Abstract
Aim:
The aim of this in vitro study was to evaluate and the compare antibacterial efficacy of four dentin bonding system against Streptococcus mutans, Streptococcus salivarius and Lactobacillus acidophilus over a period of three months using agar disk diffusion test.
Methodology:
All the three standard bacterial strains were inoculated into BHI broth and incubated at 37°C for 48 hours. A 100 μl of broth suspension containing aliquots of S. mutans, S. salivarius and L. acidophilus were spread onto M-H agar medium using sterile cotton swabs. The experimental groups were as follows: GROUP A: Test conducted for evaluation of antimicrobial efficacy against S. mutans MTCC 497, GROUP B: Test conducted for evaluation of antimicrobial efficacy against S. salivarius MTCC 1938, and GROUP C: Test conducted for evaluation of antimicrobial efficacy against L. acidophilus MTCC 447. For sample preparation, 20 μl bonding agent was dropped with micropipettes on paper disks, and blown dry for 10 seconds. Then it was light-cured at 2 mm for 20 sec using a QTH visible light curing unit. For first reading, the sample disks were placed over the freshly inoculated agar plates and then incubated at 37°C for 48 hours. The rest of the paper disks were stored in dark, submerged in distilled water at 37°C. They were placed on freshly inoculated spread plates after specific time intervals- 1 day, 1 week, 2 weeks, 1 month and 3 months after ageing in PBS. After incubation, the diameters of zones of inhibition around the plates were measured. The experiment was performed twice in triplicate. The data was then statistically analysed using Two way ANOVA test and post hoc.
Results:
Results showed that Xeno III had the maximum antibacterial efficacy over a period of three months, followed by XP bond. This antibacterial activity was maximum against Streptococcus mutans, followed by Lactobacillus acidophilus and least against Streptococcus salivarius. Adper Easy One and G bond had minimal effect against the test bacteria during the test period.
Conclusion:
The antibacterial effect decreased over a period of three months for all the dentin bonding systems.
Keywords: Antibacterial properties, dentine bonding systems, Lactobacillus acidophilus, Streptococcus mutans, Streptococcus salivarius
INTRODUCTION
The microorganisms streptococcus and lactobacillus are the main human dental caries pathogens. Streptococcus mutans is the primary etiologic agent contributing to dental caries.[1] Streptococcus salivarius generally has a stronger affinity for the oral soft tissues, but its strains are also found in dental plaque. Lactobacillus acidophilus is also associated with dental caries,[2] mainly in later stages. Several claims have been made regarding the antibacterial effects of restorative materials to prevent recurrent caries. Apart from this, it has been recommended to use an antimicrobial cavity cleanser after tooth preparation and before tooth restoration.[3] Because not much information is available regarding the antibacterial effects of dentin bonding systems, two new seventh generation (one component single-step self-etch) bonding systems – Adper Easy One and G bond, one sixth generation (two component single-step self-etch) bonding system – Xeno III and one fifth generation (universal total-etch self-priming) bonding system – XP bond were compared for their antibacterial effects.
MATERIALS AND METHODS
In this study, four dentin bonding systems, Adper Easy One (3M ESPE), G bond (GC company, USA, Xeno III (Dentsply, Tulsa, Oklahoma, USA)) and XP bond (Dentsply, Tulsa, Oklahoma, USA), were tested against three bacteria over a period of three months.
The experimental groups were divided according to the test conducted for the antibacterial effects of four bonding systems and control against Streptococcus mutans Microbial Type Culture Collection (MTCC) 497 (group A), Streptococcus salivarius MTCC 1938 (group B) and L. acidophilus MTCC 447 (group C).
The sample size for each group was six (n = 6). The study was conducted on a total of 108 Mueller Hinton agar Petri plates. Five absorbent filter paper disks of 5 mm diameter were used in each Petri plate – four disks with dentin bonding agents and one blank disk as control. of each dentin bonding agent was dropped with the respective micropipettes on the paper disk and blown dry for 10 s using a chip blower at a distance of 5 mm to remove excess material and solvents. Then, the bonding agent was light-cured at a distance of 2 mm for 20 s using Quartz Tungsten Halogen (QTH) visible light curing unit (Translux Curing Light, Kulzer). This procedure was performed in a sterile chamber under laminar air flow.
The bacterial strains used in this study were S. mutans, S. salivarius, and L. acidophilus. The bacterial suspensions were prepared in sterile brain heart infusion broth at 37°C for 24 h until a turbidity of 0.5 on the MacFarland scale was obtained. For each reading, 100 μl of the bacterial suspension was spread evenly on Mueller Hinton agar plates using sterile cotton swabs.
After the surface of the plates dried, the disks were placed immediately over the freshly inoculated agar plates using sterile forceps and then incubated at 37°C for 48 h for the first reading. The rest of the paper disks were stored in dark, submersed in distilled water at 37°C. They were placed on freshly inoculated spread plates after specific time intervals – 1 day, 1 week, 2 weeks, 1 month and 3 months – after ageing in phosphated buffer saline. After incubation, the diameter of the zones of inhibition around the disks was measured at two locations using a millimeter scale and the average was calculated.
Descriptive statistical analysis was carried out in the present study. The data were recorded in terms of the average diameter of inhibition zone (in mm) and subjected to the following statistical analysis: ANOVA test and post hoc Tukey test.
RESULTS
The dentin bonding system that exhibited the maximum diameter of zone of inhibition was considered as having the most efficient antimicrobial activity. Samples from triplicate trials showed consistent results, which are depicted in graphs 1, 2 and 3. The results revealed that:
Graph 1.
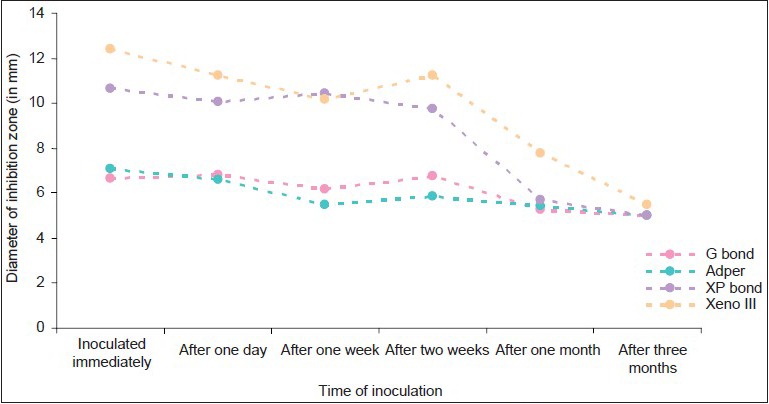
Antibacterial effects of bonding agents against samples inoculated at different time periods in group A
Graph 2.
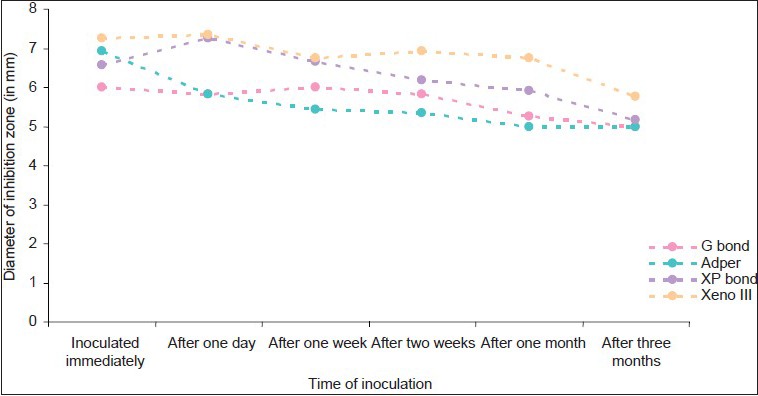
Antibacterial effects of bonding agents against samples inoculated at different time periods in group B
Graph 3.
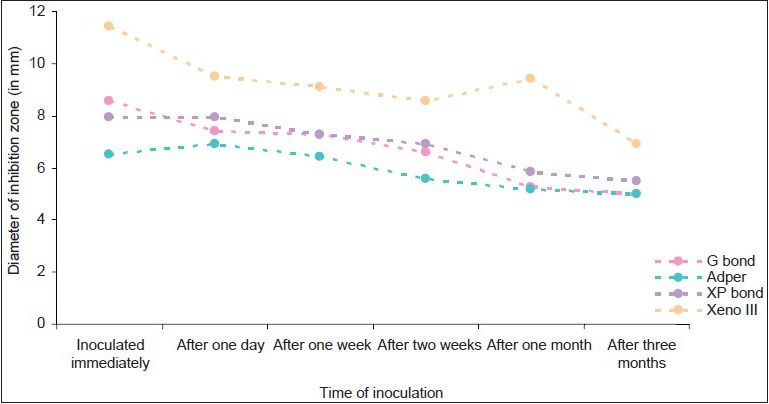
Antibacterial effects of bonding agents against samples inoculated at different time periods in group C
Adper Easy One and G bond had minimal effect against the all the test bacteria during the test period(P<0.005)
Xeno III had the maximum antibacterial efficacy over a period of 3 months(P<0.005), followed by XP bond. This antibacterial activity was maximum against S. mutans, followed by L. acidophilus, and least against S. salivarius.
XP bond showed inhibition of the test bacteria for up to 2 weeks(P, 0.005). G bond and Adper Easy One showed minimal size of inhibition zones against Streptococci. They showed significant size of inhibition zones against L. acidophilus.
DISCUSSION
Operative work in restorative dentistry is now moving away from complete caries removal to an ultraconservative approach, preserving tooth structure and preventing pulpal injury.[4] However, if there is a decreased removal of the tooth structure, it is possible that some active residual bacteria may be left behind in the minimally excavated lesions.[5,6]
S. mutans is the most cariogenic, and is responsible for the initiation of enamel caries and development of secondary caries.
S. salivarius is found abundantly in the dental plaque. L. acidophilus is the main pathogen responsible for the progression of dental caries, especially deep.[2] In vitro studies have shown that the tooth-restoration interface created when using the dentin bonding agents does not eliminate microleakage[7] and bacterial penetration.[8] This may lead to invasion of cariogenic bacteria and their toxins into the dentin. Then, their proliferation occurs and the bacteria may permeate the dentinal tubules, which leads to secondary caries.[7] Thus, dentin bonding is an adjunct in preventing secondary caries as it may stop or slow the bacteria that invade the tooth-restoration interface via microleakage. These materials provide adjunct treatment, contributing to suppression of residual infection, and increase the longevity of the restored tooth.[9] This biological, hermetic seal can be obtained by application of the dentin bonding systems directly to the dentin surface to obtain a resistant structure against bacterial invasion.[10,11]
The decrease in bacterial counts in the oral cavity is associated with decreased caries incidence. But, once a carious lesion is established, caries removal and an adequate tooth preparation are required as part of the restorative treatment.[12] Three of these were self-etching systems that simplified the application procedure and reduced the technique sensitivity and risk of the primed surface being contaminated.[2] For the etch and rinse bonding systems, like XP bond, the etchant application exerts considerable antibacterial effect.
The antibacterial effect might also be due to the release of residual monomers present in the oxygen-inhibited layer.[1]
The current dentin bonding systems available are one-, two- or three-step systems, depending on how the three cardinal steps of etching, priming, and bonding to the tooth structure are accomplished or simplified.
The recently introduced, seventh generation all-in-one adhesives have further combined these three bonding procedures into a single-step application.[13]
The self-etching dentin bonding systems used in this study demonstrated antibacterial action to varying degrees. Xeno III showed maximum antibacterial activity among all the tested dentin bonding systems. G bond does not show much antibacterial activity, and it did not differ significantly from the control group. Adper Easy One, having a pH of 3.5, also does not show much antibacterial activity. This result is in agreement with a study that found that the benefit of the low pH environment exhibited by dentin bonding systems should be considered as being limited.[14]
The difference noted in the antibacterial activity of adhesives against the bacteria could be related to either their acidity or their chemical composition. It may also be related to the viscosity, diffusion capacity and presence of antibacterial agents in the bonding systems.
Previous studies concerning the antibacterial effect of commercially marketed dentin bonding systems have generally attributed this effect to their low pH environments.[11] Harper and Loesche in 1984 reported that the pH values of denting bonding systems, which cause 100% killing over a 3-h-period, is 3.0 for S. mutans.[2] Thus, Adper Easy One, having a pH of 3.5, does not show appreciable antibacterial activity against it, while Xeno III, whose self-etch primer has a pH of 1.4, showed considerable antibacterial property against it. A recent review on self-etching dentin bonding systems has attributed their antibacterial properties to their low pH or to specific antimicrobial components such as glutaraldehyde and 12-methacryloyloxydodecylpyridinium bromide.[6,14,15]
The inhibitory property of Xeno III has also been attributed to the liquid B of this adhesive system, which contains fluoride among other components. The high viscosity of this adhesive due to the presence of Urethanedimethacrylate in its formulation, which is a hydrophobic resin monomer with high molecular weight, may modulate its antibacterial activity due to its slow diffusion.[1]
Considering the observed variations of inhibition zones between the dentin bonding systems used, it may be speculated that their antibacterial effects depends on components that are originally incorporated to promote adhesion. Bacterial binding to the tooth/restoration interface or leakage through a marginal gap might not occur for a specific period of time when using these materials.[12] Thus, the size of inhibition zones was measured for up to 3 months() against the caries-causing bacteria.
Equally important is the effect that the tested materials have on residual bacteria left in the tooth preparation prior to the restorative procedure per se. In a clinical setting, the materials are freshly applied to the cavity preparation that might contain some residual bacteria and then light cured before the final restoration is placed.[12] Thus, the inhibition zones were measured after immediate placement of the bonding agent.
Lastly, a few points have to be kept in mind as limitations of this study. First, as it was an in vitro study, the interactions that the adhesive components have with dentin will be different than from the experimental setting, which might affect their possible antimicrobial effect.[12] Second, the diffusion co-efficient in agar gel and the ability of the material to wet the agar surface might not correspond to what occurs clinically.[12] Finally, different amounts of the adhesive systems remained in the paper discs after removing excess material by air blowing. All the materials had similar-sized droppers for dispensing material but, due to the more viscous nature of Adper Easy One, more material was present on its discs as compared with the other three adhesive systems. These differences might have also affected the end results to some extent and should be considered when comparisons are made among these materials.
CONCLUSIONS
The antimicrobial efficacy of Xeno III was statistically superior, followed by XP bond, while G bond and Adper Easy One showed the least antibacterial activity over a period of 3 months
The maximum antibacterial activity was seen against S. mutans, followed by L. acidophilus, and least against S. salivarius
There was a statistically significant difference between the antibacterial properties of all the four dentin bonding agents against the test bacteria
The antibacterial effect decreased over a period of 3 months for all the dentin bonding systems. This decrease was significant after 2 weeks
The data presented here relate to in vitro conditions. The in vivo conditions, such as the presence of smear layer containing dentin, the amount and concentration of components released from dentin bonding systems, might modify the antimicrobial efficacy of the dentin bonding systems.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Gondim JO, Duque C, Hebling J, Giro EM. Influence of human dentine on the antibacterial activity of self-etching adhesive systems against cariogenic bacteria. J Dent. 2008;36:241–8. doi: 10.1016/j.jdent.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Başeren M, Yazici AR, Ozalp M, Dayangaç B. Antibacterial activity of different generation dentin-bonding systems. Quintessence Int. 2005;36:339–44. [PubMed] [Google Scholar]
- 3.Settembrini L, Boylan R, Strassler H, Scherer W. A comparison of antimicrobial activity of etchants used for a total etch technique. Oper Dent. 1997;22:84–8. [PubMed] [Google Scholar]
- 4.Vaidyanathan M, Sheehy EC, Gilbert SC, Beighton D. Antimicrobial properties of dentine bonding agents determined using in vitro and ex vivo methods. J Dent. 2009;37:514–21. doi: 10.1016/j.jdent.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent Mater. 2006;22:527–32. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Imazato S, Kaneko T, Takahashi Y, Noiri Y, Ebisu S. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent. 2004;29:369–75. [PubMed] [Google Scholar]
- 7.Boeckh C, Schumacher E, Podbielski A, Haller B. Antibacterial activity of restorative dental biomaterials in vitro. Caries Res. 2002;36:101–7. doi: 10.1159/000057867. [DOI] [PubMed] [Google Scholar]
- 8.Slutzky H, Matalon S, Weiss EI. Antibacterial surface properties of polymerized single-bottle bonding agents: Part II. Quintessence Int. 2004;35:275–9. [PubMed] [Google Scholar]
- 9.Ozer F, Karakaya S, Unlü N, Erganiş O, Kav K, Imazato S. Comparison of antibacterial activity of two dentin bonding systems using agar well technique and tooth cavity model. J Dent. 2003;31:111–6. doi: 10.1016/s0300-5712(03)00006-x. [DOI] [PubMed] [Google Scholar]
- 10.Cehreli ZC, Stephan A, Sener B. Antimicrobial properties of self-etching primer-bonding systems. Oper Dent. 2003;28:143–8. [PubMed] [Google Scholar]
- 11.Atac AS, Cehreli ZC, Sener B. Antibacterial activity of fifth-generation dentin bonding systems. J Endod. 2001;27:730–3. doi: 10.1097/00004770-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Walter R, Duarte WR, Pereira PN, Heymann HO, Swift EJ, Jr, Arnold RR. In vitro inhibition of bacterial growth using different dental adhesive systems. Oper Dent. 2007;32:388–93. doi: 10.2341/06-115. [DOI] [PubMed] [Google Scholar]
- 13.Hegde MN, Hegde P, Shetty V, Sampath PB. Assessment of antibacterial activity of self-etching dental adhesive systems: An in vitro study. J Conserv Dent. 2008;11:150–3. doi: 10.4103/0972-0707.48838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuerstein O, Matalon S, Slutzky H, Weiss EI. Antibacterial properties of self-etching dental adhesive systems. J Am Dent Assoc. 2007;138:349–54. doi: 10.14219/jada.archive.2007.0167. [DOI] [PubMed] [Google Scholar]
- 15.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26:267–71. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]


