Abstract
CONTEXT:
Oocyte quality may be a governing factor in influencing in vitro fertilization (IVF) outcomes. However, morphological evaluation of oocyte quality is difficult in conventional IVF cycles. Follicular-fluid (FF), the site for oocyte growth and development, has not yet been sufficiently explored to obtain a marker indicative of oocyte quality. Anti-Mullerian hormone (AMH) is produced by granulosa cells of preantral and early-antral follicles and is released in FF.
AIM:
To investigate AMH as a biochemical indicator of functional viability/quality of oocyte produced in the FF micro-environmental milieu.
SETTINGS AND DESIGN:
Prospective study involving 132 cycles of conventional IVF-embryo transfer (ET) in infertile women.
SUBJECTS AND METHODS:
AMH concentration was estimated in pooled FF on day of oocyte pickup. Cycles were sorted into low and high groups according to median (50 th centile) values of measurement. Main outcome measure was oocyte viability, which included morphological assessment of oocyte quality, fertilization rate, clinical pregnancy, and implantation rates.
STATISTICAL ANALYSIS:
Graph-pad Prism 5 statistical package.
RESULTS:
Low FF AMH group shows significantly higher percentage of top-quality oocytes (65.08 ± 24.88 vs. 50.18 ± 25.01%, P =0.0126), fertilization (83.65 ± 18.38 vs. 75.78 ± 21.02%, P =0.0171), clinical pregnancy (57.57 vs. 16.67%, P >0.0001), and embryo implantation rates (29.79 vs. 7.69%, P >0.0001) compared to high FF AMH group. FF AMH shares an inverse correlation with FF E2 (Pearson r = −0.43, r2 = 0.18) and clinical pregnancy (Pearson r = −0.46, r2 = 0.21). Threshold value of FF AMH for pregnancy is >1.750 ng/mg protein.
CONCLUSION:
FF AMH is a plausible biochemical indicator of functional viability of oocyte in conventional IVF cycles.
KEY WORDS: IVF, FF AMH, functional viability of oocyte, implantation rate, oocyte quality
INTRODUCTION
The successful outcome of an in vitro fertilization (IVF) cycle depends on several factors including oocyte quality. However, determination of oocyte quality through morphological evaluation is difficult in conventional (non-Intra Cytoplasmic Sperm Injection ICSI) IVF cycles where oocytes are not denuded of their cumulus complex. Therefore, in such IVF cycles, a biochemical indicator of functional viability of oocyte could offer a better alternative than a morphological marker for oocyte quality.
Follicular-fluid (FF) microenvironment, the site for oocyte growth and development, has been explored by several workers to study the oocyte developmental potential. Elevated FF levels of estradiol[1] and inhibin B[2,3] have been reported to be associated with high fertilization and pregnancy rates. However, results contradicting these findings have also been reported by certain workers.[4,5] Thus, a specific FF marker, directly indicating functional viability of oocyte and predicting pregnancy outcome in conventional IVF cycles, is still awaited.
Anti-Mullerian hormone (AMH), a glycoprotein dimer of the transforming growth factor-β super family, is exclusively produced by granulosa cells of preantral and early antral ovarian follicles in the adult female[6] and exuded into the FF.[7] In a comprehensive review article, Feyereisen et al.,[8] implicated AMH in influencing ovarian follicular status and follicle quality. Indeed, Ebner et al.,[9] associated basal serum AMH levels with oocyte quality in ICSI cycles. However, there have been very few studies on the direct putative correlation between FF AMH levels and oocyte quality in stimulated IVF cycles. Recent studies[3,10,11,12] relating FF AMH with fertilization rates and pregnancy outcome presented diverse results. Thus, ambiguity in results obtained from these studies prompts for a more holistic approach toward FF AMH evaluation so as to correlate it with functional viability/quality of oocyte.
The present study was based on the contention that granulosa cells of preovulatory follicles which are in an advanced stage of luteinization, would presumably release lesser amounts of AMH in the FF, thus reflecting the quality and viability of oocyte therein. Therefore, FF AMH may be a potent biochemical indicator of functional viability of oocyte in conventional IVF cycles. It was envisaged that estimation of AMH levels in a collective pool of FF obtained from a cohort of multiple follicles from which an oocyte had been retrieved in each cycle would be a more practical and feasible approach than estimations in isolated monofollicular fluid. Moreover, studies in mono-follicular fluids are cumbersome and have their limitations[13] and FF specimens collected from single dominant follicles may not truly reflect granulosa or theca cell production.[14] Hence, pooled FF may comprehensively reflect the dynamic microenvironmental milieu in which oocytes are produced. The limitations of conventional IVF procedure in assessment of oocyte quality prompted us to introduce an all-inclusive term “functional viability” which may include: (a) Morphological assessment of oocyte quality as permissible in non-ICSI IVF cycles; (b) fertilization rate; (c) Clinical pregnancy rate; and (d) Embryo implantation rate.
SUBJECTS AND METHODS
Subjects
This prospective study included 158 IVF cycles comprising of normoovulatory women (menstrual cycle length range: 25-32 days, mean age 32.22 ± 4.25 years, body mass index 23.97 ± 4.53, [Waist/Hip (W/H) ratio 0.88 ± 0.06] with both ovaries present, deprived of morphological abnormalities and with no clinical signs of hyperandrogenism. None of the women under treatment had any current or past diseases affecting ovaries or gonadotropin or sex steroid secretion, clearance or excretion. A total of 26 cycles were abandoned either due to no oocytes retrieved (12 cycles) or fertilization failure (14 cycles). Finally, 132 cycles were considered for oocyte quality assessment, fertilization, and clinical pregnancy. Informed consent was sought from all patients for participation in the study. The study protocol was approved by the local hospital ethical committee and was in conformity with the provisions of Declaration of Helsinki (as revised in Edinburgh 2000).
Exclusion criteria
Women older than 40 years of age
Women with polycystic ovary syndrome (PCOS as defined according to the Rotterdam consensus)
Women with endometriosis as diagnosed by laparoscopy
Cycles where no oocytes were retrieved on the day of aspiration
Male factor abnormality patients who were considered for ICSI (normal morphology >5%, immotile sperm, azoospermia).
Main outcome measure
Functional viability of oocyte comprising of the following:
Morphological assessment of oocyte quality
Fertilization rate
Clinical pregnancy: Gestational sac with positive cardiac activity observed at ultrasound at around 6th week of amenorrhea was defined as confirmation of clinical pregnancy.
-
Implantation rate:
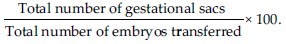
Cycle monitoring
Downregulation protocol
Pituitary desensitization involving treatment with Gonadotropin Releasing Hormone (GnRH) agonists (500 μg/day of leuprolide acetate) was started in the midluteal phase of the menstrual cycle 7 days prior to the earliest expected date of menstruation. A comprehensive downregulation was confirmed by measurement of serum Follicle Stimulating Hormone (FSH) and estradiol (E2) levels below 1.0 mIU/mL and >20 pg/mL respectively, either on the day of onset of menstruation or 1/2 days at the most, after onset.
Ovarian stimulation treatment
After confirmation of comprehensive down regulation with GnRH agonist, a standard long protocol was used for controlled ovarian hyperstimulation. Ovarian stimulation was effected with daily administration of recombinant FSH (Recagon 200 IU/day). Transvaginal ultrasound scan was done on days 8 and 10 of ovarian stimulation and every 1 or 2 days thereafter, as required. Final oocyte maturation and trigger for ovulation was induced by administering human Chorionic Gonadotropin (hCG) 5000 IU, when there was at least one leading follicle reaching a mean diameter of 18 mm and at least 2 to 4 other follicles reaching mean diameter of 16 mm.
Oocyte pickup and insemination
Transvaginal ultrasound-guided oocyte retrieval under patient sedation was done between 34 and 36 h after hCG administration. Insemination was done after 4 h of oocyte incubation.
FF collection
FF was aspirated from follicles (≥16 mm) under transvaginal ultrasound guidance and maintained at steady 37°C temperature conditions. Only the original follicular aspirate was collected in the few instances wherein oocyte was retrieved in the flush. In every cycle, FF from each follicle was collected separately and equal volume of FF from individual follicles from which an oocyte had been obtained, was pooled together. FF was then centrifuged at 3000 g for 15 min at 4°C to eliminate cellular elements. Clear supernatant obtained was used for estimations. FF from oocyte pickup (OPU) failure cycles was not used in this study.
Hormonal estimations
FF obtained on the day of oocyte retrieval was estimated for AMH levels by enzyme-linked immunosorbent assay technique using diagnostic kits. Protocol was followed as per manufacturer's instructions. Theoretical sensitivity or lowest detection limit was 0.006 ηg/mL. FF levels obtained in ηg/mL were expressed as a ratio of their corresponding total protein content to remove any bias due to volume variability. Protein estimation was performed by folin phenol reagent method described by Lowry et al.[15] The original method was scaled down to accommodate micro quantities of sample and reagents.
Estradiol levels were measured in FF by radioimmunoassay kits. Estimations were done as per manufacturer's protocol. Values were expressed as pg/mL with theoretical sensitivity or lowest detection limit 4.7 pg/mL.
Assessment of oocyte quality
A close approximation of oocyte quality was obtained by morphological evaluation of radiating corona/cumulus mass present around the oocyte.[16] Accordingly, we graded oocytes as:
Grade 1 (top-quality oocytes): Expanded cumulus mass with a sun-flare corona radiata
Grade 2 (average quality oocytes): Less expanded, intermediate size cumulus, and corona complex
Grade 3 (poor-quality oocytes): Very thin or absent cumulus-corona complex.
Fertilization assessment
Fertilization rate, which was assessed 16-18 h after insemination, was characterized by the presence of two pronuclei and two polar bodies. The position of pronuclei (whether centric or eccentric), number, and alignment of nucleoli/nucleolar precursor bodies at junction of two pronuclei, granulation of cytoplasm, and presence of halo (indicative of nuclear membrane breakdown) were also noted.
Embryo development
Embryo development was monitored daily and cleavage stage embryos were assessed before transfer.[17,18]
Embryo transfer
Trans-cervical transfer of day three cleavage stage embryos was performed using a soft-tipped embryo transfer (ET) catheter. Immediately after ET, catheter was flushed and the media was examined microscopically to confirm absence of embryos.
Micronized progesterone 200 mg twice daily was administered to support luteal phase starting from evening of day ET until day 14 of ET. On d14 ET, serum β-hCG concentration <50 mIU/mL was considered as positive indicator of pregnancy. Clinical pregnancy was confirmed by presence of gestational sac with positive cardiac activity.
Definition of study groups
Cycles were sorted into conception and nonconception cycles depending on establishment of clinical pregnancy
Cycles were then sorted into low and high groups according to FF AMH concentrations. Cut-offs for defining low and high AMH concentrations corresponded to round value of the median (50th centile) of each measurement. Hence, the FF AMH groups were: Low FF AMH (≤1.720 ng/mg protein) High FF AMH (<1.720 ng/mg protein)
Cycles where there was fertilization failure were included only for FF AMH assessment though this group was obviously not included in the study of pregnancy outcome.
Statistical analysis
Data was statistically analyzed for significant difference using Graph Pad Prism Version 5.0 statistical package. Student's t test was used to assess difference between means and to obtain statistical significance. Comparisons between continuous variables from more than two groups were performed using one-way analysis of variance (ANOVA) when data distribution was normal. Receiver-operating characteristics (ROC) curve analysis data was obtained to get cutoff values. Correlation was obtained and expressed as Pearson correlation coefficient (r). All values are expressed as mean ± SD (standard deviation). In all cases, P > 0.05 was considered statistically significant.
RESULTS
In our study, out of 132 cycles studied, total 49 pregnancies were obtained (clinical pregnancy rate 37.12%). Further, our results indicated significantly lower levels of FF AMH in conception (n = 49) cycles compared to nonconception (n = 83) cycles (1.41 ± 0.77 vs. 2.83 ± 1.62 ng/mg protein; P > 0.0001). A noteworthy finding was that the fertilization failure cycles (n = 14) showed significantly much higher level of FF AMH (3.78 ± 1.12 ng/mg protein) than both, conception and nonconception cycles (one-way ANOVA, P > 0.0001).
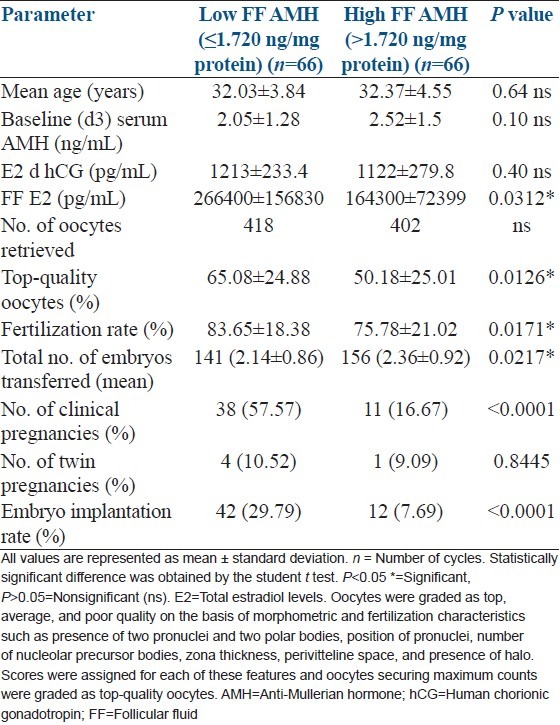
Since there was an extremely significant difference in FF AMH levels between conception and nonconception cycles, they were subsequently classified into low and high FF AMH groups. Table 1 shows comparison of hormone and embryology data between low and high FF AMH groups. The total (mean) number of embryos transferred [141 (2.14 ± 0.86) vs. 156 (2.36 ± 0.92) P = 0.0217] differed significantly between the two groups. It is also evident from Table 1 that low FF AMH group showed significantly higher rates of fertilization, more number of top-quality oocytes, and higher clinical pregnancy and embryo implantation rates than high FF AMH group. However, the twin pregnancy rates were comparable and did not differ significantly between the two groups. Pertinently, we observed that the incidence of fertilization failure was significantly lower (3/14: 21.43%) in the low FF AMH group than in high FF AMH group (11/14: 78.57%).
Table 1.
Hormone and embryology data in low vs. high FF AMH groups
The ROC curve threshold value of FFAMH for pregnancy was found to be >1.750 ng/mg protein [Table 2a]. Interestingly, and as anticipated, FF levels of E2 were found to be significantly higher in the low FF AMH group than in the high FF AMH group. Infact, FF AMH shared a strong inverse correlation with FF E2 [Table 2b]. We also obtained a strong inverse correlation between FF AMH and clinical pregnancy [Table 2b].
Table 2a.
ROC curve analysis of FF AMH

Table 2b.
Correlation with FF E2 and clinical pregnancy

DISCUSSION
The present study has for the first time taken a holistic approach towards AMH measurement by estimating its levels in pooled fluid obtained from a cohort of follicles from which an oocyte had been retrieved in each cycle. This seems logical not only because it is practically more feasible, but also because it is a more comprehensive reflection of the dynamic milieu that the FF microenvironment represents. Moreover, the practice of carrying studies in individual lead follicles may be too time-consuming, cumbersome, and unfeasible for most laboratories where OPUs are routinely done following a conventional downregulation/stimulation protocol involving multi-follicular growth.
Our results, associating lower FF AMH levels on day of OPU with conception cycles and indicating an inverse correlation of FF AMH with clinical pregnancy, completely conform to and are in conjunction with earlier reports of a progressive decline in AMH levels during ovarian stimulation, hence confirming the decreased ability of maturing follicles to produce AMH.[6,8] A recent study carried out in monofollicular fluid (FF obtained from each individual follicle) of stimulated cycles by Takahashi et al.,[11] reported correlation of higher FF AMH levels with higher rates of fertilization. However, they could not associate it with pregnancy outcome. Moreover, their study involved comparison of FF AMH between two broad groups namely fertilization success versus fertilization failure. Wunder et al.,[3] correlated higher FF AMH with reproductive outcome in IVF-ICSI cycles. Another study carried out by Fanchin et al.,[10] in monodominant follicles (single lead follicle) of unstimulated cycles, reported correlation of FF AMH with implantation rates and pregnancy outcome but not with fertilization rates. In yet another recent study, Aflatoonian et al.,[12] correlated FF AMH with fertilization and embryo quality.
Thus, all these studies seem to have contrasting results as regards association of FF AMH with fertilization rates and pregnancy outcome. Interestingly, however, all three studies correlated higher FF AMH levels with either higher rates of implantation/clinical pregnancy or fertilization or embryo quality, respectively. These findings seem paradoxical and incongruous with earlier statements by the same authors that corpus lutea, large antral follicles, oocytes and thecal, and interstitial cells express little or no AMH.[11] Rather, its production by granulosa cells of large follicles has been reported to interfere with follicular maturation and luteinization.[19] Fanchin et al's.,[19] results could partly be explained by the fact that the study was carried out in unstimulated monodominant follicle cycles where GnRH antagonist and human menopausal gonadotropin was administered only to prevent a premature leuteinizing hormone (LH) surge and to control recruitment of additional secondary follicles. Experiments in rats have indicated that FSH administration reduces AMH levels. Extrapolating from murine data, La Marca et al.,[20] reported that in women, the reduction in AMH levels may be due to the supraphysiological increase in E2 levels observed when exogenous FSH is administered. Therefore, it may be argued that in the absence of FSH stimulation for multiple follicular growth, AMH levels were found to be higher in Fanchin et al.'s study. However, Fanchin et al.,[19] in the same study refer to the presumable FSH independent production of AMH[21,22] . Fanchin et al., have also made reference to an observation by Salmon et al.,[23] that in the mice preovulatory follicles, AMH gene expression is directly activated by the oocyte, which may facilitate fertilization. However, no such claim has yet been made in the human preovulatory follicle to substantiate the presence of higher levels of AMH in FF. The results of aforementioned studies, therefore, seem confusing and contradictory.
In our study, the association of low FF AMH with higher fertilization, embryo implantation, and clinical pregnancy rates is also supported and substantiated by the observation of significantly higher levels of FF E2 [Table 1] in the low FF AMH group compared to high FF AMH group. This finding corroborates and is in conformity with the fact that as the follicle matures to the preovulatory stage, the granulosa cells undergo developmental changes which transform the follicle from being steroidogenically quiescent to one that is capable of producing large quantities of oestrogen. From the midfollicular phase, with its increased capacity to aromatize androstenedione, the follicle destined to ovulate begins to synthesize estradiol. The limiting step in follicular oestrogen biosynthesis is the acquisition of aromatase by the granulosa granulosa cells which enables these cells to convert the thecally produced androgens to estrogens. AMH is reported to have an inhibitory effect on aromatase activity and estrogen production.[24] In cultured fetal rodent ovaries, AMH has been shown to suppress the expression of cytochrome P450 aromatase,[25] the enzyme in granulosa cells that converts androgens to E2. The intrafollicular androgen to estrogen ratio acts on oocyte function; and AMH appears to play a major role in the regulation of this ratio. Animal studies have revealed that not only does AMH decrease aromatase activity of FSH-stimulated granulosa cells, but it also decreases the number of LH receptors, and regulates testosterone production in theca cells.[26,27,28,29] Moreover, many workers have associated higher levels of FF E2 with better chances of achieving pregnancy.[1,30,31,32,33,34,35,36,37] It is, therefore, logical that FF levels of AMH and E2 share an inverse relationship as demonstrated by our study [Table 2b].
The foregoing discussion, therefore, makes it clear that the mature preovulatory follicle will have minimal AMH production and maximal aromatase activity facilitating higher production of estrogen. Thus, FF AMH levels may be an index of oocyte maturity and therefore its quality. Lower FF AMH might allow for the aromatization of substrate androgens to be converted to estrogens, also necessary for preparing the endometrium for the ensuing embryo implantation process. This will reflect on the overall viability of the oocyte, thus influencing its quality, fertilization rate as well as clinical pregnancy and embryo implantation rates. This is, thus, the first ever study that has successfully explored AMH in the pooled FF microenvironmental milieu to establish it as a potent biochemical indicator of oocyte viability in conventional IVF cycles.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Botero-Ruiz W, Laufer N, DeCherney AH, Polan ML, Haseltine FP, Behrman HR. The relationship between follicular fluid steroid concentration and successful fertilization of human oocytes in vitro. Fertil Steril. 1984;41:820–6. doi: 10.1016/s0015-0282(16)47892-1. [DOI] [PubMed] [Google Scholar]
- 2.Ocal P, Aydin S, Cepni I, Idil S, Idil M, Uzun H, et al. Follicular fluid concentrations of vascular endothelial growth factor, Inhibin A and Inhibin B in IVF cycles: Are they markers for ovarian response and pregnancy outcome? Eur J Obstet Gynecol Reprod Biol. 2004;115:194–9. doi: 10.1016/j.ejogrb.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Wunder DM, Guibourdenche J, Birkhäuser MH, Bersinger NA. Anti-Müllerian hormone and inhibin B as predictors of pregnancy after treatment by in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2008;90:2203–10. doi: 10.1016/j.fertnstert.2007.10.078. [DOI] [PubMed] [Google Scholar]
- 4.Messinis IE, Templeton AA. Relationship between intrafollicular levels of prolactin and sex steroids and in-vitro fertilization of human oocytes. Hum Reprod. 1987;2:607–9. doi: 10.1093/oxfordjournals.humrep.a136598. [DOI] [PubMed] [Google Scholar]
- 5.Wen X, Tozer AJ, Butler SA, Bell CM, Docherty SM, Iles RK. Follicular fluid levels of inhibin A, inhibin B, and activin A levels reflect changes in follicle size but are not independent markers of the oocyte's ability to fertilize. Fertil Steril. 2006;85:1723–9. doi: 10.1016/j.fertnstert.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 6.Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, et al. Anti-Mullerian hormone and anti-Mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136:4951–62. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- 7.Fallat ME, Siow Y, Marra M, Cook C, Carrillo A. Mullerian-inhibiting substance in follicular fluid and serum: A comparison of patients with tubal factor infertility, polycystic ovarian syndrome, and endometriosis. Fertil Steril. 1997;67:962–5. doi: 10.1016/s0015-0282(97)81417-3. [DOI] [PubMed] [Google Scholar]
- 8.Feyereisen E, Mendez Lozano DH, Taieb J, Hesters L, Frydman R, Fanchin R. Anti-Mullerian hormone: Clinical insights into a promising biomarker of ovarian follicular status. Reprod Biomed Online. 2006;12:695–703. doi: 10.1016/s1472-6483(10)61081-4. [DOI] [PubMed] [Google Scholar]
- 9.Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of anti-Mullerian hormone is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21:2022–6. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 10.Fanchin R, Mendez Lozano DH, Frydman N, Gougeon A, di Clemente N, Frydman R, et al. Anti-Mullerian hormone concentrations in the follicular fluid of the preovulatory follicle are predictive of the implantation potential of the ensuing embryo obtained by in vitro fertilization. J Clin Endocrinol Metab. 2007;92:1796–802. doi: 10.1210/jc.2006-1053. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi C, Fujito A, Kazuka M, Sugiyama R, Ito H, Isaka K. Anti-Müllerian hormone substance from follicular fluid is positively associated with success in oocyte fertilization during in vitro fertilization. Fertil Steril. 2008;89:586–91. doi: 10.1016/j.fertnstert.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 12.Aflatoonian A, Mashayekhy M, Mohamadian F, Moghaddam FM. The correlation between follicular fluid anti-mullerian hormone levels and fertilization and embryo quality in ART cycles. Iran J Reprod Med. 2010;8:157–60. [Google Scholar]
- 13.Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7:40. doi: 10.1186/1477-7827-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi YS, Ku SY, Jee BC, Suh CS, Choi YM, Kim JG, et al. Comparison of follicular fluid IGF-I, IGF-II, IGFBP-3, IGFBP-4 and PAPP-A concentrations and their ratios between GnRH agonist and GnRH antagonist protocols for controlled ovarian stimulation in IVF-embryo transfer patients. Hum Reprod. 2006;21:2015–21. doi: 10.1093/humrep/del091. [DOI] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 16.Veeck LL. The morphological assessment of human oocytes and early concepti. In: Keel BA, Webster BW, editors. Handbook of the Laboratory Diagnosis and Treatment of Infertility. Boston, Boca Raton: CRC Press; 1990. pp. 353–69. [Google Scholar]
- 17.Veeck LL. Atlas of the human oocyte and early conceptus. Baltimore: Williams and Wilkins; 1991. Pre embryo grading; pp. 121–49. [Google Scholar]
- 18.Ebner T, Moser M, Sommergruber M, Tews G. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: A review. Hum Reprod Update. 2003;9:251–62. doi: 10.1093/humupd/dmg021. [DOI] [PubMed] [Google Scholar]
- 19.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–7. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 20.La Marca A, Malmusi S, Giulini S, Tamaro LF, Orvieto R, Levratti P, et al. Anti-Müllerian hormone plasma levels in spontaneous menstrual cycle and during treatment with FSH to induce ovulation. Hum Reprod. 2004;19:2738–41. doi: 10.1093/humrep/deh508. [DOI] [PubMed] [Google Scholar]
- 21.Bath LE, Wallace WH, Shaw MP, Fitzpatrick C, Anderson RA. Depletion of ovarian reserve in young women after treatment for cancer in childhood: Detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18:2368–74. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 22.Eldar-Geva T, Ben-Chetrit A, Spitz IM, Rabinowitz R, Markowitz E, Mimoni T, et al. Dynamic assays of inhibin B, anti-Mullerian hormone and estradiol following FSH stimulation and ovarian ultrasonography as predictors of IVF outcome. Hum Reprod. 2005;20:3178–83. doi: 10.1093/humrep/dei203. [DOI] [PubMed] [Google Scholar]
- 23.Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Mullerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266:201–8. doi: 10.1016/j.ydbio.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Vigier B, Forest MG, Eychenne B, Bezard J, Garrigou O, Robel P, et al. Anti-Muellerian hormone produces endocrine sex reversal of fetal ovaries. Proc Natl Acad Sci U S A. 1989;86:3684–8. doi: 10.1073/pnas.86.10.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.di Clemente N, Ghaffari S, Pepinsky RB, Pieau C, Josso N, Cate RL, et al. A quantitative and interspecific test for biologic activity of anti-Mullerian hormone: The fetal ovary aromatase assay. Development. 1992;114:721–7. doi: 10.1242/dev.114.3.721. [DOI] [PubMed] [Google Scholar]
- 26.Cook CL, Siow Y, Taylor S, Fallat ME. Serum müllerian-inhibiting substance levels during normal menstrual cycles. Fertil Steril. 2000;73:859–61. doi: 10.1016/s0015-0282(99)00639-1. [DOI] [PubMed] [Google Scholar]
- 27.Josso N, Racine C, di Clemente N, Rey R, Xavier F. The role of anti-Mullerian hormone in gonadal development. Mol Cell Endocrinol. 1998;145:3–7. doi: 10.1016/s0303-7207(98)00186-5. [DOI] [PubMed] [Google Scholar]
- 28.Josso N, di Clemente N, Gouedard L. Anti-Mullerian hormone and its receptors. Mol Cell Endocrinol. 2001;179:25–32. doi: 10.1016/s0303-7207(01)00467-1. [DOI] [PubMed] [Google Scholar]
- 29.Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimullerian hormone/mullerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82:1323–9. doi: 10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 30.Tarlatzis BC, Laufer N, DeCherney AH, Polan ML, Haseltine FP, Behrman HR. Adenosine 3’,5’-monophosphate levels in human follicular fluid: Relationship to oocyte maturation and achievement of pregnancy after in vitro fertilization. J Clin Endocrinol Metab. 1985;60:1111–5. doi: 10.1210/jcem-60-6-1111. [DOI] [PubMed] [Google Scholar]
- 31.Kreiner D, Liu HC, Itskovitz J, Veeck L, Rosenwaks Z. Follicular fluid estradiol and progesterone are markers of preovulatory oocyte quality. Fertil Steril. 1987;48:991–4. [PubMed] [Google Scholar]
- 32.Lee MS, Ben-Rafael Z, Meloni F, Mastroianni L, Jr, Flickinger GL. Relationship of human oocyte maturity, fertilization, and cleavage to follicular fluid prolactin and steroids. J In Vitro Fert Embryo Transf. 1987;4:168–72. doi: 10.1007/BF01555465. [DOI] [PubMed] [Google Scholar]
- 33.Reinthaller A, Deutinger J, Bieglmayer C, Riss P, Müller-Tyl E, Fischl F, et al. Hormonal parameters in follicular fluid and the fertilization rate of in vitro cultured oocytes. Arch Gynecol. 1987;240:207–10. doi: 10.1007/BF02134069. [DOI] [PubMed] [Google Scholar]
- 34.Subramanian MG, Sacco AG, Moghissi KS, Magyar DM, Hayes MF, Lawson DM, et al. Human follicular fluid: Prolactin is biologically active and ovum fertilization correlates with estradiol concentration. J In Vitro Fertil Embryo Transfer. 1988;5:129–33. doi: 10.1007/BF01131174. [DOI] [PubMed] [Google Scholar]
- 35.Tarlatzis BC, Pazaitou K, Bili H, Bontis J, Papadimas J, Lagos S, et al. Growth hormone, oestradiol, progesterone and testosterone concentrations in follicular fluid after ovarian stimulation with various regimes for assisted reproduction. Hum Reprod. 1993;8:1612–6. doi: 10.1093/oxfordjournals.humrep.a137900. [DOI] [PubMed] [Google Scholar]
- 36.Artini PG, Battaglia C, D’Ambrogio G, Barreca A, Droghini F, Volpe A, et al. Relationship between human oocyte maturity, fertilization and follicular fluid growth factors. Hum Reprod. 1994;9:902–6. doi: 10.1093/oxfordjournals.humrep.a138614. [DOI] [PubMed] [Google Scholar]
- 37.Teissier MP, Chable H, Paulhac S, Aubard Y. Comparison of follicle steroidogenesis from normal and polycystic ovaries in women undergoing IVF: Relationship between steroid concentrations, follicle size, oocyte quality and fecundability. Hum Reprod. 2000;15:2471–7. doi: 10.1093/humrep/15.12.2471. [DOI] [PubMed] [Google Scholar]


