Abstract
Introduction:
As the search for a better biocompatible medicament is on, aim of the present study was to evaluate the pulpal response to collagen particles impregnated in antibiotics (Biofil-AB™) and new commercially available cement (Pulpotec) that can be used as pulpal medicament.
Materials and Methods:
Total sample of 40 teeth from 20 children in the age group of 7-10 years which are noncarious having bilateral retained primary teeth were enrolled for the study. Nine teeth each were treated with collagen particles (group I) and Pulpotec cement (group II), and the remaining samples were discarded due to various reasons. Both groups were randomly subdivided into three teeth each that were extracted after 7, 15, and 30 days intervals and examined histologically.
Results:
Moderate to severe inflammatory cells with newly formed blood vessels and disorganized odontoblastic cell layer was observed in group I after all three intervals with dentinal bridge formation in two specimens. On contrary, none of the specimens in group II showed any signs of inflammation, but there was a discontinuity in the odontoblastic layer lining along the dentin walls.
Conclusion:
Both materials were proven to be promising alternatives as pulp medicaments. However, collagen was found to be a better material.
Keywords: Collagen, pulpotomy, Pulpotec® cement
INTRODUCTION
Quest is on for newer pulpal medicaments that are biocompatible and capable of healing the dental pulp by producing reparative dentin and/or dentinal bridge in response to various stimuli and surgical exposure. Collagen has a proven rate of success in the field of dentistry as guided tissue regeneration, root conditioning, hemostatic, and wound dressing agent. It has inherent properties like low immune response and toxicity, ability to promote cellular growth and attachment, homeostasis, and added advantage of antibiotic incorporation.[1] Pulpotec is commercially available as resinous cement that contains polyoxymethylene, iodoform, dexamethasone acetate, formaldehyde, phenol, guaiacol, and excipient. Its mode of action is by cicatrization of the pulpal stump at the chamber-canal interface, while maintaining the structure of underlying pulp.[2] So the purpose of present study was to evaluate the pulpal response to collagen particles impregnated in antibiotics (Biofil-AB™) and new commercially available cement (Pulpotec) that can be used as pulpal medicament.
MATERIALS AND METHODS
A randomized, controlled, prospective 2-year clinical study was done after obtaining the institutional ethical committee approval and an informed written consent from the parents was attained. A sample of 40 teeth from 20 children in the age group of 7-10 years without sex predilection, having bilaterally retained/primary teeth which were noncarious and having at least two-thirds of the root, and indicated for orthodontic extraction were enrolled in the study from the outpatients. Out of 20 children, five of them were excluded due to uncooperativeness. In the remaining 15 children, pulpotomy procedure was carried out on 15 teeth with collagen particles, (Biofil-AB™, Eucare Pharmaceuticals Pvt. Ltd, Chennai, India) on right side (group I) and the rest with PulpotecR cement, (Produits Dentaires, SA, Vevey, Switzerland) on the left side (group II). The teeth were anesthetized and standard pulpotomy procedure was carried out under rubber dam isolation. For group I, collagen particles were mixed with saline into paste like consistency, carried into the pulp chamber and compressed using a damp cotton pellet; for group II, the constituents were mixed into putty-like consistency and were carried into the pulp chamber. The teeth were later restored with zinc oxide eugenol cement and finally with glass ionomer cement (GC Type IX™, GC Corporation, Japan). The patients were recalled after 7, 15, and 30 days time interval and five teeth in each group were extracted and subjected for decalcification procedure. Immediately after post extraction, the teeth were fixed in 10% formalin for 48 h and placed in 10% nitric acid solution till the specimens were completely decalcified. After processing, these specimens were embedded in paraffin wax and sections of 5 μm thickness were obtained using soft tissue microtome which were further stained using hematoxylin and eosin solutions.[3,4] Two samples in each time interval (7, 15, and 30 days) from both the groups were discarded due to procedural or processing errors. The remaining sections were examined under research microscope and digital photomicrographs were obtained under × 40 and × 100 magnifications. The photographs were evaluated using the histological evaluation criteria given by Barbosa et al.[5] [Table 1]. To avoid bias, the findings were reevaluated by two oral pathologists who were unaware of the study design. The scoring thus obtained were tabulated [Table 2] and subjected to statistical analysis.
Table 1.
Histological evaluation criteria used in the study
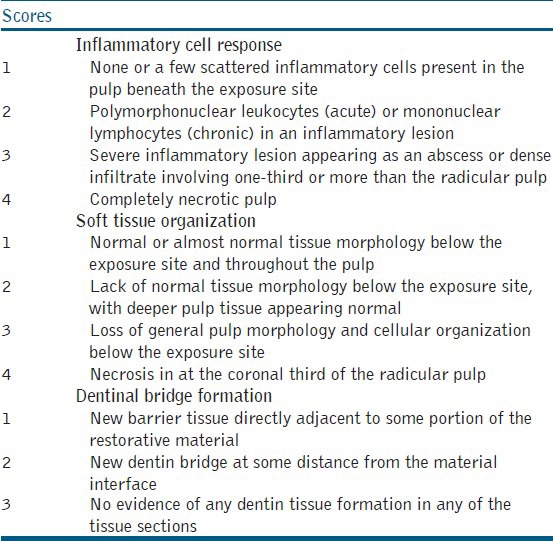
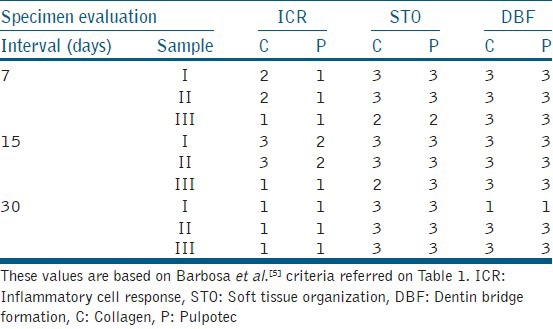
Table 2.
The absolute values for inflammatory cell response, soft tissue organization, and dentin bridge formation
RESULTS
Group I (collagen): After 7 days interval, the specimens showed moderate diffuse chronic inflammatory cells and numerous blood vessels with extravasated red blood corpuscles (RBCs) and obliteration of` odontoblastic layer was observed along the dentinal surface [Figure 1a]. At 15 days, the pulp tissue was accompanied by large quantity of extravasated erythrocytes and a large number of newly formed blood vessels in all the samples [Figure 1b] and severe inflammation within the pulp tissue was observed in two samples. At 30 days interval, there was disorganization of odontoblastic cell layer along the dentinal surface with no inflammatory cells in all the samples [Figure 1c] and dentin bridge formation was observed in two of the specimens [Figure 1d].
Figure 1.
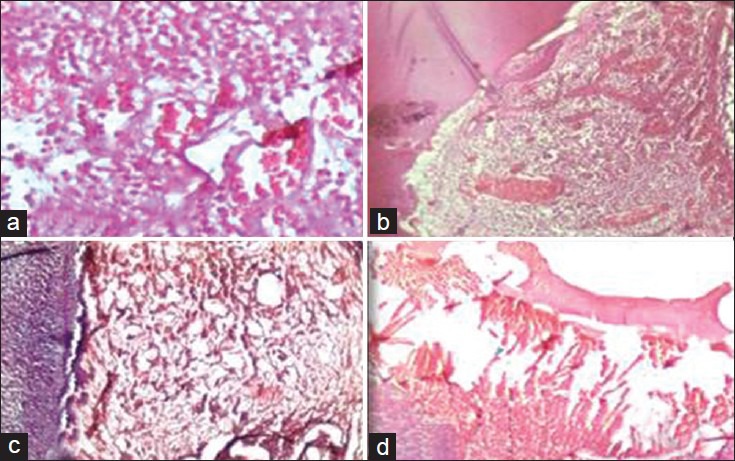
(a) Group I (collagen) at 7 days interval showing moderate inflammation. (b) Group I at 15 days interval showing extravasated erythrocytes and more number of newly formed blood vessels. (c) Group I at 30 days interval showing no inflammation with odontoblastic layer disorganization. (d) Group I at 30 days interval showing dentinal bridge formation
Group II (Pulpotec): None of the specimens showed any signs of inflammation, [Figure 2a] but there was a discontinuity in the odontoblastic layer lining along the dentin walls after 7 days [Figure 2b]. At 14 days interval, pulp tissue showed moderate inflammation in two samples and a discontinuity in odontoblastic cell layer lining the dentinal walls was noted in all the samples. [Figure 2c]. At 30 days interval, pulp tissue did not show any inflammatory changes and discontinuity in the odontoblastic layer lining the dentinal walls was observed in all the samples [Figure 2d].
Figure 2.
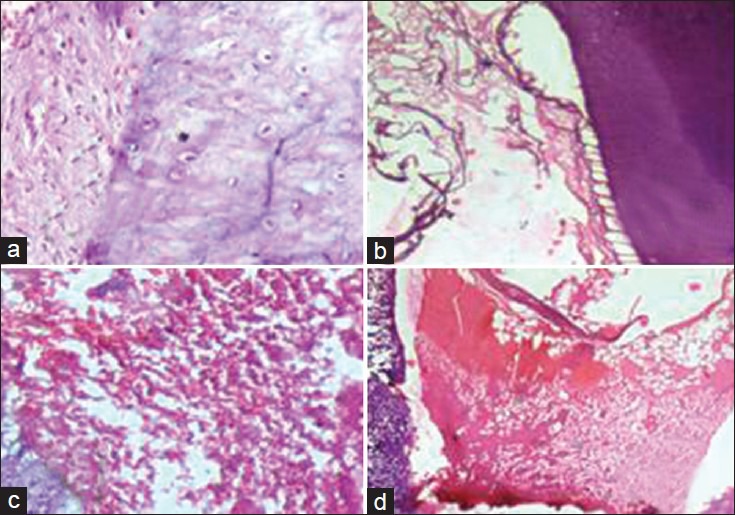
(a) Group II (Pulpotec) at 7 days interval showing no inflammatory cells. (b) Group II at 7 days interval showing odontoblastic layer disorganization. (c) Group II at 15 days interval showing moderate inflammation. (d) Group II at 30 days interval showing disorganized odontoblastic layer with no inflammatory cells
The data was analyzed by descriptive statistics according to the features observed in each experimental group. Statistics have been done using unpaired t-test which showed no significant difference (P < 0.05) for chosen parameters in the study.
DISCUSSION
In spite of wide array of materials and techniques, the gold standard material for pulpotomies of primary teeth till today is formocresol, due to its bacteriostatic and superior fixative property and a success rate of 70-90%.[6] The International Agency for Research on Cancer classified formaldehyde, one of the components of Buckley's formocresol as carcinogenic to humans. Concerns have been raised regarding its systemic absorption, increased prevalence of hypoplastic and/or hypomineralization defects and necrosis/sloughing of the tissue when it comes in contact with the gingiva.[7] Recently the National Institute for Occupational Safety and Health in the United States and the Organization for Economic Cooperation and Development have stated that “formaldehyde is not likely to be a potent carcinogen to humans when used under low exposure conditions.”[8] Therefore, Pulpotec has been selected for this study as it contains formaldehyde at a very low concentration.
Collagen that has a potent hemostatic property and the ability to aggregate platelets, that facilitates wound maturation by enhancing initial blood clot and fibrin linkage formation. It is chemotactic for fibroblasts and improves their migration and attachment through its fibrillar structure. This property may enhance cell migration into the space between collagen membrane and the pulp wound. These collagen fibers are able to induce mineral formation and orient hydroxyapatite crystals.[9] Considering the above mentioned positive properties, collagen particles (BIOFIL™-AB), impregnated with antibiotics like metronidazole and mupirocin has been selected for the study.
Findings in the specimens treated with collagen particles is a perfect indication of strong vascularization, thus resulting in increased number of fibroblasts and a tendency of forming the granulation tissue. These findings show the regenerative characteristics of collagen and are similar to the study conducted by Marsan et al.,[9] where collagen, bioresorbable membrane (Bio-Gide®) was used as a pulp capping material in mongrel dogs. After 6 weeks, the pulp tissue preserved its vitality with a better blood supply in the pulp and an increased number of blood vessels; however, none of the tested samples showed reparatory bridge formation. Thus, the authors stated that collagen bioresorbable membranes showed preservation of the morphology of all histological structures.
In the present study, moderate to severe inflammation is seen in the pulp architecture of samples treated with collagen which has been reduced subsequently. Postlewaithe and Kang[2] demonstrated that topically placed collagen can initiate wound healing by activating inflammatory cells and promoting increased vascularization of the healing tissue. Formation of dentin bridge with collagen can be considered as a sign of pulp healing. The same findings were noticed when Bimstein and Shoshan[10] used collagen as a direct pulp capping agent. In 1-month period, the pulp of tested teeth remained vital and a thin, newly created dentinal bridge was found. Fuks et al.,[11] found dentinal bridge formation in 73% of the tested monkey teeth which were subjected to vital amputation, and later treated with collagen solution.
Conversely, studies conducted by Rutherford et al.,[12] tested the pulpal reaction to human osteogenic protein where collagen matrix was used as a carrier. Results showed that human osteogenic protein had good characteristics as a direct pulp capping material, but the collagen matrix itself did not initiate mineralization nor create the dentinal bridge. It has been demonstrated that some kind of a stimulative chemical factor is necessary for the development of protective reaction of traumatized dental pulp, where mere contact with biocompatible nonstimulative matter is not sufficient. Both Bimstein and Shoshan[10] and Fuks et al.,[11] used an enriched collagen solution which contained some vitamins, amino acids and other nutritive factors; whereas study conducted by Rutherford et al.,[12] used only plain collagen. Thus, the secondary matters are more significant initiators of positive pulp reaction than the collagen itself. Hence, type I collagen particles (fish origin), impregnated with metronidazole are effective against anaerobic infections, mainly gram positive bacteria[13] and mupirocin shown to be quite effective against Staphylococcus aureus[14] can be considered as promising material for use in pulpotomy procedure, especially in the primary dentition.
In group II, pulp tissue treated with Pulpotec cement showed destruction of odontoblastic cell layer along the dentin walls and moderate inflammation limited to the coronal third of radicular pulp. Chronic inflammatory cell infiltration composed mainly of lymphocytes and plasma cells. The reduced inflammation in Pulpotec group may be attributed to the presence of corticosteroid, dexamethasone, which has a potent anti-inflammatory action. The findings of this study are similar to the study conducted by Khattab et al.,[15] who evaluated the histological response of dental pulp to Pulpotec cement in primary teeth at postoperative intervals of 1, 7, and 14 days. Moderate inflammation limiting to the coronal third of radicular pulp with congestion of the pulp vessels were noticed after 1-day interval. After 7 days, there are areas of pulpal necrosis and 14 days later, increased area of pulpal necrosis with destruction of odontoblastic cell layer and chronic inflammatory cell infiltration was noticed.
Histological evaluation following the usage of Pulpotec cement for pulpotomy procedure by Satygo[16] revealed three zones next to the pulp-cement interface which is similar to formocresol. Even though the histological findings are similar, usage of Pulpotec cement is advisable when compared to formocresol because of the minimal concentration of formaldehyde as recent research about formaldehyde metabolism, pharmacokinetics, and carcinogenicity indicate that formaldehyde is probably not a potent human carcinogen under conditions of low exposure.[8]
The synergetic action of other ingredients in the Pulpotec cement like dexamethasone, a potent synthetic member of the glucocorticoid class of steroid drugs has an anti-inflammatory and immunosuppressant property.[17] Phenol has anti-inflammatory, antiviral, antibacterial, antiatherogenic and anticarcinogenic properties.[18] Iodoform; a pale yellow, crystalline, volatile substance used as a disinfectant makes it more patient friendly.[19]
Regeneration of pulp tissue is observed with collagen and devitalization of the pulp tissue with Pulpotec in the present study. Based on the results of this histological study, both collagen and Pulpotec cement showed promising results as a valuable material for use as pulpotomy material especially in primary dentition.
To overcome the shortages of this study; long-term clinical, radiological, and histological studies have to be carried out in large sample size with both infected and healthy pulp tissue to draw proper conclusions. Even though the use of collagen in different forms has been proven in the field of medicine and dentistry, its use as pulpal medicament is very limited. The results of the present study may vary as the pulpal response was noticed after placing the medicaments on healthy pulp rather than compromised pulp tissue.
CONCLUSION
Based on the outcome, it may be considered that collagen and Pulpotec cement used for covering the radicular pulp remnant after pulpotomy were proven to be promising alternatives as a pulp medicament. Both the materials were found to be neither toxic nor biologically incompatible. Collagen, the biomaterial known for its excellent wound healing properties, increased vascularization, pulp healing capacity, and biocompatibility is found to be a better alternative to Pulpotec cement.
ACKNOWLEDGMENT
We extend our gratitude to Dr. L Krishna Prasad, Dr. T R Saraswathi and Dr. K Kiran Kumar for their guidance during the study. Our special thanks to Dr. N Siva kumar for extending his help during the preparation of this manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Da Silva LA, Leonardo MR, Nelson-Filho P, Medeiros AS, Rossi MA. Pulp response of anionic lyophilized collagen matrix with or without hydroxyapatite after pulpotomy in dog's teeth. Mater Res. 2006;9:175–80. [Google Scholar]
- 2. [Last accessed on 2012 Mar 7]. Available from: http://www.eucareindia.com/images/Biofil%20AB%20Particles.pdf .
- 3.Culling CF, Allison RT. 3rd ed. Oxford: Butterworth; 1974. Hand Book of Histopathological and Histochemical Techniques; pp. 29–217. [Google Scholar]
- 4.Bancroft JD, Gamble M. 5th ed. Amsterdam: Elseiver Health Sciences; 2002. Theory and Practice of Histological Techniques; pp. 63–279. [Google Scholar]
- 5.Barbosa AV, Sampaio GC, Gomes FA, de Oliveira DP, de Albuquerque DS, Sobral AP. Short-term analysis of human dental pulps after direct capping with portland cement. Open Dent J. 2009;3:31–5. doi: 10.2174/1874210600903010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patchett CL, Srinivasan V, Waterhouse PJ. Is there life after Buckley's formocresol? Part II-Development of a protocol for the management of extensive caries in the primary molar. Int J Paediatr Dent. 2006;16:199–206. doi: 10.1111/j.1365-263X.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- 7.IARC Classifies Formaldehyde as carcinogenic to humans. International Agency for Research on Cancer, World Health Organization, Press Release No 153, 15 June 2004. [Last accessed on 2012 Mar 7]. Available from: http://www.iarc.fr/en/media-centre/pr/2004/pr153.html .
- 8.Milnes AR. Is formocresol obsolete? A fresh look at the evidence concerning safety issues. J Endod. 2008;34:S40–6. doi: 10.1016/j.joen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Marsan T, Prpic-Mehicic G, Karlovic I, Sostaric B, Anic I, Karlovi Z. Pulpal response to direct pulp capping with collagen bio-resorbable membrane. Acta Stomat Croat. 2003;37:59–62. [Google Scholar]
- 10.Bimstein E, Shoshan S. Enhanced healing of tooth-pulp wounds in the dog by enriched collagen solution as a capping agent. Arch Oral Biol. 1981;26:97–101. doi: 10.1016/0003-9969(81)90077-7. [DOI] [PubMed] [Google Scholar]
- 11.Fuks AB, Michaeli Y, Sofer-Saks B, Shoshan S. Enriched collagen solution as a pulp dressing in pulpotomized teeth in monkeys. Pediatr Dent. 1984;6:243–7. [PubMed] [Google Scholar]
- 12.Rutherford RB, Wahle J, Tucker M, Rueger D, Charette M. Induction of reparative dentine formation in monkeys by recombinant human osteogenic protein-1. Arch Oral Biol. 1993;38:571–6. doi: 10.1016/0003-9969(93)90121-2. [DOI] [PubMed] [Google Scholar]
- 13.Lofmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50(Suppl 1):16–23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 14.Palungwachira P, Palungwachira P. An open study of mupirocin in Thai patients with skin infections. Med J Srinakharinwirot. 2000;7:20–7. [Google Scholar]
- 15.Khattab NM, El-Shehaby FA, Madany NM. A histological and bacteriological evaluation of pulpotec as a pulp medicament for pulpotomized primary teeth. Egypt Dent J. 2010;56:591. [Google Scholar]
- 16. [Last accessed on 2012 Mar 7]. Available from: http://www.pulpotec.com/PAGES/main.php?
- 17.McMaster A, Ray DW. Modelling the glucocorticoid receptor and producing therapeutic agents with anti-inflammatory effects but reduced side-effects. Exp Physiol. 2007;92:299–309. doi: 10.1113/expphysiol.2006.036194. [DOI] [PubMed] [Google Scholar]
- 18.Merkl R, Hradkova I, Filip V, Smidrkal J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J Food Sci. 2010;28:275–9. [Google Scholar]
- 19.Estrela C, Estrela CR, Hollanda AC, Decurcio Dde A, Pecora JD. Influence of iodoform on antimicrobial potential of calcium hydroxide. J Appl Oral Sci. 2006;14:33–7. doi: 10.1590/S1678-77572006000100007. [DOI] [PMC free article] [PubMed] [Google Scholar]


