Abstract
We have evaluated the specificity of Cre recombinase activity in transgenic mice expressing Cre under the control of the synatonemal complex protein 1 (Sycp1) gene promoter. Sycp1Cre mice were crossed with the ROSA26 reporter line R26R, to monitor the male germ cell stage-specificity of Cre activity as well as to verify that Cre was not active previously during development of other tissues. X-gal staining detected Cre-mediated recombination only in testis. Detailed histological examination indicated that weak Cre-mediated recombination occurred as early as in zygotene spermatocytes at stage XI of the cycle of the seminiferous epithelium. Robust expression of X-gal was detected in early to mid-late spermatocytes at stages V–VIII. We conclude that this transgenic line is a powerful tool for deleting genes of interest specifically during male meiosis.
Keywords: Cre recombinase, Sycp1Cre, ROSA26, primary spermatocytes
Introduction
Spermatogenesis is a complex developmental process involving mitotic proliferation, meiosis, and differentiation of spermatids to spermatozoa. Meiosis includes the reduction of ploidy, introduction of diversity by homologous recombination, and maintenance of genomic integrity. Although meiosis plays a crucial role in sexual reproduction, the molecular mechanisms that control the entry and progression of germ cells into their differentiation pathway are poorly defined, particularly in higher organisms. Targeted mutagenesis in embryonic stem cells has identified genes required for spermatogenesis (reviewed in Eddy, 1999), however, unequivocal evaluation of their function has frequently been impeded due to embryonic lethality or other physiological consequences of the null mutation.
The Cre/loxP approach has been used to overcome these limitations in a variety of developmental systems (reviewed in Nagy, 2000). Insertion of loxP sites into a gene of interest and targeting Cre recombinase expression to specific cell types using a cell/tissue-specific promoter makes it possible to introduce cell/tissue-restricted or conditional mutations. Conditional gene disruption only in the testis or at particular stages during spermatogenic differentiation will be very useful to dissect the molecular pathways involved in spermatogenesis.
Promoters of several genes active in early male meiotic cells, such as Hsp70-2 (Dix et al., 1996), Pdha-2 (Iannello et al., 1997), Spo11 (Baudat et al., 2000), and Sycp1 (Vidal et al., 1998; Sage et al., 1999), have been characterized in transgenic mice.
Sycp1 encodes synaptonemal complex protein 1, a major component of the central element of the synaptonemal complex. It is present exclusively from the beginning of the zygotene up to the diplotene stage, in both male and female gonads (Heyting et al., 1989; Offenberg et al., 1991; Dietrich et al., 1992; Meuwissen et al., 1992; Moens et al., 1992; Dobson et al., 1994). Sequences within the 5′ proximal 260 bp of mouse Sycp1 are sufficient to direct the expression of a lacZ transgene (Sycp1lacZ) in the testis, but are not active in the ovary (Sage et al., 1999). Transgenic mice expressing Cre recombinase driven by this Sycp1 promoter have been generated and crossed with mice carrying a RXRα gene containing loxP sites (Vidal et al., 1998). RT-PCR analysis of the putatively recombined eighth intron of the RXRα gene revealed efficient and spermatocyte-specific Cre-mediated excision (Vidal et al., 1998). However, expression was neither examined in tissues other than testis nor was the cellular specificity of expression in the testis examined in detail (Vidal et al., 1998; Rassoulzadegan et al., 2002). In the present study, the reporter transgenic lines R26R was used for monitoring the tissue, cellular and temporal specificity of transgenic mice carrying the Sycp1Cre construct.
Methods, results and discussion
To determine the tissue and cellular specificity of Sycp1-driven Cre recombinase activity in vivo, the Sycp1Cre transgenic line (Vidal et al., 1998) was crossed with the reporter line R26R mice (Soriano, 1999). R26R carries a copy of lacZ into which loxP sites have been inserted such that no functional β-galactosidase (β-gal) is made; excision of the loxP sites by Cre restores β-gal production (Zambrowicz et al., 1997; Soriano, 1999). The reporter is ubiquitously expressed, including in male germ cells, which makes it a useful model system for examining the specificity of the Sycp1Cre transgene. β-Gal activity resulting from Cre-mediated recombination of the R26R locus was monitored by X-gal staining. Male progeny from Sycp1Cre and R26R cross-breedings were collected, tail DNA was prepared using the DNeasy Tissue Kit (Qiagen, CA), and subjected to PCR analysis for the presence of Cre according to Vidal and colleagues (Vidal et al., 1998) or for lacZ using primers ROSA26-I, II, III (Soriano, 1999).
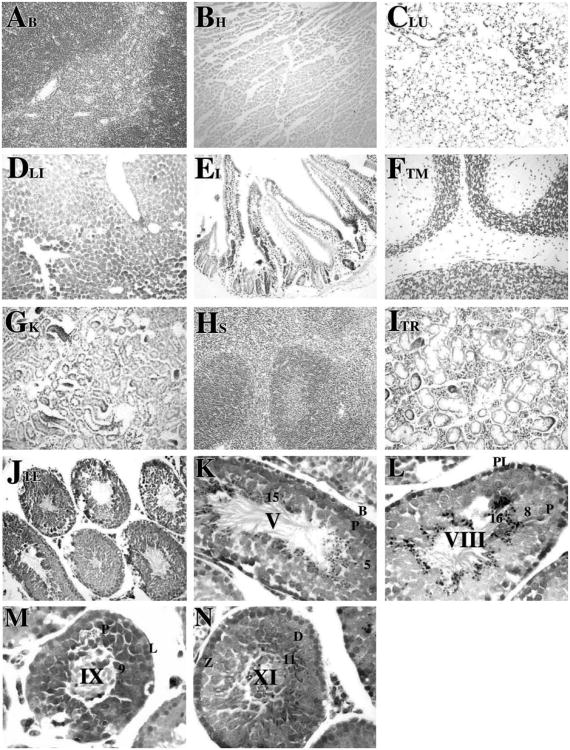
Among the tissues examined, Cre activity in the R26RSycp1Cre mice was restricted to testis. Figure 1 shows a typical X-gal staining pattern in various selected organs (Figures 1A–J), including brain, heart, lung, liver, intestine, thymus, kidney, spleen, thyroid, and testis. Slight X-gal staining was detected in the thyroid (Figure 1I), likely because of endogeneous β-gal activity. Histological testicular sections were counterstained with neutral red and tubules were staged to determine the developmental and cellular specificity of appearance of Cre recombinase activity (Figure 1K–N). Very weak Cre-mediated recombination occurred as early as in zygotene spermatocytes at stage XI (Figure 1N). As meiotic prophase progressed, β-gal was detected robustly in early to mid-late spermatocytes at stage V–VIII (Figures 1K and L, respectively). The recombined β-gal protein was apparently not turned over, since X-gal staining was detected in stages as late as step 16 spermatids (Figure 1K–N). In contrast, β-gal expression was not detected in any other testicular cell type within tubules or interstitial regions.
Figure 1.
Tissue-specific and spermatogenic cell-specific expression of β-gal following Cre recombination. Transgenic and non-transgenic control mice were euthanized, perfused with fixatives, and tissues were removed. The tissues were fixed again for 1 h at room temperature in 100 mM sodium phosphate, pH 7.3, containing 2% paraformaldehyde, 0.2% glutaraldehyde, 0.1% sodium deoxycholate, 0.2% Nonidet P-40, 5 mM EGTA and 2 mM MgCl2. The tissues were incubated overnight at 30 °C in 100 mM sodium phosphate, pH 7.3, 1.3 mM MgCl2, 3 mM K3Fe(CN)6, 3 mM K4Fe(CN)6 and 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) as described previously (Langford et al., 1991; Fire, 1992). The tissues were embedded in paraffin, sectioned at 5 μm, and mounted on Superfrost slides (Fisher Scientific, NJ). After X-gal staining, histological sections of brain (A), heart (B), lung (C), liver (D), intestine (E), thymus (F), kidney (G) spleen (H), thyroid (I), and testis (J) were obtained and counterstained with neutral red (Catalogue number N129, Fisher Scientific, NJ) according to standard procedures and viewed on a Nikon photomicroscope under bright-field optics. Photomicrographs were taken using a digital camera (Spot advanced software, Diagnostic Instruments, Inc.). Specific expression of β-galactosidase following Cre recombination was observed only in testis (J). Magnification: ×20. Histological testicular sections from R26RSycp1Cre males were examined at higher magnification (K–N, ×60) to determine cell-specific expression of Cre as detected by X-gal staining. β-gal activity was detected in zygotene spermatocytes at stage XI (N) as very weak signals. The expression was detected continuously from early primary spermatocytes to mid-late pachytene spermatocytes at stage V, VIII (K and L, respectively) to step 1–16 spermatids (K–N). β-gal activity was not detected in Sertoli cells nor in interstitial cells. Abbreviations: B, type B spermatogonia; PL, preleptotene spermatocytes; L, leptotene spermatocytes; Z, zygotene spermatocytes; D, diplotene spermatocytes; P, pachytene spermatocytes; arabic numerals, the step of elongated spermatid; Roman numerals indicated the stage of the seminiferous tubules, staged as described by Russell et al. (1990).
As presented in Table 1, several examples of Cre expression targeted to germ cells have been reported. However, ectopic Cre expression was observed in many of these mouse strains. Cre driven by 450-bp of the Pgk2 promoter, which in adult mice accurately targets Cre recombinase to male germ cells, is subject to ectopic expression during embryonic development (Bhullar et al., 2001). A larger 1.4-k bp Pgk2 fragment was reported to be specific to spermatocytes and spermatids (Ando et al., 2000), although only liver, kidney and brain of the Pgk2CreCagCatZ mice were examined. In c-kitCre transgenic mice, recombination of a floxed Neo gene was found in several tissues in which c-kit is not known to be expressed, likely resulting from an excision event in embryogenesis (Bergqvist et al., 1998). Ectopic Cre recombination had not been examined in detail for Sycp1Cre mice, but it had been noted that the endogenous Sycp1 gene is transiently active at the 2- to 4-cell stage during embryogenesis (Vidal et al., 1998). This raised the possibility that the lack of evidence for recombined alleles in various tissues in Sycp1Cre mice may be due to the method used to detect recombination events (genotyping of remaining floxed alleles) or the limited number of tissues examined (Vidal et al., 1998; Rassoulzadegan et al., 2002). Our current detailed examination of the various selected organs in R26RSycp1Cre mice by X-gal staining clearly illustrate the cellular and tissue specificity of Cre recombinase activity driven by Sycp1 promoter as well as a lack of effect of any Cre activity in early embryos, which would have been expected to donate recombined alleles to virtually all tissues.
Table 1. Summary of mouse strains carrying Cre expression targeted to male germ cells.
| Mouse strain | Target cells | References |
|---|---|---|
| Prm1-Cre | Haploid spermatid | O'Gorman et al. (1997) |
| Haploid spermatid, sterile | Schmidt et al. (2000) | |
| c-Kit-Cre | Germ cells; ubiquitous deletion during embryogenesis | Bergqvist et al. (1998) |
| Sycp1-Cre | Primary spermatocytes | Vidal et al. (1998) |
| Pgk2-Cre | Spermatocytes | Ando et al. (2000) |
| Spermatocytes; Ectopic expression during embryogenesis | Bhullar et al. (2001) | |
| TNAP-Cre | Primordial germ cells | Lomeli et al. (2000) |
| PGK-1-Cre | Diploid primordial germ cells | Lallemand et al. (1998) |
The induction of Cre expression in mouse cells is clearly an artificial situation, but is generally believed not to affect gene expression other than that of the floxed gene of interest. However, illegitimate, Cre-dependent chromosome arrangements in transgenic mouse spermatids and resulting sterility have been reported in mice carrying a transgene consisting of 4.1 kb promoter fragment of Prm1 driving Cre (Schmidt et al., 2000). Whether this reflects the vulnerable nature of DNA in spermatids to Cre-mediated reactions is not clear (Schmidt et al., 2000). However, mice generated from a truncated form of the Prm1 promoter (652 bp) driving Cre recombinase gene were fully fertile (O'Gorman et al., 1997). Further, PrmCre males can efficiently recombine a floxed RNA polymerase II locus P2Bc (Pol II, β-gal, conditional) in the male germ line of mice (O'Gorman et al., 1997).
In R26RSycp1Cre mice, loxP recombination fell to very low levels in the second generation of mice (Rassoulzadegan et al., 2002). Although Cre was still expressed during meiosis, recombination of remaining loxP sites was inhibited, putatively due to methylation of cytosines initiated within the loxP sequence and the surrounding chromosomal region (Rassoulzadegan et al., 2002). As such, Sycp1Cre males cannot be propagated with genomic loxP sites for successive generations without a rapid loss in recombination efficiency. The double transgenic Sycp1Cre/loxP genotypes could be maintained through the female germ line since the Sycp1 promoter sequence from −722 to +102 relative to the transcription start is not active during female meiosis (Sage et al., 1999; Rassoulzadegan et al., 2002). Accordingly, loxP sites would remain intact when transferred through the female germ line. Alternatively, transgenic mice expressing the tamoxifen-inducible Cre-ERT recombinase under the control of Sycp1 promoter, in which the Cre transgene is not active unless in the presence of tamoxifen, could be generated. As the Sycp1Cre mouse line is currently the only available strain that targets Cre recombinase to male meiotic prophase cells, they provide means for generating mutations during meiosis in spermatocytes, and thus, to define the role of specific genes in spermatogenesis at the zygotene to pachytene and later stages.
Acknowledgments
We thank Xiangyuan Wang for technical support in tissue processing and sectioning. This work was supported by Grants from NIH, HD34915 and P01 DK54057, Project 5 and Core B, USA to D.J.W.; a grant from Association pour la Recherche sur le Cancer, France to F.C., and fellowships from the Croucher Foundation, Hong Kong to S.S.W.C.
References
- Ando H, Haruna Y, Miyazaki J, Okabe M, Nakanishi Y. Spermatocyte-specific gene excision by targeted expression of Cre recombinase. Biochem Biophys Res Commun. 2000;272:125–128. doi: 10.1006/bbrc.2000.2762. [DOI] [PubMed] [Google Scholar]
- Baudat F, Manova K, Yuen JP, Jasin M, Keeney S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol Cell. 2000;6:989–998. doi: 10.1016/s1097-2765(00)00098-8. [DOI] [PubMed] [Google Scholar]
- Bergqvist I, Eriksson B, Eriksson M, Holmberg D. Transgenic Cre recombinase expression in germ cells and early embryogenesis directs homogeneous and ubiquitous deletion of loxP-flanked gene segments. FEBS Lett. 1998;438:76–80. doi: 10.1016/s0014-5793(98)01272-1. [DOI] [PubMed] [Google Scholar]
- Bhullar B, Schmidt JV, Truong T, Rancourt D, van der Hoorn FA. Germ cell specific promoter drives ectopic transgene expression during embryogenesis. Mol Reprod Dev. 2001;59:25–32. doi: 10.1002/mrd.1003. [DOI] [PubMed] [Google Scholar]
- Dietrich AJ, Kok E, Offenberg HH, Heyting C, de Boer P, Vink AC. The sequential appearance of components of the synaptonemal complex during meiosis of the female rat. Genome. 1992;35:492–497. doi: 10.1139/g92-072. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Rosario-Herrle M, Gotoh H, Mori C, Goulding EH, et al. Developmentally regulated expression of Hsp70-2 and a Hsp70-2/lacZ transgene during spermatogenesis. Dev Biol. 1996;174:310–321. doi: 10.1006/dbio.1996.0076. [DOI] [PubMed] [Google Scholar]
- Dobson MJ, Pearlman RE, Karaiskakis A, Spyropoulos B, Moens PB. Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci. 1994;107:2749–2760. doi: 10.1242/jcs.107.10.2749. [DOI] [PubMed] [Google Scholar]
- Eddy EM. The effects of gene knockouts on spermatogenesis. In: Gagnon C, editor. The Male Gamete: From Basic Science to Clinical Applications. Vienna, Illinois: Cache River Press; 1999. pp. 24–36. [Google Scholar]
- Fire A. Histochemical techniques for locating Escherichia coli beta-galactosidase activity in transgenic organisms. Genet Anal Tech Appl. 1992;9:151–158. doi: 10.1016/1050-3862(92)90042-4. [DOI] [PubMed] [Google Scholar]
- Heyting C, Dietrich AJ, Moens PB, Dettmers RJ, Offenberg HH, et al. Synaptonemal complex proteins. Genome. 1989;31:81–87. doi: 10.1139/g89-016. [DOI] [PubMed] [Google Scholar]
- Iannello RC, Young J, Sumarsono S, Tymms MJ, Dahl HH, et al. Regulation of Pdha-2 expression is mediated by proximal promoter sequences and CpG methylation. Mol Cell Biol. 1997;17:612–619. doi: 10.1128/mcb.17.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- Langford KG, Shai SY, Howard TE, Kovac MJ, Overbeek PA, et al. Transgenic mice demonstrate a testis-specific promoter for angiotensinconverting enzyme. J Biol Chem. 1991;266:15559–15562. [PubMed] [Google Scholar]
- Lomeli H, Ramos-Mejia V, Gertsenstein M, Lobe CG, Nagy A. Targeted insertion of Cre recombinase into the TNAP gene: excision in primordial germ cells. Genesis. 2000;26:116–117. [PubMed] [Google Scholar]
- Meuwissen RL, Offenberg HH, Dietrich AJ, Riesewijk A, van Iersel M, et al. A coiled-coil related protein specific for synapsed regions of meiotic prophase chromosomes. EMBO J. 1992;11:5091–5100. doi: 10.1002/j.1460-2075.1992.tb05616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Spyropoulos B, Dobson M, Karaiskakis A, Pearlman RE. Searching for synaptonemal complex proteins and their genes. Dev Genet. 1992;13:435–439. doi: 10.1002/dvg.1020130607. [DOI] [PubMed] [Google Scholar]
- Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- Offenberg HH, Dietrich AJ, Heyting C. Tissue distribution of two major components of synaptonemal complexes of the rat. Chromosoma. 1991;101:83–91. doi: 10.1007/BF00357057. [DOI] [PubMed] [Google Scholar]
- O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci USA. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M, Magliano M, Cuzin F. Transvection effects involving DNA methylation during meiosis in the mouse. EMBO J. 2002;21:440–450. doi: 10.1093/emboj/21.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, SinhaHikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Cache River Press; Clearwater, FL: 1990. [Google Scholar]
- Sage J, Martin L, Meuwissen R, Heyting C, Cuzin F, et al. Temporal and spatial control of the Sycp1 gene transcription in the mouse meiosis: regulatory elements active in the male are not sufficient for expression in the female gonad. Mech Dev. 1999;80:29–39. doi: 10.1016/s0925-4773(98)00191-9. [DOI] [PubMed] [Google Scholar]
- Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. Illegitimate Credependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci USA. 2000;97:13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Vidal F, Sage J, Cuzin F, Rassoulzadegan M. Cre expression in primary spermatocytes: a tool for genetic engineering of the germ line. Mol Reprod Dev. 1998;51:274–280. doi: 10.1002/(SICI)1098-2795(199811)51:3<274::AID-MRD6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, et al. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]