Abstract
Objective
To determine the effects of intratumoral injection of a hyaluronan-cisplatin nanoconjugate on local and systemic platinum concentrations and systemic toxicosis.
Animals
5 dogs with spontaneous soft tissue sarcomas (STSs).
Procedures
For each dog, approximately 1.5 mL of hyaluronan nanocarrier conjugated with 20 mg of cisplatin was injected into an external STS. Blood samples were collected immediately before (0 hours) and at 0.5, 1, 2, 3, 4, 24, and 96 hours after hyaluronan-cisplatin injection for pharmacokinetic analyses. Urine samples were obtained at 0 and at 96 hours after hyaluronan-cisplatin injection for urinalysis. Each treated STS and its sentinel lymph nodes were surgically removed 96 hours after the hyaluronan-cisplatin injection. Inductively coupled plasma mass spectrometry was used to measure platinum concentrations in blood samples, tumors, and lymph nodes.
Results
No tissue reactions were detected 96 hours after hyaluronan-cisplatin injection. Mean ± SD area under the curve, peak concentration, and terminal half-life for unbound (plasma) and total (serum) platinum were 774.6 ± 221.1 ng·h/mL and 3,562.1 ± 2,031.1 ng·h/mL, 56.5 ± 20.9 ng/mL and 81.6 ± 40.4 ng/mL, and 33.6 ± 16.1 hours and 51.2 ± 29.1 hours, respectively. Platinum concentrations ranged from 3,325 to 8,229 ng/g in STSs and 130 to 6,066 ng/g in STS-associated lymph nodes.
Conclusions and Clinical Relevance
Intratumoral injection of the hyaluronan-cisplatin nanoconjugate was well tolerated in treated dogs. Following intratumoral hyaluronan-cisplatin injection, platinum concentration was 1,000-fold and 100-fold greater within treated tumors and tumor-draining lymphatics, respectively, compared with that in plasma.
Hyaluronan is a natural polysaccharide with alternating d-glucuronic acid and N-acetyl-d-glucosamine units. Hyaluronan and its metabolites are eliminated by the lymphatics via receptor-mediated endocytosis and lysosomal degradation.1–4 Hyaluronan is part of the extracellular matrix and is also found in synovial fluid, cartilage, dermis, and vitreous humor.3,5 It is involved in multiple processes such as cell adhesion, organization of the extracellular matrix, growth, migration, tumor formation, and metastasis.3,5,6 Because hyaluronan is nonimmunogenic, it is an ideal nanocarrier for various drugs such as cisplatin, paclitaxel, doxorubicin, and mitomycin.1,5,7
Cisplatin (cis-diamminedichloroplatinum) is a chemotherapeutic agent that damages DNA and inhibits protein and rRNA synthesis.8,9 The in vitro cytotoxic effects of cisplatin include platinum binding to DNA, creation of interstrand cross-links, and formation of intrastrand bidentate N-7 adducts at d(GpG) and d(ApG).10 Cisplatin is cell cycle phase nonspecific and is eliminated via renal excretion.9 In human patients, cisplatin is used to treat many solid tumors including squamous sarcomas in the head and neck areas, lymphomas, and small cell and non-small cell lung, testicular, ovarian, gastric, esophageal, and pancreatic cancers. In veterinary medicine, cisplatin is used to treat solid tumors, including osteosarcomas, carcinomas, and sarcomas in companion animals.9,11 Common side effects associated with cisplatin administration include nephrotoxicosis (renal tubular inflammation and necrosis), leukopenia, nausea, anemia, and chronic neurotoxicosis and ototoxicosis.2,11 The toxic effects following clinical administration of cisplatin are similar for human and veterinary patients, and its use in veterinary medicine has been limited.
As peak plasma cisplatin concentration increases, the risk for systemic toxic effects increases but the therapeutic effects against the targeted tumor remain relatively stable.11 Various strategies have been proposed for cisplatin administration, including metronomic chemotherapy and local injection of cisplatin into affected tissues that can be concurrently isolated from systemic blood circulation so as to minimize peak plasma cisplatin concentration and decrease the risk of toxicosis, specifically nephrotoxicosis.11 Various routes of cisplatin administration have been evaluated in dogs. For dogs with appendicular osteosarcomas and in which limb-sparing surgery was to be performed, cisplatin administered intra-arterially in combination with radiation prior to surgery resulted in a high percentage of tumor necrosis with minimal loss of host bone viability.12 Severe toxic effects are associated with SC administration of cisplatin.13 Similar toxic effects were not detected following intracavitary (intrathoracic or intra-abdominal) adminstration of cisplatin, and this route of adminstration provided palliative treatment for pleural or abdominal effusion in 5 of 6 dogs with malignant neoplasia.14 Sustained release of cisplatin from a d, l–OPLA implant has been evaluated in dogs with nasal tumors,15 STSs,16 and osteosarcomas17–19; however, clinical improvement following administration of cisplatin via OPLA implant was equivocal. Intratumoral injection of hyaluronan nanocarriers conjugated with cisplatin represents a new modality for the administration of cisplatin that may decrease peak cisplatin concentration in plasma yet maintain cisplatin's efficacy against the targeted tumor.11
In addition to being nonimmunogenic, hyaluronan is a ligand for CD44 receptors that are located on lymphocytes and some cancer cells.11,20 Once bound to CD44, hyaluronan is catabolized, brought into the cell via receptor-mediated endocytosis, degraded in lysosomes, and then introduced into the lymphatic microcirculation.11 When cisplatin is combined with hyaluronan in a nanoconjugate, it remains inactive until the nanoconjugate comes in contact with hyaluronidase. Hyaluronidase expression is a marker for malignant cancers,21 and lymph nodes effectively catabolize hyaluronan.22 After intratumoral injection, the hyaluronan-cisplatin nanoconjugate undergoes receptor-mediated endocytosis or becomes activated by hyaluronidase, which causes cisplatin to be released from the nanoconjugate and absorbed into the peritumoral microlymphatics.3 In rodents, SC injection of a hyaluronan-cisplatin nanoconjugate resulted in increased concentrations of cisplatin in the localized tissue and lymphatics, compared with those obtained after IV administration of cisplatin.11
Soft tissue sarcomas are mesenchymal tumors that originate from connective tissue and represent approximately 8% to 17% of skin and subcutaneous tumors in dogs.23,24 These tumors are locally invasive, and surgical excision of the tumor with wide margins is generally the preferred treatment. Recurrence rate of STSs following surgery ranges from 7% to 32%.23,25,26 Adjuvant chemotherapy with cisplatin, doxorubicin, mitoxantrone, or paclitaxel has been used to treat various sarcomas, but the use of adjuvant chemotherapy for the treatment of dogs with STSs has not been fully evaluated and its effectiveness is relatively unknown.26 The clinical outcome for dogs with high-grade STSs that received doxorubicin as adjuvant chemotherapy after tumor excision did not significantly differ from that for dogs with high-grade STSs that were treated with tumor excision only.27 A protocol that used doxorubicin resulted in an overall response rate of 22% (11/51) of dogs with either naïve or recurrent sarcomas, including 8 of 34 STSs.28 The objectives of the study reported here were to assess the safety of hyaluronan as a nanocarrier for cisplatin for intratumoral injection in STSs of dogs, determine whether the hyaluronan-cisplatin nanoconjugate had preferential local lymphatic penetration, and characterize the pharmacokinetics of cisplatin after injection of the hyaluronan-cisplatin nanoconjugate. Dogs with STSs were chosen for this pilot study because STSs can be easily measured, are amenable to intratumoral drug administration, and can generally be completely excised; also, the lymph nodes draining STSs can usually be easily identified and removed. We hypothesized that the STSs of dogs would absorb the intratumoral injection of hyaluronan-cisplatin, cisplatin would become concentrated within the tumor and its associated lymphatics, the concentration of cisplatin within the tumor would be higher than that in plasma, and there would be no evidence of systemic toxicity following intratumoral injection of the hyaluronan-cisplatin nanoconjugate.
Materials and Methods
Animals
Five client-owned dogs with a spontaneously occurring STS > 2 cm in diameter and no evidence of metastasis on thoracic radiographs that were admitted to the Animal Cancer Center at Colorado State University for surgical excision of the tumor were enrolled in the study. Each dog weighed > 10 kg. For each dog, a CBC, serum biochemical analysis, and urinalysis were performed and diagnosis of the STS was confirmed on the basis of results of histologic examination of a needle core or incisional biopsy specimen. Owner consent was obtained for each dog prior to study enrollment, and all study procedures were approved by the Institutional Animal Care and Use Committee and the Veterinary Teaching Hospital Clinical Board of Colorado State University.
Synthesis of the hyaluronan-cisplatin nanoconjugate
Cisplatin was conjugated to the hyaluronan nanocarrier via a previously reported procedure.11 The ionic nanoconjugate contained approximately 20% bound platinum by weight, which was confirmed via atomic absorption spectroscopy.a
Hyaluronan-cisplatin nanoconjugate administration
For each dog, the STS and surrounding area were clipped to remove hair, and the tumor was measured with calipers in at least 2, or preferably 3, dimensions when possible. Dogs were sedated for the intratumoral injection pending individual dog disposition. The hyaluronan-cisplatin nanoconjugate (20 mg in a volume of approx 1.5 mL) was injected into the presumed center of the tumor of each dog. Ninety-six hours after the hyaluronan-cisplatin injection, the tumor was remeasured, regional lymph nodes were mapped via lymphoscintigraphy, and the tumor and its associated lymph nodes were surgically removed.
Sample collection and processing
From each dog, a urine sample was obtained via free catch or cystocentesis for urinalysis immediately before (0 hours) and 96 hours after hyaluronan-cisplatin injection. An indwelling catheter was aseptically placed into a peripheral vein of each dog for blood collection. Blood samples were collected into an evacuated tube (2 mL) containing EDTA and a serum clot tube (2 mL) at 0, 0.5, 1, 2, 3, 4, 24, and 96 hours after the hyaluronan-cisplatin injection for determination of unbound (plasma) and total (serum) platinum concentrations. A CBC and serum biochemical analysis were also performed on blood samples obtained at 0 and 96 hours after hyaluronan-cisplatin injection. Plasma or serum was removed from the blood samples, placed in individual tubes, and immediately frozen at −80°C.
Regional lymphoscintigraphy
All dogs were sedated for regional lymphoscintigraphy to identify the sentinel lymph nodes. Filtered technetium sulfur colloid (125 μCi total) was injected peritumorally in 4 quadrants. A single photon emission CT camerab was used to obtain images every 5 minutes until the first draining lymph node basin was visualized.
Surgical excision of STS and sentinel lymph nodes
All dogs were anesthetized during surgical procedures. For each dog, the STS, sentinel lymph nodes, and surrounding areas were clipped to remove hair and prepared for surgery in a routine manner. Intrathoracic and intra-abdominal sentinel lymph nodes were not removed unless they were part of the planned tumor resection. Methylene blue (0.4 mL [5 mg/mL]) was injected around the tumor in each of 4 quadrants. Tumors were excised with wide margins (2 to 3 cm) per routine clinical practice. Intraoperative lymphoscintigraphy was performed with a handheld gamma probec to identify sentinel lymph nodes. Separate surgical instruments were used for tumor and lymph node extirpation.
Once extirpated, the tumor and lymph nodes were measured. Each tumor was dissected in half and 1 half was divided again. Core samples from 1 quadrant were collected from the center of the tumor and at approximately every 2 cm radiating outward for large tumors. These samples were frozen at −80°C until analysis for platinum concentration was performed. The remaining tissue was placed in neutral-buffered 10% formalin for histologic evaluation. Lymph nodes were dissected in half; one half was frozen at −80°C until analysis for platinum concentration was performed, and the other was placed in neutral buffered 10% formalin for histo-logic evaluation.
Histologic examination and calculation of percentage tumor necrosis
Half of each tumor was dissected longitudinally into sections that were 1 cm thick. These sections, which represented the complete central longitudinal plane of the tumor, were fixed in neutral-buffered 10% formalin for 24 to 48 hours. The sections were then processed with an extended protocol, embedded in paraffin, cut into sections 4 μm thick, deparaffinized, and stained with H&E stain. All sections from each tumor were completely scanned by means of a camerad connected to a microscopee that had a mechanical stage. The scanned images were viewed simultaneously with the H&E-stained sections. On the scanned images, the tumor border was outlined as well as areas of necrosis within the tumor, which were then analyzed via image analysis softwaref to determine tumor area and the area of tumor necrosis. Area measurements from all sections of a tumor were summed to calculate the total tumor area and total area of necrosis within that tumor. Percentage necrosis for a tumor was calculated as total area of necrosis/total tumor area. Similarly, each extirpated lymph node was longitudinally dissected into sections < 1 cm thick, which represented the complete longitudinal central plane of the lymph node. The sections were fixed in neutral-buffered 10% formalin for 24 to 48 hours, processed with an extended protocol, embedded in paraffin, cut into sections 4 μm thick, deparaffinized, and stained with H&E stain.
Histologic examination of all sections was performed by a board-certified veterinary pathologist (EJE). Each STS was graded as previously defined25 on a scale of 1 to 3 on the basis of the extent of cell differentiation, mitosis, and percentage necrosis.
Determination of platinum concentration
Serum samples were thawed and centrifuged with a filter unit that had a 10,000-Da molecular weight cutoff at 7,500 × g for 20 minutes. Following centrifugation, the filter was removed, the volume of filtrate was measured, and sufficient 6% nitric acid was added to the filtrate to obtain a 1:10 dilution. Plasma samples were thawed and measured, and sufficient 6% nitric acid was added to each sample to obtain a 1:10 dilution. Tumor and lymph node specimens were thawed at room temperature (approx 22°C). The preparation method was then similar for all sample types. Each serum, plasma, or tissue (0.5 g wet weight) sample was placed in a 15-mL centrifuge tube, 0.75 mL of concentrated nitric acid (5% to 7%) was added to the sample, and the sample was heated to 95°C for 6 hours. The sample was then cooled to room temperature, 0.5 mL of 30% hydrogen peroxide was added to the sample, and it was and heated to 80°C for 30 minutes. The sample was again cooled to room temperature, 0.25 mL of concentrated hydrochloric acid (30%) was added to the sample, and it was heated to 80°C for 30 minutes. After again being cooled to room temperature, sufficient purified water was added to the sample to bring the volume to approximately 5 mL; the exact volume was determined gravimetrically. Samples were grouped into sets of approximately 20. Each set was prepared with a blank, spiked blank, standard reference,g duplicate sample, and a spiked sample. Digestates were diluted 10× in purified water and platinum concentration was determined via inductively coupled plasma mass spectrometry.h
Pharmacokinetics of platinum following intratumoral injection of hyaluronan-cisplatin nanoconjugate
Pharmacokinetic parameters were calculated via noncompartmental methods as described.29 All calculations were performed with a computerized spreadsheet programi that used standard equations for noncompartmental and system analysis.
Results
Animals
Of the 5 dogs enrolled in the study, 2 were mixed-breed dogs, 1 was a Labrador Retriever, 1 was a Golden Retriever, and 1 was a Chesapeake Bay Retriever. Three dogs were castrated males and 2 were spayed females. All 5 dogs were of similar age and weight; median age was 9 years (range, 7.5 to 10 years), and median weight was 38 kg (range, 27.1 to 43.1 kg). Each dog had 1 STS. Tumors were located on the caudal aspect of the left antebrachium, cranial aspect of the left stifle joint, medial aspect of the right hock joint, cranioventral aspect of the thorax, and the right flank. Four of the 5 tumors were > 5 cm in diameter, and the mean longitudinal tumor diameter was 8.9 cm (range, 4.4 to 13.7 cm). None of the dogs had evidence of metastasis at the time of examination at the Animal Cancer Center.
Urinalysis, CBC, and biochemical analysis results
One dog was hyposthenuric immediately before (0 hours) and 96 hours after hyaluronan-cisplatin injection, which suggested that the hyposthenuria was not related to the treatment. Evaluation of biochemical analyses revealed no clinically relevant abnormalities for kidney-related variables in any of the 5 study dogs. Within each dog, results of the CBC and serum biochemical analysis performed on samples obtained 96 hours after hyaluronan-cisplatin injection did not differ substantially from those obtained immediately prior to the hyaluronan-cisplatin injection. However, 1 dog did have a grade 1 thrombocytopenia (≤ 100,000 platelets/μL)30 at 96 hours after the hyaluronan-cisplatin injection.
Reactions to hyaluronan-cisplatin injection
Following intratumoral injection of the hyaluronan-cisplatin nanoconjugate, all tumors remained stable in size as determined by similar tumor measurements obtained at 0 and 96 hours after injection. One dog developed a grade 1 dermal reaction as defined by the Veterinary Cooperative Oncology Group30 24 hours after the hyaluronan-cisplatin injection. That reaction consisted of 2 areas of mild erythema, 1 at the injection site and the other at the distal aspect of the tumor. No other reactions were noted in any of the dogs up to 96 hours after the hyaluronan-cisplatin injection, the time at which the tumors were surgically excised.
Results of sentinel lymph node mapping and surgery
Regional lymphoscintigraphy was performed on 3 of 5 dogs prior to surgical removal of the STS. Lymph nodes draining the respective STSs were identified with lymphoscintigraphy prior to surgery and the handheld gamma probe during surgery. The STS was surgically removed from all 5 dogs, and sentinel lymph nodes were removed from 4 of 5 dogs. Prior to surgery, regional lymphoscintigraphy identified a sentinel lymph node in 1 dog, but that lymph node could not be identified with the handheld gamma probe or visualized after peritumoral injection of methylene blue for mapping purposes. For the 2 dogs that did not have regional lymphoscintigraphy performed prior to surgery, sentinel lymph nodes were identified during surgery with the handheld gamma probe and visualization following methylene blue injection and were extirpated.
Limb amputation was performed on 2 dogs to remove the STS, and each dog developed a small seroma at the amputation site 10 days after surgery. The surgical incisions of 2 dogs dehisced; in one dog, the STS was resected from the cranial aspect of the left stifle joint, and in the other, the STS was resected from the cranioventral aspect of the thorax. For the dog from which an STS (maximum diameter, 9 cm) was resected from the stifle joint, partial dehiscence developed 9 days after surgery and extended from the distal aspect of the incision proximally to the point of maximal tension over the joint (approx 8.8 cm). This incision was initially managed as an open wound and then surgically closed 5 days later (14 days after surgical resection of STS). For the dog from which an STS (maximum diameter, 7.4 cm) was removed from the cranioventral aspect of the thorax, a drain was placed when the surgical incision was originally closed and then removed an unrecorded number of days after surgery. Partial dehiscence (diameter, approx 2.2 cm) developed 22 days after surgery at the exit hole for the drain in the cranial aspect of the incision. This wound was managed as an open wound and healed. The dog from which an STS was removed from the flank developed a seroma at the incision site 14 days after surgery, which became an abscess 8 days later. The abscess was lanced; the dog was treated with systemic antimicrobials and eventually healed.
Histologic evaluation of STS and sentinel lymph nodes
Results of histologic evaluation of surgical margins confirmed complete excision of all STSs. The percentage of necrosis within the STSs ranged from 0.25% to 6.61%. The median tumor grade was 2 (range, 1 to 3). Histologic evaluation of the extirpated sentinel lymph nodes revealed reactive hyperplasia and chronic histiocytosis. No evidence of neoplasia was found in any of the lymph nodes examined.
Platinum concentration
The concentration of platinum in the STSs was greater than that in the sentinel lymph nodes or blood. Platinum concentrations ranged from 3,325 to 8,229 ng/g within the STSs, whereas platinum concentrations ranged from 130 to 6,066 ng/g within the extirpated sentinel lymph nodes. Unbound (plasma) and total (serum) concentrations of platinum over time were plotted (Figure 1). The pharmacokinetic parameters following intratumoral injection of 20 mg of cisplatin conjugated with a hyaluronan nanocarrier were summarized (Table 1). The mean ± SD Cmax for unbound and total platinum concentration was 56.5 ± 20.9 ng/mL and 81.6 ± 40.4 ng/mL, respectively. The mean ± SD t1/2 for unbound and total platinum concentration was 33.6 ± 16.1 hours and 51.2 ± 29.1 hours, respectively.
Figure 1.
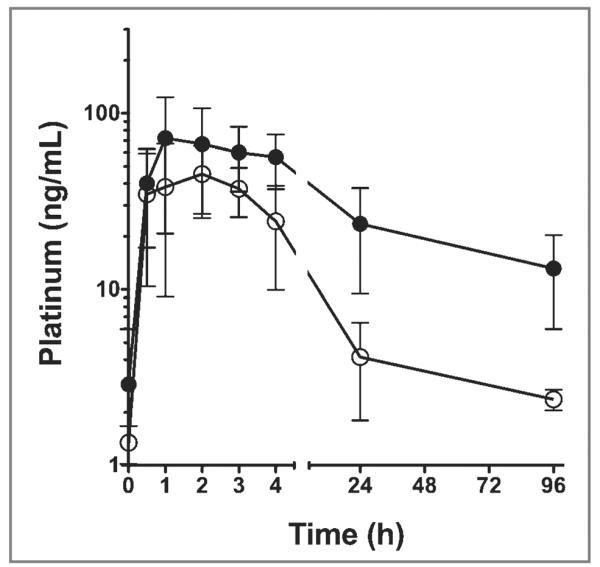
Mean ± SD unbound (plasma; white circles) and total (serum; black circles) platinum concentrations for 5 dogs with STSs immediately before (time, 0) and at 0.5, 1, 2, 3, 4, 24, and 96 hours after a single intratumoral injection of a hyaluronan nanocarrier (approx 1.5 mL) conjugated with 20 mg of cisplatin.
Table 1.
Mean ± SD and range for pharmacokinetic parameters for unbound (plasma) and total (serum) platinum after a single intratumoral injection of a hyaluronan nanocarrier (approx 1.5 mL) conjugated with 20 mg of cisplatin to 5 dogs with STSs.
| Unbound platinum |
Total platinum |
|||
|---|---|---|---|---|
| Parameter | Mean ± SD | Range | Mean ± SD | Range |
| AUC (ng•h/mL) | 774.6 ± 222.1 | 556.9−1,147.1 | 3,562.1 ± 2,031.1 | 1,457.5−6,502.4 |
| Cmax (ng/mL) | 56.5 ± 20.9 | 22.9−71.0 | 81.6 ± 40.4 | 38.0−142.0 |
| T1/2 (h) | 33.6 ± 16.1 | 24.2−62.1 | 51.2 ± 29.1 | 27.5−99.1 |
Discussion
Results of the present study indicated that administration of a single intratumoral injection of a hyaluronan nanocarrier (approx 1.5 mL) conjugated with 20 mg of cisplatin to each of 5 dogs with an STS resulted in higher platinum concentrations in the STS and sentinel lymph nodes, compared with the platinum concentrations in the blood. These results were similar to those of other studies,4,11,20 in which the same hyaluronan-cisplatin nanoconjugate was administered to rodents. In the present study, 96 hours after injection of the hyaluronan-cisplatin nanoconjugate, the platinum concentration within the STS was 1,000-fold greater than the unbound (plasma) platinum concentration and 100-fold greater than the total (serum) platinum concentration. Similarly, 96 hours after injection of the hyaluronan-cisplatin nanoconjugate, the platinum concentration within the sentinel lymph nodes was 100-fold greater than the unbound platinum concentration and 10-fold greater than the total platinum concentration. Intratumoral administration of the hyaluronan-cisplatin nanoconjugate resulted in prefer ential uptake of cisplatin by the sentinel lymph nodes, which may suggest that intratumoral injection of a hyaluronan nanoconjugate may be a beneficial route and vehicle for administration of neoadjuvant or adjuvant chemotherapy when there is a high likelihood of cancer metastasis to the lymphatics.
Results of a study11 that was conducted to evaluate the pharmacokinetics of hyaluronan-cisplatin nanoconjugates after SC and IV administration to rodents indicate that after SC injection, the AUC for platinum was increased and the plasma platinum Cmax was decreased, compared with those parameters following IV injection. For cisplatin-treated patients, the lower that the plasma Cmax for platinum is, the less likely it is that cisplatin-induced systemic toxic effects will develop. Cisplatin-induced acute renal toxicosis usually develops within 24 hours after administration.10 In the present study, no evidence of systemic toxicity was detected in any of the study dogs for up to 96 hours after injection of the hyaluronan-cisplatin nanoconjugate. The mild thrombocytopenia detected in 1 dog at 96 hours after the hyaluronan-cisplatin injection was likely not associated with the treatment because the half-life of platelets is > 96 hours.31
In the present study, all 5 dogs had various postsurgical complications at the incision site. Anecdotally, the development of a small seroma at the incision site after limb amputation in dogs is fairly common, so it was not surprising that both of the dogs that had a limb amputated developed an incisional seroma. The incision complications that developed in the other 3 dogs were most likely caused by the attempt to maintain a 2- to 3-cm margin around each tumor, even in areas where there was little or no redundant skin for wound closure. A criterion for inclusion of a dog in the present study was that the dog had to have an STS > 2 cm in diameter. Removal of relatively large tumors with the recommended 2- to 3-cm margins can result in large surgical incisions, which can make primary wound closure challenging. In dogs, seroma formation along a flank incision within 2 weeks after surgery is relatively common, as is abscess formation when a seroma becomes secondarily infected. Thus, the fact that the dog that had an STS removed from its flank developed a seroma, which subsequently became an abscess, was not unexpected. Partial dehiscence of the incision over the cranial aspect of the stifle joint in 1 dog and over the cranioventral aspect of the thorax of another dog was also not surprising because redundant skin and tissue is scarce in both locations and excessive tension applied to a primary surgical closure often results in dehiscence. The postsurgical incisional complications encountered for the dogs of the present study may also have been caused by the interference of the hyaluronan-cisplatin injection with wound healing. For the 2 dogs that each had a limb amputated, the STS was located a considerable distance away from the amputation site, which makes it unlikely that the seromas were associated with the tumor or the hyaluronan-cisplatin injection. Even though hyaluronan-cisplatin interference with wound healing was not evaluated in the studies4,11,20 involving rodents, an increased incidence of wound complications has been associated with localized administration of cisplatin in studies16,17,32 involving dogs. In studies in which OPLA-cisplatin implants were administered to dogs, 48% (38/80)17 to 60% (19/32)16 of dogs developed wound complications associated with the implant, and removal of the implant was necessary in 28% (9/32)16 of dogs. In another study32 that was conducted with dogs with STSs, a biodegradable cisplatin implant was placed at the STS resection site after tumor removal, and 16 of 19 dogs developed a wound complication. Further research is necessary to determine whether presurgical intratumoral injection of the hyaluronan-cisplatin nanoconjugate inhibits wound healing or wound complications are the result of incisional tension caused by the excision of a tumor with wide margins in an attempt to ensure complete tumor removal.
In the present study, extirpation of sentinel lymph nodes was not associated with any complications. Histologic evaluation did not reveal any evidence of STS metastasis in any of the extirpated lymph nodes. Soft tissue sarcomas generally metastasize hematogenously, with metastases most commonly developing in the lungs; STS metastasis to lymph nodes is less common.25 The risk of STS metastasis increases as tumor grade increases.23–25 The mean STS grade for the study dogs was 2. Although the exact metastatic rate for grade 2 tumors is unknown, it has been reported to be < 15%.25
In the present study, intratumoral injection of a hyaluronan-cisplatin nanoconjugate increased platinum absorption in regional lymph nodes. A similar pattern of platinum absorption by regional lymph nodes was detected in rodents that were administered a hyaluronan-cisplatin conjugate intralymphatically and was accompanied by a sustained low-level systemic release of platinum that resulted in lower peak plasma concentrations, compared with those achieved following systemic administration of cisplatin.4,11 Hyaluronan enters the microlymphatics from the interstitial space preferentially on the basis of particle size.33,34 Because many tumor cells and cells within lymph nodes express hyaluronan ligand CD44, intratumoral injection of a hyaluronan-cisplatin nanoconjugate results in intracellular uptake of the nanoconjugate by these cells.11,20 Lymph is partially comprised of interstitial fluid that enters the lymphatic circulation unidirectionally via an active process of sequential channel opening between tethered overlapping lymphatic endothelial cells, after which the fluid travels through a series of collecting lymphatic vessels and nodes.35 The hyaluronan carrier is cleaved from the nanoconjugate by lysosomal degradation, which activates the cisplatin. Thus, the hyaluronan-cisplatin nanoconjugate is a new modality to treat cancers that metastasize via the lymphatic system.11,20 Regional administration of a hyaluronan-cisplatin nanoconjugate has been used in murine models of breast cancer,20 malignant pleural mesothelioma,5 and lung cancer.2 Also, localized administration of a hyaluronan-doxorubicin nanoconjugate to rodents with xenografts of human breast cancer cells has yielded favorable results.1
The size of the STSs in the dogs of the present study remained stable for 96 hours after the intratumoral injection of the hyaluronan-cisplatin nanoconjugate. Intratumoral administration of chemotherapeutic agents has not been evaluated extensively in dogs with an STS. In the present study, the percentage necrosis in the extirpated STSs was relatively small (0.25% to 6.61%); however, substantial tumor necrosis caused by the intratumoral administration of the hyaluronan-cisplatin nanoconjugate would not be expected ≤ 96 hours after injection.
To our knowledge, the present study was the first to evaluate the pharmacokinetics of cisplatin following intratumoral administration of a hyaluronan-cisplatin nanoconjugate to dogs. In the present study, the AUC and Cmax for platinum were lower, compared with those calculated for another study11 after administration of the same hyaluronan-cisplatin nanoconjugate to rodents. The reason the AUC and Cmax for platinum were lower in the present study may be because the hyaluronan-cisplatin nanoconjugate was injected into the center of a solid tumor, whereas in the other study,11 the hyaluronan-cisplatin nanoconjugate was injected SC. Moreover, the dose (0.56 mg/kg) of cisplatin for the dogs of the present study was substantially less than that (3.3 mg/kg) for the rodents of the other study.11 The t1/2 for platinum in the dogs of the present study was greater, compared with the t1/2 for platinum in the rodents of the other study,11 but this was not surprising because dogs have slower heart rates and metabolisms than do rodents. The t1/2 for platinum in the dogs of the present study was similar to the t1/2 for platinum in human patients (30.5 hours for unbound [plasma] platinum and 130 hours for total [serum] platinum).36
The pharmacokinetics of cisplatin after intratumoral injection to dogs with oral malignant melanomas has been reported.37 The dose of cisplatin (0.25 mg/kg; approx 5 mg/m2) used in that study37 was approximately half that (0.56 mg/kg; approx 18 mg/m2) used in the present study. However, compared with the pharmacokinetic parameters for cisplatin calculated in the other study,37 administration of the hyaluronan-cisplatin nanoconjugate in the present study resulted in a 1.1-fold decrease in Cmax, 14.9-fold increase in AUC, and 50.2-fold increase in t1/2. Moreover, several of the dogs in that other study37 developed grade 2 or 3 cisplatin-induced toxic effects, whereas none of the dogs in the present study developed > grade 1 cisplatin-induced toxic effects. These findings suggested that the hyaluronan-cisplatin nanoconjugate formulation used in the present study enhanced retention of cisplatin within the tumor and resulted in a slower sustained release of cisplatin into systemic circulation with fewer toxic effects than did intratumoral injection of a lower dose of cisplatin alone. Thus, intratumoral administration of a hyaluronan-cisplatin nanoconjugate may reduce the cisplatin dose and number of cisplatin-dosing cycles required for the successful treatment of cisplatin-responsive tumors in dogs, compared with those for other routes and formulations of cisplatin.
In the present study, intratumoral administration of 1.5 mL of a hyaluronan nanocarrier conjugated with 20 mg of cisplatin to dogs with an STS resulted in higher concentrations of platinum in the tumor and sentinel lymph nodes than in plasma or serum. The STSs remained stable in size for 96 hours after the hyaluronan-cisplatin injection. No systemic cisplatin-induced toxic effects were detected in any of the 5 study dogs, although each of the dogs did have wound complications following surgical excision of the STS and its associated lymph nodes. Further investigation is needed to determine whether surgical wound complications are directly associated with the intratumoral hyaluronan-cisplatin injection; however, it is unlikely that tumor excision within 96 hours after intratumoral injection of a hyaluronan-cisplatin nanoconjugate will become standard clinical practice. Regardless, our results suggested that intratumoral injection of a hyaluronan-cisplatin nanoconjugate may be a safe and effective method for the administration of maintenance chemotherapy to dogs with an STS with preferential regional lymphatic uptake.
Acknowledgments
The authors thank Dr. Stephen J. Withrow for technical assistance.
Supported by the National Institutes of Health, American Cancer Society, NanoPharm LLC, Colorado State University Cancer Super-cluster, and Colorado State University Flint Animal Cancer Center.
Abbreviations
- AUC
Area under the curve
- Cmax
Maximum concentration
- OPLA
Open cell polylactic acid
- STS
Soft tissue sarcoma
- t1/2
Terminal half-life
Footnotes
SpectrAA spectrometer with GTA-110 graphite furnace, Varian Inc, Walnut Creek, Calif.
Millennium VG gamma camera, GE Healthcare, Pewaukee, Wis.
Neoprobe Corp, Dublin, Ohio.
AxioCam HRc camera, Carl Zeiss Industrial Metrology, Maple Grove, Minn.
Axioplan 2 microscope, Carl Zeiss Industrial Metrology, Maple Grove, Minn.
Axiovision analysis software, Carl Zeiss Industrial Metrology, Maple Grove, Minn.
DOLT-4, National Research Council Canada, Institute for National Measurement Standards, Ottawa, ON, Canada.
Elan DRC II, PerkinElmer, Waltham, Mass.
Excel 2010, Microsoft Corp, Redmond, Wash.
Presented as an oral presentation at the 2011 Annual Conference of the Veterinary Cancer Society, Albuquerque, November 2011.
Drs. Cohen and Forrest have financial interest in a company that has licensed portions of the technology used in this study.
References
- 1.Cai S, Thati S, Bagby TR, et al. Localized doxorubicin chemotherapy with a biopolymeric nanocarrier improves survival and reduces toxicity in xenografts of human breast cancer. J Control Release. 2010;146:212–218. doi: 10.1016/j.jconrel.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Y, Aillon KL, Cai S, et al. Pulmonary delivery of cisplatin-hyaluronan conjugates via endotracheal instillation for the treatment of lung cancer. Int J Pharm. 2010;392:156–163. doi: 10.1016/j.ijpharm.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong YI, Kim ST, Jin SG, et al. Cisplatin-incorporated hyaluronic acid nanoparticles based on ion-complex formation. J Pharm Sci. 2008;97:1268–1276. doi: 10.1002/jps.21103. [DOI] [PubMed] [Google Scholar]
- 4.Cai S, Xie YM, Bagby TR, et al. Intralymphatic chemotherapy using a hyaluronan-cisplatin conjugate. J Surg Res. 2008;147:247–252. doi: 10.1016/j.jss.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ampollini L, Sonvico F, Barocelli E, et al. Intrapleural polymeric films containing cisplatin for malignant pleural mesothelioma in a rat tumour model: a preliminary study. Eur J Cardiothorac Surg. 2010;37:557–565. doi: 10.1016/j.ejcts.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Kamal A, Datta K. Upregulation of hyaluronan binding protein 1 (HABP1/p32/gC1qR) is associated with cisplatin induced apoptosis. Apoptosis. 2006;11:861–874. doi: 10.1007/s10495-006-5396-4. [DOI] [PubMed] [Google Scholar]
- 7.Chen JP, Leu YL, Fang CL, et al. Thermosensitive hydrogels composed of hyaluronic acid and gelatin as carriers for the intravesical administration of cisplatin. J Pharm Sci. 2011;100:655–666. doi: 10.1002/jps.22309. [DOI] [PubMed] [Google Scholar]
- 8.Jordan P, Carmo-Fonseca M. Cisplatin inhibits synthesis of ribosomal RNA in vivo. Nucleic Acids Res. 1998;26:2831–2836. doi: 10.1093/nar/26.12.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun R, Garrett L, Vail DM. Cancer chemotherapy. In: Withrow SJ, Vail DM, editors. Withrow and MacEwen's small animal clinical oncology. 4th ed Saunders; St Louis: 2007. pp. 163–192. [Google Scholar]
- 10.Reed E, Kohn K. Platinum analogues. In: Chabner BA, Collins JM, editors. Cancer chemotherapy: principles and practice. J.B. Lippincott Co; Philadelphia: 1990. pp. 465–484. [Google Scholar]
- 11.Cai S, Xie Y, Davies NM, et al. Pharmacokinetics and disposition of a localized lymphatic polymeric hyaluronan conjugate of cisplatin in rodents. J Pharm Sci. 2010;99:2664–2671. doi: 10.1002/jps.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Withrow SJ, Thrall DE, Straw RC, et al. Intraarterial cisplatin with or without radiation in limb-sparing for canine osteosarcoma. Cancer. 1993;71:2484–2490. doi: 10.1002/1097-0142(19930415)71:8<2484::aid-cncr2820710810>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Dernell WS, Withrow SJ, Straw RC, et al. Adjuvant chemotherapy using cisplatin by subcutaneous administration. In Vivo. 1997;11:345–350. [PubMed] [Google Scholar]
- 14.Moore AS, Kirk C, Cardona A. Intracavitary cisplatin chemotherapy experience with six dogs. J Vet Intern Med. 1991;5:227–231. doi: 10.1111/j.1939-1676.1991.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 15.Lana SE, Dernell WS, Lafferty MH, et al. Use of radiation and a slow-release cisplatin formulation for treatment of canine nasal tumors. Vet Radiol Ultrasound. 2004;45:577–581. doi: 10.1111/j.1740-8261.2004.04100.x. [DOI] [PubMed] [Google Scholar]
- 16.Dernell WS, Withrow SJ, Straw RC, et al. Intracavitary treatment of soft tissue sarcomas in dogs using cisplatin in a biodegradable polymer. Anticancer Res. 1997;17:4499–4505. [PubMed] [Google Scholar]
- 17.Withrow SJ, Liptak JM, Straw RC, et al. Biodegradable cisplatin polymer in limb-sparing surgery for canine osteosarcoma. Ann Surg Oncol. 2004;11:705–713. doi: 10.1245/ASO.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Mehl ML, Seguin B, Dernell WS, et al. Survival analysis of one versus two treatments of local delivery cisplatin in a biodegradable polymer for canine osteosarcoma. Vet Comp Oncol. 2005;3:81–86. doi: 10.1111/j.1476-5810.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 19.Straw RC, Withrow SJ, Douple EB, et al. Effects of cis-diamminedichloroplatinum II released from d,l-polylactic acid implanted adjacent to cortical allografts in dogs. J Orthop Res. 1994;12:871–877. doi: 10.1002/jor.1100120615. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MS, Cai S, Xie Y, et al. A novel intralymphatic nanocarrier delivery system for cisplatin therapy in breast cancer with improved tumor efficacy and lower systemic toxicity in vivo. Am J Surg. 2009;198:781–786. doi: 10.1016/j.amjsurg.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan JX, Wang XY, Li HY, et al. HYAL1 overexpression is correlated with the malignant behavior of human breast cancer. Int J Cancer. 2011;128:1303–1315. doi: 10.1002/ijc.25460. [DOI] [PubMed] [Google Scholar]
- 22.Fraser JRE, Kimpton WG, Laurent TC, et al. Uptake and degradation of hyaluronan in lymphatic tissue. Biochem J. 1988;256:153–158. doi: 10.1042/bj2560153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis MM, McSporran KD, Bacon NJ, et al. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet Pathol. 2011;48:73–84. doi: 10.1177/0300985810388820. [DOI] [PubMed] [Google Scholar]
- 24.Stefanello D, Morello E, Roccabianca P, et al. Marginal excision of low-grade spindle cell sarcoma of canine extremities: 35 dogs (1996–2006) Vet Surg. 2008;37:461–465. doi: 10.1111/j.1532-950X.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuntz CA, Dernell WS, Powers BE, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986–1996) J Am Vet Med Assoc. 1997;211:1147–1151. [PubMed] [Google Scholar]
- 26.Dernell WS, Withrow SJ, Kuntz CA, et al. Principles of treatment for soft tissue sarcoma. Clin Tech Small Anim Pract. 1998;13:59–64. doi: 10.1016/S1096-2867(98)80029-7. [DOI] [PubMed] [Google Scholar]
- 27.Selting KA, Powers BE, Thompson LJ, et al. Outcome of dogs with high-grade soft tissue sarcomas treated with and without adjuvant doxorubicin chemotherapy: 39 cases (1996–2004) J Am Vet Med Assoc. 2005;227:1442–1448. doi: 10.2460/javma.2005.227.1442. [DOI] [PubMed] [Google Scholar]
- 28.Ogilvie GK, Reynolds HA, Richardson RC, et al. Phase-II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc. 1989;195:1580–1583. [PubMed] [Google Scholar]
- 29.Wagner J. Noncompartmental and system analysis. In: Wagner J, editor. Pharmacokinetics for the pharmaceutical scientist. Technomic; Lancaster, Pa: 1993. pp. 83–103. [Google Scholar]
- 30.Veterinary Co-operative Oncology Group—common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol. 2004;2:194–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 31.Topper M, Welles E. Hemostasis. In: Latimer K, Mahaffey E, Prasse K, editors. Duncan & Prasse's veterinary laboratory medicine clinical pathology. 4th ed Blackwell Publishing Professional; Ames, Iowa: 2003. pp. 99–135. [Google Scholar]
- 32.Havlicek M, Straw RS, Langova V, et al. Intra-operative cisplatin for the treatment of canine extremity soft tissue sarcomas. Vet Comp Oncol. 2009;7:122–129. doi: 10.1111/j.1476-5829.2009.00182.x. [DOI] [PubMed] [Google Scholar]
- 33.Zawieja DC. Lymphatic biology and the microcirculation: past, present, and future. Microcirculation. 2005;12:141–150. doi: 10.1080/10739680590900003. [DOI] [PubMed] [Google Scholar]
- 34.Hall JE, editor. Guyton and Hall textbook of medical physiology. 12th ed Saunders Elsevier; Philadelphia: 2011. The microcirculation and lymphatic system: capillary fluid exchange, interstitial fluid, and lymph flow; pp. 177–189. [Google Scholar]
- 35.Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canal P. Platinum compounds: pharmacokinetics and pharmacodynamics. In: Grochow L, Ames M, editors. A clinician's guide to chemotherapy pharmacokinetics and pharmacodynamics. Williams & Willkins; Baltimore: 1998. pp. 345–373. [Google Scholar]
- 37.Theon AP, Madewell BR, Ryu J, et al. Concurrent irradiation and intratumoral chemotherapy with cisplatin: a pilot study in dogs with spontaneous tumors. Int J Radiat Oncol Biol Phys. 1994;29:1027–1034. doi: 10.1016/0360-3016(94)90398-0. [DOI] [PubMed] [Google Scholar]


