Abstract
Aggressive nutrition support is recommended following severe burn injury. Initially, such injury results in a prolonged and persistent hypermetabolic response mediated by a 10- to 20-fold elevation in plasma catecholamines, cortisol, and inflammatory mediators. This response leads to twice-normal metabolic rates, whole-body catabolism, muscle wasting, and severe cachexia. Thus, it is relevant to review the literature on nutrition in burns to adjust/update treatment. Failure to meet the increased substrate requirements may result in impaired wound healing, multiorgan dysfunction, increased susceptibility to infection, and death. Therefore, aggressive nutrition support is essential to ensure adequate burn care, attenuate the hypermetabolic response, optimize wound healing, minimize devastating catabolism, and reduce morbidity and mortality. Here, the authors provide nutrition recommendations gained from prospective trials, retrospective analyses, and expert opinions based on the authors' practices in Galveston, Texas, and Vienna, Austria.
Keywords: adult, pediatrics, burns, critical care, trauma, wound healing, enteral nutrition
Introduction
Severe burn injury is associated with metabolic alterations that persist for up to 2 years postburn.1 Immediately after injury, patients enter a period of attenuated metabolism and decreased tissue perfusion, also referred to as the “ebb” phase. Shortly after, they enter a phase of hypermetabolic rates and hyperdynamic circulation, known as the “flow” state.2 The hypermetabolic phase is coordinated via mediators that initiate a cascade of metabolic alterations that can, in turn, prolong recovery or cause death. Management of this response can constitute a challenging endeavor to the surgeon, who is faced with the decision to implement myriad strategies that can include environmental thermoregulation, surgical excision and grafting, exercise, analgesia, anabolic hormones, and catecholamine antagonists. The global success of this effort, however, relies on the prompt commencement and maintenance of adequate nutrition support. We present recommendations gained from prospective trials, retrospective analyses, and expert opinions based on Galveston's contributions that we expect will aid in the nutrition assessment and management of severely burned adult and pediatric patients.
Prolonged and persistent hypercatabolism provokes a dreadful cascade of events, including weight loss, constitutive muscle and bone catabolism, growth retardation, immunosuppression, infection, physiologic exhaustion, and possible death.3–5 A 10% loss of total body mass leads to immune dysfunction; 20%, to decreased wound healing; 30%, to severe infections; and 40%, to death.6 In the past, severely burned, catabolic patients would routinely lose up to 25% of their total body mass. Even today, although rare, patients may still develop caloric deficits in the tens of thousands that translate into massive weight losses. The hypermetabolic and hypercatabolic response after severe burn injury requires an aggressive nutrition replacement.
Metabolic Response Postburn
The primary mediators of the hypermetabolic response postburn are catecholamines, corticosteroids, and inflammatory cytokines.7,8 Burn patients show a 10- to 20-fold elevation in catecholamines and corticosteroid levels, which may last up to 12 months postburn and a considerably altered expression of acute and constitutive proteins that last up to 2 months postinjury.8 These catabolic hormones thwart the action of insulin and establish a state of increased lipolysis, proteolysis, gluconeogenesis, and energy consumption (Figure 1). Plasma glucose and insulin levels increase and remain significantly elevated throughout the entire hospital stay.8 On one hand, the moderately increased glucose availability is beneficial to supply the heightened energy demand, aiding muscle tissue with a “protein-sparing” effect that attenuates catabolism by reducing gluconeogenesis and amino acid oxidation.8–10 Conversely, excessively high glucose levels can lead critically ill patients to poorer morbidity and mortality outcomes by increasing the risk for skin graft failure and wound infections.9,11,12 The presence of vast numbers of inflammatory mediators adds to the multiple factors that make the nutrition management in burned patients an intricate task.
Figure 1.
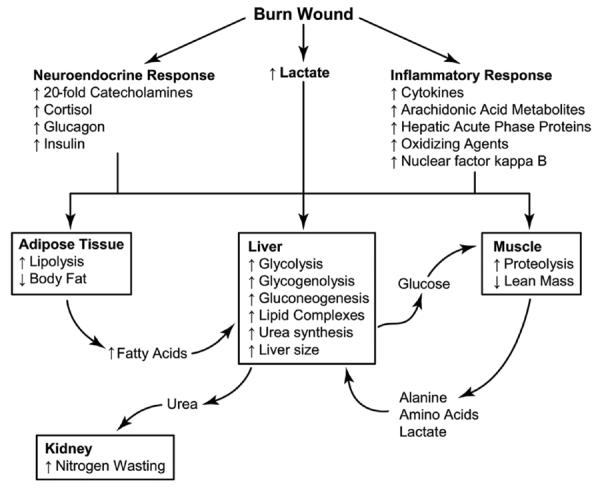
Metabolic response postburn. Severe burn injury leads to profound hypermetabolic response mediated by catecholamines, cortisol, and glucagon. Stress hormones lead to significant physiologic and metabolic derangements in every organ system. Reprinted with permission from Annals Surgery. 2008;248:387-401; Figure 1, Nutrition in Burns.
Burned children, in comparison to nonburned, have a significant and persistent increase in actual resting energy expenditure (REE) for up to 24 months postinjury. Their REE increases in a curvilinear fashion in relation to total body surface area (TBSA) burned. Thus, pediatric patients with ≤10% TBSA burns show close-to-normal percent predicted REE, and those with ≥40% TBSA burns may experience one and one-half normal percent predicted REE within the first 2 weeks postburn. It has been noted that at a neutral room temperature of 30°C, the metabolic rates of these patients approach 150% predicted REE and may decrease to 135% once the wounds have fully healed.8 Not until 2 years postburn do patients approach metabolic rates of 110%–120% predicted REE, based on the Harris-Benedict equation.1
The obligatory state to supply fuel postburn allows muscle protein to be degraded much faster than it is synthesized,8,13 causing net loss of protein soon after burn. Sustained losses lead to a decrease in lean body mass, severe muscle wasting, and failure to fully rehabilitate. The protein loss is directly related to increases in metabolic rate and may persist for up to 24 months postburn, often resulting in significantly negative whole-body and cross-leg nitrogen balances.14–16 Severely burned patients have a nitrogen loss of 20–25 g/m2 TBSA/d,15 which—if unattended—results in lethal cachexia in <30 days. In young children, protein loss leads to significant growth retardation for >1 year postinjury.17
Data obtained by stable isotope techniques reveal the significant derangements in protein turnover, urea production, and gluconeogenesis in burned patients. During the postburn hypermetabolic response, both glycolytic-gluconeogenic and triglyceride–fatty acid cycling have been reported to increase by 250% and 450%, respectively.5,18 Collectively, these changes increase glycogenolysis, gluconeogenesis, and circulation of glucogenic precursors, which translate into hyperglycemia and impaired insulin responsiveness, in turn related to postreceptor insulin resistance.4 Although glucose delivery to peripheral tissues is increased by up to 3-fold, glucose oxidation is restricted, leading to elevated fasting glucose. Increased glucose production is directed, in part, to the burn wound to support the anaerobic metabolism of fibroblasts, endothelial cells, and inflammatory cells.19 Following excessive endogenous lipolysis, the liver increases its size by 225% of normal by 2 weeks postburn18 and remains increased at discharge by 200%.8,20 The end product of anaerobic glucose oxidation—lactate—is recycled to the liver to produce more glucose via gluconeogenic pathways.15 Adequate nutrition support is an effective nonpharmacological strategy to attenuate these catastrophic metabolic responses.
Timing of Nutrition
Advances in burn care altered the magnitude of the postburn hypermetabolic response but not the nature of the response.21 A major determinant of outcome for severe burn patients is time to treatment. Any delays in resuscitation lead to poorer outcomes.22 Acutely, there is significant gut mucosal damage and increased bacterial translocation that collectively lead to decreased nutrient absorption.23,24 As such, optimal nutrition support for the severely burned patient is best accomplished by early (within 24 hours after injury) initiation of enteral nutrition (EN).25 Multiple studies demonstrate that the early institution of enteral feeding can significantly modulate the hypermetabolic response to severe burn.26 Animal studies showed significant decreases in metabolic rates by 2 weeks postburn in animals enterally fed continuously by 2 hours postburn compared to animals fed 3 days postburn, indicating the benefits of early initiation. There is significant modulation of catecholamine levels and support of gut mucosal integrity with early EN.27
In human studies, early and continuous EN has been shown to effectively deliver caloric requirements (REE) by postburn day 3, diminish the hypermetabolic response, and decrease circulating levels of catecholamines, cortisol, and glucagon.27,28 Early EN also preserves gut mucosal integrity, motility, and intestinal blood flow, which are important to prevent intestinal hypoperfusion or ileus due to delays in resuscitation or reperfusion. Because the postburn ileus primarily affects the stomach and colon,29 patients with severe burn injury can be fed through enteral tubes to the small bowel (duodenum or jejunum) 6 hours postburn, independently of total gastroduodenal function.30
Nutrition Assessment and Monitoring
Optimal nutrition assessment of the burned patient should comprise a complete review of the features in the history and physical exam that may affect nutrition management. These commonly include preexisting malnutrition, malabsorption, paralytic ileus, severe short bowel syndrome, and presence of severe shock, obstruction, or diffuse peritonitis. Additional laboratory, clinical, and metabolic examinations are not only recommended for the initial assessment but for the continued monitoring postburn. Serum proteins, nitrogen balance, anthropometric measurements, intake and output of fluids, indirect calorimetry, and tests of immune function are methods that provide a fair overview of the metabolic alterations in the postburn period. In the majority of surgical specialties, perioperative levels of serum albumin are better predictors of morbidity and mortality than other biochemical markers and even anthropomorphic measurements.31,32 Patients with serum albumin levels at a cutoff point of 21 g/L present a 30% increased risk of 30-day mortality and up to 65% risk of 30-day morbidity. However, in burned patients, weekly levels of serum prealbumin (transthyretin) are a better nutrition marker than albumin. Prealbumin levels show a maximal decrease between days 6 and 8 in all burned patients, but persistent low levels (100–150 mg/L on days 14–17 postburn) are associated with a decreased likelihood of survival.33,34 Even when patients do survive, persistently low prealbumin values are associated with a higher incidence of sepsis, lengthier stays, and a decreased ability for wound healing.33,34 Notably, prealbumin levels are inversely correlated to increased acute-phase protein levels (ie, C-reactive protein). Prealbumin, as opposed to albumin, can also be used as a sensitive tool in predicting graft take in burned patients.35
Nutrition assessment in burned children should include plotting of the patient's height and weight on percentile charts. The Centers for Disease Control and Prevention (CDC) has published revised standard gender-specific percentile charts that provide data on height for age, weight for age and body mass index (BMI). These charts are demographically representative of the U.S. population for ages 2–20 years, with charts also available for younger children. In the acute setting, these charts allow evaluation of the nutrition status of patients whose nutrition history is limited. A BMI below the 5th percentile indicates an underweight patient, whereas a trend line crossing 2 major percentile lines signifies a growth problem or failure to thrive. In the postacute setting, these charts are used to monitor the patient's long-term nutrition status. It should be noted that fluid overload not uncommonly masks a continuing loss of lean body mass. Thus, such patients can suffer significant inanition and still weigh more than at the time of admission. In addition, fluid shifts associated with infections, ventilator support, hypoproteinemia, and elevations in aldosterone and antidiuretic hormone lead to wide fluctuations in weight that have little to do with nutrition status.36 Judicious monitoring of weight trends should be a priority in the clinical management of severely burned patients. Although no single laboratory test is fully reliable in nutrition monitoring, systematic and holistic assessments are key to management of the ever-evolving physiologic response postburn.
Addressing Caloric Requirements
As part of our routine clinical practice, indirect calorimetry is performed in all patients between midnight and 5 am while asleep. Measured resting energy expenditure (MREE) is determined within 1 week of admission and weekly thereafter during their acute hospitalization. Actual MREE is expressed in units of kcal/d and is used to guide nutrition management and to estimate caloric requirements. Predicted REE, as expressed by MREE/predicted basal metabolic rate from the Harris-Benedict equation, is used as an indicator of the degree of hypermetabolism. Mlcak et al37 stratified indirect calorimetry data of pediatric burn patients (n = 100) by gender and showed that males exert predicted REE 10–20 percentage points higher than females, a significant difference that last up to 9 months postburn. Stratification in 3 age groups representing children <3, 3–9.9, and 10–18 years of age showed predicted REEs of 118% ± 10%, 139% ± 7%, and 152% ± 6%, respectively. A significant difference was shown only between the former and latter groups. In adult burn patients (≥20% TBSA burned), current reports indicate higher predicted REEs with an average of 160% and a standard deviation up to 30%.38,39
Considering the high cost of acquiring and maintaining the equipment for bedside indirect calorimetry, more centers have adapted predictive historical equations to estimate caloric requirements in burn patients. Through elaborate formulas, patient-specific factors such as age, gender, weight, temperature, and burn size are collected on a day-to-day basis to estimate caloric needs. Historically, however, adapted equations in common use such as Curreri, Harris-Benedict, Schofield-HW, and World Health Organization, have overpredicted basal metabolic rates, resulting in increased risk of overfeeding and adverse events.40,41 Not surprisingly, lack of a correcting factor in nonadapted formulas underestimates caloric needs. The adapted Toronto formula is an exception. It correlates well with MREE (r2 = 0.67) but is complicated to use.38 Studies have shown that MREE requires a correcting factor of 30% to maintain adequate body weight, obtained by the product of a 1.2–1.4 factor.41–44 The rationale for the alluded factor comes from the intention of measuring total energy expenditure (TEE), which represents the most direct assessment of caloric requirements. TEE measurement, however, requires application of a doubly labeled water technique that is invasive and not feasible for routine use. As shown by Goran et al,45 TEE assessed by this technique was significantly correlated with 1.18 ± 0.17 times MREE (r2 = 0.92) from indirect calorimetry. In other words, by supplementing 1.2× MREE kcal/d, a very close approximation to actual TEE is expected. Observational studies have shown an increase in body weight by feeding >1.4× MREE kcal/d; the gains however, were in fat deposition and thus not advocated.41,42
Ideally, optimal caloric balance should be determined from a prospective trial. Such a trial would include measures of morbidity and of functional outcome. Preservation of lean body mass should be a goal of nutrition support for severe burn victims because a major consequence of the hypermetabolic response is severe catabolism. It is our hope for the future that through further testing of these formulas and indirect calorimetry itself, an ideal method will be refined to optimally quantify requirements of nutrition support in burned children and adults. In line with above, in the treatment of severe burns, we advocate the use of indirect calorimetry to help guide caloric requirements estimated by common predictive equations (Table 1).
Table 1.
Equations Used to Estimate Caloric Requirements in Burned Patients
| Age, y | Formula Name | Formula |
|---|---|---|
| 0–1 | Galveston Infant | 2100 kcal/m2 + 1000 kcal/m2 burn |
| 1–11 | Galveston Revised | 1800 kcal/m2 + 1300 kcal/m2 burn |
| 12–16 | Galveston Adolescent | 1500 kcal/m2 + 1500 kcal/m2 burn |
| 16–59 | Curreri Formula | 25 kcal/kg of weight + (40) TBSA |
| Toronto Formula | −4343 + (10.5 × TBSA) + (0.23 × CI) + (0.84 × HBE) + (114 × T) − (4.5 × PBD) | |
| ≥60 | Curreri Formula | 20 kcal/kg of weight + (65) TBSA |
Resting energy expenditure (REE) measurements are used to guide nutrition management. Corrected 1.2–1.4× measured REE kcal/d is recommended for patients ≥3 years of age. CI, total calorie intake the previous day; HBE, Harris-Benedict estimates; PBD, number of postburn days to the day preceding the estimation; T, average of core temperatures (°C) the previous day; TBSA, burn size (percent total body surface area).
Substrate Requirements
Carbohydrates
Carbohydrates aid as fuel in wound healing and provide a protein-sparing effect that decreases the loss of lean body mass. Therefore, to adequately feed burned patients, one should first consider the minimum baseline adult requirements of carbohydrates (2 g/kg/d)46 and the maximum rate at which glucose can be assimilated in severely burned patients (7 g/kg/d).47–49 These are important values that one should keep in mind in the management of hypermetabolic patients, as they occasionally have caloric requirements that can exceed the maximum rate at which the body is able to oxidize glucose.47 In other words, severely burned patients may very well have greater needs than those that can safely be supplied. Inadequate carbohydrate delivery that fails to meet the increased demands of burned patients may lead to uncontrolled protein catabolism, whereas supplementation in excess of utilization leads to hyperglycemia, conversion of glucose into fat, glucosuria, polyuria, dehydration, and respiratory problems.
Adequate control of carbohydrate levels in critically ill patients usually goes hand in hand with watchful administration of anabolic hormones. Insulin therapy in burned patients stimulates muscle protein synthesis, increases lean body mass, and is associated with improved wound healing, without increasing hepatic triglyceride production.50,51 Severely burned patients demonstrated improved donor site wound healing after receiving 7 days of continuous infusions of insulin and glucose titrated to maintain euglycemia and plasma insulin concentrations of 400–900 μU/mL.52 In addition, patients receiving a high-carbohydrate, high-protein enteral formula and insulin infused at 1.5 μU/kg/min to maintain blood glucose levels between 100 and 140 mg/dL significantly improved lean body mass, bone mineral density, and decreased length of stay during the acute hospitalization.53 Insulin therapy, however, may also lead to hypoglycemia in some patients and should be closely monitored as hypoglycemia can quickly lead to increased morbidity and mortality.
Fats
The administration of small amounts of dietary fat (ie, 2%–3% of linoleic acid) is critical to prevent the development of essential fatty acid deficiency.54 In burned patients, the ability of the body to handle additional amounts of fat is significantly altered; thus, one should cautiously estimate the proportion of fat to be supplemented in nutrition protocols. Immediately after injury, there is an increase in peripheral fat breakdown, as well as in utilization of fat by the liver. Although, the increased β-oxidation of fat provides fuel during the hypermetabolic response, only 30% of the available free fatty acids undergo degradation; the rest undergoes reesterification and potential accumulation in the liver.54
Therefore, in burned patients, the percentage of dietary fat calories needs to be carefully considered. In our institution, we prefer EN specialty formulas with a very low fat content of 3%–15% of total calories. In patients receiving short-term (<10 days) parenteral nutrition (PN), we usually withhold entirely from using lipid emulsions.55 For patients needing longer PN periods (>10 days), we use 0.5–1 g fat/kg/d, withholding administration to once or twice weekly based on individual assessment of benefits and safety.56 In addition, the total number of nondietary fat calories is also to be considered. For example, propofol affects the total amount of fat calories administered in a given day. A 1% propofol solution has the same caloric value of a 10% intralipid emulsion (1.1 kcal/mL, 440 kcal in a 400-mL infusion) and may lead to significant metabolic alterations. Thus, serum triglyceride concentrations should be monitored in patients receiving such infusions and caloric intake corrected accordingly. One should also consider the types of dietary fat administered, as these are potentially as important as their amounts. Although there are insufficient data to decisively recommend the use of diets enriched with nutrients such as arginine and ω-3 fatty acids, some have suggested that patients with >30% TBSA third-degree burns may benefit from their use.57 Common lipid sources high in ω-6 fatty acids are metabolized to proinflammatory cytokines, which may facilitate inflammation. In contrast, diets high in ω-3 fatty acids have been associated with improved outcomes, attenuated inflammatory response, and reduced incidence of hyperglycemia.58–60
Protein
After severe burns, proteolysis is a hallmark of the hypermetabolic response and can exceed 150 g/d or almost one-half pound of skeletal muscle.61 These patients can oxidize amino acids at rates 50% higher than those seen in healthy fasting individuals. Such high breakdown rates frequently translate into significant loss of lean body mass, decreased wound healing, and immune incompetence. Therefore, attenuation of this response should be a goal of any nutrition, pharmacological, and nonpharmacological treatment regimens designed for burn patients.
Although the mechanism by which protein breakdown occurs is not yet fully understood, the human body is capable of sparing protein when adequately and timely supplemented with high-quality protein and carbohydrate. Comparably, if the amounts of supplemented protein are proportionally larger than the capacity of the protein pool in the body, increased urea production without improvements in lean body mass is anticipated.62 In clinical practice, well-accepted protein requirements are estimated at 0.8–1 g/kg/d in healthy individuals,63 at 1.5–2 g/kg/d in burned adults, and at 2.5–4.0 g/kg/d in burned children.21,64,65 Undoubtedly, even at these relatively high replacement rates, it is not rare to encounter burned patients whose neuroendocrine and proinflammatory responses lead them to persisting loss of muscle protein.
Certain amino acids have a key role in recovery following injury. Severe burn increases skeletal muscle and organ efflux of alanine (ALA), arginine (ARG), and glutamine (GLN). Available amino acids aid wound healing and supply energy to the liver66; GLN serves as a primary fuel in enterocytes and lymphocytes and plays a role maintaining small bowel integrity, preserving gut-associated immune function, and limiting intestinal permeability.67–69 Low GLN plasma concentrations have been associated with an immunodeficient state, a propensity to infection, and an increased bowel permeability. Prospective, randomized studies conducted in burned adults have shown that supplementation of EN with GLN (0.35–0.57 g L-GLN/kg of body weight/d) either intravenously or via the gastrointestinal (GI) tract is associated with decreased incidence of infections, improved visceral protein levels, decreased length of stay, and reduced mortality.69,70
Vitamins and Trace Elements
After burn injury, patients enter a period marked by extensive loss of tissue, altered metabolism, increased inflammation, and distorted cell membrane homeostasis. These, combined with a substantial redistribution of fluid and nutrients, sets the stage for the development of vitamin deficiencies and a long-lasting oxidant/antioxidant imbalance proportional to the severity of the burn.71–73 Decreased levels of vitamins A, C, and D and trace elements such as Fe, Cu, Se, and Zn have been shown to adversely affect wound healing and skeletal, neuromuscular, and immune system function.72,74 Subsequently, oxidative stress also contributes to secondary tissue damage and further impairs the immune function. Prompt replacement of vitamins is recommended. Vitamin A improves wound healing time due to an effect in epithelial growth, whereas vitamin C facilitates synthesis and cross-linking of collagen.72,75 Daily recommended intakes for vitamins A and C are listed in Table 2. The exact role of vitamin D deficiency in failure of bone density to reach that of nonburned peers is unknown.76 In a recent study, severely burned children were discharged on a multivitamin containing 400 IU of vitamin D2; serum levels of 25-hydroxyvitamin D were monitored. After 6 months, vitamin D insufficiency remained evident in all but 1 of the 8 patients studied. This finding suggests that the exact vitamin D requirement following burn injury also remains unknown.77
Table 2.
Reference Daily Intake (RDI): Vitamin and Trace Elements Requirementsa
| Age, y | Vitamin A, IU | Vitamin D, IU | Vitamin E, IU | Vitamin C, mg | Vitamin K, mcg | Folate, mcg | Cu, mg | Fe, mg | Se, mcg | Zn, mg |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–13 | ||||||||||
| Nonburned | 1300–2000 | 600 | 6–16 | 15–50 | 2–60 | 65–300 | 0.2–0.7 | 0.3–8 | 15–40 | 2–8 |
| Burned | 2500–5000 | 250–500 | 1000b | 0.8–2.8 | 60–140 | 12.5–25 | ||||
| ≥13 (includes adults) | ||||||||||
| Nonburned | 2000–3000 | 600 | 23 | 75–90 | 75–120 | 300–400 | 0.9 | 8–18 | 40–60 | 8–11 |
| Burned | 10,000 | 1000 | 1000b | 4.0 | 300–500 | 25–40 |
RDI, Reference Daily Intake refers to the daily intake level that a healthy person should achieve. Conversion based on 1 mcg of vitamin A = 3.33 IU of vitamin A; 1 mcg of calciferol = 40 IU of vitamin D; 1 mg of α-tocopherol = 1.5 IU of vitamin E.
Sources: Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin D, and Fluoride (1977); Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (1988); Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (2000); and Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001). These reports may be accessed at http://www.nap.edu.
Administered Monday, Wednesday, and Friday.
Trace elements (Fe, Cu, Se, and Zn) are involved in humoral and cellular immunity.74,78 Fe is an important cofactor in oxygen-carrying proteins.21 Zn plays a role in wound healing, DNA replication, lymphocyte function, and protein synthesis.79 Se improves cell-mediated immunity and activates the nuclear transcription factor NF-κB.74,80 Cu is necessary for collagen synthesis and wound healing. Deficiencies in Cu have been linked to fatal arrhythmias, altered immunity, and poorer outcomes.74,81 Plasma levels of these trace elements are significantly depressed for prolonged periods after the acute injury due to increased urinary excretion and significant cutaneous losses.82–85 Collectively, replacement of all these micronutrients has been found to contribute to the improvement in morbidity of severely burned patients.86–88 Daily use of multivitamins provides the recommended daily averages estimated for healthy individuals; however, further studies in burned patients are necessary to determine doses required to reach desired plasma levels (Table 3).
Table 3.
Selected Presentations of Vitamin and Trace Elements—Available in the United States
| Age, y | Enteral | Parenteral | ||
|---|---|---|---|---|
| 0–12 | ||||
| Enfamil Poly-Vi Sol (1 mL) | MVI Pediatric (5-mL vial) | |||
| Vitamin A: 1500 IU | Vitamin D: 400 IU | Vitamin A: 2300 IU | Vitamin D: 10 mcg | |
| Vitamin C: 35 mg | Vitamin E: 5 IU | Vitamin C: 80 mg | Vitamin E: 7 mg | |
| Folate: 140 mcg | Vitamin K1: 200 mcg | |||
| Vitamin A: <2 y, 2500 IU | Multitrace—4 Pediatric (3 ml vial) | |||
| 2–12 y, 5000 IU | Each mL provides: | |||
| Vitamin C: 250 mg | Zinc: 1 mg | Manganese: 25 mcg | ||
| Vitamin E: 5 mg | Copper: 0.1 mg | Chromium: 1 mcg | ||
| Folic acid: 1 mga | Selenium (sodium selenite—10 mcg/mL) | |||
| Zinc sulfate: 50–110 mg | ||||
| ≥12 | ||||
| Rx Choice Thera-Plus (5 mL) | Infuvite Adult (5-mL vial) | |||
| Vitamin A: 5000 IU | Vitamin D: 400 IU | Vitamin A: 3300 IU | Vitamin D: 200 IU | |
| Vitamin C: 35 mg | Vitamin C: 200 mg | Vitamin E: 10 IU | ||
| Folate: 600 mg | Vitamin K1: 150 mcg | |||
| Folic acid: 1 mga | Multitrace—4 (10-mL vial)Each mL provides: | |||
| Vitamin A: 10,000 IU | Zinc: 1 mg | Manganese: 100 mcg | ||
| Copper: 0.4 mg | Chromium: 4 mcg | |||
| Vitamin E: 10 mg | Selenium (sodium selenite—10 mcg/mL) | |||
| Zinc sulfate: 200 mg | ||||
IU, International Units; mcg, micrograms; mg, milligrams; mL, milliliters.
Administered Monday, Wednesday, and Friday.
Routes of Nutrition Support
Severely burned patients who become victims of inanition generally succumb to severe systemic infections or respiratory failure.89 Therefore, in the treatment of severe burns, major determining factors of success are the installation of an adequate nutrition route and a subsequent nutrition regimen. Nutrition methods that involve oral alimentation are often unsustainable because of the frequency of altered mental status, inhalation injuries, endotracheal intubation, GI dysfunction, and feeding intolerance seen in burned patients. Even in absence of these factors, studies have shown that the use of oral alimentation alone is not ideal, as it can allow patients with 40% TBSA burns to lose up to a quarter of their preadmission weight by 21 days postinjury. Oral feedings in severely burned patients are also difficult to sustain because of the large and often intolerable amounts of food necessary to manage severe catabolism.
EN is a sensible, safe, cost-effective, and widely available feeding route; it has considerably improved outcomes by mitigating the degree of catabolism. EN offers a feasible route for early installation and maintenance of nutrition support in burn patients and several other forms of trauma.26,90 EN maintains the structural and functional integrity of the gut, stimulates blood flow, and preserves first-pass nutrient delivery to the liver.90 EN reduces translocation-bacteremia and sepsis, decreases the incidence of pneumonia and central line infections, and supports IgA production in the gut-associated immunocytes.91,92 A multicenter study seeking to evaluate compliance of “early” (<24 hours from admission) installation of EN in adult burn patients (n = 153) found no significant difference in rates of hyperglycemia, abdominal compartment syndrome, or GI bleeding, but they exhibited shorter intensive care unit (ICU) length of stay (adjusted hazard ratio [HR] = 0.57; P = .03; 95% confidence interval [CI], 0.35–0.94) and reduced risk to develop wound infection (adjusted odds ratio [OR] = 0.28; P = .01; 95% CI, 0.10–0.76). Initiation of EN by 24 hours from admission is recommended as standard of care for severe burns.93
PN for the management of severe burns surfaced in the 1970s with the expectation that it would become standard of care. In the late 1980s, studies showed that use of PN, either alone or in combination with EN, was associated with overfeeding. Evident in these studies were increased incidence of liver malfunction, impaired immune response as seen by lower T cell helper/suppressor cell ratios, and heightened mortality by almost 3-fold.94,95 These factors, combined with the incidence of mechanical and infectious complications of catheters, the increase in proinflammatory cytokines, and worsening of the pulmonary function, led to further deterrence from the use of PN in the early postburn period. Nonetheless, although current practice is to preferentially use the GI tract for nutrition support, the parenteral route can be used in burned patients whose caloric requirements cannot be supplied via EN. The question of whether we should initiate PN in the first 24 hours if EN is contraindicated stands unanswered; insight can be obtained from available evidence, but a definitive recommendation will need to be further evaluated. In a large meta-analysis conducted in critically ill patients that included a small number of burned patients, a grade B evidence-based recommendation has been made for the use of PN in patients in whom EN cannot be started within the first 24 hours of admission. A modified subgroup analysis attributed a reduction in risk of mortality to trials comparing PN to delayed (>24 hours) EN, despite an association with increased infectious complications with PN.96,97 Studies specifically designed to evaluate this hypothesis in burned patients are necessary, and careful choosing of an equation to estimate caloric needs as well as the use of indirect calorimetry to guide nutrition is warranted.
Formulas
Milk became the standard of care for burn patients in the 1970s. In general, milk (44% fat, 42% carbohydrate, and 14% protein) was fairly well tolerated; however, it was considered a fat-based diet, which in certain clinical conditions did not seem to be the optimal source of energy. In the decades to follow, a debate about the roles of high-fat and high-carbohydrate diets in the care of critically ill patients was originated. In a prospective, randomized, crossover trial conducted on severely burned pediatric patients (n = 14), 2 isocaloric isnonitrogenous diets were examined, including a fat-based diet and a carbohydrate-based diet. Cross-leg stable isotope techniques detected that patients on high-carbohydrate regimens (3% fat, 15% protein, and 82% carbohydrate) had improved muscle catabolism, suggestive of a protein-sparing effect associated with higher endogenous insulin concentrations.98 When this study was published, it was suggested that high-carbohydrate diets in critically ill adults may result in hyperglycemia and may have no effect on protein metabolism.99 A prospective, randomized, multicenter trial in critically ill adults (n = 47), later examined 2 PN dietary regimens, one with a glucose/lipid ratio of 80/20 (high-carbohydrate) and the other with a 50/50 (high-fat) ratio. It was determined that the high-carbohydrate regimen had a better nitrogen-sparing effect but at the risk of altered glycemic control.100 Although this study included only 2 burned adults, a reasonable recommendation is that in acutely ill burn patients, regardless of age, a high-carbohydrate diet is preferred (Table 4).
Table 4.
Selected Enteral Nutrition Options for Burned Patients: U.S. Market
| Nutrition | kcal/mL | CHO, g/L (% Cal) | PRO, g/L (% Cal) | Fat, g/L (% Cal) | Comments |
|---|---|---|---|---|---|
| Pediatric | |||||
| Vivonex RTF | 1 | 175 (70) | 50 (20) | 12 (10) | Transitional feeding, low fat, high CHO, easily digestible |
| Vivonex TEN | 1 | 210 (82) | 38(15) | 2.8 (3) | Free AA, very low fat, high CHO.98 Severe trauma or surgery. |
| Impact Glutamine | 1.3 | 150 (46) | 78 (24) | 43 (30) | Immunonutrition, GLN, ARG, ω-3 fatty acids |
| Elecare | 0.67 | 72 (43) | 20(15) | 32 (42) | Prepared at 9.4 g/60 mL, AA-based nutrition |
| Adult | |||||
| Crucial | 1.5 | 89 (36) | 63 (25) | 45 (39) | Immune enhancing with ARG, concentrated |
| Impact | 1.0 | 130 (53) | 56 (22) | 28 (25) | Immune enhancing with ARG, GLN, fiber |
| Oxepa | 1.5 | 105 (28) | 63 (17) | 94 (55) | ALI, ARDS period (2 wk),60 concentrated |
| Glucerna | 1.0 | 96 (34) | 42 (17) | 54 (49) | For glucose intolerant or diabetic patients, low CHO |
| Nepro | 1.8 | 167 (34) | 81 (18) | 96 (48) | For CKD and patients on dialysis, concentrated |
| Osmolite 1 Cal | 1.06 | 144 (54) | 44 (17) | 35 (29) | Isotonic, for use in intolerance to hyperosmolar nutrition |
| Modular (Children/Adult) | |||||
| Benefiber Powder | 0.27 | 66 (100) | (Prepared at 4 g/60 mL) Tasteless, odorless, soluble fiber | ||
| Beneprotein | 0.83 | 200 (100) | (Prepared at 7 g/30 mL) Whey protein, mixed in foods |
AA, amino acid; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; ARG, arginine; CHO, carbohydrate; CKD, chronic kidney disease; GLN, glutamine; PRO, protein. Data extrapolated from “Enteral Product Reference Guide, by Nestle Clinical Nutrition 2010” (Minneapolis, MN) and “Abbott Nutrition Pocket Guide © 2010.”
Immune-enhancing formulas consist of macronutrients enriched with arginine, glutamine, nucleotides, and ω-3 fatty acids. These nutrition regimens have shown to be beneficial in nonburned patients in terms of neutrophil recruitment, respiratory gas exchange, cardiopulmonary function, days on mechanical ventilation, and length of hospital stay. However, little is known about their effect in burn patients. A recent report examining 19 burned pediatric patients showed a benefit resulting from the use of an ω-3 fatty acid-based diet in patients with acute lung injury or acute respiratory distress syndrome. The use of a high-fat concentrated formula (55% fat, 17% protein, and 28% carbohydrate) enriched with eicosapentaenoic acid showed to be safe and well tolerated, and it may have a role improving oxygenation and pulmonary compliance.60 Nevertheless, applicability of these findings is not recommended until prospective studies have been conducted. If such studies were to be conducted, careful attention should be rendered to the liver, where a high-fat diet may be deleterious.
Single-macronutrient formulas consist of either fiber or protein as the main source of energy. Protein is supplemented to standard or specialty formulas in clinical scenarios marked by severe loss of muscle mass and immunodeficiency, whereas fiber is added in the event of constipation, aiding to normalize bowel function. Whole-protein formulations are appropriate in most patients; peptide-based or free amino acid formulations may be considered in patients with a severely compromised GI tract or severe protein/fat malabsorption. Considering that many formulas are hyperosmolar at full strength, dilution by one-fourth to one-half is initially preferred to minimize the possibility of diarrhea from excess osmotic load and to facilitate absorption.
PN formulas, in the United States, are composed from a 70% dextrose (D70) solution, 10%–20% amino acid solutions, and 10%–20% lipid emulsions. These formulas can be individually adjusted to meet the patients' needs for the intake of calories, electrolytes, vitamins, and trace elements and administered with medications such as insulin and H2 blockers.
Complications
Overfeeding of burned patients can lead to major complications. For example, carbohydrate overfeeding may result in elevated respiratory quotients, increased fat synthesis, and increased CO2 elimination. Moreover, overfed ventilated patients become more difficult to manage or wean from ventilator support.101 Excess carbohydrate or fat can also lead to fat deposition in the liver102 and excess protein replacement to elevations in serum urea nitrogen.102 In addition, overfeeding can augment hyperglycemia, which can be difficult to treat, as both endogenous and exogenous insulin effects are often countered by the surge of catabolic hormones.103 Finally, attempting to overcompensate by providing excess calories and/or protein is ineffective and likely to increase complications such as hyperglycemia, CO2 retention, and azotemia.21 These complications are not specific to parenteral or enteral feedings but are instead due to overcompensating for the remarkably increased substrate demand experienced by burned patients.
Summary
Severe burns increase nutrition requirements because of the prolonged hypermetabolic, hypercatabolic response, which may lead to loss of lean body mass and poor outcomes. Although multiple treatment strategies have contributed to the improvements in morbidity and mortality of these patients, they have not proved sufficient to completely abate the response postinjury. Among these strategies, EN is safe, widely available, and effective in decreasing loss of lean body mass.8,104 More important, EN is beneficial in restoring and maintaining intestinal tract integrity and functionality. Therefore, it should be initiated early after admission and followed by judicious assessment and monitoring of the patients' nutrition status. As patients recover after injury, they present multiple physiological changes that make the task of nutrition assessment rather intricate. Research involving dual-energy X-ray absorptiometry, electrical bioimpedance, and indirect calorimetry allows us to determine if particular changes in weight are nutritionally significant and to evaluate if the course of action followed attained the nutrition objectives.
Clinical Relevancy Statement.
Provision of nutrition support is an essential component of burn care. Adequate assessment and management can reduce mortality and complications, optimize wound healing, and minimize the deleterious effects of the exaggerated hypermetabolic response; therefore, careful attention must be given to the tools and methods by which we estimate caloric needs. This review allows the reader to possibly change practice in burn nutrition. Furthermore, on the basis of the review data, we believe that that there should be discussion and thought about the ideal intensive care unit nutrition.
Acknowledgments
Financial disclosure: Supported by the National Institutes of Health grants R01-GM56687-11 and R01-GM56687-11S1 and Shriners Hospitals for Children grants 84309, 8640, and 9156.
References
- 1.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 2.Cuthbertson D. Post-shock metabolic response. Lancet. 1942:433–436. [PubMed] [Google Scholar]
- 3.Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress. 2007;10:109–120. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- 4.Wilmore DW, Mason AD, Jr., Pruitt BA., Jr. Insulin response to glucose in hypermetabolic burn patients. Ann Surg. 1976;183:314–320. doi: 10.1097/00000658-197603000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. JPEN J Parenter Enteral Nutr. 1999;23:160–168. doi: 10.1177/0148607199023003160. [DOI] [PubMed] [Google Scholar]
- 6.Chang DW, DeSanti L, Demling RH. Anticatabolic and anabolic strategies in critical illness: a review of current treatment modalities. Shock. 1998;10:155–160. doi: 10.1097/00024382-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Herndon DN. Mediators of metabolism. J Trauma. 1981;21:701–704. [Google Scholar]
- 8.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauglitz GG, Herndon DN, Kulp GA, Meyer WJ, III, Jeschke MG. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab. 2009;94:1656–1664. doi: 10.1210/jc.2008-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe RR, Durkot MJ, Allsop JR, Burke JF. Glucose metabolism in severely burned patients. Metabolism. 1979;28:1031–1039. doi: 10.1016/0026-0495(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 11.Tappy L, Schwarz JM, Schneiter P, et al. Effects of isoenergetic glucose-based or lipid-based parenteral nutrition on glucose metabolism, de novo lipogenesis, and respiratory gas exchanges in critically ill patients. Crit Care Med. 1998;26:860–867. doi: 10.1097/00003246-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 12.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 13.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 14.Hart DW, Wolf SE, Chinkes DL, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232:455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 16.Jahoor F, Desai M, Herndon DN, Wolfe RR. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988;37:330–337. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 17.Rutan RL, Herndon DN. Growth delay in postburn pediatric patients. Arch Surg. 1990;125:392–395. doi: 10.1001/archsurg.1990.01410150114021. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–408. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 19.Carter EA, Tompkins RG, Babich JW, Correia J, Bailey EM, Fischman AJ. Thermal injury in rats alters glucose utilization by skin, wound, and small intestine, but not by skeletal muscle. Metabolism. 1996;45:1161–1167. doi: 10.1016/s0026-0495(96)90017-7. [DOI] [PubMed] [Google Scholar]
- 20.Barrow RE, Mlcak R, Barrow LN, Hawkins HK. Increased liver weights in severely burned children: comparison of ultrasound and autopsy measurements. Burns. 2004;30:565–568. doi: 10.1016/j.burns.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 21.Saffle JR. Nutritional support of the burned patient. In: Herndon DN, editor. Total Burn Care. Saunders Elsevier; Philadelphia: 2007. pp. 398–419. [Google Scholar]
- 22.Wolf SE, Rose JK, Desai MH, Mileski JP, Barrow RE, Herndon DN. Mortality determinants in massive pediatric burns: an analysis of 103 children with > or = 80% TBSA burns (> or = 70% full-thickness) Ann Surg. 1997;225:554–569. doi: 10.1097/00000658-199705000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411–416. [PubMed] [Google Scholar]
- 24.van Elburg RM, Uil JJ, de Monchy JG, Heymans HS. Intestinal permeability in pediatric gastroenterology. Scand J Gastroenterol Suppl. 1992;194:19–24. doi: 10.3109/00365529209096021. [DOI] [PubMed] [Google Scholar]
- 25.Hart DW, Wolf SE, Chinkes DL, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–764. doi: 10.1097/01.TA.0000060260.61478.A7. [DOI] [PubMed] [Google Scholar]
- 26.Dominioni L, Trocki O, Fang CH, et al. Enteral feeding in burn hypermetabolism: nutritional and metabolic effects of different levels of calorie and protein intake. JPEN J Parenter Enteral Nutr. 1985;9:269–279. doi: 10.1177/0148607185009003269. [DOI] [PubMed] [Google Scholar]
- 27.Mochizuki H, Trocki O, Dominioni L, Alexander JW. Reduction of postburn hypermetabolism by early enteral feeding. Curr Surg. 1985;42:121–125. [PubMed] [Google Scholar]
- 28.McDonald WS, Sharp CW, Jr., Deitch EA. Immediate enteral feeding in burn patients is safe and effective. Ann Surg. 1991;213:177–183. doi: 10.1097/00000658-199102000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tinckler LF. Surgery and intestinal motility. Br J Surg. 1965;52:140–150. doi: 10.1002/bjs.1800520212. [DOI] [PubMed] [Google Scholar]
- 30.Raff T, Hartmann B, Germann G. Early intragastric feeding of seriously burned and long-term ventilated patients: a review of 55 patients. Burns. 1997;23:19–25. doi: 10.1016/s0305-4179(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 31.Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:328–340. [PubMed] [Google Scholar]
- 32.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 33.Cynober L, Prugnaud O, Lioret N, Duchemin C, Saizy R, Giboudeau J. Serum transthyretin levels in patients with burn injury. Surgery. 1991;109:640–644. [PubMed] [Google Scholar]
- 34.Beck FK, Rosenthal TC. Prealbumin: a marker for nutritional evaluation. Am Fam Physician. 2002;65:1575–1578. [PubMed] [Google Scholar]
- 35.Moghazy AM, Adly OA, Abbas AH, Moati TA, Ali OS, Mohamed BA. Assessment of the relation between prealbumin serum level and healing of skin-grafted burn wounds. Burns. 2010;36:495–500. doi: 10.1016/j.burns.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Zdolsek HJ, Lindahl OA, Angquist KA, Sjoberg F. Non-invasive assessment of intercompartmental fluid shifts in burn victims. Burns. 1998;24:233–240. doi: 10.1016/s0305-4179(98)00016-3. [DOI] [PubMed] [Google Scholar]
- 37.Mlcak RP, Jeschke MG, Barrow RE, Herndon DN. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244:121–130. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allard JP, Pichard C, Hoshino E, et al. Validation of a new formula for calculating the energy requirements of burn patients. JPEN J Parenter Enteral Nutr. 1990;14:115–118. doi: 10.1177/0148607190014002115. [DOI] [PubMed] [Google Scholar]
- 39.Dickerson R, Gervasio J, Riley M, et al. Accuracy of predictive methods to estimate resting energy expenditure of thermally-injured patients. JPEN J Parenter Enteral Nutr. 2002;26:17–29. doi: 10.1177/014860710202600117. [DOI] [PubMed] [Google Scholar]
- 40.Suman OE, Mlcak RP, Chinkes DL, et al. Resting energy expenditure in severely burned children: analysis of agreement between indirect calorimetry and prediction equations using the Bland-Altman method. Burns. 2006;32:335–342. doi: 10.1016/j.burns.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Gore DC, Rutan RL, Hildreth M, Desai MH, Herndon DN. Comparison of resting energy expenditures and caloric intake in children with severe burns. J Burn Care Rehabil. 1990;11:400–404. doi: 10.1097/00004630-199009000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Hart DW, Wolf SE, Herndon DN, et al. Energy expenditure and caloric balance after burn: increased feeding leads to fat rather than lean mass accretion. Ann Surg. 2002;235:152–161. doi: 10.1097/00000658-200201000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liusuwan RA, Palmieri TL, Kinoshita L, Greenhalgh DG. Comparison of measured resting energy expenditure versus predictive equations in pediatric burn patients. J Burn Care Rehabil. 2005;26:464–470. doi: 10.1097/01.bcr.0000185786.38365.3d. [DOI] [PubMed] [Google Scholar]
- 44.Mayes T, Gottschlich MM, Khoury J, Warden GD. Evaluation of predicted and measured energy requirements in burned children. J Am Diet Assoc. 1996;96:24–29. doi: 10.1016/s0002-8223(96)00008-9. [DOI] [PubMed] [Google Scholar]
- 45.Goran MI, Peters EJ, Herndon DN, Wolfe RR. Total energy expenditure in burned children using the doubly labeled water technique. Am J Physiol. 1990;259:E576–E585. doi: 10.1152/ajpendo.1990.259.4.E576. [DOI] [PubMed] [Google Scholar]
- 46.Bier DM, Brosnan JT, Flatt JP, et al. Report of the IDECG Working Group on lower and upper limits of carbohydrate and fat intake. International Dietary Energy Consultative Group. Eur J Clin Nutr. 1999;53(suppl 1):S177–S178. doi: 10.1038/sj.ejcn.1600759. [DOI] [PubMed] [Google Scholar]
- 47.Sheridan RL, Yu YM, Prelack K, Young VR, Burke JF, Tompkins RG. Maximal parenteral glucose oxidation in hypermetabolic young children: a stable isotope study. JPEN J Parenter Enteral Nutr. 1998;22:212–216. doi: 10.1177/0148607198022004212. [DOI] [PubMed] [Google Scholar]
- 48.Wolfe RR. Maximal parenteral glucose oxidation in hypermetabolic young children [comment] JPEN J Parenter Enteral Nutr. 1998;22:190. doi: 10.1177/0148607198022004190. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe RR, Allsop JR, Burke JF. Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism. 1979;28:210–220. doi: 10.1016/0026-0495(79)90066-0. [DOI] [PubMed] [Google Scholar]
- 50.Sakurai Y, Aarsland A, Herndon DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995;222:283–297. doi: 10.1097/00000658-199509000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aarsland A, Chinkes DL, Sakurai Y, Nguyen TT, Herndon DN, Wolfe RR. Insulin therapy in burn patients does not contribute to hepatic triglyceride production. J Clin Invest. 1998;101:2233–2239. doi: 10.1172/JCI200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierre EJ, Barrow RE, Hawkins HK, et al. Effects of insulin on wound healing. J Trauma. 1998;44:342–345. doi: 10.1097/00005373-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 53.Thomas SJ, Morimoto K, Herndon DN, et al. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002;132:341–347. doi: 10.1067/msy.2002.126871. [DOI] [PubMed] [Google Scholar]
- 54.Demling RH, Seigne P. Metabolic management of patients with severe burns. World J Surg. 2000;24:673–680. doi: 10.1007/s002689910109. [DOI] [PubMed] [Google Scholar]
- 55.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 56.Waitzberg DL, Torrinhas RS, Jacintho TM. New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr. 2006;30:351–367. doi: 10.1177/0148607106030004351. [DOI] [PubMed] [Google Scholar]
- 57.A.S.P.E.N. Consensus recommendations from the U.S. summit on immune-enhancing enteral therapy. JPEN J Parenter Enteral Nutr. 2001;25:S61–S63. doi: 10.1177/014860710102500213. [DOI] [PubMed] [Google Scholar]
- 58.Alexander JW, Saito H, Trocki O, Ogle CK. The importance of lipid type in the diet after burn injury. Ann Surg. 1986;204:1–8. doi: 10.1097/00000658-198607000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huschak G, Zur Nieden K, Hoell T, Riemann D, Mast H, Stuttmann R. Olive oil based nutrition in multiple trauma patients: a pilot study. Intensive Care Med. 2005;31:1202–1208. doi: 10.1007/s00134-005-2727-9. [DOI] [PubMed] [Google Scholar]
- 60.Mayes T, Gottschlich MM, Kagan RJ. An evaluation of the safety and efficacy of an anti-inflammatory, pulmonary enteral formula in the treatment of pediatric burn patients with respiratory failure. J Burn Care Res. 2008;29:82–88. doi: 10.1097/BCR.0b013e31815f594e. [DOI] [PubMed] [Google Scholar]
- 61.Wolfe RR, Goodenough RD, Burke JF, Wolfe MH. Response of protein and urea kinetics in burn patients to different levels of protein intake. Ann Surg. 1983;197:163–171. doi: 10.1097/00000658-198302000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patterson BW, Nguyen T, Pierre E, Herndon DN, Wolfe RR. Urea and protein metabolism in burned children: effect of dietary protein intake. Metabolism. 1997;46:573–578. doi: 10.1016/s0026-0495(97)90196-7. [DOI] [PubMed] [Google Scholar]
- 63.Hoerr RA, Matthews DE, Bier DM, Young VR. Effects of protein restriction and acute refeeding on leucine and lysine kinetics in young men. Am J Physiol. 1993;264:E567–E575. doi: 10.1152/ajpendo.1993.264.4.E567. [DOI] [PubMed] [Google Scholar]
- 64.Gottschlich MM. Nutrition in the burned pediatric patient. In: Samour PHK, Lang C, editors. Handbook of Pediatric Nutrition. A.S.P.E.N.; Gaithersbury, MD: 1999. pp. 193–511. [Google Scholar]
- 65.Norbury WB. Modulation of the hypermetabolic response after burn injury. In: Herndon DN, editor. Total Burn Care. Saunders Elsevier; Philadelphia: 2007. pp. 420–433. [Google Scholar]
- 66.Soeters PB, van de Poll MC, van Gemert WG, Dejong CH. Amino acid adequacy in pathophysiological states. J Nutr. 2004;134:1575S–1582S. doi: 10.1093/jn/134.6.1575s. [DOI] [PubMed] [Google Scholar]
- 67.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33:1125–1135. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 68.Hall JC, Dobb G, Hall J, de Sousa R, Brennan L, McCauley R. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Med. 2003;29:1710–1716. doi: 10.1007/s00134-003-1937-2. [DOI] [PubMed] [Google Scholar]
- 69.Wischmeyer PE, Lynch J, Liedel J, et al. Glutamine administration reduces gram-negative bacteremia in severely burned patients: a prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med. 2001;29:2075–2080. doi: 10.1097/00003246-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. JPEN J Parenter Enteral Nutr. 2003;27:241–245. doi: 10.1177/0148607103027004241. [DOI] [PubMed] [Google Scholar]
- 71.Gamliel Z, DeBiasse MA, Demling RH. Essential microminerals and their response to burn injury. J Burn Care Rehabil. 1996;17:264–272. [PubMed] [Google Scholar]
- 72.Gottschlich MM, Mayes T, Khoury J, Warden GD. Hypovitaminosis D in acutely injured pediatric burn patients. J Am Diet Assoc. 2004;104:931–941. doi: 10.1016/j.jada.2004.03.020. quiz 1031. [DOI] [PubMed] [Google Scholar]
- 73.Pintaudi AM, Tesoriere L, D'Arpa N, et al. Oxidative stress after moderate to extensive burning in humans. Free Radic Res. 2000;33:139–146. doi: 10.1080/10715760000300691. [DOI] [PubMed] [Google Scholar]
- 74.Berger MM, Shenkin A. Trace element requirements in critically ill burned patients. J Trace Elem Med Biol. 2007;21(suppl 1):44–48. doi: 10.1016/j.jtemb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 75.Rock CL, Dechert RE, Khilnani R, Parker RS, Rodriguez JL. Carotenoids and antioxidant vitamins in patients after burn injury. J Burn Care Rehabil. 1997;18:269–278. doi: 10.1097/00004630-199705000-00018. discussion 268. [DOI] [PubMed] [Google Scholar]
- 76.Klein GL, Langman CB, Herndon DN. Vitamin D depletion following burn injury in children: a possible factor in post-burn osteopenia. J Trauma. 2002;52:346–350. doi: 10.1097/00005373-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 77.Klein GL, Herndon DN, Chen TC, Kulp G, Holick MF. Standard multivitamin supplementation does not improve vitamin D insufficiency after burns. J Bone Miner Metab. 2009;27:502–506. doi: 10.1007/s00774-009-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berger MM, Spertini F, Shenkin A, et al. Trace element supplementation modulates pulmonary infection rates after major burns: a double-blind, placebo-controlled trial. Am J Clin Nutr. 1998;68:365–371. doi: 10.1093/ajcn/68.2.365. [DOI] [PubMed] [Google Scholar]
- 79.Selmanpakoglu AN, Cetin C, Sayal A, Isimer A. Trace element (Al, Se, Zn, Cu) levels in serum, urine and tissues of burn patients. Burns. 1994;20:99–103. doi: 10.1016/s0305-4179(06)80002-1. [DOI] [PubMed] [Google Scholar]
- 80.Hunt DR, Lane HW, Beesinger D, et al. Selenium depletion in burn patients. JPEN J Parenter Enteral Nutr. 1984;8:695–699. doi: 10.1177/0148607184008006695. [DOI] [PubMed] [Google Scholar]
- 81.Sampson B, Constantinescu MA, Chandarana I, Cussons PD. Severe hypocupraemia in a patient with extensive burn injuries. Ann Clin Biochem. 1996;33(pt 5):462–464. doi: 10.1177/000456329603300513. [DOI] [PubMed] [Google Scholar]
- 82.Cunningham JJ, Leffell M, Harmatz P. Burn severity, copper dose, and plasma ceruloplasmin in burned children during total parenteral nutrition. Nutrition. 1993;9:329–332. [PubMed] [Google Scholar]
- 83.Gosling P, Rothe HM, Sheehan TM, Hubbard LD. Serum copper and zinc concentrations in patients with burns in relation to burn surface area. J Burn Care Rehabil. 1995;16:481–486. doi: 10.1097/00004630-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 84.Shakespeare PG. Studies on the serum levels of iron, copper and zinc and the urinary excretion of zinc after burn injury. Burns Incl Therm Inj. 1982;8:358–364. doi: 10.1016/0305-4179(82)90036-5. [DOI] [PubMed] [Google Scholar]
- 85.Voruganti VS, Klein GL, Lu HX, et al. Impaired zinc and copper status in children with burn injuries: need to reassess nutritional requirements [see comment] Burns. 2005;31:711–716. doi: 10.1016/j.burns.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 86.Berger MM, Baines M, Raffoul W, et al. Trace element supplementation after major burns modulates antioxidant status and clinical course by way of increased tissue trace element concentrations. Am J Clin Nutr. 2007;85:1293–1300. doi: 10.1093/ajcn/85.5.1293. [DOI] [PubMed] [Google Scholar]
- 87.Berger MM, Binnert C, Chiolero RL, et al. Trace element supplementation after major burns increases burned skin trace element concentrations and modulates local protein metabolism but not whole-body substrate metabolism. Am J Clin Nutr. 2007;85:1301–1306. doi: 10.1093/ajcn/85.5.1301. [DOI] [PubMed] [Google Scholar]
- 88.Berger MM, Eggimann P, Heyland DK, et al. Reduction of nosocomial pneumonia after major burns by trace element supplementation: aggregation of two randomised trials. Crit Care. 2006;10:R153. doi: 10.1186/cc5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilmore DW. Nutrition and metabolism following thermal injury. Clin Plast Surg. 1974;1:603–619. [PubMed] [Google Scholar]
- 90.Mochizuki H, Trocki O, Dominioni L, Brackett KA, Joffe SN, Alexander JW. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg. 1984;200:297–310. doi: 10.1097/00000658-198409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183:390–398. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 92.Kudsk KA, Croce MA, Fabian TC, et al. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503–513. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mosier MJ, Pham TN, Klein MB, et al. Early enteral nutrition in burns: compliance with guidelines and associated outcomes in a multicenter study. J Burn Care Res. 2011;32:104–109. doi: 10.1097/BCR.0b013e318204b3be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herndon DN, Barrow RE, Stein M, et al. Increased mortality with intravenous supplemental feeding in severely burned patients. J Burn Care Rehabil. 1989;10:309–313. doi: 10.1097/00004630-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Herndon DN, Stein MD, Rutan TC, Abston S, Linares H. Failure of TPN supplementation to improve liver function, immunity, and mortality in thermally injured patients. J Trauma. 1987;27:195–204. doi: 10.1097/00005373-198702000-00018. [DOI] [PubMed] [Google Scholar]
- 96.Simpson F, Doig GS. Parenteral vs. enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med. 2005;31:12–23. doi: 10.1007/s00134-004-2511-2. [DOI] [PubMed] [Google Scholar]
- 97.Singer P, Berger MM, Van den Berghe G, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. 2009;28:387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 98.Hart DW, Wolf SE, Zhang XJ, et al. Efficacy of a high-carbohydrate diet in catabolic illness. Crit Care Med. 2001;29:1318–1324. doi: 10.1097/00003246-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 99.Bistrian BR. Fat versus carbohydrate feeding in the critically ill. Crit Care Med. 2001;29:1475–1476. doi: 10.1097/00003246-200107000-00030. [DOI] [PubMed] [Google Scholar]
- 100.Boulétreau P, Chassard D, Allaouchiche B, et al. Glucose-lipid ratio is a determinant of nitrogen balance during total parenteral nutrition in critically ill patients: a prospective, randomized, multi-center blind trial with an intention-to-treat analysis. Intensive Care Med. 2005;31:1394–1400. doi: 10.1007/s00134-005-2771-5. [DOI] [PubMed] [Google Scholar]
- 101.Askanazi J, Rosenbaum SH, Hyman AI, Silverberg PA, Milic-Emili J, Kinney JM. Respiratory changes induced by the large glucose loads of total parenteral nutrition. JAMA. 1980;243:1444–1447. [PubMed] [Google Scholar]
- 102.Klein CJ, Stanek GS, Wiles CE., III Overfeeding macronutrients to critically ill adults: metabolic complications. J Am Diet Assoc. 1998;98:795–806. doi: 10.1016/S0002-8223(98)00179-5. [DOI] [PubMed] [Google Scholar]
- 103.Turina M, Fry DE, Polk HC., Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624–1633. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 104.Jeschke MG, Mlcak RP, Finnerty CC, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]


