Abstract
CONTEXT:
Artificial reproductive techniques using seminal preparations with bacteria may cause pelvic inflammatory disease and its sequalae.
AIMS:
To assess efficacy of two sperm preparation techniques to clear bacteria and the effect of bacteriospermia on sperm recovery rates.
SETTINGS AND DESIGN:
A descriptive cross-sectional study was carried out among males of subfertile couples.
SUBJECTS AND METHODS:
Semen samples were randomly allocated into swim-up method (group S, n = 68) and density gradient method (group D, n = 50) for sperm preparation. Seminal fluid analysis and bacterial cultures were performed in each sample before and after sperm preparation.
STATISTICAL ANALYSIS:
McNemar's chi-squared test and independent samples t-test in SPSS version 16.0 were used.
RESULTS:
Organisms were found in 86 (72.88%) out of 118 samples, before sperm preparation; Streptococcus species (n = 40, 46.51% of which 14 were Group D Streptococcus species), Coagulase negative Staphylococcus species (n = 17, 19.76%), Staphylococcus aureus (n = 13, 15.11%), Coliform species (n = 11, 12.79% of which 09 were Escherichia coli) and Corynebacterium species (n = 5, 5.81%). There was a statistically significant reduction of culture positive samples in raw vs. processed samples; in group S, 49 (72.05%) vs. 16 (23.52%) and in group D, 37 (74%) vs. 18 (36%). In group S and D, mean (SD) recovery rates of culture positive vs. culture negative samples were 39.44% (SD-14.02) vs. 44.22% (SD-22.38), P = 0.39 and 52.50% (SD-37.16) vs. 49.58% (SD-40.32), P = 0.82 respectively.
CONCLUSIONS:
Both sperm preparation methods significantly reduced bacteria in semen, but total clearance was not achieved. Sperm recovery rate was not affected by bacteriospermia.
KEY WORDS: Bacteriospermia, density gradient, sperm recovery rate, swim-up
INTRODUCTION
Bacteriospermia is the presence of bacteria in seminal fluid. Detection of bacteria in semen does not necessarily signify infection as bacteriospermia may represent contamination during sample collection, bacterial colonization of the distal segment of the urethra or infection.[1,2,3] Testes, epididymis, vas deferens, ejaculatory ducts, and the proximal portion of the urethra are usually devoid of bacteria in a normal male. Fluid from the vas deferens of men undergone vasectomy has uniformly yielded negative culture results.[4] Semen that passes through the genital tract is routinely contaminated by gram-positive bacteria, usually Staphylococcus species, Streptococcus species and Corynebacterium species.[5] A significant growth of bacteria in culture; >103 colony forming units/ml (CFU/ml), detection of Neisseria gonorrhoeae, Chlamydia trachomatis, Ureaplasma urealyticam and/or significant leukocytospermia (>106 peroxidase-positive leukocytes/ml) may indicate an infection.[5,6] Prevalence of bacteriospermia and types of organisms found in seminal fluid vary depending on the populations studied and methods used for the detection of bacteria. When polymerase chain reaction (PCR)-based bacterial detection methods were used, the prevalence of bacteriospermia was significantly higher[7,8] compared to other methods used in different studies.[9,10] A recent study using molecular biological techniques showed that >104 CFU of bacteria/ml in 66% of asymptomatic subfertile men. But when using routine culture methods, significant growth was found only in 27% in the same population.[11] Chlamydia trachomatis, Mycoplasma hominis, Mycoplasma genitalium, Ureaplasma urealyticum, Ureaplasma parvum, and Gardnerella vaginalis are frequently found organisms in semen when specialized culture media and molecular biological techniques were used.[12,13]
According to Diemer et al., bacteriospermia may affect fertility and account for 15% of cases of male subfertility.[14] Infectious processes can impair fertility by different mechanisms, including male accessory sex gland dysfunction, triggering of anti-sperm antibody production, deterioration of spermatogenesis, impairment of sperm function, obstruction of the excurrent ductal system, phagocytosis, and cytokine-mediated destruction by leukocytes.[5,15,16]
Intrauterine insemination (IUI) is an artificial reproductive technique, which is widely used to treat factor subfertility. IUI with contaminated semen may cause pelvic inflammatory disease and its sequalae in women. IUI bypasses the cervical mucus and thus may be expected to have a higher incidence of infections. Stone et al., found that positive results from peritoneal cultures in five of nine women after IUI with washed sperm. But none of these women demonstrated clinical infection.[17] The incidence of clinical infection after IUI is low. The incidence of infection after IUI with no antibiotic cover and without any antibiotics added to the semen processing medium, varied 1.83 to 2.1 per 1000 patients.[18] Therefore, effective semen processing procedures should be employed to remove bacteria from semen.[19] One method of clearing the bacteria from semen is addition of antibiotics to the sperm processing media.[20] Penicillin and streptomycin are the widely used antibiotics. But some manufacturers do not provide them in a ready-to-use form. Also, there is no consensus on beneficial effects over harm to the sperm from the use of antibiotics. Use of sterile techniques in sperm processing would help to minimize or eliminate bacteria from the post-wash sperm samples.
Swim up and density gradient sperm preparation techniques vary greatly in terms of recovery rates, motility, morphology, and degree of DNA damage.[21,22] These parameters influence the fertilization rates following IUI. The ability of sperm preparation techniques to clear bacterial species from the seminal fluid and effect of bacteriospermia on recovery rates of sperms are important aspects of sperm preparation. The objectives of this study were to assess the efficacy of swim up and density gradient techniques in clearing non-specific bacteria from seminal plasma and the effect of bacteriospermia on recovery rates of sperms in males of subfertile couples.
SUBJECTS AND METHODS
A descriptive cross-sectional study was carried out from June 2012 to January 2013. Ethical clearance was obtained from the ethics review committee of the institute. All consenting males of subfertile couples were included in the study after considering the exclusion criteria [Figure 1]. Semen samples were collected into sterile wide-mouthed polystyrene containers, after two to seven days of sexual abstinence. Males were advised to pass urine half an hour prior to the collection of the sample, to wash their hands and penis thoroughly using soap, rinse away soap and dry with clean disposable towels and not to use any lubricant or saliva at the time of sample collection by masturbation. They were explained the precautions to avoid contamination and spillage and advised to hand over the sample to the laboratory immediately after collection. Samples were randomly allocated into two groups by means of simple random sampling. In the first group (Group S) sperm preparation was done with swim-up method and in the second group (Group D) sperm preparation was done with density gradient method. Assessment of volume, sperm count, and motility was performed in each sample before and after the sperm preparation, according to the WHO guidelines.[23] Aliquots (0.5 ml each) from the initial raw semen sample and processed sample were set aside for bacterial culture. Samples were sent to the microbiology laboratory immediately after sperm processing, for the microbiological culture.
Figure 1.
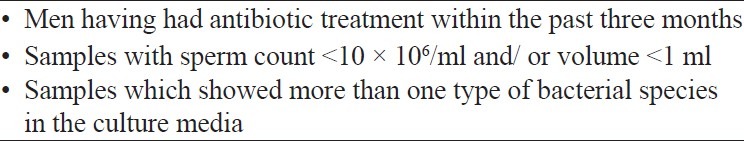
Exclusion criteria used in recruitment
Sperm preparation by swim-up and density gradients methods were done according to the procedures given in WHO guidelines, without any antibiotics added to the sperm preparation medium. One millilitre of the initial semen sample was used to process the sperms. Culture of seminal fluid samples was performed, within 2 hours of collection. The samples were inoculated in Blood Agar, Chocolate Agar and McConkey Agar, using a calibrated loop. The inoculated samples were incubated overnight at 37°C in normal air with 5% CO 2 for 24 hours. Samples which showed more than one type of bacterial species in the culture media were excluded from the study. All the procedures were carried out in strict aseptic conditions.
Data analysis was done with the use of SPSS version 16.0 software. Comparison of bacterial growth between the initial samples and the processed samples was done using the McNemar's chi-squared test. Sperm recovery rates between the sperm preparation techniques were compared using independent samples t-test.
RESULTS
The mean age of the study population was 34.2 years (Range 23-41 years). Out of 134 semen samples cultured, 102 (70.83%) showed presence of bacterial species in semen samples. Mixed growth of bacteria was observed in 16 samples, which were excluded from the study to eliminate the errors due to contamination. Semen samples with growth of a single organism (n = 86, 72.88%) and semen samples without any growth in culture media (n = 32, 27.11%) were included in the study, with the total sample size of 118. Organisms found were Streptococcus species (n = 40, 46.51% of which 14 were Group D Streptococcus species), Coagulase negative Staphylococcus species (n = 17, 19.76%), Staphylococcus aureus (n = 13, 15.11%), Coliform species (n = 11, 12.79% of which 09 were Escherichia coli) and Corynebacterium species (n = 5, 5.81%).
In group S, a positive bacterial growth was seen in 49 (72.05%) before the sperm preparation and only 16 (23.52%) after sperm preparation (P < 0.001). The specific organisms involved before and after sperm preparation were Streptococcus species excluding group D Streptococci 17 (25%) vs. 09 (13.23%): P = 0.005, Group D Streptococcus species in 05 (7.35%) vs. 01 (1.47%): P = 0.046, Coagulase negative Staphylococcus species in 10 (14.70%) vs. 02 (2.94%): P = 0.005, Staphylococcus aureus in 07 (10.29%) vs. 00 (0.00%): P = 0.008, Coliform species excluding Escherichia coli in 01 (1.47%) vs. 01 (1.47%), Escherichia coli in 06 (8.82%) vs. 03 (4.41%): P = 0.083, and Corynebacterium species in 03 (4.41%) vs. 00 (0.00%): P = 0.083. McNemar's chi-squared test was used for the comparison [Table 1].
Table 1.
Reduction of number of culture positive samples by sperm preparation using swim.up (Group S) and density gradient (Group D) methods. (n=118)
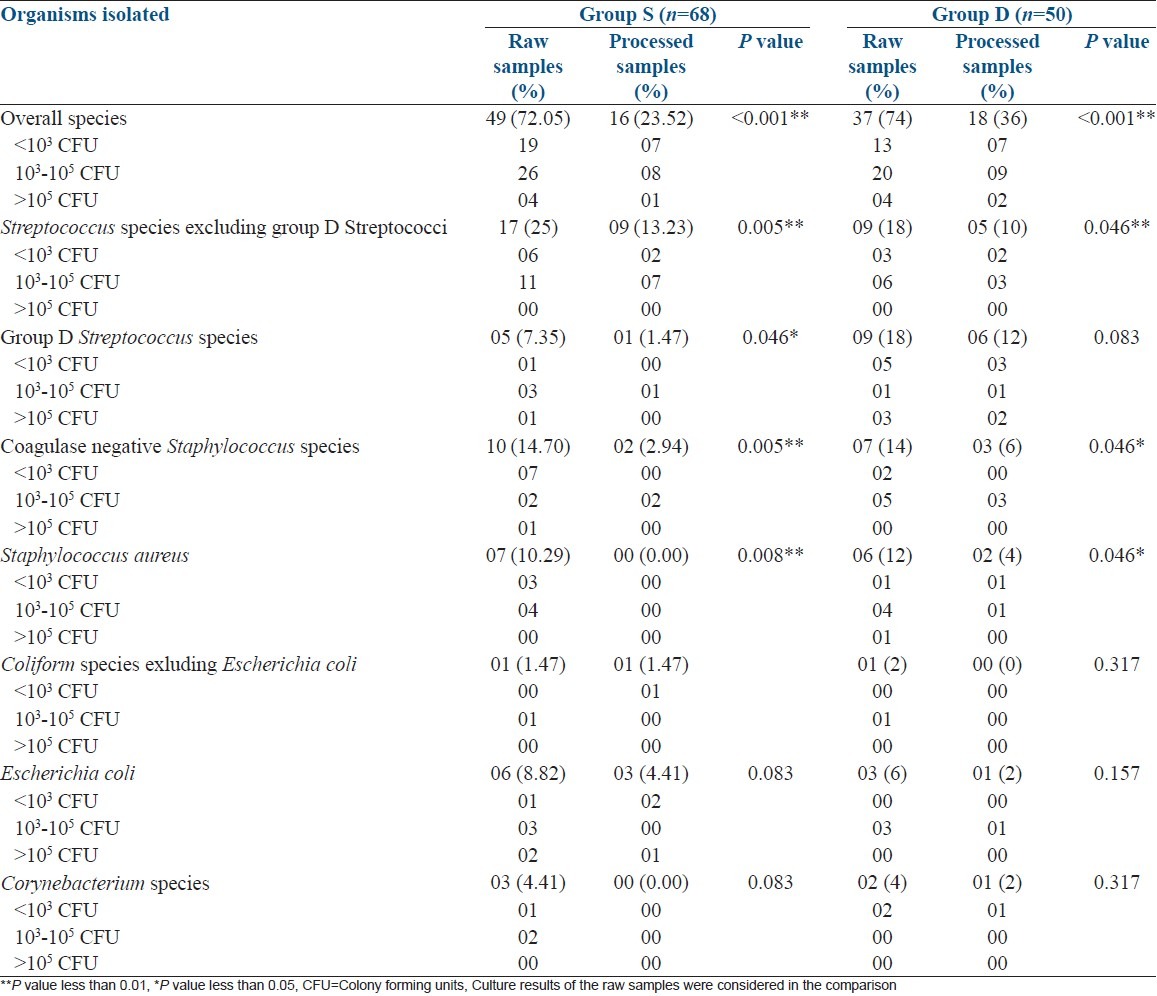
In group D, 37 samples (74%) showed a positive bacterial culture before the sperm preparation and only 18 samples (36%) were found to have a bacterial growth in culture media after sperm preparation (P < 0.001). The specific bacteria found before and after preparation of semen samples were Streptococcus species excluding group D Streptococci (9, 18%) vs. 05 (10%): P = 0.046, Group D Streptococcus species in 09 (18%) vs. 06 (12%): P = 0.083, Coagulase negative Staphylococcus species in 07 (14%) vs. 03 (06%): P = 0.046, Staphylococcus aureus in 06 (12%) vs. 02 (4%): P = 0.046, Coliform species excluding Escherichia coli in 01 (2%) vs. 00 (00%): P = 0.317, Escherichia coli in 03 (6%) vs. 01 (2%): P = 0.157 and Corynebacterium species in 02 (4%) vs. 01 (2%): P = 0.317. McNemar's chi-squared test was used for the comparison [Table 1].
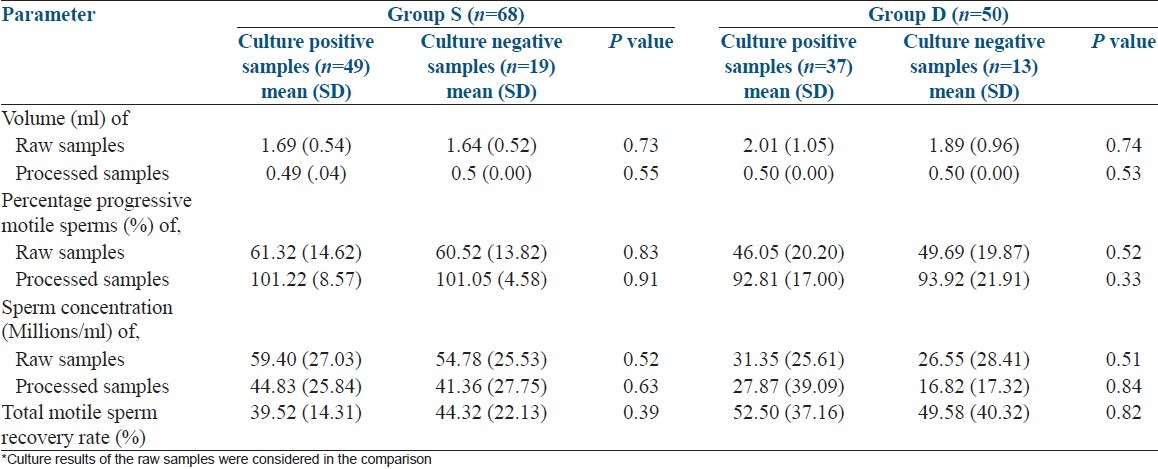
Recovery rates of sperms in culture positive vs. culture negative samples were 39.44% (SD-14.02) vs. 44.22% (SD-22.38) in group S, P = 0.39 and 52.5% (SD-37.16) vs. 49.58% (SD-40.32), P = 0.82 in group D respectively. There was no significant deference between culture positive and culture negative raw samples with regard to volume, concentration, and percentage of progressive motile sperms in either group. Independent samples t-test was used for the comparison [Table 2]. Calculation of the recovery rate was as follows:
Table 2.
Comparison of seminal fluid parameters and recovery rates between culture* positive and negative samples. (n=118)

DISCUSSION
Most frequently found bacterial species in our study were Streptococcus species, Group D Streptococci, Coagulase negative Staphylococcus species, Staphylococcus aureus, Escherichia coli and Corynebacterium species. But when PCR based methods were used, gram positive anaerobes (Peptoniphilis, Anaerococcus, Finegoldia, Peptostreptococcus species) were isolated as the most prevalent bacterial species in seminal fluid.[7,8,9] Therefore, type of organisms isolated in seminal fluid varies according to the method used for detection of bacteria. The prevalence of bacteriospermia among subfertile males has shown to be 25-100%.[10,24,25,26] It is mainly determined by the population studied and method used to identify the bacterial organisms. Organisms identified in our study are known to reduce the quality of seminal fluid.[27,28,29,30] But in our study, there was no significant difference between culture positive and culture negative samples in respect to the volume, percentage progressive motility, sperm concentration, and recovery rates. Effect of bacteriospermia on clinical pregnancy rates needs further evaluation.
Sperm preparation techniques, allowing higher sperm recovery and motility rates, have become very useful in the treatment of male infertility.[31] The recovery rates of density gradient method are higher compared with the swim-up method, which makes the density gradient method the preferred sperm preparation method regardless of the initial fresh sample concentration.[32] Density gradient centrifugation is known to clear bacteria from the seminal plasma.[19] Swim-up method is found to be more efficient in clearing bacteria from the seminal fluid compared to treatment with antibiotics of the male partner.[33] In our study, both swim-up and density gradient methods were found to be effective in clearance of non-specific bacterial species in seminal plasma. Total clearance of bacteria was not achieved in either method. In samples with Streptococcus species, excluding group D Streptococci, there was a significant reduction of bacteria by either method but complete clearance was not achieved. In samples with Group D Streptococcus species there was a significant reduction of bacteria with the swim-up method compared to the density gradient method. In samples with coagulase negative Staphylococcus species there was a significant reduction of bacteria by both methods, but complete clearance was not achieved. In samples with Staphylococcus aureus, density gradient method was capable of significantly reducing the number of post preparation samples, while swim-up method cleared the bacterium from all the samples. Even though both methods significantly cleared the non-specific bacterial species, the presence of bacteria in some post preparation samples may carry a risk of pelvic infection following intra uterine insemination (IUI). The incidence of pelvic infection following IUI with processed sperms is low. However, if pelvic inflammatory disease (PID) develops in these women that may further compromise fecundity.[34] In vitro fertilization (IVF) is also affected by presence of bacteria in seminal fluid. Contamination with seminal microorganisms may lead to oocyte degeneration, suboptimal fertilization rates and impaired embryonic development following IVF.[33,35]
Other than using sterile techniques, sperm preparation in antibiotics added media may further improve the ability to clear bacteria from seminal fluid. Enrichment of the sperm preparation media with Penicillin and Streptomycin is proven to be effective in reducing the bacterial organisms.[36] But it is well known that antibiotics are biologically active substances, which may probably affect the cell function. Antibiotics are added to the embryo culture media in IVF, to avoid contamination from micro-organisms. But evidence suggests that the absence of antibiotics in culture media is associated with an increase in embryo cell division. Indeed, the elimination of penicillin and streptomycin from the media resulted in an improved cleavage rate.[37] Faster cleaving embryos have been clearly demonstrated to be more capable of implantation in animal species.[38,39] A strong positive correlation was found between cleavage delay and chromosomal abnormalities.[40] The effect of antibiotics added media on clinical pregnancy rates is not clear with the available evidence. There is no universal agreement of adding antibiotics to the sperm preparation and/or embryo culture media in artificial reproductive techniques. Therefore, most centres in our set up, including the centre where the study was carried out, did not use antibiotic added media for sperm preparation. Therefore, more efficient methods should be implemented to improve the sperm preparation techniques to clear bacteria from the seminal plasma as the swim-up and density gradient techniques alone were inadequate to achieve complete clearance of the non-specific bacterial species from the seminal fluid.
ACKNOWLEDGMENT
We acknowledge the National Research Council of Sri Lanka (Grant No.09/69) for the financial support. The technical support extended by KAK Amaranath, CK Wijesinghe and S Abeysundara is acknowledged.
Footnotes
Source of Support: National Research Council of Sri Lanka, Grant No.09/69
Conflict of Interest: None declared.
REFERENCES
- 1.Mardh PA, Colleen S, Hovelius B. Attachment of bacteria to exfoliated cells from the urogenital tract. Invest Urol. 1979;16:322–6. [PubMed] [Google Scholar]
- 2.Willén M, Holst E, Myhre EB, Olsson AM. The bacterial flora of the genitourinary tract in healthy fertile men. Scand J Urol Nephrol. 1996;30:387–93. doi: 10.3109/00365599609181315. [DOI] [PubMed] [Google Scholar]
- 3.Marrie TJ, Lam J, Costerton JW. Bacterial adhesion to uroepithelial cells: A morphologic study. J Infect Dis. 1980;142:239–46. doi: 10.1093/infdis/142.2.239. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Chama N, Fisch H. Infection and pyospermia in male infertility. World J Urol. 1993;11:76–81. doi: 10.1007/BF00182033. [DOI] [PubMed] [Google Scholar]
- 5.Keck C, Gerber-Schäfer C, Clad A, Wilhelm C, Breckwoldt M. Seminal tract infections: Impact on male fertility and treatment options. Hum Reprod Update. 1998;4:891–903. doi: 10.1093/humupd/4.6.891. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Laboratory Manual for the Examination of Human Semen and Sperm-cervical Mucos Interaction. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 7.Jarvi K, Lacroix JM, Jain A, Dumitru I, Heritz D, Mittelman MW. Polymerase chain reaction-based detection of bacteria in semen. Fertil Steril. 1996;66:463–7. [PubMed] [Google Scholar]
- 8.Lacroixa JM, Jarvib K, Batraa SD, Heritzd DM, Mittelman MW. PCR-based technique for the detection of bacteria in semen and urine. J Microbiol Methods. 1996;26:61–71. [Google Scholar]
- 9.Moretti E, Capitani S, Figura N, Pammolli A, Federico MG, Giannerini V, et al. The presence of bacteria species in semen and sperm quality. J Assist Reprod Genet. 2009;26:47–56. doi: 10.1007/s10815-008-9283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onemu SO, Ibeh IN. Studies on the significance of positive bacterial semen cultures in male fertility in Nigeria. Int J Fertil Womens Med. 2001;46:210–4. [PubMed] [Google Scholar]
- 11.Kiessling AA, Desmarais BM, Yin HZ, Loverde J, Eyre RC. Detection and identification of bacterial DNA in semen. Fertil Steril. 2008;90:1744–56. doi: 10.1016/j.fertnstert.2007.08.083. [DOI] [PubMed] [Google Scholar]
- 12.Ison CA, Easmon CS. Carriage of Gardnerella vaginalis and anaerobes in semen. Genitourin Med. 1985;61:120–2. doi: 10.1136/sti.61.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gdoura R, Kchaou W, Ammar-Keskes L, Chakroun N, Sellemi A, Znazen A, et al. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl. 2008;29:198–206. doi: 10.2164/jandrol.107.003566. [DOI] [PubMed] [Google Scholar]
- 14.Diemer T, Huwe P, Ludwig M, Hauck EW, Weidner W. Urogenital infection and sperm motility. Andrologia. 2003;35:283–7. [PubMed] [Google Scholar]
- 15.Fisch H, Bar-Chama N, Skinner W, Naz R. Variation in antisperm antibody response following transection of male genital tract in Lewis rats. Arch Androl. 1993;30:193–9. doi: 10.3109/01485019308987756. [DOI] [PubMed] [Google Scholar]
- 16.Boitrelle F, Robin G, Lefebvre C, Bailly M, Selva J, Courcol R, et al. Bacteriospermia in Assisted Reproductive Techniques: Effects of bacteria on spermatozoa and seminal plasma, diagnosis and treatment. Gynecol Obstet Fertil. 2012;40:226–34. doi: 10.1016/j.gyobfe.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Stone SC, de la Maza LM, Peterson EM. Recovery of microorganisms from the pelvic cavity after intracervical or intrauterine artificial insemination. Fertil Steril. 1986;46:61–5. doi: 10.1016/s0015-0282(16)49458-6. [DOI] [PubMed] [Google Scholar]
- 18.Sacks PC, Simon JA. Infectious complications of intrauterine insemination: A case report and literature review. Int J Fertil. 1991;36:331–9. [PubMed] [Google Scholar]
- 19.Fourie J, Loskutoff N, Huyser C. Elimination of bacteria from human semen during sperm preparation using density gradient centrifugation with a novel tube insert. Andrologia. 2012;44:513–7. doi: 10.1111/j.1439-0272.2011.01217.x. [DOI] [PubMed] [Google Scholar]
- 20.Wong PC, Balmaceda JP, Blanco JD, Gibbs RS, Asch RH. Sperm washing and swim-up technique using antibiotics removes microbes from human semen. Fertil Steril. 1986;45:97–100. doi: 10.1016/s0015-0282(16)49104-1. [DOI] [PubMed] [Google Scholar]
- 21.Centola GM, Herko R, Andolina E, Weisensel S. Comparison of sperm separation methods: Effect on recovery, motility, motion parameters, and hyperactivation. Fertil Steril. 1998;70:1173–5. doi: 10.1016/s0015-0282(98)00352-5. [DOI] [PubMed] [Google Scholar]
- 22.Byrd W, Drobnis EZ, Kutteh WH, Marshburn P, Carr BR. Intrauterine insemination with frozen donor sperm: A prospective randomized trial comparing three different sperm preparation techniques. Fertil Steril. 1994;62:850–6. doi: 10.1016/s0015-0282(16)57015-0. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. WHO Press, World Health Organization; 2010. [Google Scholar]
- 24.Liversedge NH, Jenkins JM, Keay SD, McLaughlin EA, Al-Sufyan H, Maile LA, et al. Antibiotic treatment based on seminal cultures from asymptomatic male partners in in-vitro fertilization is unnecessary and may be detrimental. Hum Reprod. 1996;11:1227–31. doi: 10.1093/oxfordjournals.humrep.a019361. [DOI] [PubMed] [Google Scholar]
- 25.Mogra N, Dhruva A, Kothari LK. Non-specific seminal tract infection and male infertility: A bacteriological study. J Postgrad Med. 1981;27:99–104. [PubMed] [Google Scholar]
- 26.Henkel R, Schill WB. Sperm separation in patients with urogenital infections. Andrologia. 1998;301:91–7. doi: 10.1111/j.1439-0272.1998.tb02832.x. [DOI] [PubMed] [Google Scholar]
- 27.Jiang J, Lu DY. Detection of bacteria from semen of infertile males and their seminal parameters. Chin J Androl. 1996;10:196–8. [Google Scholar]
- 28.Türk S, Korrovits P, Punab M, Mändar R. Coryneform bacteria in semen of chronic prostatitis patients. Int J Androl. 2007;30:123–8. doi: 10.1111/j.1365-2605.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 29.Magnanelli S, Wilks M, Boake S, Tabaqchalli S, Wass AH. Quantitative bacteriology of seminal fluid in health and disease. Microb Ecol Health Dis. 1990;3:129–37. [Google Scholar]
- 30.Gupta S, Prabha V. Human Sperm Interaction with Staphylococcus aureus: A Molecular Approach. J Pathog. 2012;2012:816536. doi: 10.1155/2012/816536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barroso G, Chaya M, Bolaños R, Rosado Y, García León F, Ibarrola E. Prognostic value on recovery rates for the application of sperm preparation techniques and their evaluation in sperm function. Ginecol Obstet Mex. 2005;73:221–8. [PubMed] [Google Scholar]
- 32.Evliyaoðlu Y, Ciftçi U, Bozdemir N. Spermatozoa selection by the swim-up procedure and two-layer percoll gradient centrifugation. Int Urol Nephrol. 1996;28:409–18. doi: 10.1007/BF02550505. [DOI] [PubMed] [Google Scholar]
- 33.Huyser C, Fourie FL, Oosthuizen M, Neethling A. Microbial flora in semen during in vitro fertilization. J In Vitro Fert Embryo Transf. 1991;8:260–4. doi: 10.1007/BF01139781. [DOI] [PubMed] [Google Scholar]
- 34.Pavletic AJ, Wölner-Hanssen P, Paavonen J, Hawes SE, Eschenbach DA. Infertility following pelvic inflammatory disease. Infect Dis Obstet Gynecol. 1999;7:145–52. doi: 10.1002/(SICI)1098-0997(1999)7:3<145::AID-IDOG6>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steyaert SR, Leroux-Roels GG, Dhont M. Infections in IVF: Review and guidelines. Hum Reprod Update. 2000;6:432–41. doi: 10.1093/humupd/6.5.432. [DOI] [PubMed] [Google Scholar]
- 36.Karlström PO, Hjelm E, Lundkvist O. Comparison of the ability of two sperm preparation techniques to remove microbes. Hum Reprod. 1991;6:386–9. doi: 10.1093/oxfordjournals.humrep.a137346. [DOI] [PubMed] [Google Scholar]
- 37.Magli MC, Gianarolil L, Fiorentino A, Ferraretti AP, Fortini D, Panzella S. Improved cleavage rate of human embryos cultured in antibiotic-free medium. Hum Reprod. 1996;7:1520–4. doi: 10.1093/oxfordjournals.humrep.a019430. [DOI] [PubMed] [Google Scholar]
- 38.Gardner DK, Sakkas D. Mouse embryo cleavage, metabolism and viability: Role of medium composition. Hum Reprod. 1993;8:288–95. doi: 10.1093/oxfordjournals.humrep.a138039. [DOI] [PubMed] [Google Scholar]
- 39.McKiernan SH, Bavister BD. Timing of development is a critical parameter for predicting successful embryogenesis. Hum Reprod. 1994;9:2123–9. doi: 10.1093/oxfordjournals.humrep.a138403. [DOI] [PubMed] [Google Scholar]
- 40.Munné S, Lee A, Rosenwaks Z, Grifo J, Cohen J. Diagnosis of major chromosome aneuploidies in human preimplantation embryos. Hum Reprod. 1993;8:2185–91. doi: 10.1093/oxfordjournals.humrep.a138001. [DOI] [PubMed] [Google Scholar]


