Abstract
Estrogens have a multitude of effects on opioid systems and are thought to play a key role in sexually dimorphic nociception and opioid antinociception. Heretofore, classical genomic actions of estrogens are largely thought to be responsible for the effects of these steroids on nociception and opioid antinociception. The recent discovery that estrogens can also activate estrogen receptors that are located in the plasma membrane, the effects of which are manifest in seconds to minutes instead of hours to days has revolutionized our thinking concerning the ways in which estrogens are likely to modulate pain responsiveness and the dynamic nature of that modulation. This review summarizes parameters of opioid functionality and nociception that are subject to modulation by estrogens, underscoring the added dimensions of such modulation that accrues from rapid membrane estrogen receptor signaling. Implications of this mode of signaling regarding putative sources of estrogens and its degradation are also discussed.
Keywords: estrogens, estrogen receptors, antinociception, nociception, opioid, opioid receptors, sexual dimorphism, mu-opioid receptor, kappa-opioid receptor, rapid membrane estrogen receptor signaling
1. Introduction
The influence of sex on nociception and its amelioration has been extensively documented but the underlying biology remains elusive. Multiple cross validating studies reveal that women are more likely than men to experience chronic pain as well as pain of greater severity and duration [100,104,123,161,163,190,202]. Chronic pain disorders that are vastly more prevalent in women than men include migraine (2:1), irritable bowel syndrome (2:1), interstitial cystitis (9:1) and fibromyalgia (6:1). Sex-dependent differences in nociception are observed across multiple modalities of nociceptive stimuli, e.g., thermal [70], electrical [195], pressure [60]. Moreover, epidemiological studies have consistently shown that women have greater severity and frequency of visceral pain than do men [21,22,147].
Sex-dependent differences in nociceptive responsiveness and endogenous pain modulation have also been documented using laboratory animals [21,41,48,49,92,139,140]. In particular, studies with laboratory animals reveal that females have significantly lower thresholds to experimental visceral pain than do males [143]. Despite the pervasiveness of these observations, there is little mechanistic understanding of the sex-dependent experience of either chronic or acute pain. In particular, the specific factor(s) that is (are) causally associated with sex-dependent nociception have not been delineated.
Sex is also increasingly recognized to be causally associated with antinociceptive efficacy of many opioids [50,52,94,132]. There is a rapidly emerging consensus that women respond more efficaciously to opioid analgesics than do men. For example, in one study [42], females consumed significantly less morphine via patient-controlled analgesia in the first three postoperative days than was the case for males, gender being the strongest predictor for postoperative morphine requirements. The implied greater opioid antinociceptive efficacy in women vs. men was subsequently affirmed by demonstrating that women manifested greater analgesic responsiveness to three mu opioid receptor agonists (morphine, meperidine (pethidine) and hydromorphone) than their male counterparts using cold pressure as the nociceptive stimulus [203].
Estrogens have a multitude of well-documented effects on opioid systems. There is considerable evidence that they play a key role in aspects of nociception and opioid antinociception that exhibit sexual dimorphism. Our understanding of the mechanisms that could underlie estrogenic modulation has been revolutionized by the recent discovery that receptors for estrogens exist in the plasma membrane and that these membrane receptors function mechanistically and temporally in a fundamentally different manner from their nuclear counterpart. The contribution of the plasma membrane estrogen receptor (ER) to estrogenic modulation of nociception and opioid antinociception is just beginning to be delineated.
Our aim in this review is to provide selective perspective on selective components of nociception and antinociception that exhibit sexually dimorphic plasticity and the roles of estrogens in that sex-dependent modulation. This review summarizes parameters of opioid functionality and nociception that are subject to modulation by estrogens with particular emphasis on estrogenic regulation that are best explained by the involvement of its plasma membrane receptors whose signaling mirrors that of G protein coupled receptors both in mechanism and temporal profile. The importance of synthesis of estrogens by the CNS as well as its rapid degradation, necessitated by the utilization of rapid membrane ER signaling will also be covered.
2. Sexually dimorphic kappa-opioid receptor (KOR)-mediated antinociception
Perhaps the most poignant example of the influence of sex on opioid antinociception in humans is the demonstration of antithetical antinociceptive/nociceptive responsiveness of females vs. males to KOR agonists-antagonists. In these studies, which made use of a clinically relevant pain model, postoperative pain resulting from the extraction of third molar teeth, butorphanol and nalbuphine were shown to have greater analgesic efficacy in women vs. men [74]. The absence of any observable sexual dimorphism in antinociception elicited by placebo administration [75] indicated that sex-dependent differential analgesic responses to mixed KOR agonist-antagonists most likely did not result from female/male differences in psychosocial parameters.
Strikingly, sexually dimorphic differences in antinociceptive responsiveness to drugs such as butorphanol and nalbuphine were not only quantitative but qualitative as well; doses of each that resulted in antinociception in women were pronociceptive in men [75]. This suggests that mixed KOR agonist-antagonists possess antinociceptive as well as pronociceptive capabilities, the proportions of which are sex dependent. This supposition is supported by the demonstration that concomitant administration of low doses of the opioid receptor antagonist naloxone with either nalbuphine or butorphanol not only dramatically augments their antinociceptive actions in both sexes but also eliminates sex-dependent differences thereof [76]. These studies, however, did not reveal the sex-based factors that influence the relative preponderance of nociceptive vs. antinociceptive responsiveness.
Sexually dimorphic opioid analgesia has also been documented in laboratory animals. Using multiple antinociceptive assays, male rats have been shown to be markedly more sensitive to morphine antinociception than females. This sex-dependent difference cannot be explained by male female differences in pharmacokinetics [43], blood/brain levels of morphine attained following systemic application [44], number/binding affinity of MOR [152] and MOR G protein coupling [152]. One quandary that remains poorly understood is that many aspects of sexually dimorphic opioid responsiveness in humans are opposite to that observed in laboratory animals, e.g., sensitivity to morphine.
3. Sex-dependent mechanistic underpinnings of opioid antinociception
Even when opioid antinociceptive responsiveness is sexually monomorphic, underlying mechanisms can still be sexually dimorphic. For example, the antinociception produced by intrathecal morphine, which does not significantly differ in magnitude between males and females, results from the sex-based differential recruitment of spinal analgesic components [111]. In males, spinal morphine antinociception results from the exclusive activation of spinal MOR whereas in females, spinal morphine antinociception requires the concomitant activation of spinal dynorphin (Dyn)/KOR as well as MOR [111]. These observations suggest sex-dependent organizational influences of estrogens and or progesterone that will be discussed below.
4. Ovarian sex steroids and Nociception/Antinociception
The milieu of ovarian sex steroids is thought to be a major determinant of sex-dependent nociception [21,51,92,140] and opioid antinociception [140,180]. The effects of estrogens on nociception are bimodal being both pronociceptive as well as antinociceptive. Antinociceptive actions of estrogens include: (1) KOR antinociception and gene expression are enhanced by exogenous or endogenous estradiol in female [102], (2) long term (28 days) ovariectomy of adult rats induces thermal and mechanical hyperalgesia that can be reversed by estradiol replacement [169], (3) physiological concentrations of estradiol attenuate drug-induced temporomandibular joint (TMJ) pain [71,96], (4) in a rat model of calculosis, estradiol is an effective analgesic in females but not males [6], (5) in women, the follicular phase (elevated estradiol) has been associated with higher thresholds to pressure, thermal and ischemic muscle pain than later phases [162], which is consonant with studies in rats showing enhanced uretal pain sensitivity during metestrus / diestrus vs. proestrus / estrus [77]. These findings agree with reports of a greater incidence of colics in the perimenstrual period (equivalent to metestrus and diestrus in rats) in fertile women with urinary calculosis.
In contrast, estradiol has also been demonstrated to (1) mediate enhanced sensitivity of females to capsaicin-induced acute pain, consistent with potentiation by estradiol of the capsaicin receptor-mediated current in rat dorsal root ganglion (DRG) neurons [116], (2) mediate the greater sensitivity of female vs. male mice to mechanical and thermal nociceptive stimuli, which is eliminated following ablation of the genes encoding ERs [106], and (3) increase responsiveness to colorectal distention [88], which fluctuates with stage of estrous cycle being greatest during proestrus, when levels of estrogens are near maximal [89].
The widespread and multidirectional effects of estrogens on nociception/antinociception raise the possibility that the prevalence of chronic pain syndromes in women vs. men may result from malfunctioning of steroid regulation, which alters the equilibrium between nociception and antinociception but the molecular underpinnings for such impaired modulation by estradiol remain undefined. Opposing effects of estradiol on nociception, which could be of equal magnitude at particular doses although this has not been systematically investigated, could also underlie observations suggesting that in humans, contrary to experimental animals, changes in estradiol levels do not influence pain sensitivity. For example, estradiol replacement to post-menopausal women has been reported not to ameliorate fibromyalgia [179], and there was no detectable relationship between estradiol and pain in women undergoing in vitro fertilization despite the dramatic increase in plasma estradiol in these subjects [178].
While these could indicate that sex-dependent differences in pain sensitivity observed in humans do not result from acute activational actions of gonadal steroids (see section 4.1), as they appear to in experimental animals, explanations in addition to that of concomitant equivalent opposing effects on nociception should be considered. For example, there is an emerging perspective that effects of estrogens on nociception are dependent on the modality of the nociceptive stimulus and the dermatome to which it is applied. Indeed, the influence of menstrual phase on nociception was found to be influenced by segmental site. Moreover, menstrual variations of pain threshold in skin differed from those of subcutaneous and muscle tissues [78]. Dermatomal specificity of estrogenic modulation of nociception could differ between experimental animals vs. humans. Also, observed differences in effects of estradiol on nociception between laboratory animals and humans could result from differences in accessibility of peripheral estradiol to ER receptors in the CNS, which could depend on physiological state.
4.1. Modalities of gonadal steroid action
Gonadal hormonal action can be either ‘activational’ or ‘organizational‘ [154]. Acute ‘activational’ effects of gonadal hormones can be detected via their elimination following adult gonadectomy. In contrast, ‘organizational’ consequences of gonadal hormones, which result from permanent effects of hormone action during critical periods of gestation or the neonatal period, should not be affected by adult gonadal ablation. The increased sensitivity of male vs. female rats to the antinociceptive properties of morphine most likely results from organizational effects of sex steroids during critical developmental periods since castration in adult is without effect on observed sex-related differences in morphine antinociceptive efficacy [43] while gonadal ablation during the neonatal period abolished it [45,97]. Similarly, the female-specific dependence of intrathecal morphine antinociception on spinal Dyn/KOR, in addition to MOR [111], is insensitive to adult orchiectomy or ovariectomy but this component can be abolished by neonatal androgenization [111]. This could suggest, at least in part, the importance of organizational gonadal effects to sexually dimorphic mechanisms of spinal morphine antinociception. However, activational actions of sex hormones have also shown to be relevant to sex differences in nociceptive responsiveness. For example, female rats have more nociceptive responses than do males in the formalin paw withdrawal test, but such differences are not observed between gonadectomized females and males. This suggests that activational but not organizational effects of sex hormones are responsible for female vs. male differences in formalin responsiveness [73].
4.2. Anatomical correlates of estrogen effects on nociception/antinociception
There are both anatomical as well as biochemical bases for the observed ability of estradiol to modulate nociception and opioid antinociception. The α isoform of the ER is present in laminae I, II, VI and VII [9,172,199]. ERα colocalizes with enkephalin in many neurons of the superficial lamina of the spinal dorsal horn [9], consistent with the ability of estradiol to regulate synthesis and secretion of methionine-enkephalin [8,115,164]. ERα is also co-expressed by Dyn-ergic neurons in the dorsal horn of lumbar spinal cord, the numbers of which, in L6 and S1, significantly increase in the presence of pregnancy levels of estrogens and progesterone (hormone simulated pregnancy) [80]. Cells expressing the β ER isoform have also been identified in lamina II of the dorsal horn [172]. In addition to their localization in spinal cord, ERs are also present in dorsal root ganglia [177,183], which contain the cell bodies of primary afferent somatosensory and viscerosensory neurons that receive nociceptive, mechanical and proprioceptive inputs. Consonant with their presence in dorsal root ganglia, estradiol regulates ATP (believed to be a nociceptive transmitter) stimulation of P2X receptors and the resultant activation of L-type voltage gated calcium channels [35].
Numerous supraspinal areas of the central nervous system known to be involved in nociception also contain ERα and or ERβ. These include periaqueductal gray, parabrachial nuclei, and raphe nuclei, hypothalamus, limbic system, and several cortical areas [20,172–174,192]. Thus, putative modulation by estradiol of nociception and antinociception are likely to occur via effects on multiple anatomical levels.
5. Biochemical bases for effects of estrogens on nociception/antinociception
ER activation can also result in modification of signaling cascades known to mediate opioid receptor signaling. For example, ER-coupled actions can result in activation of protein kinase A [11,81,137,142], protein kinase C [156], mitogen-activated protein kinase (MAPK) [204], extracellular signal-regulated kinase 1/2 (ERK1/2) [171] and Akt proteins [193]. All of these signaling molecules are relevant to opioid receptor-mediated signaling. Interestingly, many if not all of these estradiol effects on downstream signaling events are mediated via non-classical ERs located in the plasma membrane (discussed below).
In addition to influencing known downstream components of G protein coupled signaling, ER initiated events are known to modulate opioid receptors themselves. MOR labeling in the ventrolateral preoptic area is significantly reduced following ovariectomy, which is reversed by estradiol replacement [90]. Analogously, estradiol (via ERα) [133] induces the internalization of membrane MOR in cell groups of the limbic system and hypothalamus (medial preoptic nucleus, the principal part of the bed nucleus, and the posterodorsal medial amygdala) [59]. Conversely, estradiol levels have also been shown to be causally associated with the positive regulation of the availability of MORs on GABAergic interneurons in the dentate gyrus [188]. Treatment with estradiol, alone or with progesterone, has also been shown to significantly increase agonist-stimulated [(35)S]-GTPgammaS binding (a measure of MOR G protein coupling) in the medial preoptic area as well as the caudate putamen [3].
Estradiol (in the presence of progesterone) can also qualitatively alter the functionality of opioid receptors as well as the relationship among them. For example, exogenous activation of the spinal delta opioid receptor (DOR) inhibits evoked Dyn release from control lumbar spinal cord [82]. However, the same DOR agonist enhances evoked Dyn release from lumbar spinal cord obtained from animals exposed to pregnancy levels of ovarian sex steroids [82]. This could explain, in part, why the antinociception associated with physiological gestation [79] or its hormonal simulation [53] is not only mediated by KOR and DOR [54,56] but requires their concomitant activation; blockade of either KOR or DOR, individually, abolishes the antinociception of both conditions [55]
6. Regulation by estradiol of interactions among opioid receptors
Surprisingly, treatment of orchiectomized male rats with pregnancy levels of ovarian sex steroids also produces spinally mediated opioid antinociception, the temporal profile and magnitude of which is indistinguishable from that observed in females [108]. But this antinociception results from the additive, not synergistic, contributions of spinal opioid systems [108]. In males, the antinociception resulting from ovarian steroid treatment results from the independent, parallel contributions of spinal KOR and MOR; the individual blockade of either KOR or MOR results in an incremental reduction in the overall steroid-induced antinociception whereas in females the individual blockade of KOR or DOR abolishes the entirety of the steroid-induced antinociception (Figure 1, top panel). Thus, in females, but not males, ovarian sex steroids reveal a propensity for spinal KOR to functionally partner with another type of opioid receptor. These observations not only underscore the ability of ovarian sex steroids to activate spinal opioid antinociceptive processes but also demonstrate that the effects of ovarian sex steroids on opioid antinociception are state or context dependent. Reports that treatment of gonadectomized males and females with pregnancy levels of sex hormones produce comparable opioid antinociception (albeit via different mechanisms) [55,108] resonate with analogous studies of effects of sex hormone replacement on formalin responsiveness [7,73]. These studies revealed the potential for gonadal hormone replacement in gonadectomized males and females to elicit similar effects even if the neurobiological substrates are sex-specific.
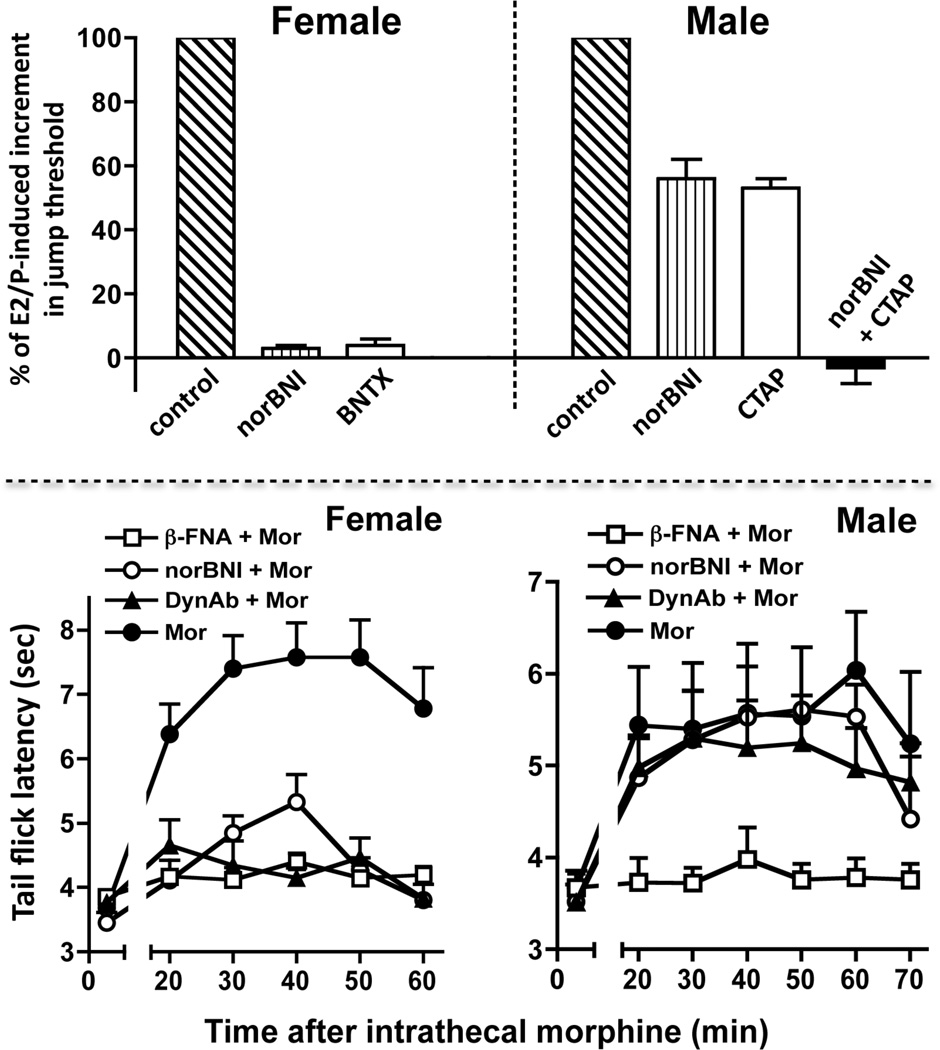
Figure 1. Interactive vs. parallel and additive contributions of spinal opioid receptors to opioid antinociception elicited by estradiol / progesterone (E2/P) in females vs. males.
Top panel: blockade of individual types of spinal opioid receptor [KOR or DOR (but not MOR, not shown)] abolished E2/P-induced antinociception in females indicating the interdependence of these types of opioid receptor [55]. In contrast, the individual blockade of spinal opioid receptors [KOR or MOR (but not DOR, not shown)] in E2/P-treated males only partially blocked the antinociception. Moreover, the decrement in antinociception produced by individually blocking KOR and MOR was additive indicating that in males, these opioid receptors functioned separately, in parallel [108]. Bottom panel illustrates that in females, the individual blockade of either MOR or KOR abolished the spinal morphine antinociception indicating that spinal KOR and MOR interdependently mediate this antinociception. In contrast, in males, intrathecal morphine produces antinociception by acting exclusively via MOR. This indicates the propensity of MOR and KOR to functionally interact in females, but not males. BNTX (7-Benzylidenenaltrexone; DOR-selective blocker); CTAP (Phe-Cys-Tyr-Trp-Arg-Thr-Pen-Thr-NH2; MOR-selective blocker).
6.1. Mechanistic underpinnings of spinal morphine antinociception in females vs. males
The sexually dimorphic propensity for spinal KOR to function in a cooperative fashion with another type of opioid receptor is also revealed by the spinal mechanisms recruited by morphine to produce antinociception in females vs. males. In both females and males, blockade of spinal MOR obliterates spinal morphine antinociception [111], as expected. Strikingly, however, in males, neither the blockade of spinal KOR [via intrathecal nor-Binaltorphimine (nor-BNI)] nor the neutralization of spinal Dyn (via intrathecal anti-Dyn antibodies) influences antinociceptive responses to intrathecal morphine. But, in females, the individual intrathecal application of either nor-BNI or anti-Dyn antibodies obliterates intrathecal morphine antinociception [111] (Figure 1, bottom panel). These results suggest that ovarian sex steroids can regulate cooperative functional interactions between KOR and MOR but they do not reveal the relevant loci of action of ovarian sex steroids (e.g., KOR, MOR or down stream signaling events, etc.) or the mechanism by which the sex steroids act.
6.2. Regulation by estrogens of KOR MOR heterodimerization
The discovery that sexually dimorphic spinal mechanisms involving the female-specific partnering of KOR and MOR mediated the equi-analgesic effects of spinal morphine in females vs. males foreshadowed the discovery that ovarian sex steroids directly influenced direct interactions between KOR and MOR [37]. In addition to existing as monomers, KOR and MOR can also exist as heterodimers (MOR/KOR). Expression levels in spinal cord of MOR/KOR heterodimers are strikingly sexually dimorphic, being approximately 4-fold higher in spinal cord of females vs. males. The spinal cord content of MOR/KOR heterodimers also varied to a similar extent between stages of the estrus cycle; spinal MOR/KOR expression levels are 4-fold higher during proestrus vs. diestrus animals suggesting its regulation by estrogens and/or progesterone [37].
7. Genomic effects of estrogens on opioid antinociception
Classically, the ER was considered to be a ligand-activated transcription factor [47,125,151]. This was based on the nuclear localization of ERs [95,198], its binding to estrogen response elements on DNA [148] and the dependence of the actions of estrogens on gene expression and protein synthesis [158]. Activation of gene expression and de novo protein synthesis by estrogens is quite complex, involving the participation of multiple co-activators and additional transcription factors [112,189,200]. This can account for a considerable diversity of effects attributed to estrogens. Genomic effects of estrogens on antinociception include alterations in expression of the gene encoding spinal cord enkephalin [8], the differential contribution of phosphorylation signaling cascades to evoked hyperalgesia in males vs. females [57], activation of descending noradrenergic transmission and synergistic interactions between spinal KOR and DOR (in females) [110] vs. additive interactions between DOR and MOR (in males) [108].
The physiological manifestation of transcriptional effects of estrogens require multiple processes (translation, post translational processing, protein trafficking, etc.), the aggregate of which requires hours to days before effects can be realized. This is dichotomous with temporal requirements for modulation by estrogens of nociception/antinociception via acute dynamic regulation of neuronal excitability or equilibria among signaling molecules. Consequently, exclusive mediation by genomic effects of estrogens of its ability to modulate nociception/antinociception would severely limit the physiological role played by estrogens in gating pain.
8. Plasma membrane ERs and nociception/antinociception
The more recently discovery that estrogens exert effects by acting at ERs located in the plasma membrane (as well as nucleus ERs) [26,157,205], the effects of which are manifest within seconds to min, instead of hours/days, enormously broadens the physiological functions that could be modulated by estrogens. This mode of action was foreshowed by the report that within seconds of application of estradiol alters excitability of neurons in the preoptic area and septum [93] as well as levels of second messenger, e.g., cAMP, in uterine tissue [181].
Knockout of ERα or ERβ, the two predominant types of nuclear ER, results in the elimination of rapid effects of estradiol on intracellular signaling in mouse brain [2]. Moreover, transfection of Chinese Hamster Ovary cells with a single cDNA encoding ERα or ERβ results in the presence of a single transcript and expression of the corresponding ER in membrane as well as nuclear compartments. Notably, the dissociation constants of membrane and nuclear ERα and ERβ are virtually identical [159]. These results indicate that nuclear ERα and ERβ also traffic to the plasma membrane (subsequent to being palmitoylated) [105], where they mediate rapid estradiol-initiated signaling. Additionally, plasma membrane ERs are also thought to include ERX [69,160,186] and an orphan G protein coupled receptor (G-protein coupled ER1, GPER, aka GPR30), which unlike ERα and ERβ, is a G protein-coupled seven membrane-spanning receptor [27,33,68,160,182,184].
Plasma membrane ERs, like G protein coupled receptors, localize to membrane micro-signaling domains known as caveolae, where caveolin-1 serves as a scaffold protein that facilitates association of signaling molecules into macromolecular signaling complexes within the caveolae domains. In fact, caveolins are essential for many membrane ERα responses [117], e.g., functional coupling of ERα to mGluR1 [28]. Stimulation of plasma membrane ERs is coupled to the activation of the same spectrum of signaling molecules that participate in most membrane initiated signaling cascades, e.g., protein kinase A, protein kinase B, protein kinase C, phospholipase C, inositol trisphosphate, MAPK, ERK, tyrosine kinases, etc. [10,23,32,81,107,122,130,136,138,144,156,159,175,181,187,197,206]. Estrogens can also modulate neuronal membrane excitability by gating calcium currents [25,35,87,103,131,141,160].
The panoply of signaling options available to membrane ERs would enable rapid membrane ER signaling to produce a myriad of nuanced effects on nociception and antinociception. Importantly, the temporal profile of physiological responses to non-genomic ER signaling (sec to min) is consistent with the varied temporal profile of the above biochemical mechanisms. For example, modulation of intracellular calcium concentrations by estradiol has latencies of sec [25,34,141,160] up to min [35,87]; the time course of estradiol-induced protein phosphorylation and kinase activation occurs within 5–30 min [23,87,118,134,197].
8.1. Plasma membrane ERs and pain regulation
ERs localized to the plasma membrane are present in small-diameter DRG neurons [183], suggesting a role in modulating nociception. Consistent with this inference, calcium currents induced by ATP, believed to be a nociceptive transmitter, are attenuated within minutes of estradiol application (via inhibition of L-type voltage gated calcium channels). Estradiol conjugated to serum albumin, which is membrane impermeable, also attenuates ATP-induced calcium currents. The ERα/ERβ blocker ICI 182780 eliminates the effects of both free as well as conjugated estradiol on ATP-induced calcium currents [35,36,183]. These aggregate characteristics are strongly indicative of estradiol modulation of DRG excitability being mediated via rapid membrane ER signaling.
TMJ pain, induced via formalin injection, can also be reduced by the application of estradiol or estradiol conjugated with bovine serum albumin into the ipsilateral TMJ (of females, but not males). ICI 182780 as well as acute inhibition of either nitric oxide synthase or guanylate cyclase eliminates antinociceptive effects of estradiol applied directly to the TMJ [67]. This confirms mediation of estradiol antinociceptive effects via a non-genomic membrane-generated second messenger mechanism.
Rapid, non-genomic effects of estrogens also influence the sexually dimorphic mechanistic underpinnings of nociception by sensory neurons. Estrogens can act directly on nociceptive neurons to influence the participation of PKCε in signaling pathways mediating mechanical hyperalgesia [86], i.e., in DRG cultures obtained from males, estradiol rapidly (onset within 1 min) eliminates translocation and activation of PKCε in response to β-adrenergic receptor activation [86]. Interestingly, this effect is mediated via activation of GPR30 [98], the non-classical ER that is an integral membrane G protein coupled receptor [27,33,68,160,182,184], which is present in a IB4-positive subset of sensory neurons [86].
Effects of GPR30 activation on PKCε activity in nociceptors and corresponding mechanical hyperalgesia can be both pronociceptive or antinociceptive. Activation of GPR30 inhibits PKCε translocation/activation and the onset of mechanical hyperalgesia when either estradiol or the GPR30-selective agonist G-1 is given subsequent to isoproterenol or an activator of down stream signaling events. However, GPR30 activation also stimulates PKCε and hyperalgesia when it is administered alone. These observations emphasize the state dependence of effects on nociception of rapid ER signaling. Furthermore, unlike ERα and ERβ, GPR30, does not function as a nuclear transcription factor, underscoring the putative relevance of rapid signaling by membrane ERs to gating pain experience. The antithetical effects of activating an ER on nociception provides a rubric for beginning to understand the myriad of opposing effects of ovarian sex steroids on pain processing that have thus far hindered full acceptance of the importance of sex in pain management and investigations thereof.
8.2. Relevance of plasma membrane ERs to heterodimerization of KOR with MOR and spinal opioid antinociception
An exemplar of the importance of rapid membrane ER signaling to opioid regulation of pain is its ability to modulate the equilibrium between monomeric KOR and KOR heterodimerized with MOR across the estrus cycle. Concomitant blockade of spinal ERα/ERβ (using ICI 182,780) substantially reduces lumbar spinal cord levels of MOR/KOR heterodimers during proestrus [109], when heterodimer levels are at their maximum. Analogous effects on spinal MOR/KOR heterodimers were observed following the individual blockade of either ERα, (using MPP), or ERβ (using PHTPP) or GPR30 (using G-15). Notably, in all cases, reductions in MOR/KOR heterodimerization could be observed within 30 min of spinal ER blockade [109].
Rapid membrane ER signaling is also essential for the mechanisms utilized by intrathecal morphine to produce antinociception in females vs. males. In both proestrus and diestrus females, spinal morphine produces a robust antinociception but only during proestrous is the morphine-induced antinociception dependent on Dyn and KOR as well as on MOR, i.e., during proestrus, intrathecal morphine antinociception is abolished by intrathecal nor-BNI (KOR-selective antagonist) and anti-Dyn antibodies as well as by β-funaltrexamine (β-FNA; MOR-selective antagonist) [111]. However, within 30 min of blocking spinal ERα + ERβ, or the individual blockade of either ERα, ERβ or GPR30, antinociceptive responses to spinal morphine are insensitive to KOR blockade; following acute blockade of spinal membrane ERs, spinal morphine antinociception was no longer dependent on KOR [109] but instead manifested the phenotypic response characteristic of diestrus females and males to intrathecal morphine [111]. The ability of acute blockade of spinal membrane ERs during proestrus to alter mechanistic underpinnings of spinal morphine antinociception not only underscores the fluidity of the antinociceptive signaling components recruited by intrathecal morphine but also the importance of rapid membrane ER signaling to their selection.
9. Integration of the effects resulting from rapid membrane ER and genomic ER signaling
It is important to realize that genomic and plasma membrane actions of estrogens are not mutually exclusive. There is a complex interaction between the functional consequences of activating ERs in different subcellular compartments that can be modulated by cell context-specific environments. This enables the fine-tuning of estradiol function. For example, one consequence of rapid membrane initiated ER signaling is the enhancement of genomic effects of estradiol function; activation of downstream kinases, such as ERK, PI3K, by estrogens acting via membrane ERα can result in the phosphorylation of nuclear ERα, which promotes its transcriptional effects [24]. Additionally, rapid membrane ER signaling often results in the phosphorylation and consequent activation of the transcription factor cAMP response element binding protein (CREB) [29,194], which in turn regulates gene expression through interaction with DNA at CREB response elements. The close interface between the signaling of plasma membrane and nuclear ERs enables the modulation of ER-coupled nuclear transcription by endogenous estrogens that depend on the particular signaling pathway(s) that they activate.
In addition to the cellular integration of effects resulting from activating membrane and nuclear ERs, respective effects can also be integrated, even when they occur in separate cells. For example, genomic actions of estradiol are presumably responsible for the increase in spinal cord content of Dyn during late gestation and its hormonal simulation [127,129]. KOR/MOR is activated by Dyn and is also expected to be elevated in spinal cord during late gestation, but as a consequence of rapid membrane ER signaling (as was demonstrated during proestrus) [109], not genomic actions of estradiol. We envision that Dyn acting via heterodimerized KOR and MOR results in augmented antinociception vs. that produced when activating monomeric KOR [109]. In other words, genomic actions of estradiol during physiological pregnancy are responsible for elevated spinal Dyn, which in turn could act via the elevated KOR/MOR that results from rapid membrane ER signaling.
10. Membrane ERs are co-expressed and act cooperatively to regulate MOR/KOR
The effects of doses of spinal ER type-selective antagonists that produced submaximal reductions in spinal MOR/KOR heterodimerization or in the KOR component of spinal morphine antinociception are not additive. Instead, reductions in either the content of MOR/KOR or the KOR component of spinal morphine antinociception produced by the individual submaximal antagonism of ERα, ERβ, or GPR30 are indistinguishable from that produced by the concomitantly blocking combinations of ERs (ERα + GPR30 and ERα + ERβ) [109]. This functional interrelatedness of membrane ERs indicates that they function cooperatively as part of a macromolecular signaling complex to regulate spinal MOR/KOR formation and phenotypic responsiveness to intrathecal morphine.
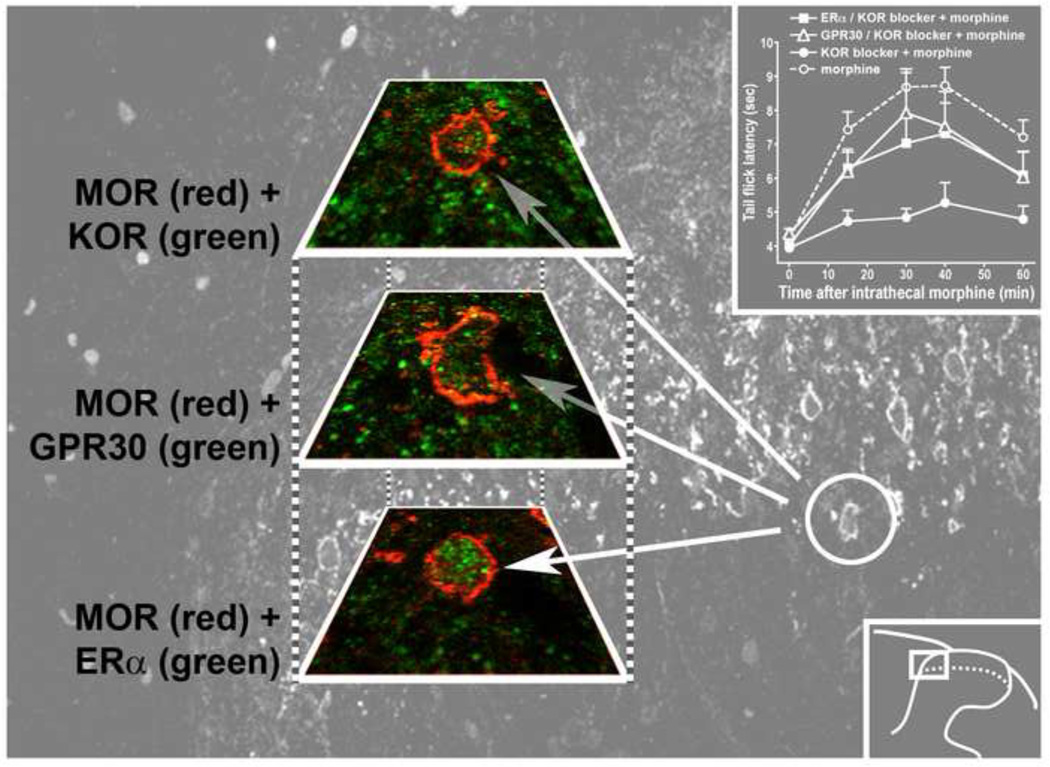
The tenability of the formulation that regulation of spinal KOR MOR heterodimerization by rapid spinal membrane ER signaling is physiologically relevant requires that participating membrane ERs as well as KOR and MOR are expressed in the same neurons. This prerequisite was satisfied using double labeling immunohistochemical analysis of tissue obtained from the L5 and L6 segments of proestrus rats [109]. In the superficial dorsal horn, KOR and MOR, MOR and ERα, and MOR and GPR30 are frequently co-expressed. Importantly, ERα could be visualized in the cytoplasm and membrane as well as the nucleus and GPR30 could be visualized in cytoplasm and in or near the plasma membrane. Moreover, by serially examining 5 µm adjacent sections, it was possible to demonstrate single cells expressing MOR, KOR, ERα, and GPR30. This provides a structural foundation for the regulation by multiple spinal membrane ERs of KOR MOR heterodimerization [109] (see figure 2).
Figure 2. An individual spinal neuron expresses a multiplicity of membrane ERs together with MOR and KOR.
The red and green images show co-expression of MOR with KOR, G protein coupled estrogen receptor (GPR30) and ERα in three serial 5 µm sections of a single cell in spinal cord. The gray background image shows the cell location; the inset on the bottom right shows the location of the background within the dorsal horn. The inset in the upper right illustrates that blockade of spinal ERα or GPR30 during proestrus rapidly relieves the dependence of intrathecal morphine antinociception on KOR, i.e., 30 min following spinal membrane ER blockade, intrathecal morphine antinociception no longer requires KOR, paralleling the loss of KOR MOR heterodimers (not shown). The coexpression of ERs, MOR and KOR enables the formation of KOR MOR heterodimers that is synchronized with the estrus cycle and mediates the estradiol-dependent KOR component of spinal morphine antinociception. Image was developed in collaboration with Martin Wessendorf, University of Minnesota, Minneapolis, MN as reported in [109].
11. Spinal membrane ERs and sexually dimorphic nociceptive/antinociceptive effects of Dyn/KOR
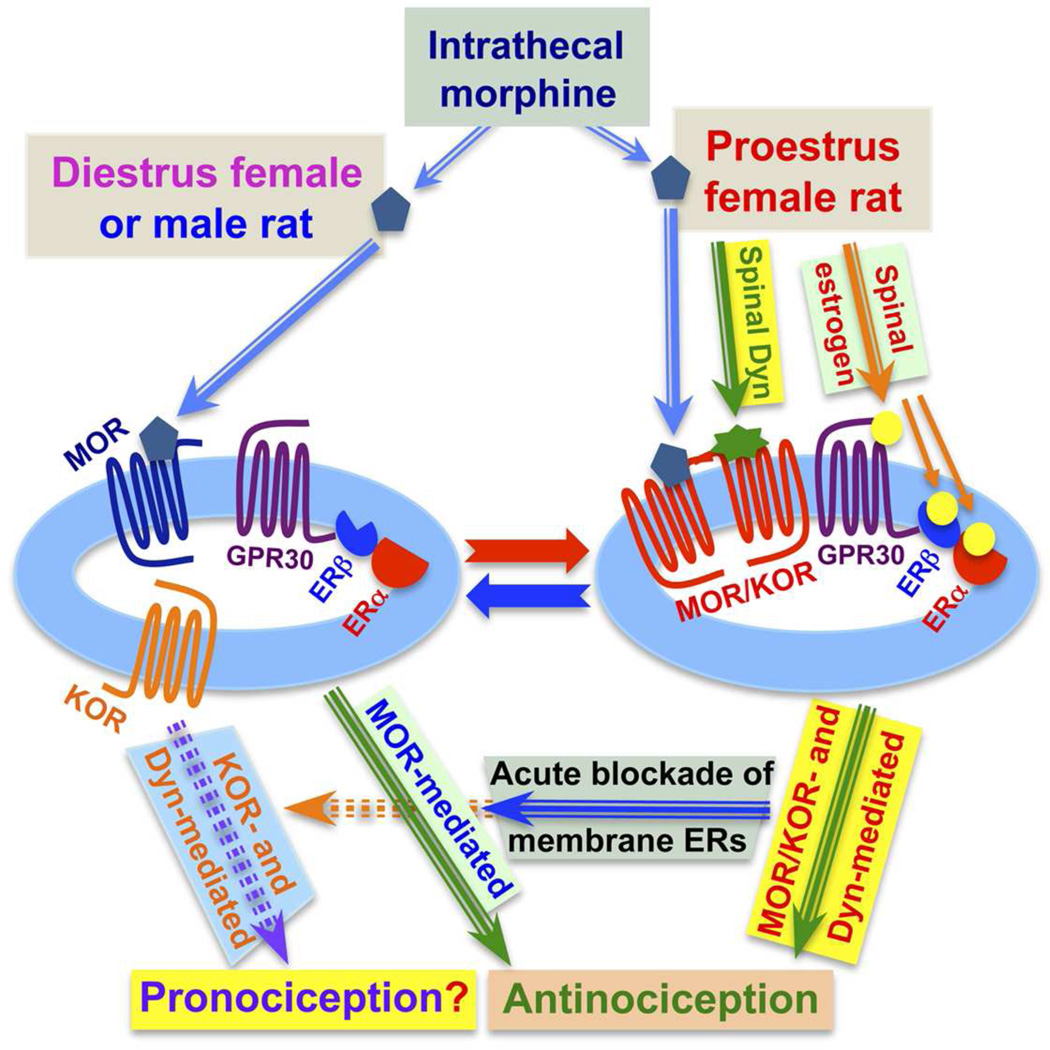
Regulation by rapid plasma membrane ER signaling of the equilibrium between monomeric KOR and KOR heterodimerized with MOR enables modulation of nociception by utilizing the bimodal functionality inherent in Dyn/KOR signaling. Dyn has long been considered to be an endogenous KOR substrate [38–40,114]. The actions of Dyn are very complex, if not contradictory (see [99] for overview). It is now well established that Dyn is pronociceptive [101,113,191] as well as antinociceptive [53,55,58,79,91,127–129,150,201], but the molecular determinants of this antithetical functionality remains obscure. It is relevant to note that although intrathecal injection of anti-Dyn antiserum blocks the increased sensitivity to noxious thermal and innocuous mechanical stimuli that commonly accompanies injury to spinal nerves, the same spinal treatment does not alter sensory thresholds in non-injured animals [119,149,196]. This suggests that pronociceptive actions of spinal Dyn are ‘state-dependent’, the molecular determinants of which are not known. We hypothesize, based on the greater expression levels of MOR/KOR in spinal cord of females vs. males, that monomeric KOR mediates nociception whereas MOR/KOR heterodimers mediate antinociception. In this formulation the equilibrium between monomeric KOR and KOR heterodimerized with MOR is a major determinant of pronociceptive vs. antinociceptive actions of KOR.
This formulation implies that, by regulating the formation of MOR/KOR, rapid signaling by membrane ERs influences the nociceptive/antinociceptive functionality of endogenous Dyn. Interference with or a malfunctioning of the ER-coupled regulation of the interaction of KOR with MOR would favor pronociceptive functions of endogenous Dyn/KOR and, therefore, result in a state of heightened nociception. The contribution of deregulated spinal membrane ERs to the pathophysiology underlying the myriad of chronic pain syndromes that are vastly more prevalent in women vs. men remains to be determined (see figure 3 for summary of the influence of the equilibrium between heterodimeric MOR/KOR and monomeric KOR on KOR-mediated nociception vs. antinociception).
Figure 3. Rapid signaling via membrane estrogen receptors influences the nature of the antinociception produced by spinal morphine as well as pronociceptive vs. antinociceptive properties of spinal Dyn.
In males and females, intrathecal morphine produces equivalent antinociception, which is abolished following blockade of spinal MOR [111]. However, in proestrus females (high levels of estradiol), but not diestrus females (low levels of estradiol) or males, blockade of either spinal KOR (via nor-BNI) or neutralization of Dyn (via the intrathecal application of anti-Dyn antibodies) also abolishes the antinociception produced by spinal morphine [109]. Abolition of spinal morphine antinociception by intrathecal anti-Dyn antibodies suggests that during proestrus, morphine acts, in part, by releasing spinal Dyn. The Dyn/KOR component of spinal morphine antinociception is mediated by a newly formed complex containing KOR that is heterodimerized with MOR (MOR/KOR) [37], the formation of which is vastly greater in the spinal cord of proestrus vs. diestrus or male animals [37]. Regulation of the heterodimerization of KOR and MOR requires rapid signaling of a complex of spinal plasma membrane ERs consisting of ERα, ERβ and GPR30 [109]. We propose that the equilibrium between monomeric KOR, hypothesized to mediate pronociception, and KOR heterodimerized with MOR, hypothesized to mediate antinociception, shifts the net effect of endogenous Dyn functionality as well as responsiveness to exogenous Dyn from pronociception to antinociception. In this fashion, rapid signaling via spinal cord plasma membrane ERs can dynamically regulate pain processing.
The regulation of KOR MOR heterodimerization in spinal cord by membrane ERs would explain the much earlier paradoxical findings that butorphanol and nalbuphine (mixed MOR and KOR opioid receptor ligands) are antinociceptive in women whereas in men they produce nociception [74,76]. In women, the equilibrium between monomeric pronociceptive KOR and antinociceptive heterodimeric MOR/KOR would favor the latter, enabling compounds such as butorphanol and nalbuphine to activate KOR within the heterodimeric MOR/KOR. This provides a mechanism for recruiting spinal KOR-mediated antinociception without activating the concomitant pronociceptive functions that monomeric KOR subserves. In contrast, in men, the equilibrium between monomeric pronociceptive KOR and antinociceptive heterodimeric MOR/KOR would favor the former making it the primary target for butorphanol and nalbuphine and thus the predominance of their nociceptive actions. It was recently demonstrated that estradiol could substantially influence the ability of spinal KOR activation to attenuate acute inflammatory pain in female rats [102]. However, the experimental design of that study assumed predominantly transcriptional actions of estradiol; effects of estradiol via rapid membrane ER signaling on spinal KOR-mediated amelioration of inflammatory pain would have gone undetected. Thus, the influence of rapid spinal ER signaling on KOR antinociception could have greater applicability than has thus far been demonstrated.
12. Aromatase and nociception/antinociception
The data mentioned above regarding the ability of rapid membrane ER signaling to modulate nociception/antinociception constitutes proof of principle and defines a specific physiological state (proestrus) in which rapid membrane ER signaling is of particular relevance to antinociception. A more generalized relevance to nociception of rapid membrane ER signaling requires their access to dynamically regulated nuanced levels of estrogens that fluctuate within a time frame comparable to that of membrane ER signaling (sec/min). This requirement cannot be met by circulating ovarian derived estrogens, which has a temporal profile of change of hours/days. In rats, the increment in plasma estradiol concentrations during proestrus occurs over several hours, peaking in approximately 12 h [4,30,176] with ovulation and sexual receptivity occurring in the ensuing 24 h [124]. While this temporal profile of change in plasma estrogens is compatible with the activation of transcription, e.g., of hypothalamic progesterone receptors and events required for mating [120,124], it is incongruent with the time frame of the dynamics of non-genomic membrane effects of estrogens. Utilization of the full capability of rapid signaling via membrane ERs in the CNS during normal physiology requires a source of estrogens that is intrinsic to it, the synthesis and degradation of which can be rapidly regulated. This criterion is met by the ability of the CNS to not only rapidly synthesize estrogens in a highly regulated fashion, but to also rapidly eliminate it.
12.1. Distribution of aromatase in the CNS
The enzyme aromatase (estrogen-synthase), which catalyzes the conversion of C19 androgens, such as testosterone, to estradiol, is present in cells scattered throughout the brain and spinal cord. In brain, aromatase is present in hypothalamus and limbic systems [18,31,165,166]. Spinal cord of both female and male quail (confirmed in rodents), contain aromatase-immunoreactive somata and fibers in the spinal dorsal horn from the upper cervical segment to the lower caudal area, predominantly in laminae I and II. [61–63,145]. Direct quantification of aromatase activity, assessed via tritiated water released from the conversion of tritiated androgens into estrogens, confirm that the substantial levels of immunoreactive aromatase protein that is present throughout the spinal cord is enzymatically active.
The cellular distribution of aromatase is compatible with its synaptic regulation. Aromatase is present in neuronal dendrites and axons, within presynaptic boutons [65,72], at the surface of synaptic vesicles [85,146,153]. Moreover, it is enriched in synaptosomal preparations [65]. All are prerequisites for the rapid production of estrogens at CNS synapses and thus the targeted activation of membrane ERs. This suggests that locally synthesized estrogens might function as a neurotransmitter/neuromodulator within the time frame of rapid membrane ER signaling with exquisite anatomical specificity and selectivity.
12.2. Regulation of CNS aromatase activity
CNS aromatase is regulated by sex steroids that can act as transcription factors to regulate aromatase expression levels [1,17,167,168,170]. But, this modality occurs on a time scale of hours to days. However, there are alternate modes of regulation, similar to those utilized to regulate many signaling enzymes, which have a time scale commensurate with that of rapid membrane ER signaling. Of particular note, activity of aromatase is dependent on its state of phosphorylation. In hypothalamic homogenates of quail, aromatase activity is rapidly (within min) down regulated under conditions in which protein phosphorylation is enhanced. This inhibition of aromatase is blocked by inhibitors of protein kinase A and C kinase [12,13]. The identification of phosphorylation consensus sequences in aromatase [83,84,126] supports the importance of phosphorylation in the rapid regulation of aromatase activity [15,16]. Aromatase activity is also rapidly (within 5 min) inhibited by K+-induced depolarization and consequent elevated intracellular calcium or by thapsigargin, which mobilizes intracellular pools of calcium [13]. Notably, inhibition of aromatase resulting from high K+ or thapsigargin is not only very rapid in onset but is fully reversible [13], suggesting physiological relevance. Additionally, there is evidence that variations in neurotransmitter activity, e.g., dopamine, glutamate, also modulate aromatase activity, presumably via effects of phosphorylation [12]. Indeed glutamatergic agonists, acting via AMPA or kainate or NMDA receptor types, rapidly and profoundly inhibit aromatase activity, which can be blocked by glutamatergic receptor antagonists. Importantly, most neuronal cells expressing aromatase are sensitive to dopamine, AMPA, kainate and NMDA [16,46]. These observations indicate the presence of mechanisms that coordinate aromatase activity with neuronal excitability.
Most studies investigating the regulation of aromatase activity have focused on the importance of phosphorylation in attenuating it. However, the converse should also be applicable. One would anticipate that rapid dephosphorylation via phosphatase would be a mechanism utilized to enhance aromatase activity. Regulation of aromatase activity by phosphorylation/dephosphorylation, one of the most commonly utilized mechanisms to regulate signal transduction in general, provides proof of principle that CNS synthesis of estrogens can be regulated in a time frame commensurate with the temporal profile of rapid membrane ER signaling and thus locally synthesized estrogens are likely to be a substrate for this type of signaling.
12.3. Relevance of CNS aromatase to nociception
Studies directly quantifying nociceptive response thresholds indicate a role for spinal cord aromatase in conjunction with rapid membrane ER signaling. Either of two structurally dissimilar inhibitors of aromatase, vorozole or 1,4,6-androstatriene-3,17-dione, increases foot withdrawal latency within one min following their intrathecal application [64]. Additionally, primary afferent transmitters such as glutamate (via NMDA or kainate receptor subtypes) or substance P, both of which are released in response to nociceptive stimuli, can reversibly depress aromatase activity [13,14,66]. Importantly, aromatase neurons in the spinal cord colocalize with neurokinin 1 receptors and are in close apposition with substance P-immunoreactive fibers suggesting a physiological role for interactions between afferent transmitters and spinal cord aromatase.
12.4. Relevance of CNS aromatase to female phenotypic responsiveness to morphine
Inhibition of spinal cord aromatase via the intrathecal application of fadrozole had the same effect on phenotypic responsiveness to spinal morphine as did blockade of spinal membrane ERs (see above) [109]. Within 1 h of its spinal application, intrathecal fadrozole eliminated the dependence of spinal morphine antinociception on KOR in proestrus rats [109]. These observations do not preclude the importance of circulating estrogens to the proestrus phenotypic responsiveness to spinal morphine since the cumulative activation of spinal membrane ERs could, at least in part, be graded, paralleling the increment in peripheral levels of estrogens. This notwithstanding, the ability of fadrozole to eliminate the KOR component of intrathecal morphine antinociception strongly underscores that locally synthesized estrogens by spinal aromatase is a major determinant of the antinociceptive mechanisms utilized by spinal morphine.
The prevalence of sexual dimorphism in nociception and antinociception is incontrovertible. Also incontrovertible is the capacity of estrogens to modulate numerous functional components of nociception and antinociception. The growing awareness and acceptance of rapid membrane-initiated signaling by ERs that utilize caveolin-associated signaling microdomains adds a new conceptual framework within which to formulate possible mechanisms by which estrogens can influence nociception. A critical constraint in the putative physiological relevance of regulation of pain and its amelioration by membrane ERs is their immediate access to dynamically regulated levels of estrogens. This makes rapid and pliable regulation of CNS aromatase, for which there is growing precedence, yet another critical component of pain regulation.
13. Rapid catabolism of estrogens
The physiological importance of rapid membrane ER signaling requires the ability to rapidly degrade estrogens in addition to the ability to acutely regulate aromatase activity. In this regard, it should be noted that the CNS contains high levels of two enzymes that can degrade estrogens. The preoptic-hypothalamic region contains 2- and 4-hydroxylases that convert estrogens to 2- and 4-hydroxyestrogens [19,185,207], which are subsequently rapidly metabolized by catecholamine-O-methyltransferases (COMT) into methoxyestrogens that have greatly diminished estrogenic activity [121]. Brain also contains appreciable levels of glucuronidase and sulfotransferase activities [5,135,155], which conjugate and inactivate estrogens. Thus, by dynamically regulating the equilibrium between phosphorylated and dephosphorylated aromatase and the presence of enzymes that inactivate estrogens, the availability of locally synthesized estrogens in the CNS can be tightly controlled with exquisite temporal resolution compatible with the dynamic effects produced by membrane ERs on nociception and antinociception.
14. Conclusion
This new-found complexity and sexual dimorphism thereof could provide solid ground for understanding the sex divide in the experience of pain and its treatment, the growing examples of which outpace our comprehension. This newly appreciated complexity could also provide a starting point for interpreting the spectrum of contradictory findings that pervade the sex/pain literature. The importance of estrogens in pain modulation can only be fully comprehended within the context of the male female dichotomy in nociception and opioid antinociception, a systematic investigation of which is mandated not only because of social considerations but also because of the translational opportunities is promises.
Rapid membrane estrogen receptor signaling influences pain regulation.
Membrane estrogen receptors modulate μ- and κ-opioid receptor heterodimerization.
Membrane estrogen receptors regulate pro- vs. anti-nociceptive functions of dynorphin
Spinal estrogen via its membrane receptor regulates dynorphin effects on nociception.
Membrane estrogen receptor activity influences sex difference in pain process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE. Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology. 1994;135:395–401. doi: 10.1210/endo.135.1.8013375. [DOI] [PubMed] [Google Scholar]
- 2.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 3.Acosta-Martinez M, Etgen AM. Estrogen modulation of mu-opioid receptor-stimulated [35S]-GTP-gamma-S binding in female rat brain visualized by in vitro autoradiography. Neuroendocrinology. 2002;76:235–242. doi: 10.1159/000065953. [DOI] [PubMed] [Google Scholar]
- 4.Ahdieh HB, Siegel HI, Wade GN. The role of the uterus in regulation of heat duration in cycling rats. Horm Behav. 1985;19:292–303. doi: 10.1016/0018-506x(85)90028-5. [DOI] [PubMed] [Google Scholar]
- 5.Albert C, Barbier O, Vallee M, Beaudry G, Belanger A, Hum DW. Distribution of uridine diphosphate-glucuronosyltransferase (UGT) expression and activity in cynomolgus monkey tissues: evidence for differential expression of steroid-conjugating UGT enzymes in steroid target tissues. Endocrinology. 2000;141:2472–2480. doi: 10.1210/endo.141.7.7583. [DOI] [PubMed] [Google Scholar]
- 6.Aloisi AM, Affaitati G, Ceccarelli I, Fiorenzani P, Lerza R, Rossi C, Pace MC, Chiefari M, Aurilio C, Giamberardino MA. Estradiol and testosterone differently affect visceral pain-related behavioural responses in male and female rats. Eur J Pain. 14:602–607. doi: 10.1016/j.ejpain.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Aloisi AM, Ceccarelli I. Role of gonadal hormones in formalin-induced pain responses of male rats: modulation by estradiol and naloxone administration. Neuroscience. 2000;95:559–566. doi: 10.1016/s0306-4522(99)00445-5. [DOI] [PubMed] [Google Scholar]
- 8.Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 9.Amandusson A, Hermanson O, Blomqvist A. Colocalization of oestrogen receptor immunoreactivity and preproenkephalin mRNA expression to neurons in the superficial laminae of the spinal and medullary dorsal horn of rats. Eur J Neurosci. 1996;8:2440–2445. doi: 10.1111/j.1460-9568.1996.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 10.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auger AP, Meredith JM, Snyder GL, Blaustein JD. Oestradiol increases phosphorylation of a dopamine- and cyclic AMP-regulated phosphoprotein (DARPP-32) in female rat brain. J Neuroendocrinol. 2001;13:761–768. doi: 10.1046/j.1365-2826.2001.00700.x. [DOI] [PubMed] [Google Scholar]
- 12.Balthazart J, Baillien M, Ball GF. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol. 2001;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 13.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:63–73. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 14.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 15.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 16.Balthazart J, Baillien M, Charlier TD, Cornil CA, Ball GF. Multiple mechanisms control brain aromatase activity at the genomic and non-genomic level. J Steroid Biochem Mol Biol. 2003;86:367–379. doi: 10.1016/s0960-0760(03)00346-7. [DOI] [PubMed] [Google Scholar]
- 17.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J, Foidart A, Harada N. Immunocytochemical localization of aromatase in the brain. Brain Res. 1990;514:327–333. doi: 10.1016/0006-8993(90)91428-j. [DOI] [PubMed] [Google Scholar]
- 19.Balthazart J, Stoop R, Foidart A, Granneman JC, Lambert JG. Distribution and regulation of estrogen-2-hydroxylase in the quail brain. Brain Res Bull. 1994;35:339–345. doi: 10.1016/0361-9230(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 20.Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Berkley KJ. Sex differences in pain. Behav. Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 22.Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 23.Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci U S A. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 25.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 26.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonini JA, Anderson SM, Steiner DF. Molecular cloning and tissue expression of a novel orphan G protein-coupled receptor from rat lung. Biochem Biophys Res Commun. 1997;234:190–193. doi: 10.1006/bbrc.1997.6591. [DOI] [PubMed] [Google Scholar]
- 28.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 31.Callard GV, Petro Z, Ryan KJ. Phylogenetic distribution of aromatase and other androgen-converting enzymes in the central nervous system. Endocrinology. 1978;103:2283–2290. doi: 10.1210/endo-103-6-2283. [DOI] [PubMed] [Google Scholar]
- 32.Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Brain Res Mol Brain Res. 2002;107:80–88. doi: 10.1016/s0169-328x(02)00449-7. [DOI] [PubMed] [Google Scholar]
- 33.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 34.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 35.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 36.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 37.Chakrabarti S, Liu NJ, Gintzler AR. Formation of {micro}-/{kappa}-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proc Natl Acad Sci U S A. 2010;107:20115–20119. doi: 10.1073/pnas.1009923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chavkin C, Goldstein A. Demonstration of a specific ynorphin receptor in guinea pig ileum myenteric plexus. Nature (Lond) 1981;291:591–593. doi: 10.1038/291591a0. [DOI] [PubMed] [Google Scholar]
- 39.Chavkin C, Goldstein A. Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc Natl Acad Sci U S A. 1981;78:6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 41.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Influences of laboratory environment on behavior. Nat Neurosci. 2002;5:1101–1102. doi: 10.1038/nn1102-1101. [DOI] [PubMed] [Google Scholar]
- 42.Chia YY, Chow LH, Hung CC, Liu K, Ger LP, Wang PN. Gender and pain upon movement are associated with the requirements for postoperative patient-controlled iv analgesia: a prospective survey of 2,298 Chinese patients. Can J Anaesth. 2002;49:249–255. doi: 10.1007/BF03020523. [DOI] [PubMed] [Google Scholar]
- 43.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- 44.Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine's antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–944. [PubMed] [Google Scholar]
- 45.Cicero TJ, Nock B, O'Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- 46.Cornil CA, Seutin V, Motte P, Balthazart J. Electrophysiological and neurochemical characterization of neurons of the medial preoptic area in Japanese quail (Coturnix japonica) Brain Res. 2004;1029:224–240. doi: 10.1016/j.brainres.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 47.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 48.Coyle DE, Sehlhorst CS, Behbehani MM. Intact female rats are more susceptible to the development of tactile allodynia than ovariectomized female rats following partial sciatic nerve ligation (PSNL) Neuroscience Letters. 1996;203:37–40. doi: 10.1016/0304-3940(95)12259-1. [DOI] [PubMed] [Google Scholar]
- 49.Coyle DE, Sehlhorst CS, Mascari C. Female rats are more susceptible to the development of neuropathetic pain using the partial sciatic nerve ligation (PSNL) model. Neuroscience Letters. 1995;186:135–138. doi: 10.1016/0304-3940(95)11304-f. [DOI] [PubMed] [Google Scholar]
- 50.Craft RM. Sex differences in opioid analgesia: "from mouse to man". Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8:397–411. doi: 10.1016/j.ejpain.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- 53.Dawson-Basoa ME, Gintzler AR. 17-b-Estradiol and progesterone modulate an intrinsic opioid analgesic system. Brain Research. 1993;601:241–245. doi: 10.1016/0006-8993(93)91716-6. [DOI] [PubMed] [Google Scholar]
- 54.Dawson-Basoa ME, Gintzler AR. Estrogen and Progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain. 1996;64:607–615. doi: 10.1016/0304-3959(96)87175-2. [DOI] [PubMed] [Google Scholar]
- 55.Dawson-Basoa ME, Gintzler AR. Gestational and ovarian sex steroid antinociception: synergy between spinal k and d opioid systems. Brain Research. 1998;794:61–67. doi: 10.1016/s0006-8993(98)00192-9. [DOI] [PubMed] [Google Scholar]
- 56.Dawson-Basoa ME, Gintzler AR. Involvement of spinal cord d opiate receptors in the antinociception of gestation and its hormal simulation. Brain Research. 1997;757:37–42. doi: 10.1016/s0006-8993(97)00092-9. [DOI] [PubMed] [Google Scholar]
- 57.Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- 58.Draisci G, Kajander KC, Dubner R, Bennett GJ, Iadarola MJ. Up-regulation of opioid gene expression in spinal cord evoked by experimental nerve injuries and inflammation. Brain Res. 1991;560:186–192. doi: 10.1016/0006-8993(91)91231-o. [DOI] [PubMed] [Google Scholar]
- 59.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellermeier W, Westphal W. Gender diferences in pain ratings and pupil reactions to painful pressure stimuli. Pain. 1995;61:435–439. doi: 10.1016/0304-3959(94)00203-Q. [DOI] [PubMed] [Google Scholar]
- 61.Evrard H, Baillien M, Foidart A, Absil P, Harada N, Balthazart J. Localization and controls of aromatase in the quail spinal cord. J Comp Neurol. 2000;423:552–564. doi: 10.1002/1096-9861(20000807)423:4<552::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 62.Evrard HC, Balthazart J. Aromatase (estrogen synthase) activity in the dorsal horn of the spinal cord: functional implications. Ann N Y Acad Sci. 2003;1007:263–271. doi: 10.1196/annals.1286.025. [DOI] [PubMed] [Google Scholar]
- 63.Evrard HC, Balthazart J. Localization of oestrogen receptors in the sensory and motor areas of the spinal cord in Japanese quail (Coturnix japonica) J Neuroendocrinol. 2002;14:894–903. doi: 10.1046/j.1365-2826.2002.00857.x. [DOI] [PubMed] [Google Scholar]
- 64.Evrard HC, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evrard HC, Harada N, Balthazart J. Immunocytochemical localization of aromatase in sensory and integrating nuclei of the hindbrain in Japanese quail (Coturnix japonica) J Comp Neurol. 2004;473:194–212. doi: 10.1002/cne.20068. [DOI] [PubMed] [Google Scholar]
- 66.Evrard HC, Willems E, Harada N, Balthazart J. Specific innervation of aromatase neurons by substance P fibers in the dorsal horn of the spinal cord in quail. J Comp Neurol. 2003;465:309–318. doi: 10.1002/cne.10854. [DOI] [PubMed] [Google Scholar]
- 67.Favaro-Moreira NC, Torres-Chavez KE, Fischer L, Tambeli CH. Peripheral estradiol induces temporomandibular joint antinociception in rats by activating the nitric oxide/cyclic guanosine monophosphate signaling pathway. Neuroscience. 2009;164:724–732. doi: 10.1016/j.neuroscience.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 68.Feng Y, Gregor P. Cloning of a novel member of the G protein-coupled receptor family related to peptide receptors. Biochem Biophys Res Commun. 1997;231:651–654. doi: 10.1006/bbrc.1997.6161. [DOI] [PubMed] [Google Scholar]
- 69.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 70.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 71.Fischer L, Torres-Chavez KE, Clemente-Napimoga JT, Jorge D, Arsati F, de Arruda Veiga MC, Tambeli CH. The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. J Pain. 2008;9:630–638. doi: 10.1016/j.jpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 73.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- 74.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. 1996;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- 75.Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345. doi: 10.1016/s0304-3959(99)00119-0. [DOI] [PubMed] [Google Scholar]
- 76.Gear RW, Miaskowski C, Gordon NC, Paul SM, Helter PH, Levine JD. Action of Naloxone on Gender-Dependent Analgesic and Antianalgesic Effects of Nalbuphine in Humans. J. Pain. 2000;1(2):122–127. [Google Scholar]
- 77.Giamberardino MA, Affaitati G, Valente R, Iezzi S, Vecchiet L. Changes in visceral pain reactivity as a function of estrous cycle in female rats with artificial ureteral calculosis. Brain Res. 1997;774:234–238. doi: 10.1016/s0006-8993(97)81711-8. [DOI] [PubMed] [Google Scholar]
- 78.Giamberardino MA, Berkley KJ, Iezzi S, de Bigontina P, Vecchiet L. Pain threshold variations in somatic wall tissues as a function of menstrual cycle, segmental site and tissue depth in non-dysmenorrheic women, dysmenorrheic women and men. Pain. 1997;71:187–197. doi: 10.1016/s0304-3959(97)03362-9. [DOI] [PubMed] [Google Scholar]
- 79.Gintzler AR. Endorphin-mediated increases in pain threshold during pregnancy. Science. 1980;210:193–195. doi: 10.1126/science.7414330. [DOI] [PubMed] [Google Scholar]
- 80.Gintzler AR, Schnell SA, Gupta DS, Liu NJ, Wessendorf MW. Relationship of spinal dynorphin neurons to delta-opioid receptors and estrogen receptor alpha: anatomical basis for ovarian sex steroid opioid antinociception. J Pharmacol Exp Ther. 2008;326:725–731. doi: 10.1124/jpet.108.139816. [DOI] [PubMed] [Google Scholar]
- 81.Gu Q, Moss RL. 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta DS, Kelson AB, Polgar WE, Toll L, Szucs M, Gintzler AR. Ovarian sex steroid-dependent plasticity of nociceptin/orphanin FQ and opioid modulation of spinal dynorphin release. J Pharmacol Exp Ther. 2001;298:1213–1220. [PubMed] [Google Scholar]
- 83.Harada N. Cloning of a complete cDNA encoding human aromatase: immunochemical identification and sequence analysis. Biochem Biophys Res Commun. 1988;156:725–732. doi: 10.1016/s0006-291x(88)80903-3. [DOI] [PubMed] [Google Scholar]
- 84.Hickey GJ, Krasnow JS, Beattie WG, Richards JS. Aromatase cytochrome P450 in rat ovarian granulosa cells before and after luteinization: adenosine 3',5'-monophosphate-dependent and independent regulation. Cloning and sequencing of rat aromatase cDNA and 5' genomic DNA. Mol Endocrinol. 1990;4:3–12. doi: 10.1210/mend-4-1-3. [DOI] [PubMed] [Google Scholar]
- 85.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hucho TB, Dina OA, Kuhn J, Levine JD. Estrogen controls PKCepsilon-dependent mechanical hyperalgesia through direct action on nociceptive neurons. Eur J Neurosci. 2006;24:527–534. doi: 10.1111/j.1460-9568.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- 87.Improta-Brears T, Whorton AR, Codazzi F, York JD, Meyer T, McDonnell DP. Estrogen-induced activation of mitogen-activated protein kinase requires mobilization of intracellular calcium. Proc Natl Acad Sci U S A. 1999;96:4686–4691. doi: 10.1073/pnas.96.8.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ji Y, Murphy AZ, Traub RJ. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Joshi D, Billiar RB, Miller MM. Modulation of hypothalamic mu-opioid receptor density by estrogen: a quantitative autoradiographic study of the female C57BL/6J mouse. Brain Res Bull. 1993;30:629–634. doi: 10.1016/0361-9230(93)90093-q. [DOI] [PubMed] [Google Scholar]
- 91.Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 1990;11:719–728. doi: 10.1016/0196-9781(90)90187-a. [DOI] [PubMed] [Google Scholar]
- 92.Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res. 1996;742:352–354. doi: 10.1016/s0006-8993(96)01108-0. [DOI] [PubMed] [Google Scholar]
- 93.Kelly MJ, Moss RL, Dudley CA. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res. 1976;114:152–157. doi: 10.1016/0006-8993(76)91017-9. [DOI] [PubMed] [Google Scholar]
- 94.Kest B, Sarton E, Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93:539–547. doi: 10.1097/00000542-200008000-00034. [DOI] [PubMed] [Google Scholar]
- 95.King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- 96.Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150:3680–3689. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- 98.Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. Eur J Neurosci. 2008;27:1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 99.Lai J, Ossipov MH, Vanderah TW, Malan TP, Jr, Porreca F. Neuropathic pain: the paradox of dynorphin. Mol Interv. 2001;1:160–167. [PubMed] [Google Scholar]
- 100.Larijani GE, Goldberg ME, Gratz I, Warshal DP. Analgesic and hemodynamic effects of a single 7.5-mg intravenous dose of morphine in patients with moderate-to-severe postoperative pain. Pharmacotherapy. 2004;24:1675–1680. doi: 10.1592/phco.24.17.1675.52335. [DOI] [PubMed] [Google Scholar]
- 101.Laughlin TM, Vanderah TW, Lashbrook J, Nichols ML, Ossipov M, Porreca F, Wilcox GL. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72:253–260. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 102.Lawson KP, Nag S, Thompson AD, Mokha SS. Sex-specificity and estrogen-dependence of kappa opioid receptor-mediated antinociception and antihyperalgesia. Pain. 2010;151:806–815. doi: 10.1016/j.pain.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee DY, Chai YG, Lee EB, Kim KW, Nah SY, Oh TH, Rhim H. 17Beta-estradiol inhibits high-voltage-activated calcium channel currents in rat sensory neurons via a non-genomic mechanism. Life Sci. 2002;70:2047–2059. doi: 10.1016/s0024-3205(01)01534-x. [DOI] [PubMed] [Google Scholar]
- 104.Lee OY, Mayer EA, Schmulson M, Chang L, Naliboff B. Gender-related differences in IBS symptoms. Am J Gastroenterol. 2001;96:2184–2193. doi: 10.1111/j.1572-0241.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- 105.Levin ER. G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology. 2009;150:1563–1565. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li L, Fan X, Warner M, Xu XJ, Gustafsson JA, Wiesenfeld-Hallin Z. Ablation of estrogen receptor alpha or beta eliminates sex differences in mechanical pain threshold in normal and inflamed mice. Pain. 2009;143:37–40. doi: 10.1016/j.pain.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 107.Lieberherr M, Grosse B, Kachkache M, Balsan S. Cell signaling and estrogens in female rat osteoblasts: a possible involvement of unconventional nonnuclear receptors. J Bone Miner Res. 1993;8:1365–1376. doi: 10.1002/jbmr.5650081111. [DOI] [PubMed] [Google Scholar]
- 108.Liu N-J, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 109.Liu NJ, Chakrabarti S, Schnell S, Wessendorf M, Gintzler AR. Spinal Synthesis of Estrogen and Concomitant Signaling by Membrane Estrogen Receptors Regulate Spinal {kappa}- and {micro}-Opioid Receptor Heterodimerization and Female-Specific Spinal Morphine Antinociception. J Neurosci. 2011;31:11836–11845. doi: 10.1523/JNEUROSCI.1901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu NJ, Gintzler AR. Gestational and ovarian sex steroid antinociception: relevance of uterine afferent and spinal alpha(2)-noradrenergic activity. Pain. 1999;83:359–368. doi: 10.1016/s0304-3959(99)00120-7. [DOI] [PubMed] [Google Scholar]
- 111.Liu NJ, von Gizycki H, Gintzler AR. Sexually dimorphic recruitment of spinal opioid analgesic pathways by the spinal application of morphine. J Pharmacol Exp Ther. 2007;322:654–660. doi: 10.1124/jpet.107.123620. [DOI] [PubMed] [Google Scholar]
- 112.Lonard DM, Tsai SY, O'Malley BW. Selective estrogen receptor modulators 4-hydroxytamoxifen and raloxifene impact the stability and function of SRC-1 and SRC-3 coactivator proteins. Mol Cell Biol. 2004;24:14–24. doi: 10.1128/MCB.24.1.14-24.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Long JB, Petras JM, Mobley WC, Holaday JW. Neurological dysfunction after intrathecal injection of dynorphin A (1–13) in the rat. II. Nonopioid mechanisms mediate loss of motor, sensory and autonomic function. J Pharmacol Exp Ther. 1988;246:1167–1174. [PubMed] [Google Scholar]
- 114.Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature (Lond) 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- 115.Low KG, Nielson CP, West NB. Proenkephalin gene expression in the primate uterus: regulation by estradiol in the endometrium. Mol. Endocrinol. 1989;3:852–857. doi: 10.1210/mend-3-5-852. [DOI] [PubMed] [Google Scholar]
- 116.Lu YC, Chen CW, Wang SY, Wu FS. 17Beta-estradiol mediates the sex difference in capsaicin-induced nociception in rats. J Pharmacol Exp Ther. 2009;331:1104–1110. doi: 10.1124/jpet.109.158402. [DOI] [PubMed] [Google Scholar]
- 117.Luoma JI, Boulware MI, Mermelstein PG. Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol. 2008;290:8–13. doi: 10.1016/j.mce.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Ando S. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–27016. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- 119.Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86:185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- 120.Mani SK, Blaustein JD, O'Malley BW. Progesterone receptor function from a behavioral perspective. Horm Behav. 1997;31:244–255. doi: 10.1006/hbeh.1997.1393. [DOI] [PubMed] [Google Scholar]
- 121.Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 122.Marino M, Pallottini V, Trentalance A. Estrogens cause rapid activation of IP3-PKC-alpha signal transduction pathway in HEPG2 cells. Biochem Biophys Res Commun. 1998;245:254–258. doi: 10.1006/bbrc.1998.8413. [DOI] [PubMed] [Google Scholar]
- 123.Mayer EA, Naliboff B, Lee O, Munakata J, Chang L. Review article: gender-related differences in functional gastrointestinal disorders. Aliment Pharmacol Ther. 1999;13(Suppl 2):65–69. doi: 10.1046/j.1365-2036.1999.00008.x. [DOI] [PubMed] [Google Scholar]
- 124.McCarthy MM, Becker JB. Neuroendocrinology of sexual behavior in the female. Cambridge: MIT press; 2002. [Google Scholar]
- 125.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 126.Means GD, Mahendroo MS, Corbin CJ, Mathis JM, Powell FE, Mendelson CR, Simpson ER. Structural analysis of the gene encoding human aromatase cytochrome P-450, the enzyme responsible for estrogen biosynthesis. J Biol Chem. 1989;264:19385–19391. [PubMed] [Google Scholar]
- 127.Medina VM, Dawson-Basoa ME, Gintzler AR. 17-b-estradiol and progesterone positively modulate spinal cord dynorphin: relevance to the analgesia of pregnancy. Neuroendocrinology. 1993;58:310–315. doi: 10.1159/000126555. [DOI] [PubMed] [Google Scholar]
- 128.Medina VM, Gupta D, Gintzler AR. Spinal cord dynorphin precursor intermediates decline during late gestation: implications for enhanced processing as an adaptive mechanism in dynorphin neurons. J Neurochem. 1995;65(3):1374–1380. doi: 10.1046/j.1471-4159.1995.65031374.x. [DOI] [PubMed] [Google Scholar]
- 129.Medina VM, Wang L, Gintzler AR. Spinal cord dynorphin: positive region-specific modulation during pregnancy and parturition. Brain Research. 1993;623:41–46. doi: 10.1016/0006-8993(93)90007-a. [DOI] [PubMed] [Google Scholar]
- 130.Mendoza C, Soler A, Tesarik J. Nongenomic steroid action: independent targeting of a plasma membrane calcium channel and a tyrosine kinase. Biochem Biophys Res Commun. 1995;210:518–523. doi: 10.1006/bbrc.1995.1690. [DOI] [PubMed] [Google Scholar]
- 131.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miaskowski C, Levine JD. Does opioid analgesia show a gender preference for females? Pain Forum. 1999;8:34–44. [Google Scholar]
- 133.Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 134.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab. 2002;87:5760–5768. doi: 10.1210/jc.2002-020670. [DOI] [PubMed] [Google Scholar]
- 136.Minami T, Oomura Y, Nabekura J, Fukuda A. 17 beta-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- 137.Mize AL, Alper RH. Rapid uncoupling of serotonin-1A receptors in rat hippocampus by 17beta-estradiol in vitro requires protein kinases A and C. Neuroendocrinology. 2002;76:339–347. doi: 10.1159/000067583. [DOI] [PubMed] [Google Scholar]
- 138.Mobbs CV, Kaplitt M, Kow LM, Pfaff DW. PLC-alpha: a common mediator of the action of estrogen and other hormones? Mol Cell Endocrinol. 1991;80:C187–C191. doi: 10.1016/0303-7207(91)90136-g. [DOI] [PubMed] [Google Scholar]
- 139.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 140.Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 141.Morley P, Whitfield JF, Vanderhyden BC, Tsang BK, Schwartz JL. A new, nongenomic estrogen action: the rapid release of intracellular calcium. Endocrinology. 1992;131:1305–1312. doi: 10.1210/endo.131.3.1505465. [DOI] [PubMed] [Google Scholar]
- 142.Moss RL, Gu Q. Estrogen: mechanisms for a rapid action in CA1 hippocampal neurons. Steroids. 1999;64:14–21. doi: 10.1016/s0039-128x(98)00092-0. [DOI] [PubMed] [Google Scholar]
- 143.Murphy AZ, Suckow SK, Johns M, Traub RJ. Sex differences in the activation of the spinoparabrachial circuit by visceral pain. Physiol Behav. 2009;97:205–212. doi: 10.1016/j.physbeh.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986;233:226–228. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- 145.Naftolin F, Horvath TL, Balthazart J. Estrogen synthetase (aromatase) immunohistochemistry reveals concordance between avian and rodent limbic systems and hypothalami. Exp Biol Med (Maywood) 2001;226:717–725. doi: 10.1177/153537020222600802. [DOI] [PubMed] [Google Scholar]
- 146.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63:149–155. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 147.Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 148.Nawaz Z, Tsai MJ, McDonnell DP, O'Malley BW. Identification of novel steroid-response elements. Gene Expr. 1992;2:39–47. [PMC free article] [PubMed] [Google Scholar]
- 149.Nichols ML, Lopez Y, Ossipov MH, Bian D, Porreca F. Enhancement of the antiallodynic and antinociceptive efficacy of spinal morphine by antisera to dynorphin A (1–13) or MK-801 in a nerve-ligation model of peripheral neuropathy. Pain. 1997;69:317–322. doi: 10.1016/S0304-3959(96)03282-4. [DOI] [PubMed] [Google Scholar]
- 150.Ossipov MH, Kovelowski CJ, Wheeler-Aceto H, Cowan A, Hunter JC, Lai J, Malan TP, Jr, Porreca F. Opioid antagonists and antisera to endogenous opioids increase the nociceptive response to formalin: demonstration of an opioid kappa and delta inhibitory tone. J Pharmacol Exp Ther. 1996;277:784–788. [PubMed] [Google Scholar]
- 151.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 152.Peckham EM, Barkley LM, Divin MF, Cicero TJ, Traynor JR. Comparison of the antinociceptive effect of acute morphine in female and male Sprague-Dawley rats using the long-lasting mu-antagonist methocinnamox. Brain Res. 2005;1058:137–147. doi: 10.1016/j.brainres.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 153.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 155.Purinton SC, Wood CE. Ovine fetal estrogen sulfotransferase in brain regions important for hypothalamus-pituitary-adrenal axis control. Neuroendocrinology. 2000;71:237–242. doi: 10.1159/000054541. [DOI] [PubMed] [Google Scholar]
- 156.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rai D, Frolova A, Frasor J, Carpenter AE, Katzenellenbogen BS. Distinctive actions of membrane-targeted versus nuclear localized estrogen receptors in breast cancer cells. Mol Endocrinol. 2005;19:1606–1617. doi: 10.1210/me.2004-0468. [DOI] [PubMed] [Google Scholar]
- 158.Rainbow TC, Davis PG, McEwen BS. Anisomycin inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1980;194:548–555. doi: 10.1016/0006-8993(80)91240-8. [DOI] [PubMed] [Google Scholar]
- 159.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 160.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 161.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 162.Riley JL, 3rd, Robinson ME, Wise EA, Price DD. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235. doi: 10.1016/S0304-3959(98)00258-9. [DOI] [PubMed] [Google Scholar]
- 163.Rollman GB, Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–24. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 164.Romano GJ, Harlan RE, Shiverst BD, Howels RD, Pfaff DW. Estrogen increases proenkephalin messenger ribonucleic acid levels in the ventromedial hypothalamus of the rat. Mol. Endocrinol. 1988;2:1320–1328. doi: 10.1210/mend-2-12-1320. [DOI] [PubMed] [Google Scholar]
- 165.Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- 166.Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J Steroid Biochem Mol Biol. 2001;79:247–253. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 167.Roselli CE, Resko JA. Sex differences in androgen-regulated expression of cytochrome P450 aromatase in the rat brain. J Steroid Biochem Mol Biol. 1997;61:365–374. [PubMed] [Google Scholar]
- 168.Roselli CE, Resko JA. Testosterone regulates aromatase activity in discrete brain areas of male rhesus macaques. Biol Reprod. 1989;40:929–934. doi: 10.1095/biolreprod40.5.929. [DOI] [PubMed] [Google Scholar]
- 169.Sarajari S, Oblinger MM. Estrogen effects on pain sensitivity and neuropeptide expression in rat sensory neurons. Exp Neurol. 2010;224:163–169. doi: 10.1016/j.expneurol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. [DOI] [PubMed] [Google Scholar]
- 171.Setalo GJ, Singh M, Guan X, Toran-Allerand CD. Estradiol-induced phosphorylation of ERK1/2 in explants of the mouse cerebral cortex: the roles of heat shock protein 90 (Hsp90) and MEK2. J Neurobiol. 2002;50:1–12. doi: 10.1002/neu.10000. [DOI] [PubMed] [Google Scholar]
- 172.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 173.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 174.Simonian SX, Delaleu B, Caraty A, Herbison AE. Estrogen receptor expression in brainstem noradrenergic neurons of the sheep. Neuroendocrinology. 1998;67:392–402. doi: 10.1159/000054338. [DOI] [PubMed] [Google Scholar]
- 175.Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 177.Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stening KD, Berg G, Hammar M, Voster H, Eriksson O, Amandusson A, Blomqvist A. Influence of estrogen levels on thermal perception, pain thresholds, and pain tolerance: studies on women undergoing in vitro fertilization. J Pain. 13:459–466. doi: 10.1016/j.jpain.2012.01.446. [DOI] [PubMed] [Google Scholar]
- 179.Stening KD, Eriksson O, Henriksson KG, Brynhildsen J, Lindh-Astrand L, Berg G, Hammar M, Amandusson A, Blomqvist A. Hormonal replacement therapy does not affect self-estimated pain or experimental pain responses in post-menopausal women suffering from fibromyalgia: a double-blind, randomized, placebo-controlled trial. Rheumatology (Oxford) 50:544–551. doi: 10.1093/rheumatology/keq348. [DOI] [PubMed] [Google Scholar]
- 180.Sternberg WF, Mogil JS, Kest B, Page GG, Leong Y, Yam V, Liebeskind JC. Neonatal testosterone exposure influences neurochemistry of non-opioid swim stress-induced analgesia in adult mice. Pain. 1995;63:321–326. doi: 10.1016/0304-3959(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 181.Szego CM, Davis JS. Adenosine 3',5'-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci U S A. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 183.Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res. 1999;57:603–615. [PubMed] [Google Scholar]
- 184.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 185.Timmers RJ, Granneman JC, Lambert JG, van Oordt PG. Estrogen-2-hydroxylase in the brain of the male African catfish, Clarias gariepinus. Gen Comp Endocrinol. 1988;72:190–203. doi: 10.1016/0016-6480(88)90202-x. [DOI] [PubMed] [Google Scholar]
- 186.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Toran-Allerand CD, Singh M, Setalo G., Jr Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 188.Torres-Reveron A, Williams TJ, Chapleau JD, Waters EM, McEwen BS, Drake CT, Milner TA. Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp Neurol. 2009;219:319–327. doi: 10.1016/j.expneurol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Uht RM, Anderson CM, Webb P, Kushner PJ. Transcriptional activities of estrogen and glucocorticoid receptors are functionally integrated at the AP-1 response element. Endocrinology. 1997;138:2900–2908. doi: 10.1210/endo.138.7.5244. [DOI] [PubMed] [Google Scholar]
- 190.Unruh AM. Gender variations in clinical pain experience {Review} Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 191.Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, Malan TP, Jr, Porreca F. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;68:275–281. doi: 10.1016/s0304-3959(96)03225-3. [DOI] [PubMed] [Google Scholar]
- 192.VanderHorst VG, Meijer E, Schasfoort FC, Van Leeuwen FW, Holstege G. Estrogen receptor-immunoreactive neurons in the lumbosacral cord projecting to the periaqueductal gray in the ovariectomized female cat. Neurosci Lett. 1997;236:25–28. doi: 10.1016/s0304-3940(97)00750-7. [DOI] [PubMed] [Google Scholar]
- 193.Vertes Z, Lengyel F, Oszter A, Kornyei JL, Sumegi B, Vertes M. Effect of estradiol on expression and activation of Akt protein in rat hypothalamus exposed to chronic [D-Met2, Pro5]-enkephalinamide treatment. Steroids. 2004;69:263–270. doi: 10.1016/j.steroids.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 194.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5'-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 195.Walker JS, Carmody JJ. Experimental pain in healthy human subjects: gender diferences in nociception and in response to ibuprofen. Anesthesia & Analgesia. 1998;86:1257–1262. doi: 10.1097/00000539-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 196.Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 198.Welshons WV, Lieberman ME, Gorski J. Nuclear localization of unoccupied oestrogen receptors. Nature. 1984;307:747–749. doi: 10.1038/307747a0. [DOI] [PubMed] [Google Scholar]
- 199.Williams SJ, Papka RE. Estrogen receptor-immunoreactive neurons are present in the female rat lumbosacral spinal cord. J Neurosci Res. 1996;46:492–501. doi: 10.1002/(SICI)1097-4547(19961115)46:4<492::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 200.Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A. 2000;97:6379–6384. doi: 10.1073/pnas.120166297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Terman GW, Chavkin C. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J Neurosci. 2004;24:4576–4584. doi: 10.1523/JNEUROSCI.5552-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yunus MB. The role of gender in fibromyalgia syndrome. Curr Rheumatol Rep. 2001;3:128–134. doi: 10.1007/s11926-001-0008-3. [DOI] [PubMed] [Google Scholar]
- 203.Zacny JP. Gender differences in opioid analgesia in human volunteers: cold pressor and mechanical pain. NIDA Research Monograph. 2002;182:22–23. [Google Scholar]
- 204.Zhang Z, Maier B, Santen RJ, Song RX. Membrane association of estrogen receptor alpha mediates estrogen effect on MAPK activation. Biochem Biophys Res Commun. 2002;294:926–933. doi: 10.1016/S0006-291X(02)00348-0. [DOI] [PubMed] [Google Scholar]
- 205.Zheng J, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17beta-estradiol-[125I]bovine serum albumin conjugate. J Steroid Biochem Mol Biol. 1997;62:327–336. doi: 10.1016/s0960-0760(97)00037-x. [DOI] [PubMed] [Google Scholar]
- 206.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 207.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]