Abstract
Strict control of systolic blood pressure is known to slow progression of chronic kidney disease (CKD). Here we compared audit-based education (ABE) to guidelines and prompts or usual practice in lowering systolic blood pressure in people with CKD. This 2-year cluster randomized trial included 93 volunteer general practices randomized into three arms with 30 ABE practices, 32 with guidelines and prompts, and 31 usual practices. An intervention effect on the primary outcome, systolic blood pressure, was calculated using a multilevel model to predict changes after the intervention. The prevalence of CKD was 7.29% (41,183 of 565,016 patients) with all cardiovascular comorbidities more common in those with CKD. Our models showed that the systolic blood pressure was significantly lowered by 2.41 mm Hg (CI 0.59–4.29 mm Hg), in the ABE practices with an odds ratio of achieving at least a 5 mm Hg reduction in systolic blood pressure of 1.24 (CI 1.05–1.45). Practices exposed to guidelines and prompts produced no significant change compared to usual practice. Male gender, ABE, ischemic heart disease, and congestive heart failure were independently associated with a greater lowering of systolic blood pressure but the converse applied to hypertension and age over 75 years. There were no reports of harm. Thus, individuals receiving ABE are more likely to achieve a lower blood pressure than those receiving only usual practice. The findings should be interpreted with caution due to the wide confidence intervals.
Keywords: blood pressure, clinical trial, glomerular filtration rate, hypertension, kidney disease, quality of health care
Chronic kidney disease (CKD) can be managed in primary care
Internationally, the management of stages 1–3 CKD is carried out in primary care; however, there is scope to improve the coordination and quality of care.1, 2, 3 CKD is more common with increasing age, and in females but the proportion of males increases with declining renal function;4, 5 with males more likely to develop proteinuria.6 CKD differs across ethnic groups,7, 8 and with increased deprivation.9 It is also associated with heart disease,10 heart failure, hypertension (HT), and diabetes.11 Intervention in CKD is needed because untreated CKD associated with an increased risk of cardiovascular morbidity and mortality,12, 13, 14 hospitalization,15 and progression to renal failure.16, 17 Strict control of systolic blood pressure (SBP) is known to slow progression;18, 19 and may be cost effective.20, 21 CKD was added to the UK's pay-for-performance (P4P) (Quality Outcomes Framework) scheme for primary care in April 2006. This scheme uses routine data to determine the level of case ascertainment, on a disease register, and sets financially incentivized quality indicators. The CKD indicator includes a treatment target of keeping blood pressure (BP) below 140/85 mm Hg preferentially using angiotensin-modulating drugs in the presence of proteinuria.
Uncertainty about CKD and its management
Despite all that is known about CKD, there is uncertainty about how to improve the quality of care. A systematic review of interventions in CKD concludes that other than for people with diabetes, there is a lack of research evidence about how best to develop services. Primary care clinicians feel they lack knowledge and confidence in its management. CKD is usually diagnosed using a four-item formula (age, gender, serum creatinine, and ethnicity) to estimate glomerular filtration rate; and from the presence of proteinuria or albuminuria. The condition has a range of different underlying pathological and ageing processes leading some clinicians to speculate that is merely a biochemical construction rather than a disease per se.22, 23, 24 A Cochrane review of the use of angiotensin-modulating drugs, the main recommended treatment, in early CKD reports uncertainty about their value,25, 26 other than for people with CKD and diabetes.27 In addition, a systematic review of interventions in CKD concludes that again, other than for people with diabetes, there is a lack of research as to how services to support management should be developed.28, 29
An appropriate quality improvement (QI) intervention
Audit-based education (ABE) is a QI intervention developed over the last 15 years, which provides education, peer support, and documents the gap between achievement and guidelines. This is a complex, non-judgmental, educational intervention underpinned by the use of information technology to extract and make comparisons between practices and against evidence-based guidelines; details in Box 1.30 In observational studies, this intervention improves the quality of cardiovascular disease management.31, 32 The theoretical basis for this intervention is that audit and feedback, and professional meetings are known to have small but positive effects on quality.33, 34 Any change that takes place might be explained by control theory,35 which suggests that this is most likely if feedback is accompanied by a target or action plan,36 ideally in writing.37
Box 1. Components of audit-based education (ABE).
- Anonymized extraction of the data set required to report any whether there was any quality improvement. The usual components are:
- Denominator to allow standardization of prevalence.
- Subset of people with the target condition—to create a virtual disease register.
- Clinically relevant comorbidities, risk factors, and treatment.
Processing that data to make it informative and providing comparative feedback combined with academic detailing. A key feature is presenting comparative feedback comparing practices at twice yearly meetings held within a locality/primary care organization. These meetings are called Data Quality Workshops (DQW), generally locally led with a consultant of the relevant discipline attending as a specialist resource.
- In addition to the presentation at the DQW practices are provided two additional printed aids:
- ‘Laminate'—a single laminated A4 page summary of the practice demographics and case ascertainment compared with others who attended the DQW. This is for the practice notice board or other prominent location (we recommend wherever they take their breaks).
- Workbook—a slide by slide explanation of the DQW presentation—and what the data means for their practice, compared with their peers and any evidence-based guidance.
Running local searches in the practices to provide lists of patients that need to be targeted for intervention. These lists are usually generated by individual GP. Experiential learning is that audit lists of up to 150 per 10,000 registered patients result in change.
Supporting education about the evidence-base and providing coding or other training is provided as required.
Participants have been encouraged to contribute to the future development of the ABE program.
ABE is an intervention developed over 10 years ago; its aim is to provide feedback about performance against guidance. ABE includes feedback about quality compared with peers in a workshop setting usually led by a local GP with a specialist available as an expert resource, and also supported by academic detailing. ABE usually also identified lists of patients within the practices needing intervention.
Rationale for the study
A systematic review identified very few studies involving QI interventions to lower SBP in CKD, and those there were largely focused on high-risk groups, including ethnic minorities with interventions largely carried out by nurses and pharmacists.18 A subsequent diagnostic analysis, using focus groups, found primary care teams are uncertain about whether CKD was really a disease, disliked its diagnosis based on estimate glomerular filtration rate and found it very difficult to explain the condition to patients.22
Aim
We carried out this study to determine whether ABE might be effective in addressing the quality gap in CKD management using reduction in SBP as the primary outcome measure of improved management.
RESULTS
Recruitment and sample size
We over-recruited into the trial, anticipating that more practices would drop out than did. The registered practice populations consisted of 691,504 registered people. The mean age of the population was 41.1 years (s.d. 22.36 years) and the mean index of multiple deprivation 17.4 (s.d. 13.66, Supplementary Table S6 online). There were 30 practices in the ABE arm with a mean list size of 9082; 32 practices in the guidelines and prompts (G&P) arm with a mean list size of 6992; and 31 practices in usual practice (UP) arm with a mean list size of 6300 (Table 1). During the course of the study, 10.6% of the population died or left the trial practices. Ethnicity was recorded for around half the population and 69% of these had white ethnicity (Table 1). There were statistically significant differences between the population arms in demographics and proportions with cardiovascular comorbidities (Supplementary data files online).
Table 1. Baseline characteristics of the population in each study arm.
| Audit-based education | Guidelines and prompts | Usual practice | All practices total | Statistical test | |
|---|---|---|---|---|---|
| List sizes for trial practices | NPar χ2 | ||||
| Trial population | |||||
| Patients | 272,467 (39.4%) | 223,730 (32.4%) | 195,307 (28.24%) | 691,504 (100%) | P<0.001 |
| Mean list size | 9082 | 6992 | 6300 | 7436 | |
| Adult population | |||||
| Patients | 223,847 (39.6%) | 181,318 (32.1%) | 159,851 (28.29%) | 565,016 (100%) | P<0.001 |
| Mean list size | 7462 | 5666 | 5156 | 6075 | |
| Demographics of population | |||||
| Age (years) | |||||
| n | 272,467 (39.4%) | 223,730 (32.4%) | 195,307 (28.24%) | 691,504 (100%) | ANOVA |
| Mean | 41.4 | 40.1 | 41.8 | 41.1 | P<0.001 |
| s.d. | 22.3 | 22.2 | 22.6 | 22.4 | |
| Gender | |||||
| Female | 135,305 (49.7%) | 110,600 (49.4%) | 98,457 (50.4%) | 344,362 (49.8%) | Pearson χ2 |
| Male | 137,162 (50.3%) | 113,130 (50.6%) | 96,850 (49.6%) | 347,142 (50.2%) | P<0.001 |
| Multiple deprivation index score | |||||
| n | 250,832 (38.9%) | 208,577 (32.3%) | 185,651 (28.8%) | 645,060 (100%) | ANOVA |
| Mean | 16.1 | 20.5 | 15.6 | 17.4 | P<0.001 |
| s.d. | 13.1 | 14.7 | 12.6 | 13.7 | |
| Ethnicity for population | Pearson χ2 | ||||
| Not recorded or not stated | 155,035 (56.9%) | 96,946 (43.3%) | 104,723 (53.6%) | 356,704 (51.6%) | P<0.001 |
| White | 75,249 (27.6%) | 82,851 (37.0%) | 71,547 (36.6%) | 229,647 (33.2%) | |
| Mixed | 2607 (1.0%) | 3737 (1.7%) | 1483 (0.8%) | 7827 (1.1%) | |
| Asian or Asian British | 25,088 (9.2%) | 26,925 (12.0%) | 8289 (4.2%) | 60,302 (8.7%) | |
| Black or black British | 11,875 (4.4%) | 10,306(4.6%) | 6857 (3.5%) | 29,038 (4.2%) | |
| Chinese or other ethnicity | 2613 (1.0%) | 2965 (1.3%) | 2408 (1.2%) | 7986 (1.2%) | |
| Trial practices (n) | 272,467 (100.0%) | 223,730 (100.0%) | 195,307 (100.0%) | 691,504 (100.0%) | |
| Comorbidities | Pearson χ2 | ||||
| Diabetes | 10,969 (4.9%) | 9465 (5.2) | 7322 (4.6) | 27,756 (4.9) | P<0.001 |
| Hypertension | 35,513 (15.9) | 27,216 (15.0) | 25,680 (16.1) | 88,409 (15.6) | P<0.001 |
| Heart failure | 1659 (0.7) | 1581 (0.9) | 1313 (0.8) | 4553 (0.8) | P<0.001 |
| Peripheral vascular disease | 1341 (0.6) | 1342 (0.7) | 1147 (0.7) | 3830 (0.7) | P<0.001 |
| Ischemic heart disease | 8491 (3.8) | 6654 (3.7) | 6266 (3.9) | 21,411 (3.8) | P<0.001 |
| Cerebrovascular disease | 2472 (1.1) | 2302 (1.3) | 1994 (1.2) | 6768 (1.2) | P<0.001 |
Abbreviation: ANOVA, analysis of variance.
Complete case analysis including deaths and leavers during the trial.Bold values indicate the total of the three columns to the left.
Class of CKD by study arm
The crude prevalence of CKD for the trial population (aged 18 years and over) was 9.84% for women, 4.74% for men, and 7.29% for the population. The age-standardized rates (based on the 2001 UK National Census) were 9.84% (95% confidence interval (CI) 9.73–9.95%), 3.69% (95% CI 4.66–4.81%), and 7.29% (95% CI 7.22–7.36%), respectively.
The CKD cases with before and after BP readings, included in our analysis, (n=23,311) had a mean age of 75.1 years: 75.1 years in the ABE arm (n=9333); 74.7 years in G&P (n=6871); 75.3 years in the UP arm (n=7107, Table 2).
Table 2. Baseline characteristics of the CKD cohort with repeat SBP data.
| |
Audit-based education |
Guidelines and prompts |
Usual practice |
All practicestotal |
Statistical test |
| List sizes for trial practices | NPar χ2 | ||||
| Trial population | |||||
| Patients | 272,467 | 223,730 | 195,307 | 691,504 | P<0.001 |
| Mean list size | 9082.2 | 6991.6 | 6300.2 | 7435.5 | |
| Adult population | |||||
| Patients | 204,124 | 159,261 | 140,822 | 504,207 | P<0.001 |
| Mean list size | 6804.1 | 4976.9 | 4542.6 | 5421.6 | |
| Demographics of CKD cohort | |||||
| Age (years) | |||||
| n | 9333 (40.04%) | 6871 (29.48%) | 7107 (30.49%) | 23,311 (100%) | ANOVA |
| Mean | 75.08 | 74.69 | 75.32 | 75.04 | P=0.079 |
| s.d. | 11.85 | 11.92 | 11.68 | 11.82 | |
| Gender | |||||
| Female | 6145 (65.84%) | 4506 (65.58%) | 4760 (66.98%) | 15,411 (66.11%) | Pearson χ2 |
| Male | 3188 (34.16%) | 2365 (34.42%) | 2347 (33.02%) | 7900 (33.89%) | P=0.023 |
| Multiple Deprivation Index score | |||||
| n | 9333 (40.04%) | 6871 (29.48%) | 7107 (30.49%) | 23,311 (100%) | ANOVA |
| Mean | 15.38 | 17.76 | 14.38 | 15.77 | P<0.001 |
| s.d. | 12.45 | 13.47 | 10.61 | 12.30 | |
| Ethnicity for CKD cohort | Pearson χ2 | ||||
| Not recorded or not stated | 4416 (47.32%) | 2069 (30.11%) | 2701 (38.00%) | 9186 (39.41%) | P<0.001 |
| White | 3878 (41.55%) | 3863 (56.22%) | 4011 (56.44%) | 11,752 (50.41%) | |
| Mixed | 45 (0.48%) | 85 (1.24%) | 27 (0.38%) | 157 (0.67%) | |
| Asian or Asian British | 565 (6.05%) | 550 (8.00%) | 143 (2.01%) | 1258 (5.40%) | |
| Black or black British | 386 (4.14%) | 279 (4.06%) | 204 (2.87%) | 869 (3.73%) | |
| Chinese or other ethnicity | 43 (0.46%) | 25 (0.36%) | 21 (0.30%) | 89 (0.38%) | |
| Trial practices (n) | 30 (32.26%) | 32 (34.41%) | 31 (33.33%) | 93 (100%) | |
| Comorbidities CKD cohort | Pearson χ2 | ||||
| Diabetes | 1814 (19.44%) | 1405 (20.45%) | 1263 (17.77%) | 4482 (19.23%) | P<0.001 |
| Hypertension | 6725 (72.06%) | 4949 (72.03%) | 4979 (70.06%) | 16,653 (71.44%) | P<0.001 |
| Heart failure | 527 (5.65%) | 444 (6.46%) | 373 (5.25%) | 1344 (5.77%) | P<0.001 |
| Peripheral vascular disease | 347 (3.72%) | 296 (4.31%) | 293 (4.12%) | 936 (4.02%) | P=0.001 |
| Ischemic heart disease | 1973 (21.14%) | 1485 (21.61%) | 1428 (20.09%) | 4886 (20.96%) | P=0.011 |
| Cerebrovascular disease | 550 (5.89%) | 444 (6.46%) | 468 (6.59%) | 1462 (6.27%) | P<0.001 |
Abbreviations: ANOVA, analysis of variance; CKD, chronic kidney disease; SBP, systolic blood pressure.Bold values indicate the total of the three columns to the left.
The mean deprivation score for the CKD cases was 15.8 and ethnicity was recorded for 60.6% there were statistically significant differences in these between the study arms. The proportion of people with HT and stroke was not significantly different between the arms of the study, but the proportions of other comorbidities were significantly different (Table 1). All the cardiovascular comorbidities were more common in the CKD cases than in the general population. The prevalence of diabetes was 4.9% in the practices' overall adult population and 19.2% in the CKD cases; HT 15.6% versus 71.4% heart failure 0.8% versus 5.8% peripheral vascular disease 0.7% versus 4.0% cerebrovascular disease (stroke or transient ischemic attack (TIA) 1.2% versus 6.3% for the overall population and CKD cases population respectively.
Fall in SBP between study arms
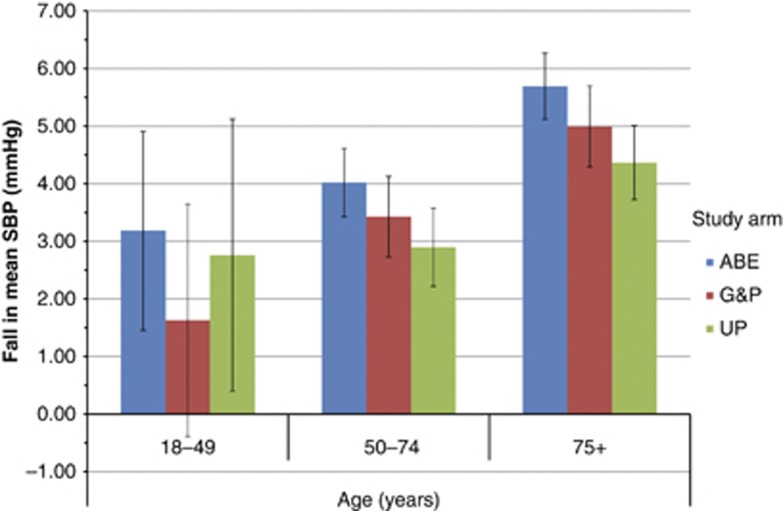
Mean SBP fell by 4.91 (95% CI 4.51–5.32) mm Hg in the ABE arm, by 4.20 (95% CI 3.71–4.68) mm Hg in G&P, and 3.71 (95% CI 3.25–4.17) mm Hg in UP (Table 3). The fall was greatest with increasing age, the biggest reduction being in people over 75 years. Across all age bands the reduction in BP was greatest in the ABE arm of the study (Figure 1). When we compared the reduction in the ABE arm with pooled data from the other two arms the reduction in SBP is 0.96 mm Hg (95% CI 0.439–1.429). There was no difference in the time period between observations between the SBP measures in the three arms of the study. The median and interquartile range between the BP readings by study arm were 943 days (833–1035), 941 days (829–1036), and 937 days (826–1031) for ABE, G&P, and UP arms, respectively.
Table 3. Change in systolic BP by arm of study.
| Systolic BP | n | Mean (mm Hg) | s.d. (mm Hg) | s.e.m. (mm Hg) |
|---|---|---|---|---|
| ABE | ||||
| Before | 9333 | 138.98 | 17.73 | 0.18 |
| After | 134.06 | 16.21 | 0.17 | |
| Change | 4.91 | 19.96 | 0.21 | |
| G&P | ||||
| Before | 6871 | 139.05 | 18.81 | 0.23 |
| After | 134.85 | 16.54 | 0.20 | |
| Change | 4.20 | 20.61 | 0.25 | |
| UP | ||||
| Before | 7107 | 139.37 | 18.20 | 0.22 |
| After | 135.66 | 16.42 | 0.19 | |
| Change | 3.71 | 19.81 | 0.24 | |
| Total | ||||
| Before | 23,311 | 139.12 | 18.20 | 0.12 |
| After | 134.78 | 16.38 | 0.11 | |
| Change | 4.33 | 20.12 | 0.13 | |
Abbreviations: ABE, audit-based education; BP, blood pressure; G&P, guidelines and prompts; UP, usual practice.
Figure 1.
Fall in mean blood pressure (BP) by study arm between before and after time periods with increasing age. ABE, audit-based education; G&P, guidelines and prompts; UP, usual practice.
A greater proportion of people in the ABE arm (12.3%) were at target post intervention compared with G&P (9.2%) and UP (9.3%, Table 4). The proportion of people with a >5 mm Hg reduction in SBP was 47.1% in ABE, 45.9% in G&P, and 45.3% in the UP arm. The odds ratio (OR) of achieving a >5 mm Hg reduction in ABE compared with UP, was 1.24 (95% CI 1.053–1.450, P=0.010). People with ischemic heart disease (IHD) also, had an increased OR 1.132 (95% CI 1.01–1.27; P=0.033); whereas people treated with ‘other' antihypertensives (i.e., non-angiotensin-modulating drugs) and those over 75 years old had OR suggesting they were less likely to achieve a >5 mm Hg reduction in SBP (Table 5).
Table 4. Proportion of people reaching BP target by arm.
|
Status afterward |
||||||||
|---|---|---|---|---|---|---|---|---|
| Off target | On target | Missing | Total | Before (% at target) | After (% at target) | Difference (% difference) | ||
| Audit-based education | ||||||||
| Status before | Off target | 2327 | 2376 | 1167 | 5870 | 49.0 | 61.1 | 12.0 |
| On target | 1267 | 3258 | 1317 | 5842 | ||||
| Missing | 531 | 927 | 1886 | 3344 | ||||
| Total | 4125 | 6561 | 4370 | 15,056 | ||||
| Guidelines and prompts | ||||||||
| Status before | Off target | 1729 | 1678 | 567 | 3974 | 50.2 | 59.5 | 9.2 |
| On target | 1046 | 2393 | 663 | 4102 | ||||
| Missing | 410 | 620 | 1292 | 2322 | ||||
| Total | 3185 | 4691 | 2522 | 103,98 | ||||
| Usual practice | ||||||||
| Status before | Off target | 1977 | 1740 | 654 | 4371 | 47.6 | 56.8 | 9.3 |
| On target | 1084 | 2287 | 680 | 4051 | ||||
| Missing | 451 | 680 | 1278 | 2409 | ||||
| Total | 3512 | 4707 | 2612 | 10,831 | ||||
Abbreviations: ABE, audit-based education; BP, blood pressure; G&P, guidelines and prompts; UP, usual practice.
In all, 12.3% more are at target post intervention with ABE, 9.2% with G&P, and 9.3% with UP.
Table 5. A multilevel logistic model to predict impact of arm of study and other factors on reduction in systolic BP >5 mm Hg.
|
Model performance |
|
AIC=11,851 |
BIC=11,916 | Log likelihood=−5916 | Deviance=−5916 | ROC C stat=0.625 |
|---|---|---|---|---|---|---|
| Random effects: |
|
|
|
|
|
|
| Groups | Name | Variance | s.d. | |||
| National practice ID | (Intercept) | 0.043 | 0.206 | |||
| Fixed effects | Estimate | s.e. | Pr(>|z|) | OR | Lower 95% CI | Upper 95% CI |
| (Intercept) | 1.035 | 0.127 | 0.000 | 2.815 | 2.193 | 3.613 |
| Study arms: audit-based education | 0.211 | 0.082 | 0.010 | 1.235 | 1.053 | 1.450 |
| Study arms: guidelines and prompts | 0.098 | 0.085 | 0.250 | 1.103 | 0.933 | 1.303 |
| Systolic BP (z scored) | 1.193 | 0.036 | <0.001 | 3.297 | 3.072 | 3.539 |
| Gender: male | 0.086 | 0.048 | 0.071 | 1.090 | 0.993 | 1.198 |
| IHD | 0.124 | 0.058 | 0.033 | 1.132 | 1.010 | 1.268 |
| Non-angiotensin-modulating antihypertensive drugs | −0.118 | 0.052 | 0.024 | 0.889 | 0.802 | 0.985 |
| Aged over 75 | −0.288 | 0.115 | 0.012 | 0.750 | 0.598 | 0.939 |
Abbreviations: AIC, Akaike's information criterion; BIC, Bayesian information criterion; BP, blood pressure; CI, confidence interval; CVA, cerebrovascular accident; IHD, ischemic heart disease; IMD, index of multiple deprivation; OR, odds ratio; PVD, peripheral vascular disease; ROC C stat, receiver operating characteristic area under the curve statistic; TIA, transient ischemic attack.
The estimate represents the change in SBP because of study arm or other variable.
Not in the model: IMD quartile, PVD, CVA, TIA, hypertension, heart failure, angiotensin-modulating drugs, Afro-Caribbean ethnicity, general practice list size.
Change in process and outcome measures
Within the ABE arm, there was a greater switch to using angiotensin-modulating antihypertensive therapy (angiotensin-converting enzyme inhibitors (ACE) and angiotensin receptor blockers), and a lower rate of mortality and onset of cardiovascular disease compared with the other two arms. 6.5% (n=1058) in the ABE arm; 6.9% (n=712) in G&P arm; and 5.8% (n=623) people were switched to from non-ACE to ACE antihypertensive medicines. The annualized incidence of new cardiovascular disease by arm was 2.4% (n=364), 2.7% (n=285), and 3.0% (n=325) for ABE, G&P, and UP arms, respectively (χ2 P=0.015). Renal function improved marginally more in the ABE arm, mean difference 1.99 ml/min; compared with an improvement of 1.95 and 1.96 ml/min in G&P and UP arms. Median estimate glomerular filtration rate improved from 53 to 54 ml/min. The mortality by arm was: 5.0% (n=828), 7.8% (n=954), and 6.6% (n=812) for ABE, G&P, and UP, respectively.
Multilevel model to explore reduction in SBP by study arm
The multilevel models using linear mixed models (LMMs) showed a statistically significant greater likelihood of lowering BP by around 2.4 mm Hg in the ABE arm of the trial compared with the UP arm (Table 6). The changes within the G&P arm compare with UP crossed parity, and were not significant. The interclass cluster correlation (ICC) value of 0.061 for the model was higher than that (ICC+0.03) used in the sample size calculation reported in our protocol.38
Table 6. A multilevel model to predict impact of arm of study and other factors on final SBP.
| Model performance | |||||
|---|---|---|---|---|---|
|
AIC=87,638 |
BIC=87,732 |
Log likelihood=−43,806 |
Deviance=87,617 |
REML deviance=87,612 |
ICC=0.05 |
| Random effects |
|
|
|
|
|
| Groups | Name | Variance | s.d. | ||
| National practice ID | (Intercept) | 10.878 | 3.2982 | ||
| Residual |
|
241.097 |
15.5273 |
|
|
| Fixed effects | Estimate | Lower 95% CI | Upper 95% CI | s.e. | Pr(|x|>0) |
| (Intercept) | 136.565 | 134.517 | 138.608 | 1.063 | <0.001 |
| Study arms: audit-based education | −2.408 | −4.285 | −0.593 | 0.979 | 0.012 |
| Study arms: guidelines and prompts | −0.925 | −2.845 | 0.963 | 0.984 | 0.329 |
| Previous systolic BP (z scored) | 2.856 | 2.558 | 3.164 | 0.155 | <0.001 |
| Gender: male | −1.208 | −1.856 | −0.559 | 0.328 | 0.000 |
| IHD | −0.841 | −1.632 | −0.049 | 0.403 | 0.038 |
| Hypertension | 1.359 | 0.576 | 2.163 | 0.401 | 0.001 |
| Heart failure | −1.651 | −3.117 | −0.153 | 0.743 | 0.026 |
| Non-angiotensin-modulating antihypertensive drugs | 1.245 | 0.434 | 2.033 | 0.409 | 0.002 |
| Afro-Caribbean | 2.548 | 0.635 | 4.386 | 0.964 | 0.009 |
| Aged over 75 | 2.097 | 0.546 | 3.631 | 0.794 | 0.008 |
Abbreviations: AIC, Akaike's information criterion; BIC, Bayesian information criterion; BP, blood pressure; CI, confidence interval; CVA, cerebrovascular accident; IHD, ischemic heart disease; ICC, interclass cluster correlation; IMD, index of multiple deprivation; OR, odds ratio; PVD, peripheral vascular disease; REML, restricted maximum likelihood; TIA, transient ischemic attack.
The estimate represents the change in SBP because of study arm or other variable.
Not in the model: IMD quartile, PVD, CVA, TIA, angiotensin-modulating drugs, general practice list size.
The LMM suggested that ABE lowered SBP by 2.41 mm Hg (95% CI 0.593–4.285, Table 6). Deprivation quartile, peripheral vascular disease, cerebrovascular accident and transient ischemic attack, angiotensin-modulating drugs, and general practice list size were excluded from the models as they had no significant impact. In addition to ABE, male gender, IHD, and chronic heart failure were independently associated with an increased lowering SBP; whereas the converse effect was found in people with a diagnosis of HT, and in people treated with non-angiotensin-modulating HT therapy (non-ACE HT Px), Afro-Caribbean ethnicity, and age over 75 years old. All other age bands were excluded as they had no significant influence on the model.
DISCUSSION
Principal findings
We found that practitioners exposed to the ABE intervention are more likely to achieve a greater reduction SBP in their patients with CKD than those practitioners exposed to UP; patients in the ABE arm also had a greater chance of achieving a >5 mm Hg reduction in SBP. A higher proportion of people in the ABE were changed to angiotensin-modulating antihypertensive therapy. Also, people in the ABE arm had fewer cardiovascular events and lower mortality. There was no evidence that G&P improved care or changed practice. G&P was not associated with having a significant reduction in BP. However, the differences between the arms were small, and much lower than the anticipated reduction. We also found that use of other antihypertensive therapy (i.e., which does not act on the angiotensin system) was associated with a higher SBP at the end of the trial; and being less likely to achieve ⩾5 mm Hg reduction in SBP.
Across all three arms people aged over 75, of Afro-Caribbean ethnicity, those treated with other (non-angiotensin-modulating) hypertensive therapy, and with a diagnosis of HT were less likely to achieve SBP target and reduce SBP. Conversely, ABE, male gender, IHD, chronic heart failure, and larger practices were associated with a higher chance of reaching target.
The study was over-recruited, and no practices withdrew post-randomization. Generally, there was a much higher prevalence of cerebrovascular disease among people with CKD.
Implications of the findings
Despite widespread skepticism among practitioners as to the significance of a CKD diagnosis in older people practices continued to allow access to their records even if they did not fully participate in their study arm intervention for the duration of the study. The participant general practices appeared willing to join and maintain involvement with improvement science projects. The reduction in SBP achieved, although small, under 3 mm Hg, is likely to have a significant clinical impact at the population level. Although this reduction in SBP is small and around a third of that achieved by treatment with BP lowering therapy, it is known that at the population level a reduction of 5 mm Hg produces a reduction of about 34% in stroke and 21% in IHD.39
The study outcomes should lead practitioners to reflect on how they treat different patient groups. People with IHD and chronic heart failure may have been perceived to be at higher risk and treated more aggressively as a result. There are large numbers of older people with HT and early CKD many of whom are female who may not be classified as high risk by many general practitioners. It is possible that clinicians were treating these people to the P4P target for HT (SBP of 145 mm Hg) ignoring their CKD.
Caution should be exercised in the interpretation of these findings because the CIs around the finding of benefit from ABE were wide. However, we have demonstrated that ABE appears to be effective in improving the proportion of people who achieve a reduction in SBP in primary care. If a similar effect is confirmed in other studies then we would be more optimistic of the effectiveness of this intervention. The use of ACE was promoted within the ABE, and it is possible that accounted for the improvement in this arm of the trial. Although we noted a lower incidence of vascular events and mortality we have not demonstrated any effect related to variables rejected from the model.
ABE should be considered as an intervention by those seeking to improve quality. Sending out academic detailing in G&P did not appear to change practice. Based on these findings, it cannot be recommended as a QI strategy.
Comparison with the literature
Although the trial set out to follow the CONSORT recommendations for randomized controlled trials40 we did not achieve an even distribution of practice characteristics, demographic, and comorbidities between study arms. Our simple block randomization led to there being differences between the study arms. In a future study, we would use allocation techniques that achieve a better baseline balance.41
The findings contrast with the findings of a study exploring the variation in incidence of renal replacement therapy. This study showed that deprivation, non-white ethnicity, diabetes, and non-achievement of P4P BP target in CKD, were predictor variables of progression to renal replacement.42
A number of other interventions to improve CKD management have been reported but these have mainly looked at their impact on referral rather than on BP management.43
ABE has many of the characteristics of an effective intervention to improve quality in primary care. It is tailored, educational, and multifaceted.44 ABE also includes some of the features of the chronic care model: principally better use of practice information systems to prepare proactive practice teams looking to engage and change their quality of service delivery.45 As more information about how to effect change becomes available, ABE may be developed further, or alternatively more likely candidates to drive change may be adopted.46
Limitations of the method
We did not fully implement ABE as designed. A general practitioner from the study team was not always present at the feedback meetings, although where this happened we tried to at least give individual feedback to that practice. The majority of attendees at ABE sessions were general practitioners or practice nurses from non-neighboring practices so the data were fed back to groups from practices who were meeting for the first time. No ‘local queries' were run to identify individual patients requiring intervention for practices. This important aspect of the intervention was omitted as a decision of the wider study team who were concerned about the delays in ethics and in recruitment early in the study. However, this omission is likely to have lessened the effect of the intervention: a study of HT management, which compared audit-based feedback with audit plus details of patients risk achieved a greater reduction in BP in the latter group.47 We could also have examined pulse pressure rather than SBP the latter may be a better predictor of progression in CKD.48
It is possible that changes in end-digit preference in recording BP may have influenced the recording of BP49 and repeated measures may result in regression to the mean;50 but these effects would be expected to have an equal effect on each arm of the study. Also, the use of only two BP readings, the two furthest apart in the study period, may have resulted in a loss of fidelity compared with using more. However, this maximized the number of people we could include in the study.
Proteinuria is an independent risk factor for cardiovascular risk in CKD and an important effect modifier for intervention; incomplete recording in people with CKD meant we could not look at this as an additional variable.51
The power of the analysis was restricted by inter-practice variation in demographics and cardiovascular comorbidity. We cannot report yet on the cost effectiveness of ABE as an intervention but are due to conduct an economic analysis.
Call for further research
Further studies are needed to test the effectiveness of ABE, perhaps in those people with CKD at highest risk, for example, those with proteinuria or declining renal function. It may have been better to have chosen a stepped wedge design. This would have been ethically simpler, as all arms are exposed to the same intervention components, and may have overcome some of the initial delays in recruitment.52
CONCLUSIONS
ABE is a responsive tool to feedback clinically led customized analyses to improve quality. Here we demonstrate, in the first trial of an educational intervention underpinned by information technology, its potential to improve chronic disease management in primary care. Further work is required to determine the generalizability and cost-effectiveness of this approach.
MATERIALS AND METHODS
Trial design
The quality improvement in CKD (QICKD) trial was a three-arm cluster randomized study with an intervention period of 2 years,38 approved by research ethics committees and registered with a clinical trials database.53 The QICKD trial compared two QI interventions G&P and ABE, with UP.
Setting
We carried out this study in UK primary care. This is a setting that lends itself to this type of research.54 There is a registration-based system (patients only register with one practice). Practices are computerized and electronic patient record (EPR) systems are used almost universally at the point of care.55 Repeat prescribing data are complete and electronic links to pathology labs means that test results are sent directly into practice EPR systems. The UK primary care P4P scheme rewards quality based on routinely collected data measures; this in turn has further improved data quality.56 P4P was first introduced in April 2004, mainly targeted on vascular disease, with CKD domain added in 2006. The provision of a common data extraction platform for the different brands of EPR systems (MIQUEST—Morbidity Information Query and Export Syntax) make conducting this type of study more straightforward. We became involved with CKD in collaboration with renal specialists interested in identifying people with CKD from general practice computer records.57 We demonstrated this process was valid58 and could be used to define the United Kingdom prevalence of CKD.59 The reliability of the diagnosis improving after 2006 when national quality control system was put in place,60 although there may be some disparity in creatinine testing.61
Participants
The study participants were health-care professionals who managed people with CKD in the study practices. Between December 2007 and May 2008, we recruited practices that had the same EPR system for at least 5 years. Where they changed EPR system we were able to map patients from one system to the next by anonymous data linkage based on year of birth, gender, date of registration, and date of last BP recording. Two rounds of follow-up data collections were conducted after the intervention: between 1 May 2009 and 29 September 2009; and between 1 April 2010 and 29 July 2010. We defined the people with CKD as those with an estimated glomerular filtration rate of <60 ml/min (stages 3–5 CKD) based on two readings at least 90 days apart, whenever available. This fits with international guidance and smoothes the effect of creatinine fluctuation.5 We used the Modified Diet in Renal Disease four-item equation. We restricted our analysis to the group of adults, people over 18 years old, who fulfilled these criteria between 1 July 2007 and 30 June 2008 7.29% (41,183/565,016; 95% CI 7.22–7.36%) of the registered population at the start of the study were labeled the ‘CKD cases.' We included in the analysis people with CKD who had raised BP, or a diagnosis of HT, or cardiovascular comorbidities treated using antihypertensive agents.
Interventions
G&P involved the sending of academic detailing,62 printed information containing local guidelines on CKD management;63 and providing access to an information website. This was subsequently revised to the provision of national guidance following the publication of CKD guidelines by the National Institute for Health and Clinical Excellence (NICE). This intervention was designed to be typical of low cost methods used by the NHS used to prompt practitioners about quality. ABE (Box 1) was largely implemented as planned. However, we did not include ‘local queries', which provide practices with lists of patients with CKD who are suboptimally managed; additionally this arm did not receive ABE in addition to G&P as originally planned, until the second year. In our protocol, we intended that ABE would be provided in addition to G&P rather than as an alternative.38
Sample size/power calculation
Our intention was to recruit a sample to finish the study with 25 practices per arm. We identified practices for the study by first recruiting renal centers willing to take part in the study. This was because the G&P intervention and ABE arms required the agreement and participation of the local renal center. We identified renal centers wishing to participate in Leicester, Birmingham, Cambridge, southwest London, and Surrey and Sussex. We then recruited practices in these areas via a range of sources including through dedicated practice liaison managers, teaching practice networks, and the local Primary Care Research Networks.
The study was powered to detect a >3 mm Hg difference in SBP between the group. Using a sample data set of 30 practices, we estimated that the variation between practice means has a s.d. of 3.77 mm Hg. Assuming this sample of 30 practices to be representative of the study practices in terms of their size and number of CKD patients, we estimated that a sample size of 25 practices per intervention group was required to detect a difference of 3 mm Hg at the 5% level with a power of 80%.
Randomization (including blinding)
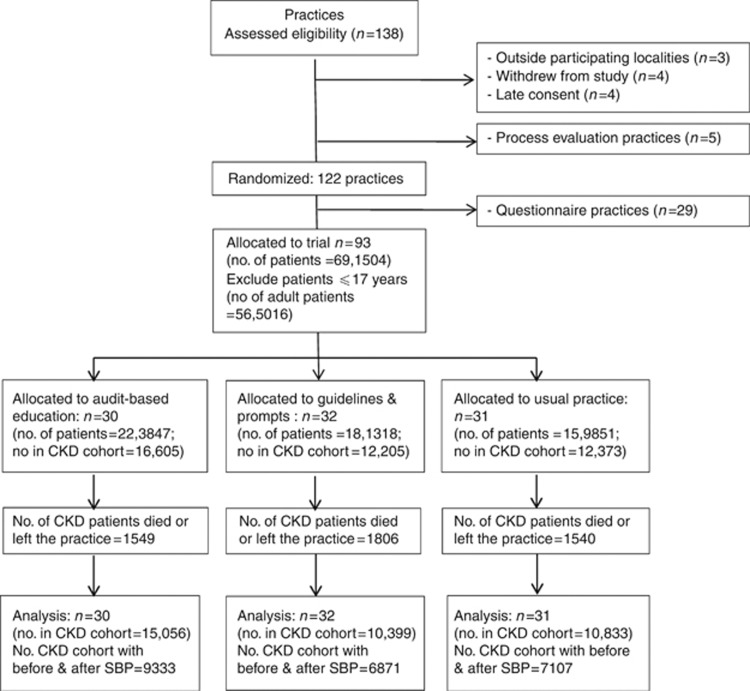
A total of 138 practices expressed interest in the project although 16 did not progress to randomization (Figure 2). The reasons for this were: outside the participating health service locality (n=3); not consented before we closed recruitment (n=4); withdrew pre-randomization (n=4). Five practices were allocated to an in-depth process evaluation arm. A randomization sequence was created in Microsoft Excel. As practices were recruited a centralized recruitment list was appended on a weekly basis and practices assigned a unique sequential study number. Newly recruited practices were then allocated to each of the three study arms at random in blocks of nine. Once each successive block was filled, the practices were informed of their allocation. Once the total study practice cohort recruitment was completed 10 practices from each arm were randomly allocated per study arm to become questionnaire practices. These practices received questionnaires about their confidence and competence in managing CKD. They were not included in this analysis. Investigators were blinded to the study arm allocation during analysis, and arms were designated by a number within the database.
Figure 2.
CONSORT (2010) flow diagram of practice recruitment and exclusion in the quality improvement in chronic kidney disease (QICKD) trial. SBP, systolic blood pressure.
Data
We extracted data from general practice EPR systems using MIQUEST64 and aggregated the data using well-established methods.65 We have made our data dictionary, an online lookup tool, which lists every variable extracted online, publicly available.66
From within the total population (N=951,764), we identified deaths and leavers from the practices (n=109,701, 11.5%) and excluded them from the study (Figure 2).
We report the completeness of recording of study variables (Supplementary data file), demographics, and key comorbidities. Demographics include: age, index of multiple deprivation score,67 and ethnicity by study arm. We mapped ethnicity codes to the National Statistics 5+1 categories using a mapping process developed in-house.68 We additionally captured any coding suggestive of African-Caribbean ethnicity as this group has a special correction factor in the formula used to estimate kidney function.69 We also record the proportion of people at baseline with diabetes having corrected miscoding, and misdiagnosis;70 as the thresholds for SBP are different for those with diabetes. We also report the prevalence of five other cardiovascular co morbidities: IHD, HT, chronic heart failure, peripheral vascular disease, and cerebrovascular disease.
Outcomes
We report the effect of the intervention arm, compared with UP and other likely predictor variables on reduction in SBP over the period of the study. We compared the earliest BP measure in the first year of the study with the latest recorded in the last. We checked to see if there was any difference in the time interval between the earliest and latest SBP reading by arm, reporting median and interquartile range.
We used ‘Z-score' to transform BP. This is a standard methodology for transforming a continuous variable by (x-mean(x))/s.d.(x). The resultant variable has a mean of zero and a s.d. of 1; making SBP more readily combined with variables with binary values. We also reported incident cases of cardiovascular disease and HT, and any change in renal function as measured using estimate glomerular filtration rate.
Statistical methods
We report data using the mean and s.d. and s.e.m. where appropriate. For non-normally distributed data, we report the median and interquartile range. We quote prevalence as a percentage of the population aged 18 years and over with 95% CIs. We used Pearson's χ2 to report any differences in the proportion of people in each study arm.
The secondary outcome measure is a binary outcome, whether a patient achieved the target BP according to NICE guidelines63 or a reduction of ⩾5 mm Hg we implemented a simple multilevel logistic regression model,71 using ORs as a measure of effect size.
We developed multilevel models using LMMs fitted using restricted maximum likelihood; we applied our models to the whole study arm with CKD and paired BPs. These models were used to explore the influence of the study arms whilst controlling for age, deprivation, sex, ethnicity, diabetes, and cardiovascular disease and differences in practice size between arms. Our approach included checking for collinearity between variables. This approach was adopted instead of the originally planned analysis of variance because of difference in baseline characteristics between the study arms in particular in the age–sex distribution. These baseline differences included a difference in practice size, which we have included in the analysis, as this might affect quality achievement.72
The LMM looked at the impact of potential predictor variables on reduction of SBP. These LMM were developed using R Statistical package software 2.14 (www.r-project.org) with the lme4 add in.73 By default, lme4 fits the model using restricting maximum likelihood. Across all arms of the trial, patients were nested within their general practice using a random intercept. Model selection was performed following Maindonald and Braun's approach, which aims to maximize the log likelihood, by backward stepwise elimination of variables.74 We used Markov Chain Monte Carlo methods to estimate the 95% credible interval for the estimates obtained by this method; these are broadly similar to confidence intervals, and we report them as such.
Acknowledgments
The following patients and practices contributed to the study. Bernie Stribling initially, then Jo Moore, project managers. Gabreilla Gomez (GG) for initial support with the application. Michael Nation (MN) Director Kidney Research UK for input into the initial protocol development and support throughout the study. Nigel Hague, who wrote all the initial study data extraction queries and attended some ABE workshops. Azhar Farooqi for leadership of the northern ABE; Iain Crinson for methodological advice for the process evaluation; Fiona Reid for statistical support and Andre Ring for data collection. Nigel Mehdi and Mark Bradley of Mehdi-Ward for their assistance with setting up the database and assistance with databases upload and query utilities; Antonios Ntasioudis who worked extensively with the project data. This work was funded by the Health Foundation and Edith Murphy Trust; National Institute for Health Research (NIHR) research portfolio supported participant practices; Edith Murphy Foundation. The Joint Research Office at St George's, University of London and St George's Healthcare Trust for supporting the application and sponsorship of the research.
SdeL led the expert reference group for the development of the Quality and Outcomes Framework (QOF) CKD indicator. A P4P chronic disease management program. Subsequently lead author for NHS Employers/British Medical Association Frequently Asked Questions about CKD, now in its third edition; and General Practitioner Advisor to NICE for CKD indicator. Funding to write three articles and give two invited conference lectures in last 5 years. HG was an expert advisor to QOF, co-author CKD FAQs, honoraria for lectures to General Practitioners. AT co-authored CKD frequently asked questions. NT was deputy on NICE clinical guideline development group for CKD guidance 2008. Funding to write eight articles and develop on-line learning materials for early management of CKD in last 5 years. KH co-authored for NHS Employers/British Medical Association Frequently Asked Questions about CKD, now in its third edition; Member of NICE CKD clinical guideline develop group; Honoraria received for lectures to General Practitioners on CKD. The remaining authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Table S1. Data collection dates from participating practices.
Table S2. Number of practices, total population, and mean list for study joiner, deaths and leavers and questionnaire and process evaluation practices.
Table S3. Proportion of practice population complete data.
Table S4. Proportion of practice population with records in the key diagnostic and management variables.
Table S5. Proportion of chronic kidney disease (CKD) cases with records in the key diagnostic and management variables.
Table S6. Population mean age by arm of study, and mean deprivation index.
Table S7. Population ethnicity by study arm.
Table S8. Prevalence of common cardiovascular comorbidities by study arm in the trial practices.
Table S9. Ethnicity recording in the adult population in the trial practices.
Table S10. a–b Baseline characteristics of the chronic kidney disease (CKD) cohort.
Table S11. a–d Incidence of hypertension by study arm and for the chronic kidney disease (CKD) cohort by study arm.
Table S12. a–b Renal function recording.
Table S13. Change in renal function (measured as estimate glomerular filtration rate (eGFR)) between study arms.
Table S14. Incident cases of cardiovascular disease by study arm for the population and chronic kidney disease (CKD) cases.
Table S15. a–d Change to taking an angiotensin-modulating antihypertensive medicine.
Table S16. a–c Death and leavers.
Table S17. Difference in time between first and last blood pressure (BP) reading by arm of study.
Table S18. a–d Absolute change in systolic blood pressure (SBP).
Table S19. Baseline characteristics of each study arm population.
Table S20. a–b Change in diastolic blood pressure (BP).
Table S21. a–b Change in pulse pressure.
Table S22. Multilevel model using pulse pressure as an alternative primary outcome measure.
Table S23. a–b Sensitivity/missing values—simulation of patients achieving a reduction of 5 mm Hg in systolic blood pressure.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
SdeL conceived the idea for a smaller scale trial of ABE as a QI intervention in CKD with GG. Worked with NM, to develop the final successful funding application. Lead author for the protocol, supported HG with the ethics application. PI, strongly engaged in all parts of the trial and the lead author of the final write-up. HG worked with the principal investigator to develop initial funding application, co-authored protocol, led ethics application, senior study investigator strongly engaged in all parts of the trial, and made significant contribution to papers. SJ assisted with overall design of the analysis plan. Supervision and direction of the final analysis for this paper and conducted some of the analyses; including the development of the multilevel models. A significant contribution to the papers. TC worked with the principal investigator to design the data set and queries, led data analysis team, organized, and developed the academic input into ABE and made significant contribution to papers. JvV conducted much of the data processing and contributed to the design of the database and data handing methods. Conducted the block randomization. Some basic descriptive results for the analysis and supporting SJ in developing and testing the multilevel models. AT is an active member of the study team throughout. Assistance with recruitment, ABE in the south of England. Major input into the process evaluation, and the reliability of the interventions. Contribution to the analysis and papers. NT is an active member of the study team throughout. Recruited and liaised with participating practices in southern localities, organized, and ran ABE interventions in southern localities, reviewed manuscript. NJ is an active member of the study team throughout. Recruited and liaised with participating practices in northern localities, organized, and ran ABE interventions in northern localities, reviewed manuscript and contributed to final write-up. OD contributed as a member of the local study team, using clinical knowledge to interpret and quality assure study findings and outputs. IR active member of the study team throughout. Assistance with recruitment, ABE in the south of England. Contribution to the analysis and papers. AM extensive assistance with the statistical review and response to reviewers' comments. KH worked with the principal investigator to develop initial funding application, co-authored protocol, senior study investigator strongly engaged in all parts of the trial, and made significant contribution to papers. SdeL is the guarantor for this paper. The Joint Research Office of St George's, University of London and St George's Healthcare Trust were the research sponsors. They fulfilled the role of the sponsor as required to submit an NHS research ethics application ensuring that the research team had appropriate arrangements in place. We are able to make available the table of results used to carry out the analysis reported in this paper.
Supplementary Material
References
- Levin A. The need for optimal and coordinated management of CKD. Kidney Int Suppl. 2005;99:S7–10. doi: 10.1111/j.1523-1755.2005.09902.x. [DOI] [PubMed] [Google Scholar]
- Gomez GB, de Lusignan S, Gallagher H. Chronic kidney disease: a new priority for primary care. Br J Gen Pract. 2006;56:908–910. [PMC free article] [PubMed] [Google Scholar]
- Herget-Rosenthal S, Quellmann T, Linden C, et al. Management of advanced chronic kidney disease in primary care—current data from Germany. Int J Clin Pract. 2006;60:941–948. doi: 10.1111/j.1742-1241.2006.01028.x. [DOI] [PubMed] [Google Scholar]
- Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69:375–382. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- de Lusignan S, Tomson C, Harris K, et al. Creatinine fluctuation has a greater effect than the formula to estimate glomerular filtration rate on the prevalence of chronic kidney disease. Nephron Clin Pract. 2011;117:c213–c224. doi: 10.1159/000320341. [DOI] [PubMed] [Google Scholar]
- O'Seaghdha CM, Hwang SJ, Upadhyay A, et al. Predictors of incident albuminuria in the Framingham Offspring cohort. Am J Kidney Dis. 2010;56:852–860. doi: 10.1053/j.ajkd.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce MA, Beech BM, Crook ED, et al. Association of socioeconomic status and CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2010;55:1001–1008. doi: 10.1053/j.ajkd.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly SE, Burrows NR, Chen SC, et al. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the Kidney Early Evaluation Program (KEEP) Clin J Am Soc Nephrol. 2011;6:1858–1865. doi: 10.2215/CJN.00500111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qaoud TM, Nitsch D, Wells J, et al. Socioeconomic status and reduced kidney function in the Whitehall II Study: role of obesity and metabolic syndrome. Am J Kidney Dis. 2011;58:389–397. doi: 10.1053/j.ajkd.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EH, Lim SY, Cho KH, et al. GFR and cardiovascular outcomes after acute myocardial infarction: results from the Korea acute myocardial infarction registry. Am J Kidney Dis. 2012;59:795–802. doi: 10.1053/j.ajkd.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Levey AS, Astor BC, Stevens LA, et al. Chronic kidney disease, diabetes, and hypertension: what's in a name. Kidney Int. 2010;78:19–22. doi: 10.1038/ki.2010.115. [DOI] [PubMed] [Google Scholar]
- Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- Glynn LG, Buckley B, Reddan D, et al. Multimorbidity and risk among patients with established cardiovascular disease: a cohort study. Br J Gen Pract. 2008;58:488–494. doi: 10.3399/bjgp08X319459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- Weiss JW, Johnson ES, Petrik A, et al. Systolic blood pressure and mortality among older community-dwelling adults with CKD. Am J Kidney Dis. 2010;56:1062–1071. doi: 10.1053/j.ajkd.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar TH, Stark PC, Schmid CH, AIPRD Study Group et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Gallagher H, de Lusignan S, Harris K, et al. Quality-improvement strategies for the management of hypertension in chronic kidney disease in primary care: a systematic review. Br J Gen Pract. 2010;60:e258–e265. doi: 10.3399/bjgp10X502164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards N, Harris K, Whitfield M, et al. Primary care-based disease management of chronic kidney disease (CKD), based on estimated glomerular filtration rate (eGFR) reporting, improves patient outcomes. Nephrol Dial Transplant. 2008;23:549–555. doi: 10.1093/ndt/gfm857. [DOI] [PubMed] [Google Scholar]
- Klebe B, Irving J, Stevens PE, et al. The cost of implementing UK guidelines for the management of chronic kidney disease. Nephrol Dial Transplant. 2007;22:2504–2512. doi: 10.1093/ndt/gfm248. [DOI] [PubMed] [Google Scholar]
- Couser WG, Remuzzi G, Mendis S, et al. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- Crinson I, Gallagher H, Thomas N, et al. How ready is general practice to improve quality in chronic kidney disease? A diagnostic analysis. Br J Gen Pract. 2010;60:403–409. doi: 10.3399/bjgp10X502100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles PD, Fitzmaurice DA. Formula estimation of glomerular filtration rate: have we gone wrong. BMJ. 2007;334:1198–1200. doi: 10.1136/bmj.39226.400694.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winearls CG, Lamb EJ. Chronic kidney disease: the CKD-EPI equation to estimate GFR-better alchemy. Nat Rev Nephrol. 2011;7:127–128. doi: 10.1038/nrneph.2010.190. [DOI] [PubMed] [Google Scholar]
- Navaneethan SD, Nigwekar SU, Sehgal AR, et al. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2009. p. CD007004. [DOI] [PubMed]
- Sharma P, Blackburn RC, Parke CL, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease. Cochrane Database Syst Rev. 2011. p. CD007751. [DOI] [PubMed]
- Strippoli GF, Bonifati C, Craig M, et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev. 2006. p. CD006257. [DOI] [PMC free article] [PubMed]
- Black C, Sharma P, Scotland G, et al. Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess. 2010;14:1–184. doi: 10.3310/hta14210. [DOI] [PubMed] [Google Scholar]
- Fink HA, Ishani A, Taylor BC, et al. Chronic Kidney Disease Stages 1–3: Screening, Monitoring, and Treatment [Internet] Agency for Healthcare Research and Quality (US): Rockville, MD, USA; 2012. [PubMed] [Google Scholar]
- de Lusignan S. An educational intervention, involving feedback of routinely collected computer data, to improve cardiovascular disease management in UK primary care. Methods Inf Med. 2007;46:57–62. [PubMed] [Google Scholar]
- de Lusignan S, Hague N, Brown A, et al. An educational intervention to improve data recording in the management of ischaemic heart disease in primary care. J Public Health (Oxf) 2004;26:34–37. doi: 10.1093/pubmed/fdh104. [DOI] [PubMed] [Google Scholar]
- de Lusignan S, Belsey J, Hague N, et al. Audit-based education to reduce suboptimal management of cholesterol in primary care: a before and after study. J Public Health (Oxf) 2006;28:361–369. doi: 10.1093/pubmed/fdl052. [DOI] [PubMed] [Google Scholar]
- Forsetlund L, Bjørndal A, Rashidian A, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2009. p. CD003030. [DOI] [PMC free article] [PubMed]
- Jamtvedt G, Young JM, Kristoffersen DT, et al. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2006. p. CD000259. [DOI] [PubMed]
- Francis JJ, Stockton C, Eccles MP, et al. Evidence-based selection of theories for designing behaviour change interventions: using methods based on theoretical construct domains to understand clinicians' blood transfusion behaviour. Br J Health Psychol. 2009;14 (Pt 4:625–646. doi: 10.1348/135910708X397025. [DOI] [PubMed] [Google Scholar]
- Gardner B, Whittington C, McAteer J, et al. Using theory to synthesise evidence from behaviour change interventions: the example of audit and feedback. Soc Sci Med. 2010;70:1618–1625. doi: 10.1016/j.socscimed.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care. 2009;47:356–363. doi: 10.1097/MLR.0b013e3181893f6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lusignan S, Gallagher H, Chan T, et al. The QICKD study protocol: a cluster randomised trial to compare quality improvement interventions to lower systolic BP in chronic kidney disease (CKD) in primary care. Implement Sci. 2009;4:39. doi: 10.1186/1748-5908-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7:1–94. doi: 10.3310/hta7310. [DOI] [PubMed] [Google Scholar]
- Ivers NM, Taljaard M, Dixon S, et al. Impact of CONSORT extension for cluster randomised trials on quality of reporting and study methodology: review of random sample of 300 trials, 2000-8. BMJ. 2011;343:d5886. doi: 10.1136/bmj.d5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab GM, Butcher I. Balance in cluster randomized trials. Stat Med. 2001;20:351–365. doi: 10.1002/1097-0258(20010215)20:3<351::aid-sim797>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Dhoul N, de Lusignan S, Dmitrieva O, et al. Quality achievement and disease prevalence in primary care predicts regional variation in renal replacement therapy (RRT) incidence: an ecological study. Nephrol Dial Transplant. 2011;27:739–746. doi: 10.1093/ndt/gfr347. [DOI] [PubMed] [Google Scholar]
- Stevens P, de Lusignan S, Farmer C, et al. Engaging primary care in CKD initiatives: the UK experienceSubmitted for publication NDTNephrol Dial Transplant 201227iii5–11. [DOI] [PubMed] [Google Scholar]
- Hulscher ME, Wensing M, van Der Weijden T, et al. Interventions to implement prevention in primary care. Cochrane Database Syst Rev. 2001. p. CD000362. [DOI] [PubMed]
- Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–1779. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- Weir MC, Ryan R, Mayhew A, et al. The Rx for Change database: a first-in-class tool for optimal prescribing and medicines use. Implement Sci. 2010;5:89. doi: 10.1186/1748-5908-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E, Sullivan F, Grimshaw JM, et al. Improving management of hypertension in general practice: a randomised controlled trial of feedback derived from electronic patient data. Br J Gen Pract. 2005;55:94–101. [PMC free article] [PubMed] [Google Scholar]
- Arulkumaran N, Diwakar R, Tahir Z, et al. Pulse pressure and progression of chronic kidney disease. J Nephrol. 2010;23:189–193. [PubMed] [Google Scholar]
- Mo R, Omvik P, Lund-Johansen P. The Bergen Blood Pressure Study. Estimated prevalence of postural hypotension is influenced by the alerting reaction to blood pressure measurement. J Hum Hypertens. 1994;8:171–176. [PubMed] [Google Scholar]
- Alsanjari ON, de Lusignan S, van Vlymen J, et al. Trends and transient change in end-digit preference in blood pressure recording: studies of sequential and longitudinal collected primary care data. Int J Clin Pract. 2012;66:37–43. doi: 10.1111/j.1742-1241.2011.02781.x. [DOI] [PubMed] [Google Scholar]
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- Mdege ND, Man MS, Taylor Nee Brown CA, et al. Systematic review of stepped wedge cluster randomized trials shows that design is particularly used to evaluate interventions during routine implementation. J Clin Epidemiol. 2011;64:936–948. doi: 10.1016/j.jclinepi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Current controlled trials URL http://www.controlled-trials.com/ISRCTN56023731/ .
- de Lusignan S, van Weel C. The use of routinely collected computer data for research in primary care: opportunities and challenges. Fam Pract. 2006;23:253–263. doi: 10.1093/fampra/cmi106. [DOI] [PubMed] [Google Scholar]
- Schade CP, Sullivan FM, de Lusignan S, et al. e-Prescribing, efficiency, quality: lessons from the computerization of UK family practice. J Am Med Inform Assoc. 2006;13:470–475. doi: 10.1197/jamia.M2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lusignan S, Chan T. The development of primary care information technology in the United kingdom. J Ambul Care Manage. 2008;31:201–210. doi: 10.1097/01.JAC.0000324664.88131.d2. [DOI] [PubMed] [Google Scholar]
- de Lusignan S, Chan T, Stevens P, et al. Identifying patients with chronic kidney disease from general practice computer records. Fam Pract. 2005;22:234–241. doi: 10.1093/fampra/cmi026. [DOI] [PubMed] [Google Scholar]
- Anandarajah S, Tai T, de Lusignan S, et al. The validity of searching routinely collected general practice computer data to identify patients with chronic kidney disease (CKD): a manual review of 500 medical records. Nephrol Dial Transplant. 2005;20:2089–2096. doi: 10.1093/ndt/gfi006. [DOI] [PubMed] [Google Scholar]
- Stevens PE, O'Donoghue DJ, de Lusignan S, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72:92–99. doi: 10.1038/sj.ki.5002273. [DOI] [PubMed] [Google Scholar]
- Lamb EJ, Vickery S, Dalton RN, et al. Estimating GFR with ID-MS traceable creatinine assays. Ann Clin Biochem. 2006;43 (Pt 4:327. doi: 10.1258/000456306777695663. [DOI] [PubMed] [Google Scholar]
- de Lusignan S, Nitsch D, Belsey J, et al. Disparities in testing for renal function in UK primary care: cross-sectional study. Fam Pract. 2011;28:638–646. doi: 10.1093/fampra/cmr036. [DOI] [PubMed] [Google Scholar]
- Soumerai SB, Avorn J. Principles of educational outreach ('academic detailing') to improve clinical decision making. JAMA. 1990;263:549–556. [PubMed] [Google Scholar]
- National Collaborating Centre for Chronic Conditions: Chronic kidney disease: national clinical guideline for early identification and management in adults in primary and secondary care2008. URL http://www.nice.org.uk/cg73 . [PubMed]
- Michalakidis G, Kumarapeli P, Ring A, et al. A system for solution-orientated reporting of errors associated with the extraction of routinely collected clinical data for research and quality improvement. Stud Health Technol Inform. 2010;160 (Pt 1:724–728. [PubMed] [Google Scholar]
- van Vlymen J, de Lusignan S, Hague N, et al. Ensuring the Quality of Aggregated General Practice Data: Lessons from the Primary Care Data Quality Programme (PCDQ) Stud Health Technol Inform. 2005;116:1010–1015. [PubMed] [Google Scholar]
- Clinical Informatics. QICKD trial data dictionary ‘QICKD Dictionary.' URL http://www.clininf.eu/qickd-data-dictionary.html .
- UK Statistics Authority. Index of Multiple Deprivation (IMD)2004. URL http://data.gov.uk/dataset/imd_2004 .
- Kumarapeli P, Stepaniuk R, de Lusignan S, et al. Ethnicity recording in general practice computer systems. J Public Health (Oxf) 2006;28:283–287. doi: 10.1093/pubmed/fdl044. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- de Lusignan S, Khunti K, Belsey J, et al. A method of identifying and correcting miscoding, misclassification and misdiagnosis in diabetes: a pilot and validation study of routinely collected data. Diabet Med. 2010;27:203–209. doi: 10.1111/j.1464-5491.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- Omar R, Thompson S. Analysis of a cluster randomized trial with binary outcome data using a multi-level model. Stat Med. 2000;19:2675–2688. doi: 10.1002/1097-0258(20001015)19:19<2675::aid-sim556>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Saxena S, Car J, Eldred D, et al. Practice size, caseload, deprivation and quality of care of patients with coronary heart disease, hypertension and stroke in primary care: national cross-sectional study. BMC Health Serv Res. 2007;7:96. doi: 10.1186/1472-6963-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comprehensive R Archive Network (CRAN). lme4: Linear mixed-effects models using S4 classes. Fit linear and generalized linear mixed-effects models. URL http://cran.us.R-project.org .
- Maindonald J, Braun J.Data Analysis and Graphics Using R3rd EdnCambridge University Press:: Cambridge; 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.