Abstract
We have recently identified Dlk1/FA1 (Delta-like 1/FA1) as a novel regulator of bone mass that functions to mediate bone loss, under estrogen deficiency, in mice. In this report, we investigated the effects of estrogen (E)-deficiency and E replacement on serum (s) levels of Dlk1/FA1 (s-Dlk1FA1) and its correlation with bone turnover markers. s-Dlk1/FA1 and bone turnover markers (s-CTx and s-osteocalcin), were measured in two cohorts: a group of pre- and postmenopausal women (n=100) and a group of postmenopausal women, where half had received estrogen replacement therapy (ERT) (n=166). s-Dlk1/FA1, and s-CTX were elevated in postmenopausal E-deficient compared to premenopausal E-replete women (both; P<0.001). s-Dlk1/FA1 was correlated with s-CTX (r=0.30, P<0.01). ERT, in postmenopausal women, decreased s-Dlk1/FA1, as well as s-CTX and s-osteoclacin (all; P<0.0001). Changes in s-Dlk1 were significantly correlated with those observed in s-CTx (r=0.18, P<0.05) and s-osteocalcin (r=0.28, P<0.001). In conclusion, s-Dlk1/FA1 is influenced by E-deficiency and is correlated with bone turnover. Increased levels of s-Dlk1/FA1 in post-menopausal women may be a mechanism mediating the effects estrogen deficiency on bone turnover.
Introduction
A major part of age-related bone-loss in women is related to estrogen deficiency in the postmenopausal period.(1) Estrogen deficiency leads to increased bone turnover and increased osteoclastic bone resorption due to enhanced production of a number of pro-inflammatory and osteoclastogenic cytokines.(2)
Delta-like 1/preadipocyte factor 1 (Dlk1/Pref-1) is an imprinted gene encoding a transmembrane protein that belongs to the EGF-like repeat-containing family of proteins. (3) The Dlk1 protein exists both as a membrane bound protein and as a soluble factor (known as fetal antigen 1, FA1) released from the cell membrane through proteolytic cleavage by ADAM 17.(4) The importance of Dlk1 in normal skeletal physiology has been demonstrated by the presence of growth disturbances as well as skeletal abnormalities in gene-modified mice(5;6) (7). Also, in the human syndrome of maternal uniparental disomy (matUPD14) where Dlk1/Pref-1 is silent, the patients exhibit obesity, hypotonia, premature puberty, macrocephaly, short stature, and small hands (8;9), while patients with paternal uniparental disomy (patUPD14) where Dlk1/Pref-1 is overexpressed, the patients exhibit narrow thorax with abnormally curved ribs, facial dysmorphism and mild hypoplasia of the ilia (10).
We have previously reported that circulating s-Dlk1/FA1 functions as an endocrine factor that regulates bone mass in adult mice.(11) Recently, we have reported that a high level of circulating s-Dlk1/FA1 was associated with bone loss in mice caused by enhancing bone resorption and inhibition of bone formation. In addition, Dlk1 deficient mice were protected from ovariectomy (ovx)-induced bone loss.(7) To further investigate the role of Dlk1/FA1 in human physiology, we examined the effects of estrogen (E)-deficiency and E replacement on serum levels of Dlk1/FA1 and its correlation with other known bone turn over markers.
Materials and Methods
Human cohort 1: Healthy pre- and postmenopausal women
Fifty healthy premenopausal women (age: 30 to 40 yrs, mean age 35 yrs) and 50 healthy untreated postmenopausal women (age 48–73 yrs, mean 59 yrs) were included. All premenopausal women had regular cyclic menses. All postmenopausal women were amenorrhoeic for at least five years. A fasting serum sample was collected before 9 am and stored at a temperature below −70°C until analyzed. Institution’s ethical review board (IRB) approved the study.
Human cohort 2: Postmenopausal women receiving estrogen replacement therapy
Study subjects were recruited from an age-stratified random sample of Rochester, Minnesota.(12) Mayo IRB approved the study and informed consent was obtained from all subjects. The cohort included 166 postmenopausal women consisting of two groups: The estrogen replacement therapy (ERT) group consisted of 83 postmenopausal women; mean (SD) age and BMI were 63.3 (10.1) and 27.2 (5.8), respectively, and a control group consisting of 83 subjects who did not receive ERT, selective estrogen receptor modulators (SERM) or bisphosphonates; mean (SD) age and BMI were 63.4 (10.1) and 27.2 (54.8), respectively. The estrogen regimen in the study subjects included either estrogen and progesterone combination therapy or estrogen alone (if hysterectomized) for varying durations of 1 year to more than 10 years and two subjects who had been on estrogen for less than 1 year duration. There are 4 women (3 ERT women and 1 control) who have serum creatinine > 1.2 (mg/dL). Fasting-state serum samples were obtained and were stored at −70° C until analyzed. We did not specifically exclude women based on vitamin D levels.
Measurements of serum Dlk1/FA1 and biochemical markers of bone turnover
Quantification of human Dlk1/FA1 (hFA1) was performed as previously described using a sandwich ELISA technique.(13) Both the intra- and interassay coefficient of variation (CV-%) were <5 %. The bone resorption marker CTX-I (epitope: EKAHDGGR), which is cleaved and released from the C-terminal of type I collagen(14) was measured using a commercial kit: CrossLaps® ELISA (Nordic Bioscience, Herlev, Denmark), intra- and inter assay variations were 7 % and 9 % (CV-%), respectively. Human serum osteocalcin was measured by a commercial radioimmunoassay ELISA-OST-NAT that recognizes both the N- and C-terminal of the osteocalcin molecule (Cis BioInternational, Bedford, MA, USA).(15) The intra- and inter-assay CV were < 6%.
Statistical analysis
The differences in Dlk1/FA1 levels in the human case-control study were assessed by the Mann-Whitney test. The correlations between Dlk1/FA1 and serum bone markers were examined using linear regression analysis and the Pearson correlation coefficient (r).
Results
We measured s-Dlk1/FA1 and s-CTX-I in a cohort of healthy premenopausal (n=50) and postmenopausal (n=50) women. Both markers were significantly elevated in the postmenopausal women compared with the premenopausal women (Table 1) and s-Dlk1/FA1 was positively correlated with s-CTX-I (Fig. 1A). To investigate the ability of ERT to inhibit the observed increase in s-Dlk1/FA1 in postmenopausal women, we compared s-Dlk1/FA1 levels in untreated and E- treated postmenopausal women (n = 83 per group). Table (1) demonstrates that s-Dlk1/FA1, s-CTX-I and s-osteocalcin were all significantly decreased in postmenopausal women receiving ERT. In addition, the two bone turnover markers were correlated with each other (r=0.7581, P<0.0001), each of the bone markers was positively correlated with the s-Dlk1/FA1 (Fig. 1B&C).
Table 1.
Comparison of the serum levels of Dlk1/FA1, CTX-I or osteocalcin between groups within the two human cohorts
| Dlk1/FA1 | P | CTX-I | P | Osteocalcin | P | |
|---|---|---|---|---|---|---|
| Cohort 1 (N=100) | ||||||
| Premenopausal women | 23.0 (20.6, 25.5) | < 0.0001 | 0.298 (0.262, 0339) | < 0.0001 | - | |
| Postmenopausal women | 41.9 (37.0, 46.8) | 0.456 (0.403, 0.509) | - | |||
| Cohort 2 (N=166) | ||||||
| Postmenopausal women + ERT | 29.9 (27.2, 32.7) | < 0.0001 | 0.388 (0.321, 0.455) | < 0.0001 | 16.4 (14.5, 18.2) | < 0.0001 |
| Postmenopausal women | 41.5 (37.9, 45.1) | 0.647 (0.573, 0722) | 22.9 (20.6, 25.3) | |||
Figure 1.
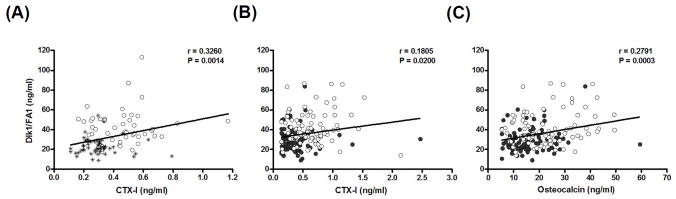
Measurement of s-Dlk1/FA1 and bone turnover markers. Asterisk indicates premenopausal women; closed circles indicate postmenopausal given estrogen replacement therapy (ERT), and; open circles indicates postmenopausal women. A) Correlation between serum levels of Dlk1/FA1 and CTX-I in cohort 1 (see Material and Methods). C) Correlation between serum levels of Dlk1/FA1 and CTX-I in cohort 2. (see Material and Methods) D) Correlation between serum levels of Dlk1/FA1 and osteocalcin in cohort 2.
Discussion
In the present study we have demonstrated that E-deficiency in humans is associated with increased serum levels of Dlk1/FA1 that were normalized by ERT. Additionally, changes of s-Dlk1/FA1 were positively correlated with changes in serum bone turnover markers suggesting a role in mediating E action on the skeleton. These results were obtained from two population cohorts. Cohort 1 revealed an association between E status and s-FA1 and cohort 2 was an intervention study that provided a causal relationship between E levels and s-FA1.
The range of s- FA1 in normal adults is 12.3–46.6 ng/ml (age range: 19–60) (16) and its levels reflect a balance between production and clearance rate. During development, Dlk1/FA1 is widely expressed in various organs; however, in adults, Dlk1/FA1 is produced by neuroendocrine organs e.g. adrenal glands, pituitary gland, β-cells in pancreas, male and female gonads and the hypothalamus as well as other distinct nuclei in CNS.(17) s-Dlk1/FA1 is cleared through the kidneys.(16) s-Dlk1/FA1 is elevated in several human diseases including neurofibromatosis,(18) small cell lung cancer patients,(19) in patients with anorexia nervosa (20) and renal failure.(16)
The effects of estrogen on s-Dlk1/FA1 can take place at several levels: transcription/translation, proteolysis/shedding or clearance. Since estrogen receptors are widely distributed in different tissues, including the neuroendocrine tissues,(21) it is plausible that estrogen affects the production of s-Dlk1/FA1. We have identified several possible cell types that produce Dlk1/FA1 in the bone marrow and are known targets for E action including osteoprogenitors/stromal cells, pre-adipocytes, B-cells and T-cells.(22). Thus, our working hypothesis is that E deficiency results in upregulation of the level of Dlk1/FA1 in the bone microenvironment which will, in turn, activate NF-κB signalling pathway and increase the production of pro-inflammatory cytokines and that will lead to increase in bone turnover, enhance bone resorption and inhibit bone formation leading to bone loss.(11) (7) In support of this hypothesis is the finding of partial protection of ovariectomy-induced bone loss in Dlk1-deficient mice.(7) It is also possible that E causes changes in s-Dlk1/FA1 indirectly through other hormonal changes. Menopause and ERT lead to changes serum growth hormone (GH) (23) which is known to lead to changes in s-Dlk1/FA1. (24)
In conclusion, targeting Dlk1/FA1 is potentially a novel approach to prevent the deleterious effects of estrogen deficiency on the skeleton.
Acknowledgments
This work was supported by grants from the Lundbeck foundation, The Novo Nordisk foundation, by a grant from the local government of Southern Denmark, and NIH AR027065. The technical help of Ms Bianca Jørgensen and the advice of Dr Charlotte H. Jensen regarding the ELISA assay are acknowledged.
This study was supported by grants from the Lundbeck foundation, the Danish Medical Research Council, the Novo Nordisk Foundation and NIH grant AR027065.
Footnotes
Conflict of interest: All authors have no conflicts of interest.
Contributor Information
B. M. Abdallah, Email: babdallah@health.sdu.dk.
A. Bay-Jensen, Email: acbj@Nordicbioscience.com.
B. Srinivasan, Email: Bhuma984@gmail.com.
N. C. Tabassi, Email: Nadine.Charni@synarc.com.
P. Garnero, Email: patrick.garnero@synarc.com.
J. Delaissé, Email: Jean-Marie.Delaisse@vgs.regionsyddanmark.dk.
S. Khosla, Email: khosla.sundeep@mayo.edu.
M. Kassem, Email: mkassem@health.sdu.dk.
Reference List
- 1.Riggs BL, Khosla S, Melton L. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 2.Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research. 1996;11:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- 3.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Sul HS. Ectodomain shedding of preadipocyte factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and Inhibition of Adipocyte Differentiation. Mol Cell Biol. 2006;26:5421–5435. doi: 10.1128/MCB.02437-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J Clin Invest. 2003;111:453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, Sul HS. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdallah BM, Ditzel N, Mahmood A, Isa A, Traustadottir GA, Schilling AF, Ruiz-Hidalgo M, Laborda J, Amling M, Kassem M. DLK1 is a novel regulator of bone mass that mediates estrogen-deficiency induced bone loss in mice. J Bone Miner Res. 2011 doi: 10.1002/jbmr.346. [DOI] [PubMed] [Google Scholar]
- 8.Kotzot D. Abnormal phenotypes in uniparental disomy (UPD): fundamental aspects and a critical review with bibliography of UPD other than 15. Am J Med Genet. 1999;82:265–274. [PubMed] [Google Scholar]
- 9.Berends MJ, Hordijk R, Scheffer H, Oosterwijk JC, Halley DJ, Sorgedrager N. Two cases of maternal uniparental disomy 14 with a phenotype overlapping with the Prader-Willi phenotype. Am J Med Genet. 1999;84:76–79. doi: 10.1002/(sici)1096-8628(19990507)84:1<76::aid-ajmg16>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Kurosawa K, Sasaki H, Sato Y, Yamanaka M, Shimizu M, Ito Y, Okuyama T, Matsuo M, Imaizumi K, Kuroki Y, Nishimura G. Paternal UPD14 is responsible for a distinctive malformation complex. Am J Med Genet. 2002;110:268–272. doi: 10.1002/ajmg.10404. [DOI] [PubMed] [Google Scholar]
- 11.Abdallah BM, Ding M, Jensen CH, Ditzel N, Flyvbjerg A, Jensen TG, Dagnaes-Hansen F, Gasser JA, Kassem M. Dlk1/FA1 is a novel endocrine regulator of bone and fat mass and its serum level is modulated by growth hormone. Endocrinology. 2007;148:3111–3121. doi: 10.1210/en.2007-0171. [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL, Melton IL, III, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 13.Jensen CH, Krogh TN, Hojrup P, Clausen PP, Skjodt K, Larsson LI, Enghild JJ, Teisner B. Protein structure of fetal antigen 1 (FA1). A novel circulating human epidermal-growth-factor-like protein expressed in neuroendocrine tumors and its relation to the gene products of dlk and pG2. Eur J Biochem. 1994;225:83–92. doi: 10.1111/j.1432-1033.1994.00083.x. [DOI] [PubMed] [Google Scholar]
- 14.Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C. Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clin Chem. 1994;40:2022–2025. [PubMed] [Google Scholar]
- 15.Price PA, Parthemore JG, Deftos LJ. New biochemical marker for bone metabolism. Measurement by radioimmunoassay of bone GLA protein in the plasma of normal subjects and patients with bone disease. J Clin Invest. 1980;66:878–883. doi: 10.1172/JCI109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen CH, Krogh TN, Stoving RK, Holmskov U, Teisner B. Fetal antigen 1 (FA1), a circulating member of the epidermal growth factor (EGF) superfamily: ELISA development, physiology and metabolism in relation to renal function. Clin Chim Acta. 1997;268:1–20. doi: 10.1016/s0009-8981(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 17.Bauer M, Szulc J, Meyer M, Jensen CH, Terki TA, Meixner A, Kinkl N, Gasser T, Aebischer P, Ueffing M. Delta-like 1 participates in the specification of ventral midbrain progenitor derived dopaminergic neurons. J Neurochem. 2008;104:1101–1115. doi: 10.1111/j.1471-4159.2007.05037.x. [DOI] [PubMed] [Google Scholar]
- 18.Jensen CH, Schroder HD, Teisner B, Laursen I, Brandrup F, Rasmussen HB. Fetal antigen 1, a member of the epidermal growth factor superfamily, in neurofibromas and serum from patients with neurofibromatosis type 1. Br J Dermatol. 1999;140:1054–1059. doi: 10.1046/j.1365-2133.1999.02906.x. [DOI] [PubMed] [Google Scholar]
- 19.Harken JC, Drivsholm L, Laursen I, Teisner B. Elevated serum levels of fetal antigen 1, a member of the epidermal growth factor superfamily, in patients with small cell lung cancer. Tumour Biol. 1999;20:256–262. doi: 10.1159/000030072. [DOI] [PubMed] [Google Scholar]
- 20.Fazeli PK, Bredella MA, Misra M, Meenaghan E, Rosen CJ, Clemmons DR, Breggia A, Miller KK, Klibanski A. Preadipocyte factor-1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95:407–413. doi: 10.1210/jc.2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 22.Laborda J. The role of the epidermal growth factor-like protein dlkin cell differentiation. Histol Histopathol. 2000;15:119–129. doi: 10.14670/HH-15.119. [DOI] [PubMed] [Google Scholar]
- 23.Moe KE, Prinz PN, Larsen LH, Vitiello MV, Reed SO, Merriam GR. Growth hormone in postmenopausal women after long-term oral estrogen replacement therapy. J Gerontol A Biol Sci Med Sci. 1998;53:B117–B124. doi: 10.1093/gerona/53a.2.b117. [DOI] [PubMed] [Google Scholar]
- 24.Andersen M, Jensen CH, Stoving RK, Larsen JB, Schroder HD, Teisner B, Hagen C. Fetal antigen 1 in healthy adults and patients with pituitary disease: relation to physiological, pathological, and pharmacological GH levels. J Clin Endocrinol Metab. 2001;86:5465–5470. doi: 10.1210/jcem.86.11.7990. [DOI] [PubMed] [Google Scholar]


