. 2001;13:1–2. [PubMed] [Google Scholar]
- 33.Salgueiro MJ, Zubillaga M, Lysionek A, Cremaschi G, Goldman CG, Caro R, et al. Zinc status and immune system relationship: A review. Biol Trace Elem Res. 2000;76:193–205. doi: 10.1385/BTER:76:3:193. [DOI] [PubMed] [Google Scholar]
- 34.Turk S, Bozfakioglu S, Ecder ST, Kahraman T, Gurel N, Erkoc R, et al. Effects of zinc supplementation on the immune system and on antibody response to multivalent influenza vaccine in hemodialysis patients. Int J Artif Organs. 1998;21:274–8. [PubMed] [Google Scholar]
- 35.Licastro F, Mocchegiani E, Masi M, Fabris N. Modulation of the neuroendocrine system and immune functions by zinc supplementation in children with Down's syndrome. J Trace Elem Electrolytes Health Dis. 1993;7:237–9. [PubMed] [Google Scholar]
- 36.Haase H, Mazzatti DJ, White A, Ibs KH, Engelhardt G, Hebel S, et al. Differential gene expression after zinc supplementation and deprivation in human leukocyte subsets. Mol Med. 2007;13:362–70. doi: 10.2119/2007-00049.Haase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase H, Mocchegiani E, Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. 2006;7:421–8. doi: 10.1007/s10522-006-9057-3. [DOI] [PubMed] [Google Scholar]
- 38.Overbeck S, Rink L, Haase H. Modulating the immune response by oral zinc supplementation: A single approach for multiple diseases. Arch Immunol Ther Exp (Warsz) 2008;56:15–30. doi: 10.1007/s00005-008-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta RP, Verma PC, Garg SR. Effect of experimental zinc deficiency on immunological responses in Salmonella-infected guinea-pigs. J Comp Pathol. 2000;123:1–6. doi: 10.1053/jcpa.2000.0376. [DOI] [PubMed] [Google Scholar]
- 40.Izdebska-Szymona K, Drela N, Kozlowska E, Kowalczyk R, Konopka E. Zinc affects humoral and cellular response in mice. Arch Immunol Ther Exp (Warsz) 1991;39:13–7. [PubMed] [Google Scholar]
- 41.Kruse-Jarres JD. The significance of zinc for humoral and cellular immunity. J Trace Elem Electrolytes Health Dis. 1989;3:1–8. [PubMed] [Google Scholar]
- 42.Wieringa FT, Dijkhuizen MA, West CE, van der Ven-Jongekrijg J, van der Meer JW. Reduced production of immunoregulatory cytokines in vitamin A- and zinc-deficient Indonesian infants. Eur J Clin Nutr. 2004;58:1498–504. doi: 10.1038/sj.ejcn.1601998. [DOI] [PubMed] [Google Scholar]
- 43.Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 44.Kreft B, Fischer A, Kruger S, Sack K, Kirchner H, Rink L. The impaired immune response to diphtheria vaccination in elderly chronic hemodialysis patients is related to zinc deficiency. Biogerontology. 2000;1:61–6. doi: 10.1023/a:1010077622172. [DOI] [PubMed] [Google Scholar]
- 45.Provinciali M, Montenovo A, Di Stefano G, Colombo M, Daghetta L, Cairati M, et al. Effect of zinc or zinc plus arginine supplementation on antibody titre and lymphocyte subsets after influenza vaccination in elderly subjects: A randomized controlled trial. Age Ageing. 1998;27:715–22. doi: 10.1093/ageing/27.6.715. [DOI] [PubMed] [Google Scholar]
- 46.Apgar J, Everett GA. Low zinc intake affects maintenance of pregnancy in guinea pigs. J Nutr. 1991;121:192–200. doi: 10.1093/jn/121.2.192. [DOI] [PubMed] [Google Scholar]
- 47.Graham TW, Thurmond MC, Gershwin ME, Picanso JP, Garvey JS, Keen CL. Serum zinc and copper concentrations in relation to spontaneous abortion in cows: Implications for human fetal loss. J Reprod Fertil. 1994;102:253–62. doi: 10.1530/jrf.0.1020253. [DOI] [PubMed] [Google Scholar]


