Abstract
Tendons are composed of fibroblasts and collagen fibrils. The fibrils are organized uniaxially and grouped together into fibers. Collagen VI is a non-fibrillar collagen expressed in developing and adult tendons. Human collagen VI mutations result in muscular dystrophy, joint hyperlaxity and contractures. The purpose of this study is to determine the functional roles of collagen VI in tendon matrix assembly. During tendon development, collagen VI was expressed throughout the extracellular matrix, but enriched around fibroblasts and their processes. To analyze the functional roles of collagen VI a mouse model with a targeted inactivation of Col6a1 gene was utilized. Ultrastructural analysis of Col6a1−/− versus wild type tendons demonstrated disorganized extracellular micro-domains and collagen fibers in the Col6a1−/− tendon. In the col6a1−/− tendon, fibril structure and diameter distribution was abnormal compared to wild type controls. Col6a1−/− fibrils had smaller diameters and the diameter distributions were shifted significantly toward the smaller diameters. An analysis of fibril density (number/μm2) demonstrated an ~2.5 fold increase in the Col6a1−/− versus wild type tendons. In addition, the fibril arrangement and structure was aberrant in the peri-cellular regions of Col6a1−/− tendons with frequent very large fibrils and twisted fibrils observed restricted to this region. The biomechanical properties were analyzed in mature tendons. A significant decrease in cross sectional area was observed. The percent relaxation, maximum load, maximum stress, stiffness and modulus were analyzed and Col6a1−/− tendons demonstrated a significant reduction in maximum load and stiffness compared to wild type tendons. An increase in matrix metalloproteinase activity was suggested in the absence of collagen VI. This suggests alterations in tenocyte expression due to disruption of cell-matrix interactions. The changes in expression may result in alterations in the peri-cellular environment. In addition, the absence of collagen VI may alter the sequestering of regulatory molecules such as leucine rich proteoglycans. These changes would result in dysfunctional regulation of tendon fibrillogenesis indirectly mediated by collagen VI.
Keywords: Collagen VI, Tendon, Development, Fibrillogenesis, Tendon Biomechanics, Collagen VI-null mouse
1. Introduction
Tendons are connective tissues that connect muscle to bone and transmit mechanical forces. Tendons are composed of columns of fibroblasts (tenocytes) and the major structural element is the collagen fibril. The fibrils are organized uniaxially and grouped together into fibers. The fibers together with the tenocytes are organized into fascicles; and the fascicles are bound together by connective tissue sheaths resulting in the mature, weight-bearing tendon (Kastelic et al. 1978;Birk and Trelstad 1986;Birk and Mayne 1997;Zhang et al. 2005).
Collagen VI is ubiquitously distributed in extracellular matrices including tendons, ligaments, muscles, skin, cornea and cartilage (Keene et al. 1988). The expression of collagen VI is detected early in development and in the adult tendon. It is assembled into several different tissue forms, including beaded microfibrils, broad banded structures and hexagonal networks and is often enriched in peri-cellular regions (Furthmayr et al. 1983;von der Mark et al. 1984;Bruns et al. 1986;Wiberg et al. 2002). In tendon, it is found as a network of beaded filaments (Bruns et al. 1986). Collagen VI interacts with a large number extracellular matrix molecules including: collagens I, II, IV, XIV, microfibril-associated glycoprotein (MAGP-1), perlecan, decorin, biglycan, hyaluronan, heparin and fibronectin as well as integrins and the cell-surface proteoglycan NG2. Based on the tissue-localization and large number of potential interactions, collagen VI has been proposed to integrate different components of the extracellular matrix, including cells (Kielty and Grant 2002). This suggests roles in the development of tissue-specific extracellular matrix structure and function.
The best characterized form of collagen VI is a heterotrimer composed of α1(VI), α2(VI) and α3(VI) chains (Chu ML et al. 1987;Bonaldo et al. 1990;Chu et al. 1990;Kielty and Grant 2002).The monomer has a 105 nm triple helical domain with flanking N- and C-terminal globular domains. The molecular mass of the N–terminal domain is mainly derived from the α3(VI) chain and is approximately twice that of the C-terminal domain. The α3(VI) chain can be processed extracellularly and the α3(VI) N–terminal domain has alternatively spliced domains. Three additional α chains have been described, α4(VI), α5(VI), α6(VI) (Fitzgerald et al. 2008;Gara et al. 2008). These chains have high homology with the α3(VI) chain and may form additional isoforms.
Collagen VI mutations in humans are the cause of Ullrich’s congenital muscular dystrophy and Bethlem myopathy. These diseases are characterized not only by muscular dystrophy, but also by joint hyperlaxity and contractures (Lampe and Bushby 2005). A mouse model with a targeted inactivation of Col6a1 gene has been generated (Bonaldo et al. 1998). This model is null for all forms of collagen VI since all isoforms contain an α1(VI) chain. These mice develop an early onset myopathic phenotype, as well as spontaneous apoptosis and ultrastructural defects of mitochondria and sarcoplasmic reticulum in myogenic cells (Irwin et al. 2003). In addition, there are alterations in the peri-cellular environment of chondrocytes and development of osteoarthritis that may be associated with joint laxity (Alexopoulos et al. 2009). An increase of α1(VI) mRNA expression during the collagen fibril growth period in tendon development suggested a role for collagen VI in tendon extracellular matrix assembly (Nurminskaya and Birk 1996). Therefore, this mouse model was utilized to elucidate the functional roles of collagen VI in the tendon.
In the present study, we have demonstrated abnormal tendon fibrillogenesis in collagen VI null mice. Collagen VI was enriched in the peri-cellular region of tendon fibroblasts during normal tendon development, growth and maturation. In the collagen VI null tendons there was a disruption in cellular organization, fiber assembly and fibril formation associated with decreased mechanical properties. In addition, an alteration in fibroblast expression of matrix metalloproteinases was observed. Therefore, we hypothesized that collagen VI maintains fibroblast shape, organization which mediates expression. In addition, collagen VI regulates the availability of regulatory matrix molecules which is crucial for regulation of collagen fibrillogenesis in the tendon.
2. Materials and methods
2.1. Animals
The Col6a1 deficient mice have been described (Bonaldo et al. 1998). The mice were rederived and backcrossed into a CD1 background. All animal studies were performed in compliance with IACUC approved animal protocols.
2.2. Immuno-localization
Flexor digitorum longus (FDL) tendons were dissected from Col6a1−/− and Col6a1+/+ mice at P1, P7, P16 and P30. Tissues were embedded in OCT medium, quick frozen in liquid nitrogen and stored at −80°C. Immunofluorescence localization was performed as previously described (Zhang et al. 2009). Rabbit anti-human type VI collagen polyclonal antibody was used (Tillet et al. 1994). The secondary antibody was an Alexa Fluor 568-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR). Vectashield mounting solution with DAPI (Vector Laboratories, Inc., Burlingame, CA) was used as a nuclear marker. Negative control samples were incubated identically; except the primary antibody was excluded. Images were captured using a Leica DM5500 fluorescence microscope. Identical conditions and set integration times were used to facilitate comparisons between samples.
2.3 Transmission electron microscopy
FDL samples from Col6a1−/− and wild-type mice between P1 and P30 were analyzed by transmission electron microscopy. Briefly, tendons were dissected and fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate, pH 7.4, with 8.0 mM CaCl2 at 4°C for 2 hours (Birk and Trelstad 1986). This was followed by post-fixation with 1% osmium tetroxide, dehydration in an ethanol series, followed by propylene oxide and the tissue samples were infiltrated and embedded in a mixture of EMbed 812, nadic methyl anhydride, dodecenylsuccinic anhydride, and DMP-30 (Electron Microscopy Sciences, Hatfield, PA). Thin sections were post-stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. Cross sections of FDL were examined at 80 kV using a Tecnai 12 transmission electron microscope equipped with a Gatan Ultrascan US100 2K digital camera.
2.4. Fibril Diameter Distribution
Three tendons form 3 different animals for each genotype were analyzed at P30. Digital images at a magnification of 65,000X were randomly selected from the area adjacent to the surface of the tendon fibroblasts and from an area peripheral to the fibroblast near the center of the fiber. Three images of each area were analyzed for each animal. Fibril diameter was measured using a RM Biometrics-Bioquant Image Analysis System (Nashville, TN). The fibril diameters and fibril density were measured in a ROI with a constant area of 3μm2. Means for the fibril diameters were calculated based on the mean values in each RO1, n=9. There were between 1156 and 1122 fibrils in the wild type tendons versus 2776 and 2786 fibrils analyzed in the 2 mutant distributions.
2.5. Extraction of MMPs from Flexor digitorum longus tendons
To extract MMPs, FDL tendons were dissected from P30 Col6a1−/− and Col6a1+/+ mice. The extraction of soluble proteins was described previously (Jung et al. 2009). Extracts were analyzed using zymography and immuno-blotting for MMPs. Samples were normalized to total protein. Protein concentration was determined using a BCA protein assay reagent (Pierce, Rockford, USA).
2.6. Zymography
FDL tendon extracts were analyzed by zymography according to the manufacturer’s instructions. Briefly, equal amount of tendon extracts, normalized for protein (10 μg), were separated by electrophoresis under non-reducing conditions in 10% Zymogram Gels containing 1% gelatin (Invitrogen). After electrophoresis, the gels were incubated in Zymogram Developing Buffer (Invitrogen) at 37°C overnight. The gels were stained with SimplyBlue Safestain (Invitrogen) an activity was visualized as unstained bands.
2.7. Immuno-blotting
Immuno-blotting analysis was performed using the FDL extracts obtained from P30 Col6a1−/− and Col6a1+/+ mice described above. According to the manufacture’s instruction, the extract proteins were eluted in SDS sample buffer with heating at 70°C for 10 min, and then were electrophoresed in SDS–PAGE under non-reducing condition. Beta actin detected by a mouse monoclonal antibody (Sigma) was used as a loading control.
2.8. Mechanical testing
Mechanical testing was performed on FDLs obtained from 6 Col6a1+/+ and 6 Col6a1−/− P90 mice. Cross-sectional area of the P90 FDLs was measured using a custom built device consisting of LVDTs, a CCD laser, and translation stages. Briefly, the specimen was translated beneath the laser while displacement data was simultaneously acquired in two directions from two LVDTs, as well as thickness data from a CCD laser displacement sensor (Favata 2006). Each end of the FDL was glued (cyanoacylate) to sand paper 5mm apart. Stain lines were placed 2.5mm apart within the mid-substance to track strain optically (Derwin et al. 1994). Tendons were placed in custom grips and loaded in an Instron 5543 (Instron Corp., Canton, MA). Each FDL underwent the following protocol while immersed in a 37°C PBS bath—preloaded to a nominal load, preconditioned for 10 cycles at a rate of 0.1%/s, and held for 300s. Immediately following, a stress relaxation experiment was performed by elongating the tendon to a strain of 5% at a rate of 5%/s, followed by a relaxation for 600s. Finally, a ramp to failure was applied at a rate of 0.1%/s. Percent relaxation was calculated from the peak and equilibrium stresses during stress relaxation. Maximum load and stress were determined and stiffness and modulus were calculated using linear regression from the near-linear region of the curve.
2.9. Statistical analysis
Student’s t test was used to compare Col6a1+/+ and Col6a1−/− samples. The difference was judged to be statistically significant for p<0.05 as represented by single asterisk and for p<0.01 by double asterisks. All the numeral data in the results were presented as means ± sd unless otherwise indicated.
Results
3.1. Collagen VI is enriched in the peri-cellular region of tendon fibroblasts
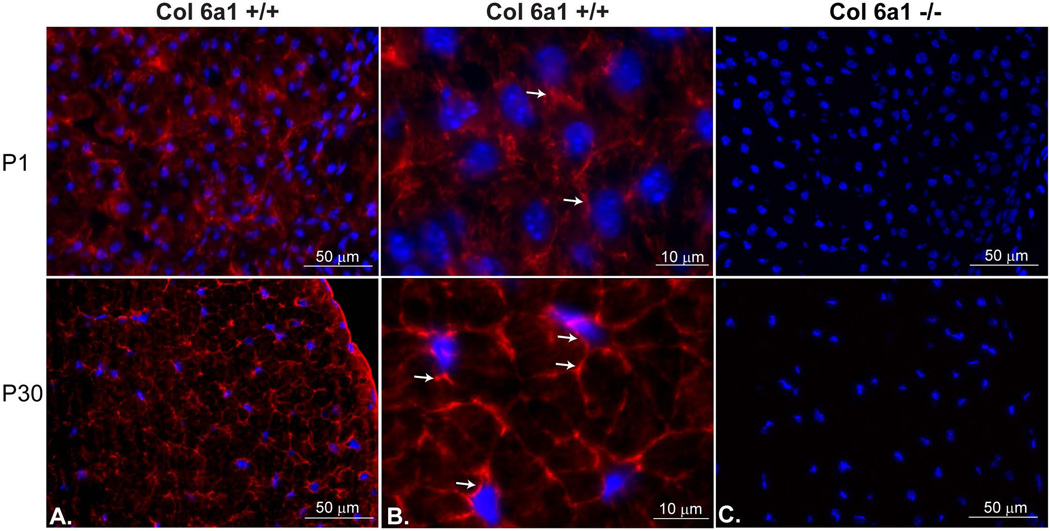
During normal tendon development, collagen VI was expressed throughout the developing extracellular matrix. Collagen VI was immuno-localized in wild type FDL tendons at day P1, P7, P16 and P30 of development (Fig.1 and data not shown). Collagen VI reactivity was enriched in the peri-cellular region of tendon fibroblasts. In high magnification micrographs, collagen VI was localized around tendon fibroblasts and extended along with the processes of tendon fibroblasts. Similar expression patterns of collagen VI were detected at all the ages. This suggests that collagen VI has a function associated with fibroblast-matrix interface during tendon development.
Fig. 1. Collagen VI is enriched in the peri-cellular region of tendon fibroblasts.
Collagen VI immuno-reactivity was localized throughout the tendon extracellular matrix in P1 and P30 wild type mice (A,B). No reactivity was observed in the Col6a1−/− control mice (C). At higher magnifications (B) reactivity was enriched in the peri-cellular regions around tendon fibroblasts in both P1 and P30 mice (arrows). Nuclei were labeled with DAPI.
3.2. Collagen VI deficiency results in altered tendon fibroblast shape and fiber organization
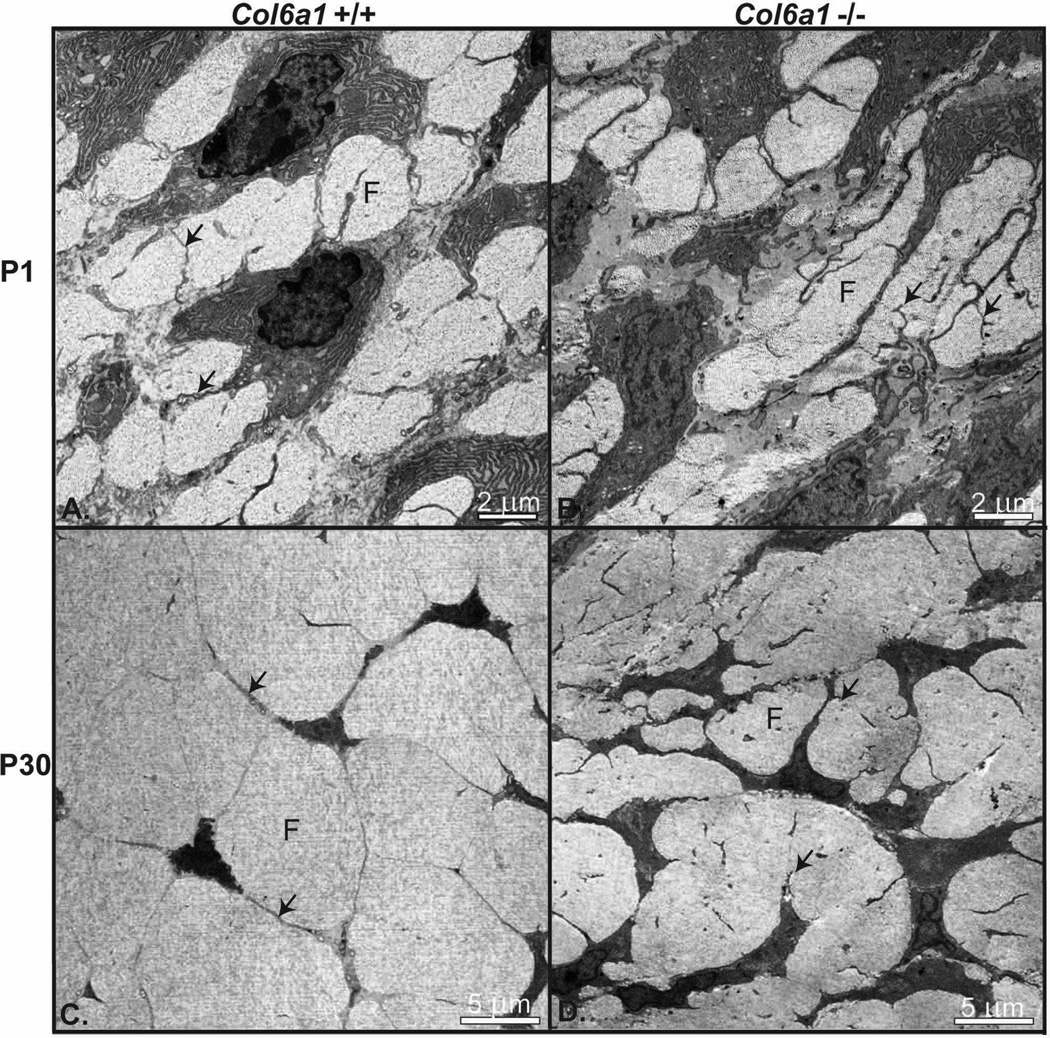
The morphological phenotypes of Col6a1−/− and wild type control (Col6a1+/+) developing tendons were analyzed using transmission electron microscopy of FDL tendons at P1 and P30. At an early stage of tendon development (P1) wild type tendon fibroblasts were well organized with well defined fibers. Adjacent tendon fibroblasts were connected via extended processes, which formed micro-domains containing collagen fibers (Fig. 2). In contrast, this organization was disrupted in P1 Col6a1−/− tendons. Col6a1−/− tendon fibroblasts had less regular cell shapes and organization. In addition, their processes had numerous thin branches. In addition, the adjacent fibroblasts were sometimes more closely localized than in the wild type tendons. Overall, this resulted is a disorganized micro-domain and fiber structure in the Col6a1−/− tendons.
Fig. 2. Altered tendon fibroblast, extracellular micro-domains and fiber organization in collagen VI null tendons.
Transmission electron micrographs of FDL tendons at P1 and P30 from Col6a1+/+ and Col6a1−/− mice. (A) At P1, wild type tendon fibroblasts showed a well organized arrangement with extended processes defining extracellular micro-domains containing small collagen fibers (fibril bundles). (B) In contrast, Col6a1−/− tendon fibroblasts had altered cell shape, and their processes had numerous thin branches resulting in disorganized micro-domains and fibers. (C) At P30, the wild type tendon fibroblasts defined well organized micro-domains and larger organized fibers. (D) In contrast, P30 Col6a1−/− tendon fibroblasts show altered shape, arrangement and organization with abnormal micro-domain organization and disrupted fiber formation. Fibers (F); Fibroblast processes (arrows).
As tendon development progressed (P30), fibroblast organization, micro-domain structure and fiber organization in Col6a1−/− tendons remained aberrant compared to the wild type control tendons (Fig. 2). Col6a1−/− tendons demonstrated a severe disruption of fibroblast organization compared to wild type controls. In the collagen VI null tendons, the fibroblasts and their processes were less attenuated and the microdomains were smaller and less organized compared to wild type tendons. This was associated with the development of smaller, less organized fibers in the Col6a1−/− tendons. These results suggest that collagen VI has crucial roles for maintaining the cell shape, micro-domain structure and fiber organization during tendon development that may be associated with fibrils formation, assembly and growth.
3.3. Collagen VI null tendons have altered fibril formation in the peri-cellular region and increased numbers of small diameter
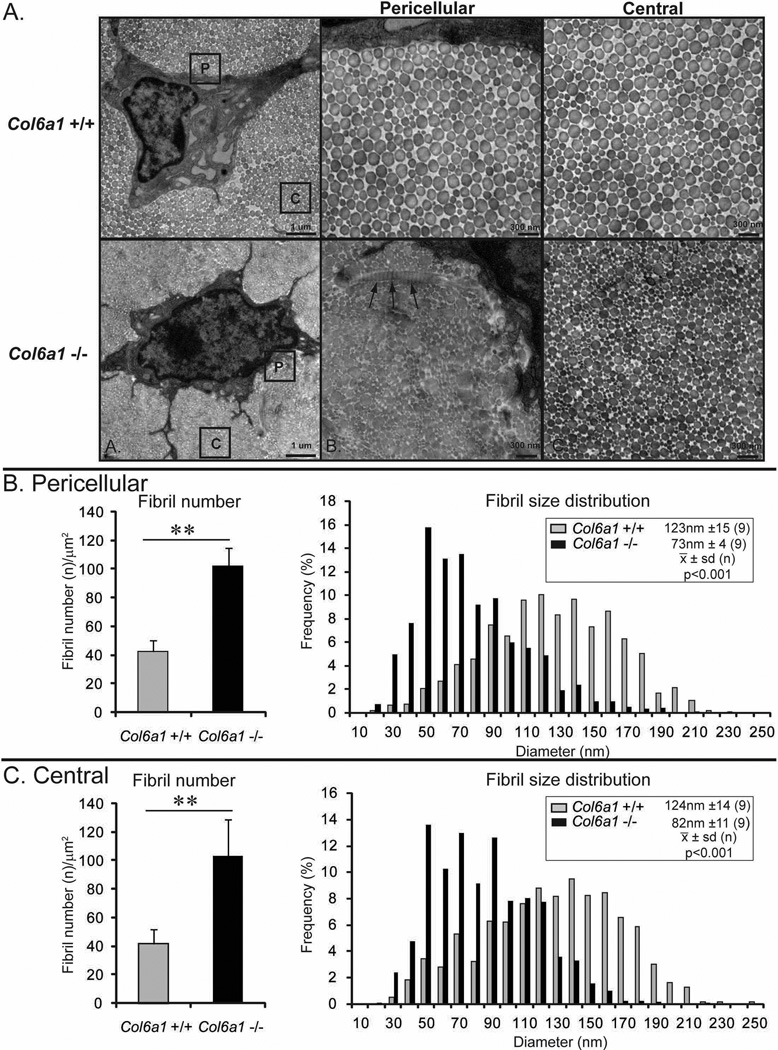
Collagen fibril assembly in Col6a1−/− and wild type control tendons was analyzed using transmission electron microscopy. At P1, wild type and Col6a1−/− tendon collagen fibrils both were of uniform size with normal circular cross-sectional profiles throughout the tendon extracellular matrix (data not shown). In contrast, P30 wild type and Col6a1−/− tendons had distinctly different fibril phenotypes (Fig. 3). Fibril arrangement and structure was aberrant in the peri-cellular regions of Col6a1−/− tendons. The fibrils were structurally abnormal with irregular cross-sectional profiles. In addition, there were frequent very large fibrils and twisted fibrils detected in the peri-cellular region. However, this alteration in fibril structure and organization was restricted to the pericellular regions and not observed in areas displaced from the fibroblast surface in the center of fibers. The wild type tendon contained fibrils with normal circular cross sectional profiles throughout the tendon.
Fig. 3. Dysfunctional tendon fibrillogenesis in collagen VI null mice.
(A) Transmission electron micrographs of P30 FDL tendons form Col6a1+/+ and Col6a1−/− mice. Wild type fibrils have normal circular cross sectional profiles and a broad heterogeneous distribution of fibrils in both peri-cellular and central areas (labeled P and C). In contrast, Col6a1−/− tendons have smaller diameter fibrils. In the peri-cellular area, very large, structurally aberrant, twisted fibrils are frequently observed (arrows). In central area, structurally aberrant fibrils are not observed. (B,C) Histograms of fibril number (density) and fibril diameter distributions in the peri-cellular area and central area from P30 Col6a1+/+ and Col6a1−/− mice. Fibril number is significantly increased in Col6a1−/− tendons in both peri-cellular and central areas compared to wild type controls. The fibril diameter distributions were shifted toward smaller diameters in peri-cellular and central areas of Col6a1−/− tendons compared to wild type controls with a significant difference in mean fibril diameter in both regions (p<0.001).
Fibril structure and diameter distribution was analyzed in the peri-cellular region and regions peripheral to the cell in the center of the fiber in wild type and Col6a1−/− mice. The wild type tendon contained a heterogeneous distribution of large diameter collagen fibrils in both regions and the fibril diameter distribution was comparable throughout the wild type tendon. In contrast, in Col6a1−/− tendons, fibril structure and diameter distribution were distinctly different from that observed in the wild type controls. Fibrils in the Col6a1−/− tendons had smaller diameters than the wild type tendons. In the pericellular region the mean fibril diameter was significantly smaller than in the wild type tendon, 72nm ± 4 (9) versus 123nm ± 15(9) [mean ± sd(n)] respectively (p<0.001). Likewise, mean diameters were significantly decreased in the central region as well. The respective diameters were 82nm ± 11(9) versus 124nm ± 14(9), p<0.001.
The diameter distributions in both the peri-cellular and central regions of the Col6a1−/− tendon were shifted toward the smaller diameters compared to the wild type tendon. The median fibril diameter and intra-quartile ranges (Q3-Q1) for Col6a1−/− and wild type distributions were 121nm (59nm) versus 65nm (42nm) for the peri-cellular region and 124nm (59nm) versus 76nm (47nm) for the central region. An analysis of fibril density (number/μm2) demonstrated a ~2.5 fold increase in both peri-cellular and central areas of Col6a1−/− tendons compared to wild type controls. The increased number is consistent with the decreased diameters compared to wild type tendons. These results indicate that collagen VI deficiency results in abnormal regulation of initial fibril assembly in peri-cellular region and regulation of normal lateral fibril growth is altered resulting in maintenance of smaller diameter fibrils.
3.4. Collagen VI null tendons display reduced biomechanical strength and stiffness
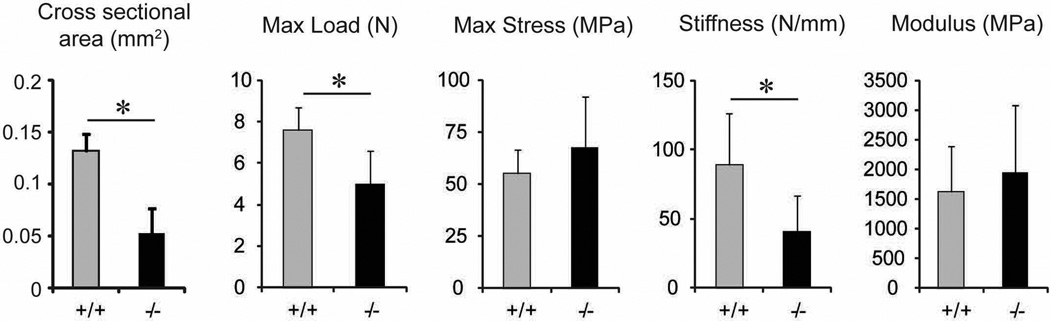
The biomechanical properties of Col6a1−/− and wild type control tendons were analyzed in mature tendons (P90) (Fig. 4). Cross-sectional area was determined and the collagen VI null tendons were significantly smaller than the wild type control tendons. The percent relaxation, maximum load, maximum stress, stiffness and modulus were analyzed. The Col6a1−/− tendons demonstrated a significant reduction in maximum load and stiffness compared to wild type tendons. However, percent relaxation (data not shown), maximum stress and modulus were comparable in both genotypes. This demonstrates decreased tensile strength and stiffness associated with abnormal tendon structure in the null mice.
Fig. 4. Altered biomechanical properties of tendon in collagen VI null mice.
Cross-sectional area, maximum load, maximum stress, stiffness and modulus were measured in P30 FDL tendons from Col6a1+/+ and Col6a1−/− mice. Cross sectional areas are significantly decreased in Col6a1−/− tendons compared to wild type control tendons. There is a significant reduction in maximum load and stiffness in Col6a1−/− tendons compared to wild type control tendons. However, maximum stress and modulus are comparable in both genotypes. Asterisk; P<0.05.
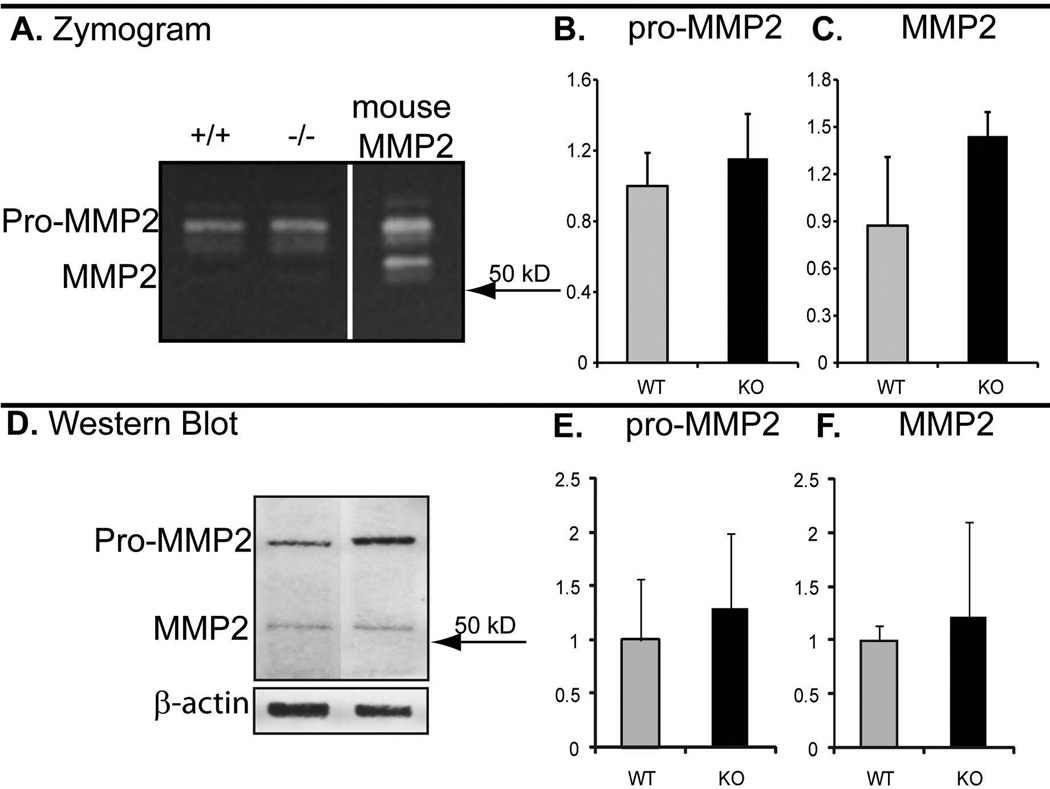
3.4. Collagen VI null tendons have increased MMP2 activity
To address the regulatory roles of collagen VI in the peri-cellular region of tendon fibroblasts, the expression and activity of matrix metalloprotease-2 (MMP-2) was examined in P30 wild type and Col6a1−/− FDL tendons using zymography and immunoblots (Fig. 5). A consistent small increase in both proMMP-2 and MMP-2 activity was observed in Col6a1−/− tendons compared to wild type controls. Immuno-blots confirmed that the activity was due to proMMP-2/MMP-2 and demonstrated a modest increase in Col6a1−/− compared to control tendons. The observed increases were consistent, but not statistically significant. This data support the hypothesis that the absence of collagen VI alters tenocyte expression due to disruption of cell-matrix interactions.
Fig. 5. Increased MMP-2 in collagen VI null tendons.
Collagen VI deficiency tends to increase MMP2 activation. Tendon extracts of P30 FDL tendons from Col6a1+/+ and Col6a1−/− mice were analyzed using zymography (A-C) and western blotting (D-F). Tendon extracts from both genotypes contained proMMP-2 and MMP-2 activity (A). The activity of proMMP-2 (B) and MMP-2 (C) tends to increase in Col6a1−/− tendons compared to wild type controls. Western blotting analysis of the same extracts used for zymography confirms the expression of proMMP-2 and MMP-2 (D). The expression of proMMP-2 (E) and MMP-2 (F) tends to increase in Col6a1−/− tendon extracts consistent with zymography data. The amount of extract loaded was normalized to β-actin.
3. Discussion
The current study demonstrates a dysfunctional regulation of tendon fibrillogenesis in the collagen VI null mouse model. In wild type tendons collagen VI was localized to the peri-cellular region surrounding tendon fibroblasts, indicating an important role(s) for this cell-matrix interface in tendon fibril formation. A disruption of cell and extracellular micro-domain organization was demonstrated in the absence of collagen VI. This was associated with abnormal fiber formation within the micro-domains. Finally, in the absence of collagen VI there was abnormal fibril assembly in the peri-cellular region and an assembly of abnormally small diameter fibrils throughout the tendon. The abnormal structure of the tendon fibrillar matrix in the absence of collagen VI was associated with altered function, i.e., decreased tensile strength and stiffness.
The assembly and deposition of collagen fibrils with tendon-specific structures involves a series of events that occur in both intracellular and extracellular compartments. Extracellularly, the relationship between the extracellular domain structure and the assembly of collagen fibrils and fibers has been extensively studied in the developing tendon (Birk et al. 1989a;Birk and Linsenmayer 1994;Canty and Kadler 2002;Zhang et al. 2005). In developing tendon, fibril assembly begins in deep recesses or channels defined by the fibroblast surface (Trelstad and Hayashi 1979;Birk and Trelstad 1986;Canty et al. 2004;Canty and Kadler 2005). In these micro-domains, precursor suprastructures, the protofibrils, are assembled (Birk and Trelstad 1986;Birk et al. 1989b). Once the protofibrils are deposited into the extracellular matrix there is an additional level of compartmentalization where fibrils are organized into fibers. The extracellular micro-domains provide a mechanism for the fibroblast to exert control over the extracellular steps of fibrillogenesis.
Our data demonstrated a disruption of the micro-domain structure in Col6a1−/− tendons. This coupled with the normal peri-cellular localization of collagen VI in the wild type tendon suggest a disruption in fibroblast-substrate interactions. We suggest that the establishment of micro-domains and their organization is compromised by the altered cell-substrate interactions in the absence of collagen VI. This abnormal cell behavior would be analogous to the altered interaction of myocytes with their substrates seen in Ullrich congenital muscular dystrophy and Bethlem myopathy with altered collagen VI expression (Lampe and Bushby 2005). The altered tendon micro-domain structure then results in dysfunctional fiber formation observed in the mutant tendons.
A disruption of the peri-cellular environment can also lead to altered fibroblast expression. The absence of a matricellular protein in thrombospondin 2-null mouse results in an abnormal increase in MMP-2 activity. This altered expression pattern is associated with abnormal collagen fibril structure in the developing tendon (Bornstein et al. 2000;Yang et al. 2001;Bornstein et al. 2004). The fibrils have larger diameters and irregular contours (Kyriakides et al. 1998). This is comparable to the abnormal fibrils we observe in the peri-cellular region of Col6a1−/− tendons. Our data, also suggest an abnormal up-regulation of MMP-2 by the collagen VI null tendon fibroblasts. We suggest that altered expression of matrix degrading enzymes could disrupt the regulatory interactions of matrix molecules with the fibril surface during the initial stages in fibril assembly. This would lead to structurally aberrant fibrils like the large diameter fibrils with cauliflower profiles as well as the twisted fibrils observed in the peri-cellular region.
Structurally aberrant fibrils are also observed in the dermis of patients deficient in collagen VI with Ullrich’s myopathy (Kirschner et al. 2005). Fibrils with cauliflower cross-sectional profiles were observed in some patients. In addition, alterations in fibril structure and organization were demonstrated comparable to the tendon data presented here; dermal fibrils were disorganized with smaller and variable diameters. This general fibril phenotype is seen in some forms of Ehlers Danlos Syndrome and suggests common pathophysiological pathways in a range of connective tissue disorders.
Our data indicate that collagen VI influences tendon fibrillogenesis. We suggest that this is the result of cell-matrix interactions directing fibroblast behavior and expression. Collagen VI interacts with a large number of extracellular molecules including small leucine rich proteoglycans (SLRPs) and collagen XIV (Kielty and Grant 2002). Collagen XIV (Ansorge et al. 2009) and SLRPs are involved in the normal regulation of the later stages in tendon fibrillogenesis (Ezura et al. 2000;Ameye and Young 2002;Ameye et al. 2002;Zhang et al. 2005;Zhang et al. 2006). In mouse models with targeted deletions in SLRPs, often there is a disruption in fibril growth generating resulting in larger diameter and structurally abnormal fibrils as well as altered tendon function (Danielson et al. 1997;Ezura et al. 2000;Ameye et al. 2002;Zhang et al. 2005;Zhang et al. 2006). In contrast, our data demonstrated an abnormal maintenance of small diameter fibrils in the Col6a1−/− tendon relative to the wild type control. One explanation for this is that collagen VI normally binds SLRPs and in the absence of collagen VI the equilibrium is disrupted with higher SLRP concentrations and more is bound to the fibril surface, therefore preventing normal lateral fibril growth. These fibril-associated regulators turnover regularly and the collagen VI network may serve as a reservoir within the fiber. In contrast, in the peri-cellular region, an increase in MMP-2 activity or membrane type matrix metalloproteinases may increase the turnover of SLRPs resulting in a focal dysfunctional fibril assembly. Together, the altered fibril and fiber structure would explain the functional defects seen in the biomechanical analysis. Similar biomechanical changes are observed when fibril-associated regulators are disrupted (Robinson et al. 2005;Zhang et al. 2005;Ansorge et al. 2009).
A decrease in maximum load and stiffness was observed in the Col6a1−/− tendons. It is interesting to note that a similar decrease in maximum load and stiffness has been observed in other mouse models with targeted deletions in regulatory molecules. Both Col6a1−/− d fibromodulin-null (with up-regulated lumican expression) mice have smaller tendon fibril diameters as opposed to increased fibril diameters observed in decorin-, biglycan-, collagen XIV- and compound fibromodulin/lumican-null mice (Svensson et al. 1999;Ezura et al. 2000;Ameye et al. 2002;Jepsen et al. 2002;Zhang et al. 2006;Ansorge et al. 2009). This demonstrates that decreased strength can be associated with both increased and decreased fibril diameters. Also, unlike collagen XIV- and decorin-null mice, Col6a1−/− FDLs did not exhibit a significant decrease in the material properties maximum stress or modulus. This indicates that decreased structural properties may be due to the decreased size of the mice and therefore decreased cross-sectional area of the null tendons. Interestingly, the role of collagen VI in the maintenance of myocyte/muscle function may have an impact on the tendon. The small tendons with decreased structural mechanical properties could be due to decreased loading from impaired musculature and not necessarily due to a dysfunction in the tendon itself. In addition, the decreased stiffness demonstrated in the current study supports using Col6a1−/− as a model for Bethlem myopathy and Ullrich congenital muscular dystrophy which include joint laxity as a feature.
Acknowledgments
We thank Bao Zuo for expert assistance with the transmission electron microscopy and Sheila Adams for help in preparation of the figures.
Footnotes
This study was supported by NIH/NIAMS grants AR044745 (DEB), AR053251 (MLC), NIH/NIAMS grant AR050950, supporting the Penn Center for Musculoskeletal Disorders (LJS) and Telethon grant GGP08107 (PB).
Reference List
- Alexopoulos LG, Youn I, Bonaldo P, Guilak F. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 2009;60:771–779. doi: 10.1002/art.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107R–116R. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- Ansorge HL, Meng X, Zhang G, Veit G, Sun M, Klement JF, Beason DP, Soslowsky LJ, Koch M, Birk DE. Type XIV Collagen Regulates Fibrillogenesis: premature collagen fibril growth and tissue dysfunction in null mice. J Biol Chem. 2009;284:8427–8438. doi: 10.1074/jbc.M805582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Linsenmayer TF. Collagen fibril assembly, depostion, and organization into tissue-specific matrices. In: Yurchenco PD, Birk DE, Mecham RP, editors. Extracellular Matrix Assembly and Structure. NY: Academic Press; 1994. pp. 91–128. [Google Scholar]
- Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol. 1997;72:352–361. [PubMed] [Google Scholar]
- Birk DE, Southern JF, Zycband EI, Fallon JT, Trelstad RL. Collagen fibril bundles: a branching assembly unit in tendon morphogenesis. Development. 1989a;107:437–443. doi: 10.1242/dev.107.3.437. [DOI] [PubMed] [Google Scholar]
- Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Zycband EI, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proc Natl Acad Sci U S A. 1989b;86:4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM. Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet. 1998;7:2135–2140. doi: 10.1093/hmg/7.13.2135. [DOI] [PubMed] [Google Scholar]
- Bonaldo P, Russo V, Bucciotti F, Doliana R, Colombatti A. Structural and functional features of the alpha 3 chain indicate a bridging role for chicken collagen VI in connective tissues. Biochemistry. 1990;29:1245–1254. doi: 10.1021/bi00457a021. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Bornstein P, Kyriakides TR, Yang Z, Armstrong LC, Birk DE. Thrombospondin 2 modulates collagen fibrillogenesis and angiogenesis. J Investig Dermatol Symp Proc. 2000;5:61–66. doi: 10.1046/j.1087-0024.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- Bruns RR, Press W, Engvall E, Timpl R, Gross J. Type VI collagen in extracellular, 100-nm periodic filaments and fibrils: identification by immunoelectron microscopy. J Cell Biol. 1986;103:393–404. doi: 10.1083/jcb.103.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Collagen fibril biosynthesis in tendon: a review and recent insights. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:979–985. doi: 10.1016/s1095-6433(02)00212-x. [DOI] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ML, Mann K, Deutzmann R, Pribula-Conway D, Hsu-Chen CC, Bernard MP, Timpl R. Characterization of three constituent chains of collagen type VI by peptide sequences and cDNA clones. Eur J Biochem. 1987;168:309–317. doi: 10.1111/j.1432-1033.1987.tb13422.x. [DOI] [PubMed] [Google Scholar]
- Chu ML, Pan TC, Conway D, Saitta B, Stokes D, Kuo HJ, Glanville RW, Timpl R, Mann K, Deutzmann R. The structure of type VI collagen. Ann N Y Acad Sci. 1990;580:55–63. doi: 10.1111/j.1749-6632.1990.tb17917.x. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derwin KA, Soslowsky LJ, Green WD, Elder SH. A new optical system for the determination of deformations and strains: calibration characteristics and experimental results. J Biomech. 1994;27:1277–1285. doi: 10.1016/0021-9290(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata M. Bioengineering. Vol. 216. Philadelphia, PA: University of Pennsylvania; 2006. Scarless healing in the fetus: Implications and strategies for postnatal tendon repair. Ref Type: Thesis/Dissertation. [Google Scholar]
- Fitzgerald J, Rich C, Zhou FH, Hansen U. Three novel collagen VI chains, alpha4(VI), alpha5(VI), and alpha6(VI) J Biol Chem. 2008;283:20170–20180. doi: 10.1074/jbc.M710139200. [DOI] [PubMed] [Google Scholar]
- Furthmayr H, Wiedemann H, Timpl R, Odermatt E, Engel J. Electron-microscopical approach to a structural model of intima collagen. Biochem J. 1983;211:303–311. doi: 10.1042/bj2110303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gara SK, Grumati P, Urciuolo A, Bonaldo P, Kobbe B, Koch M, Paulsson M, Wagener R. Three novel collagen VI chains with high homology to the alpha3 chain. J Biol Chem. 2008;283:10658–10670. doi: 10.1074/jbc.M709540200. [DOI] [PubMed] [Google Scholar]
- Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, Oldberg A, Birk DE, Chakravarti S. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- Jung JC, Wang PX, Zhang G, Ezura Y, Fini ME, Birk DE. Collagen fibril growth during chicken tendon development: matrix metalloproteinase-2 and its activation. Cell Tissue Res. 2009;336:79–89. doi: 10.1007/s00441-009-0755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Keene DR, Engvall E, Glanville RW. Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol. 1988;107:1995–2006. doi: 10.1083/jcb.107.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty CM, Grant ME. The collagen family: structure, assembly, and organization in the extracellular matrix. In: Royce PM, Steinmann B, editors. Connective tissue and its heritable disorders. New York: Wiley-Liss; 2002. pp. 159–222. [Google Scholar]
- Kirschner J, Hausser I, Zou Y, Schreiber G, Christen HJ, Brown SC, nton-Lamprecht I, Muntoni F, Hanefeld F, Bonnemann CG. Ullrich congenital muscular dystrophy: connective tissue abnormalities in the skin support overlap with Ehlers-Danlos syndromes. Am J Med Genet A. 2005;132A:296–301. doi: 10.1002/ajmg.a.30443. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005;42:673–685. doi: 10.1136/jmg.2002.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminskaya MV, Birk DE. Differential expression of fibromodulin mRNA associated with tendon fibril growth: isolation and characterization of a chicken fibromodulin cDNA. Biochem J. 1996;317:785–789. doi: 10.1042/bj3170785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PS, Huang TF, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ. Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng. 2005;127:181–185. doi: 10.1115/1.1835363. [DOI] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- Tillet E, Wiedemann H, Golbik R, Pan TC, Zhang RZ, Mann K, Chu ML, Timpl R. Recombinant expression and structural and binding properties of alpha 1(VI) and alpha 2(VI) chains of human collagen type VI. Eur J Biochem. 1994;221:177–185. doi: 10.1111/j.1432-1033.1994.tb18727.x. [DOI] [PubMed] [Google Scholar]
- Trelstad RL, Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev Biol. 1979;71:228–242. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- von der Mark H, Aumailley M, Wick G, Fleischmajer R, Timpl R. Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem. 1984;142:493–502. doi: 10.1111/j.1432-1033.1984.tb08313.x. [DOI] [PubMed] [Google Scholar]
- Wiberg C, Heinegard D, Wenglen C, Timpl R, Morgelin M. Biglycan organizes collagen VI into hexagonal-like networks resembling tissue structures. J Biol Chem. 2002;277:49120–49126. doi: 10.1074/jbc.M206891200. [DOI] [PubMed] [Google Scholar]
- Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]