Abstract
Rationale: Air pollution is a known asthma trigger and has been associated with short-term asthma symptoms, airway inflammation, decreased lung function, and reduced response to asthma rescue medications.
Objectives: To assess a causal relationship between air pollution and childhood asthma using data that address temporality by estimating air pollution exposures before the development of asthma and to establish the generalizability of the association by studying diverse racial/ethnic populations in different geographic regions.
Methods: This study included Latino (n = 3,343) and African American (n = 977) participants with and without asthma from five urban regions in the mainland United States and Puerto Rico. Residential history and data from local ambient air monitoring stations were used to estimate average annual exposure to five air pollutants: ozone, nitrogen dioxide (NO2), sulfur dioxide, particulate matter not greater than 10 μm in diameter, and particulate matter not greater than 2.5 μm in diameter. Within each region, we performed logistic regression to determine the relationship between early-life exposure to air pollutants and subsequent asthma diagnosis. A random-effects model was used to combine the region-specific effects and generate summary odds ratios for each pollutant.
Measurements and Main Results: After adjustment for confounders, a 5-ppb increase in average NO2 during the first year of life was associated with an odds ratio of 1.17 for physician-diagnosed asthma (95% confidence interval, 1.04–1.31).
Conclusions: Early-life NO2 exposure is associated with childhood asthma in Latinos and African Americans. These results add to a growing body of evidence that traffic-related pollutants may be causally related to childhood asthma.
Keywords: air pollution, minority, children, asthma
At a Glance Commentary
Scientific Knowledge on the Subject
Air pollution has been associated with asthma prevalence. However, few studies look at early-life exposures before the development of childhood asthma and many are limited to populations of mostly European descent.
What This Study Adds to the Field
Using the largest pediatric gene–environment study of asthma in Latinos and African Americans in the United States, we found that exposure during infancy to NO2, a traffic-related air pollutant, was associated with increased risk for subsequent development of childhood asthma. Our results suggest that air pollution may contribute to the higher prevalence of asthma, especially in some minority children exposed to higher levels of air pollution.
Asthma is the most common chronic disease in American children (1). In 2011, there were approximately 25.9 million Americans with asthma, 7.1 million of those being children (2). In 2008, asthma resulted in 14.4 million missed school days and 14.2 million lost work days, costing $56.0 billion in direct and indirect costs (3). A substantial proportion of asthma cases can be prevented, underscoring the unnecessary burden of this disease. According to the World Health Organization, up to 44% of the asthma burden can be attributed to modifiable environmental factors (4), such as air pollution.
Current exposure to air pollution has been associated with short-term asthma outcomes including emergency hospitalization (5–7), reduced lung function (8–11), poor asthma control (12), and reduced response to asthma rescue medications (13). Whereas it is generally accepted that air pollution can aggravate existing asthma, it is less clear whether exposure to air pollutants plays a causal role in the development of asthma. Cross-sectional studies have found an association between current air pollution levels and asthma prevalence, implying that pollution may increase the risk for developing asthma (14–23). However, studies that measure the association of air pollution exposures that occur before asthma onset are necessary to evaluate the causality of this association.
Fewer studies have been conducted using early-life air pollution exposures and asthma incidence in children, in part because it requires costly longitudinal follow-up of a cohort, or the means to measure an exposure that has already occurred. Furthermore, most studies focus on a single geographic region with participants primarily of European descent. A meta-analysis of 17 population-based cohort studies found a statistically significant odds ratio (OR) of 1.07 (95% confidence interval [CI], 1.02–1.13) for every 10-μg/m3 increase in nitrogen dioxide (NO2) (24). However, both child and adult cohorts were used in this meta-analysis and many (11 of 17) were based in Europe. A similar meta-analysis with 19 studies conducted exclusively in children found a statistically significant association between the incidence of childhood asthma and NO2 (OR, 1.14; 95% CI, 1.06–1.24, per 10-μg/m3 increase) (16). A review of these and other studies concluded that traffic-related pollution may play a role in the development of asthma, especially in individuals living near high-volume roadways (25).
Latino and African American populations often live in neighborhoods with high levels of air pollution (26), and some of these groups have the highest prevalence of asthma in the United States (27). Puerto Ricans (16.6%) and African Americans (11.1%) have among the highest prevalence, significantly higher than that of white individuals (7.8%). Interestingly, Mexicans have one of the lowest prevalence rates (4.9%), which challenges the practice of grouping Puerto Ricans and Mexicans together as “Hispanic/Latino.” Despite the fact that African Americans and some Latino subgroups have a high prevalence of asthma and a disproportionate exposure to air pollution, little research has been conducted in these populations (28, 29). To adequately assess the relationship between traffic-related air pollution and childhood asthma, it is important to study this association in high-risk racial/ethnic minorities, and to our knowledge no previous study has been conducted exclusively in these groups.
The Genes–environments and Admixture in Latino Americans (GALA II) and the Study of African Americans, Asthma, Genes and Environments (SAGE II) are parallel case–control studies of Latino and African American children. Together, GALA II and SAGE II represent the largest gene–environment study of asthma of minority children in the United States. Here, we seek to leverage the geographic and ethnic diversity in these two studies to examine the relationship between early-life air pollution exposure and the subsequent onset of asthma.
Methods
Study Population
The GALA II and SAGE II studies recruited Latino and African American children with and without asthma. GALA II recruited Latinos from urban regions in the mainland United States (Chicago, IL; Bronx, NY; Houston, TX; San Francisco Bay Area, CA) and Puerto Rico, using a combination of community and clinic-based recruitment. SAGE II recruited African Americans from the San Francisco Bay Area only. All participants were 8 to 21 years old and had no history of other lung or chronic illnesses (other than atopy and allergy-related diseases in the case subjects). Participants were eligible to participate in GALA II or SAGE II if they self-identified as Latino or African American and had four Latino or African American grandparents, respectively. Case subjects were defined as those with physician-diagnosed asthma plus two or more symptoms of coughing, wheezing, or shortness of breath in the 2 years before recruitment. Eligible control subjects had no reported history of asthma, lung disease, or chronic illness, and no reported symptoms of coughing, wheezing, or shortness of breath in the 2 years before enrollment. Control subjects were 1:1 frequency matched within each recruitment center by age (within 1 yr). Case subjects and control subjects were recruited from similar geographic regions (see Figure E2 in the online supplement). Those in the third trimester of pregnancy, current smokers, and those with an at least a 10 pack-year smoking history were not eligible. All local institutional review boards approved the study and all parents/participants provided signed written consent and assent as appropriate.
Exposure Assessment
Trained bilingual (English–Spanish) interviewers administered questionnaires to the parents/caretakers of the participants to collect basic demographic information, medical histories, and environmental exposure–related information. Self-reported residential histories from birth were collected and assigned geographic coordinates for each residence, using TomTom/Tele Atlas EZ-Locate software (TomTom, Amsterdam, The Netherlands). Regional ambient air pollution data were acquired from the U.S. Environmental Protection Agency Air Quality System. To average out yearly temporal changes, annual average exposures to ozone (O3), NO2, sulfur dioxide (SO2), particulate matter not greater than 10 μm in diameter (PM10), and particulate matter not greater than 2.5 μm in diameter (PM2.5) were calculated for each calendar year of life. Pollution exposures were estimated by calculating the inverse distance-squared weighted average from the four closest air pollution monitoring stations within 50 km of the residence. If a participant moved during the course of the year, their pollutant exposure assignments were weighted on the basis of the number of months spent at each residence. Exposures over the first 3 years of life were calculated by averaging all available pollutant values from birth to age 3.
Statistical Analysis
To account for regional characteristics, we used a two-stage analysis, allowing us to measure the between-region heterogeneity and to obtain a representative estimate across all regions. In the first stage, associations for each pollutant were determined separately for each study and region. Unadjusted logistic regression models and models adjusted for age, sex, ethnicity, and composite socioeconomic status (SES) were used to calculate the association between pollutant exposures during the first 3 years of life and subsequent asthma diagnosis as a dichotomous outcome. The SES variable was calculated for each participant by assigning a low, medium, or high score for income, level of education, and insurance type, and then by taking the sum of these three values. These covariates were included in the adjusted model if they resulted in a 10% or greater change in the β coefficient, or were standard in similar analyses. An interaction variable was used to test for effect modification between air pollution and ethnicity, SES, and sex. We also performed a sensitivity analysis examining additional potential covariates for maternal in utero smoking, environmental tobacco smoke in the household between 0 and 2 years old, and maternal language of preference (as an indicator of acculturation). These variables were included in the final site-specific adjusted models if the sample size was large enough and their inclusion improved the fit of the model as indicated by the Akaike Information Criterion (AIC). The analyses were repeated, limiting the exposures to the first year of life to ensure assessment of the air pollution–asthma relationship during critical periods of early infant lung and immunological development (30). The average pollution values were scaled to represent a 5-ppb (or μg/m3) increase in O3, NO2, and PM10, and a 1-ppb (or μg/m3) increase in PM2.5 and SO2, based on the range of pollution exposures reported.
In the second stage, the regression coefficients for each region were combined, using a random-effects meta-analysis with a restricted maximum-likelihood estimator to generate a summary OR for each pollutant. Heterogeneity between the study regions for each pollutant was evaluated using the I2 statistic, which estimates the percentage of between-study variation due to heterogeneity. Analyses with I2 less than 50% were considered to have acceptable heterogeneity.
We performed three stratified analyses: with or without family history of asthma, male or female sex, and high or low total IgE (above/below 200 IU/ml, the approximate median among case subjects). The analyses stratified by family history of asthma (defined as at least one parent ever diagnosed with asthma) was conducted to examine the association among children who were more or less likely to be genetically predisposed to asthma. The analyses stratified by sex addressed the mixed findings of previous studies (31–34). The analyses stratified by high/low total IgE were intended as a proxy for the risk of atopic and nonatopic asthma (35). All analyses were performed in STATA 11 (StataCorp, College Station, TX) and R version 2.15 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The GALA II and SAGE II studies have enrolled 4,157 and 1,281 participants, respectively, from 2006 to 2011. Participants were excluded if there was no self-reported residential history (n = 674) or were missing essential covariate data (n = 1,118). The final analytical sample size was 4,320, including 3,343 Latinos from GALA II (1,688 case subjects; 1,655 control subjects) and 977 African Americans from SAGE II (603 case subjects; 374 control subjects) (Table 1). Case subjects who reported an age of diagnosis during the exposure history window (i.e., the first year or first 3 yr of life) were omitted from the respective analyses to guarantee that exposures preceded the development of asthma. Additional participants were excluded from pollutant-specific analyses if the exposure data were unavailable.
TABLE 1.
DEMOGRAPHIC INFORMATION FOR CHILDREN INCLUDED IN THIS STUDY*
| Case Subjects (n = 2,291) | Control Subjects (n = 2,029) | Total (n = 4,320) | |
|---|---|---|---|
| Recruitment center |
|
|
|
| Chicago |
302 (13%) |
323 (16%) |
625 (14%) |
| Houston |
204 (9%) |
158 (8%) |
362 (8%) |
| New York |
208 (9%) |
195 (10%) |
403 (9%) |
| Puerto Rico |
674 (29%) |
662 (33%) |
1,336 (31%) |
| San Francisco Bay Area |
903 (39%) |
691 (34%) |
1,594 (37%) |
| Sex |
|
|
|
| Female |
1,033 (45%) |
1,141 (56%) |
2,174 (50%) |
| Male |
1,258 (55%) |
888 (44%) |
2,146 (50%) |
| Age at recruitment, yr | |||
| 8–9 |
580 (25%) |
320 (16%) |
900 (21%) |
| 10–14 |
1,115 (49%) |
982 (48%) |
2,097 (49%) |
| 15–19 |
530 (23%) |
623 (31%) |
1,153 (27%) |
| ≥20 |
66 (3%) |
104 (5%) |
170 (4%) |
| Child ethnicity |
|
|
|
| Mexican |
582 (25%) |
645 (32%) |
1,227 (28%) |
| Puerto Rican |
783 (34%) |
729 (36%) |
1,512 (35%) |
| Other Latino† |
323 (14%) |
281 (14%) |
604 (14%) |
| African American |
603 (26%) |
374 (18%) |
977 (23%) |
| SES composite score‡ |
|
|
|
| 1–3 |
13 (1%) |
33 (2%) |
46 (1%) |
| 4–6 |
1,481 (65%) |
1,299 (64%) |
2,780 (64%) |
| 7–9 |
797 (35%) |
697 (34%) |
1,494 (35%) |
| Birth year |
|
|
|
| 1986–1990 |
167 (7%) |
194 (10%) |
361 (8%) |
| 1991–1995 |
725 (32%) |
768 (38%) |
1,493 (35%) |
| 1996–2000 |
1,149 (50%) |
931 (46%) |
2,080 (48%) |
| 2001–2005 |
250 (11%) |
136 (7%) |
386 (9%) |
| Family history§ |
|
|
|
| Yes |
1,073 (47%) |
399 (20%) |
1,472 (34%) |
| No |
1,035 (45%) |
1,469 (72%) |
2,504 (58%) |
| Missing |
183 (8%) |
162 (8%) |
345 (8%) |
| Place of birth |
|
|
|
| United States |
1,476 (64%) |
1,075 (53%) |
2,551 (59%) |
| Puerto Rico |
665 (29%) |
655 (32%) |
1,320 (31%) |
| Other |
106 (5%) |
272 (13%) |
378 (9%) |
| Missing |
44 (2%) |
27 (1%) |
71 (2%) |
| Age at onset, yr | 2.0 (0.75–5.0) | — | — |
Definition of abbreviation: SES = socioeconomic status.
Reported as n (%), or median (IQR), for subjects with complete data on age, sex, ethnicity, and SES.
“Other Latino” reports either one Latino ethnicity other than Mexican or Puerto Rican, or reports more than one Latino ethnicity.
Composite based on the level of maternal education, annual household income, and type of medical insurance.
Participants had a family history of asthma if either parent was ever diagnosed with asthma.
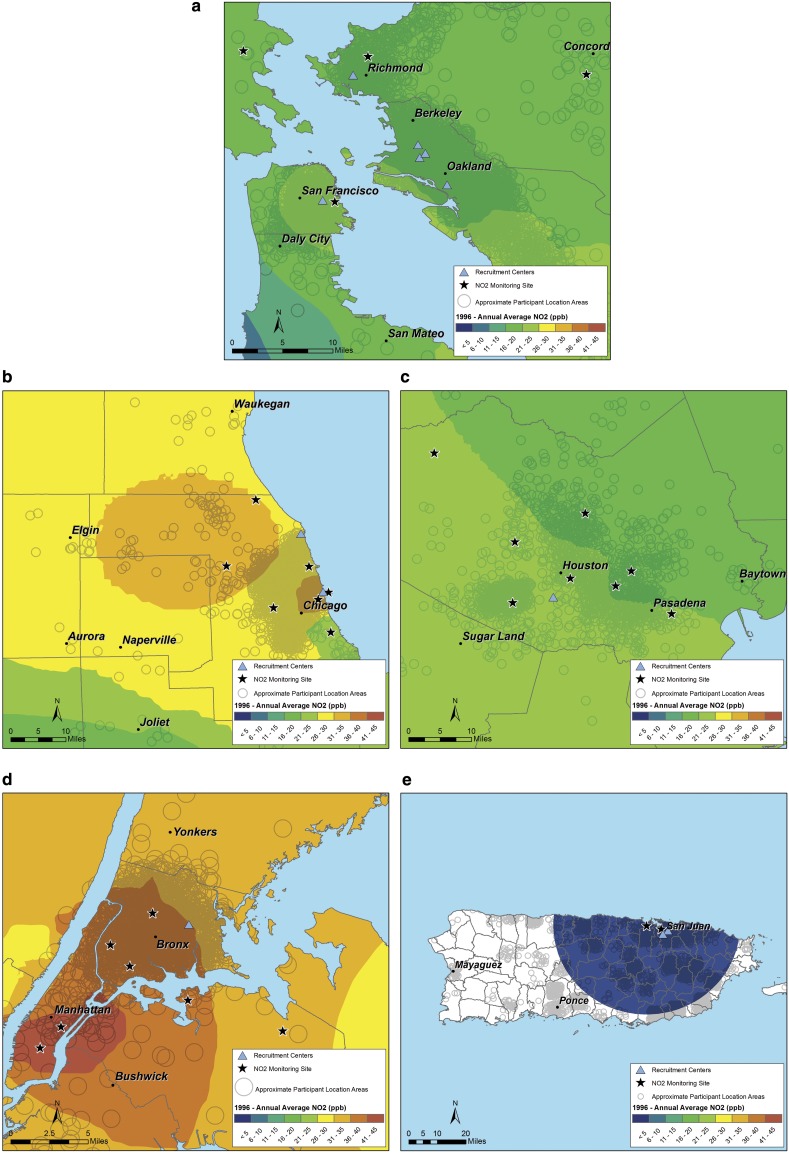
The various study regions had different levels and mixtures of pollutants, reflecting the differing geography, weather, and pollution sources (Table 2 and Table E1; and Figure 1 and Figure E1). For example, whereas Puerto Rican subjects were exposed to much lower levels of NO2 and PM10, they had some of the highest levels of O3, surpassed only by Houston. Participants from the San Francisco Bay Area had the lowest exposures to O3, PM10, and SO2. Participants from Chicago and New York City generally had the highest level of traffic-related pollutants (NO2, PM2.5, and PM10). In the median birth year for subjects in this study (1996), all regions were in compliance with the U.S. Environmental Protection Agency (EPA) National Ambient Air Quality Standards for NO2 and SO2. However, Houston, Chicago, and New York were designated by the EPA as nonattainment areas for O3. Chicago, New York, and a portion of Puerto Rico were designated as nonattainment areas for PM10. Within each region, pollution levels remained relatively constant during the study period (Figure E3).
TABLE 2.
AVERAGE POLLUTION IN 1996 (MEDIAN YEAR OF BIRTH),* COMPARED WITH VARIOUS AIR QUALITY STANDARDS
| Chicago | Houston | New York | Puerto Rico | SF Bay Area | All | EPA National Air Quality Standard | Cal EPA Air Quality Standard | WHO Air Quality Guidelines | |
|---|---|---|---|---|---|---|---|---|---|
| NO2, ppb (24-h ave) |
|
||||||||
| Mean (SD) |
26.9 (3.4) |
19.2 (4.6) |
32.1 (5.7) |
9.9 (2.9) |
18.9 (3.9) |
19.3 (8.0) |
53† |
30† |
20.9† |
| 25th percentile |
25.4 |
15.6 |
30.4 |
8.7 |
16.9 |
12.7 |
|
|
|
| 50th percentile |
27.0 |
19.4 |
32.4 |
9.0 |
18.7 |
18.7 |
|
|
|
| 75th percentile |
28.4 |
21.9 |
35.1 |
10.1 |
20.9 |
24.0 |
|
|
|
| O3, ppb (1-h max ave) |
|
|
|
|
|
|
|
|
|
| Mean (SD) |
34.8 (4.8) |
48.0 (5.0) |
35.6 (3.6) |
36.9 (9.2) |
32.0 (7.2) |
34.3 (7.7) |
NAS |
NAS |
NAS |
| 25th percentile |
31.9 |
44.8 |
33.6 |
28.8 |
26.3 |
29.1 |
|
|
|
| 50th percentile |
34.2 |
47.7 |
35.2 |
37.0 |
30.5 |
33.8 |
|
|
|
| 75th percentile |
36.6 |
51.5 |
36.9 |
45.9 |
36.5 |
37.5 |
|
|
|
| O3, ppb (8-h max ave) |
|
|
|
|
|
|
|
|
|
| Mean (SD) |
28.6 (4.0) |
38.1 (4.3) |
28.6 (3.2) |
30.2 (9.4) |
25.5 (6.4) |
27.6 (6.6) |
NAS |
NAS |
NAS |
| 25th percentile |
25.8 |
35.1 |
26.8 |
21.7 |
20.1 |
23.0 |
|
|
|
| 50th percentile |
28.3 |
38.0 |
28.0 |
30.3 |
24.6 |
27.3 |
|
|
|
| 75th percentile |
30.6 |
41.1 |
29.8 |
39.1 |
30.1 |
30.9 |
|
|
|
| PM10, μg/m3 (24-h ave) |
|
|
|
|
|
|
|
|
|
| Mean (SD) |
34.1 (4.5) |
30.1 (6.6) |
27.5 (4.1) |
29.1 (4.8) |
24.5 (5.5) |
27.8 (6.0) |
NAS |
20† |
20† |
| 25th percentile |
31.2 |
25.8 |
25.0 |
25.6 |
21.1 |
23.6 |
|
|
|
| 50th percentile |
33.8 |
29.3 |
27.1 |
28.4 |
23.48 |
27.1 |
|
|
|
| 75th percentile |
36.8 |
32.6 |
28.8 |
32.1 |
26.97 |
31.4 |
|
|
|
| PM2.5, μg/m3 (24-h ave) |
|
|
|
|
|
|
|
|
|
| Mean (SD) |
17.0 (1.6) |
13.2 (1.2) |
14.4 (2.0) |
8.1 (1.5) |
12.3 (1.5) |
11.8 (3.6) |
12‡ |
12† |
10† |
| 25th percentile |
16.1 |
12.3 |
14.2 |
7.2 |
11.3 |
8.5 |
|
|
|
| 50th percentile |
17.1 |
13.0 |
14.6 |
7.7 |
12.4 |
11.9 |
|
|
|
| 75th percentile |
18.0 |
12.9 |
15.4 |
9.1 |
12.8 |
14.5 |
|
|
|
| SO2, ppb (24-h ave) |
|
|
|
|
|
|
|
|
|
| Mean (SD) |
5.1 (1.2) |
3.6 (1.4) |
11.7 (3.3) |
4.1 (2.0) |
1.6 (0.9) |
4.0 (3.4) |
30† |
NAS |
3§ |
| 25th percentile |
4.6 |
2.7 |
10.1 |
2.9 |
1.3 |
1.6 |
|
|
|
| 50th percentile |
5.0 |
3.5 |
11.2 |
3.5 |
1.5 |
3.0 |
|
|
|
| 75th percentile | 5.5 | 4.4 | 14.0 | 4.8 | 1.9 | 5.0 | |||
Definition of abbreviations: ave = average; Cal EPA = California Environmental Protection Agency; EPA = U.S. Environmental Protection Agency; max = maximum; NAS = no annual standard; NO2 = nitrogen dioxide; O3 = ozone; PM2.5 = particulate matter < 2.5 μm in diameter; PM10 = particulate matter < 10 μm in diameter; ppb = parts per billion; SF = San Francisco; SO2 = sulfur dioxide; WHO = World Health Organization.
With the exception of PM2.5, where 2000 was used because of insufficient observations in 1996; reported as average (SD).
Annual mean.
Annual mean, over 3 years.
The WHO does not have an annual SO2 guideline because they regard their 7-ppb (20-μg/m3) 24-hour standard sufficiently protective for the annual average. In the GALA II regions, an annual average of about 3 ppb SO2 is comparable to a daily maximal concentration of 7 ppb.
Figure 1.
(a) NO2 exposure in 1996 (median birth year) for the San Francisco Bay Area. Each circle represents the participant’s residence (open circles with random noise are used to prevent determination of the participant’s address). Major cities are included to provide a reference for the nearby urban centers. Solid stars represent the location of monitoring sites. Blue triangles represent recruitment centers. NO2 = nitrogen dioxide. (b–e) NO2 exposure in 1996 in Chicago (b), Houston (c), New York (d), and Puerto Rico (e).
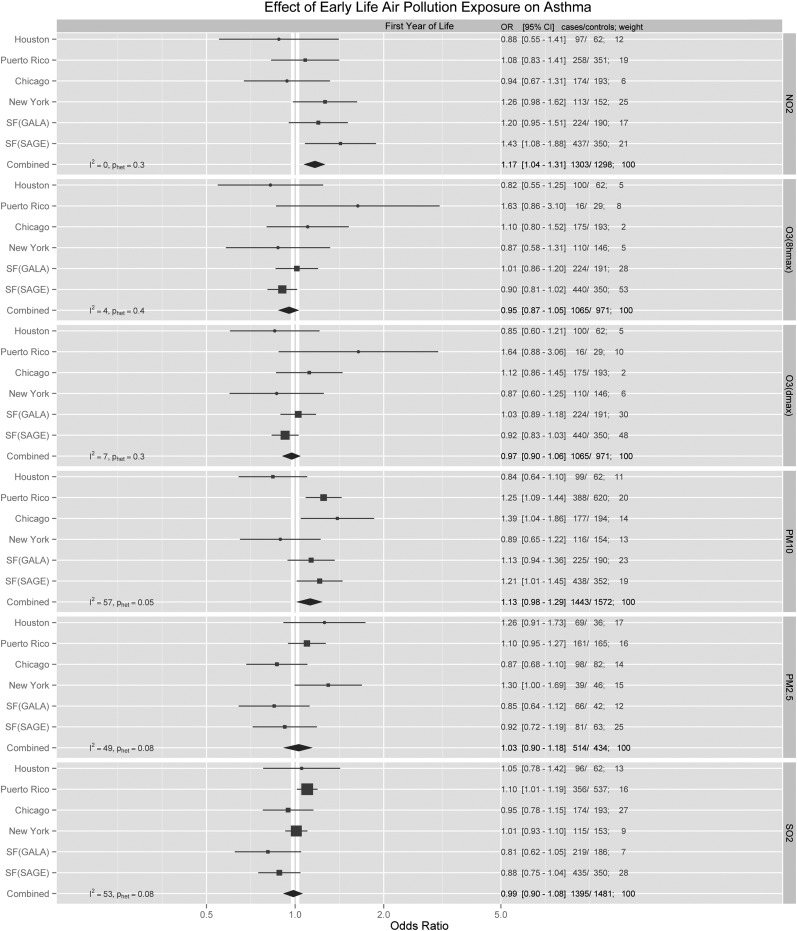
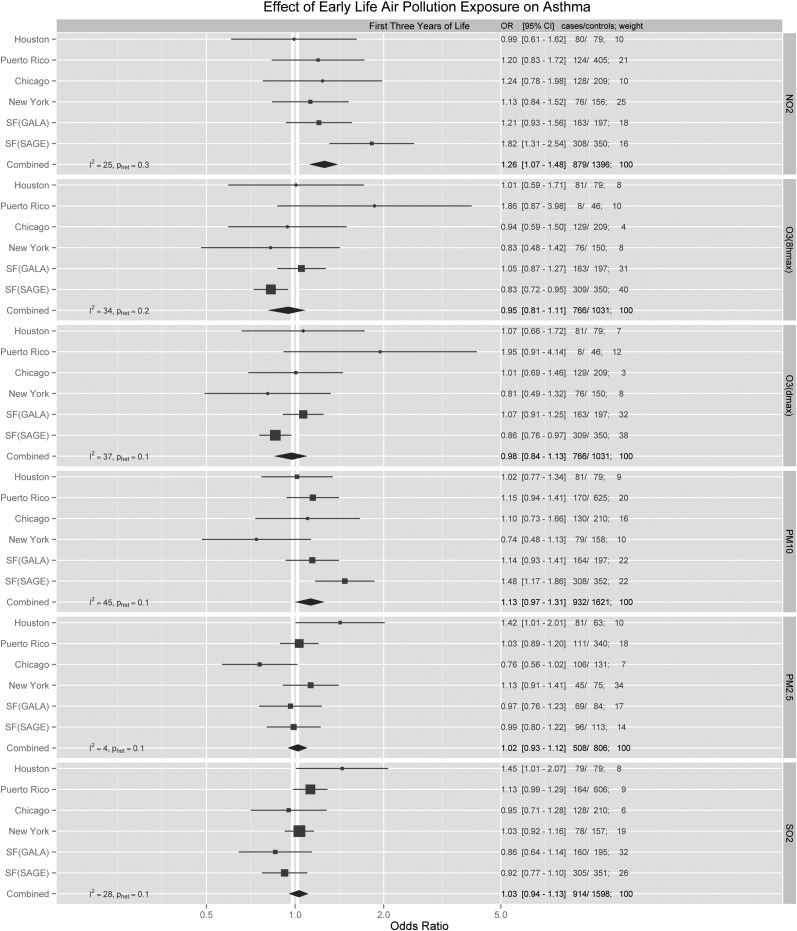
In the combined second-stage analysis, the model adjusting for age, sex, ethnicity, and SES did not differ significantly from the model using AIC model selection. In the AIC models, NO2 exposure during the first year and first 3 years of life were both associated with a statistically significant increase in the odds for developing childhood asthma, with summary ORs of 1.17 (95% CI, 1.04–1.31) and 1.26 (95% CI, 1.07–1.48), respectively (Figures 2 and 3; for NO2, 5 ppb is equivalent to 9.4 μg/m3). Many first-stage region-specific results were not statistically significant, presumably due to the relatively small sample size. However, some region-specific effects were detected. For example, asthma was associated with first year of life exposure to PM10 in Chicago (OR, 1.39; 95% CI, 1.04–1.86) and Puerto Rico (OR, 1.25; 95% CI, 1.09–1.44). An association with SO2 was also found in Puerto Rico (OR, 1.10; 95% CI, 1.01–1.19). Among African Americans from the San Francisco Bay Area, first year of life exposures to NO2 (OR, 1.43; 95% CI, 1.08–1.88) and PM10 (OR, 1.21; 95% CI, 1.01–1.45) were found to have the strongest effect compared with any other region. Analyses stratified by sex, high/low IgE, and family history (Tables E2–E4) did not reveal any statistically significant associations.
Figure 2.
Adjusted region-specific and summary odds ratio (OR) estimates for all pollutants during the first year of life. Region-specific analyses were adjusted for age, SES, income, and race/ethnicity, and then pooled using a random-effects model. For NO2 and O3 (1-h max, 8-h max), ORs were calculated for every 5-ppb change. For PM10, ORs were calculated for every 5-μg/m3 change. For PM2.5, ORs were calculated for every 1-μg/m3 change. For SO2, ORs were calculated for every 1-ppb change. CI = confidence interval; GALA = Genes–environments and Admixture in Latino Americans; NO2 = nitrogen dioxide; O3 = ozone; OR = odds ratio; PM2.5 = particulate matter < 2.5 μm in diameter; PM10 = particulate matter < 10 μm in diameter; ppb = parts per billion; SAGE = Study of African Americans, Asthma, Genes, and Environments; SO2 = sulfur dioxide.
Figure 3.
Adjusted region-specific and summary odds ratio (OR) estimates for all pollutants during the first 3 years of life. Region-specific analyses adjusted for age, SES, income, and race/ethnicity, and then pooled using a random-effects model. For NO2 and O3 (1-h max, 8-h max), ORs were calculated for every 5-ppb change. For PM10, ORs were calculated for every 5-μg/m3 change. For PM2.5, ORs were calculated for every 1-μg/m3 change. For SO2, ORs were calculated for every 1-ppb change. CI = confidence interval; GALA = Genes–environments and Admixture in Latino Americans; NO2 = nitrogen dioxide; O3 = ozone; OR = odds ratio; PM2.5 = particulate matter < 2.5 μm in diameter; PM10 = particulate matter < 10 μm in diameter; ppb = parts per billion; SAGE = Study of African Americans, Asthma, Genes, and Environments; SES = socioeconomic status; SO2 = sulfur dioxide.
Discussion
In this study, early-life exposure to NO2, a motor vehicle pollutant, was associated with an increased risk for subsequent asthma in Latino and African American children across five geographic regions in the United States and Puerto Rico. Our results are consistent with previous findings from two meta-analyses that support a relationship between NO2 and asthma incidence (16, 24). Some studies included in these meta-analyses lacked accurate measurements of the exposure before the onset of asthma or used community-level data rather than residential addresses to determine pollutant levels (36–38). Inaccuracies introduced by these methods may explain the inconsistencies seen in the literature. The current study overcomes these limitations by using residential addresses to determine pollutant exposures and limiting the analyses to participants whose exposure predated their asthma diagnosis.
In addition, even though all participants lived in regions that met the current EPA annual air quality standard for NO2, our results were statistically significant. This indicates that a risk still exists for levels of NO2 pollution below the EPA annual standard, and suggests that this standard may not sufficiently protect children’s health.
As expected, air pollution exposures differed greatly by region in our study. Although only the SAGE II participants in San Francisco showed a statistically significant association between NO2 and asthma in the region-specific analysis, most regions showed nominally positive associations that were broadly consistent with one another, with little between-study heterogeneity (I2 for the first year and first 3 yr of life were 0 and 25%, respectively), suggesting that the association between NO2 and asthma is generalizable across geographic regions.
For other pollutants, there was greater region-specific variability. For example, Puerto Rico showed associations between asthma and PM10 and SO2, pollutants that are associated with emissions from vehicles and industrial processes using sulfur-containing fuels (coal and petroleum). The African Americans from the San Francisco Bay Area showed associations with NO2 and PM10, which are primarily traffic-related pollutants. The region-specific results suggest that susceptibility to asthma due to air pollution may not be uniform throughout the nation and could be dependent on local characteristics, such as varying proportions of different racial/ethnic groups and differing pollution sources and/or weather patterns. The I2 statistic estimating heterogeneity was greater than 50% for PM10 and SO2 (I2, 57 and 53%, respectively), principally because of non–statistically significant inverse associations in Houston, San Francisco and New York. By using a random-effects model to combine the individual findings, our two-stage analysis allows us to take into account these interregional differences.
Because asthma susceptibility has a genetic contribution, we performed analyses stratified by family history of asthma. Those with a family history did not show an association between NO2 and asthma, and those without a family history showed a similar OR compared with the combined analysis, but the finding was not statistically significant because of the loss of power associated with the subgroup analysis. One study previously documented a stronger relationship between traffic exposures and asthma among children without a family history of asthma, whereas their results for children with a family history of asthma were not statistically significant (39). However, they did not measure individual pollutants or limit exposure measurements to periods before asthma diagnosis.
Our analyses stratified by sex showed some differences in pollution-associated asthma risk. However, the effect modification interaction P values were not statistically significant. The analyses stratified by high/low IgE were also not statistically significant. There are few previous relevant studies and those that exist report contradictory findings. Additional studies are required to determine whether family history, sex, or atopy increases the risk for pollution-associated asthma.
Overall, our results suggest that the timing of exposure to air pollution may play a role in the development of asthma. Most lung and immunological development is believed to occur in the first few years of life. Children have narrower airways and generally breathe more air per pound of body weight than adults, making them particularly susceptible to air pollution (16). One study reported that maternal exposure to NO2 and PM10 was associated with altered blood lymphocyte subpopulations in fetal cord blood, suggesting that the immune system may be compromised by pollutant exposure in utero, which potentially increases the risk for developing asthma or allergies later in life (40).
Air pollution is hypothesized to alter biological processes through multiple pathways, such as inducing epigenetic changes resulting in gene dysregulation, oxidative stress resulting in a heightened inflammatory response, and airway wall remodeling resulting in physiological impairment (25). Exposure to PM2.5 has been associated with a decrease in global methylation (41), hypermethylation of the FOXP3 gene, reduced population of regulatory T cells, and more severe asthma (42). Many chemicals found in vehicle emissions are oxidants or capable of reacting to form reactive oxygen species (ROS) (43). ROS can oxidize nearby macromolecules, resulting in cellular damage or “oxidative stress,” which is thought to modify the pulmonary inflammatory response (44). There is also evidence that underlying genetic traits may alter the susceptibility to asthma in the presence of air pollution (45). Many of the previously identified gene variants are related to immune cell responses (46) and prevention of oxidative damage from ROS (47). For example, the null mutations of GSTM1 have been shown to increase the risk of asthma, especially among children with high levels of O3 exposure (48).
To our knowledge, this is the largest and only study on the impact of air pollution on childhood asthma in U.S. minorities. Because geocoded childhood residences were used to extrapolate air pollutant concentrations from multiple nearby monitors before the development of asthma, this study benefits from high-quality estimates of pollution exposures and addresses three of the Bradford Hill Viewpoints for Causality, including demonstrating a temporal relationship between the exposure and outcome, establishing consistency by measuring the effect in multiple racial/ethnic populations from different regions, and providing evidence for biological plausibility. Uniform data collection was guaranteed by using a standardized questionnaire, diagnostic criteria, and exposure assessment in all recruitment regions, which ensures the generalizability of the results.
There are several limitations of this study that should be acknowledged. Many prior studies have used PM2.5 as another indicator of traffic-related pollution, though a meta-analysis reported nonsignificant results for this pollutant (24). We also found no significant associations with PM2.5. However, regional monitoring for this pollutant was less complete during the window of exposure for our study population, resulting in a smaller sample size compared with other pollutants. Thus, the nonsignificant results may be due to reduced power, rather than the absence of an association. A second limitation is that Puerto Rico has only two monitoring stations, which may reduce the accuracy of the pollutant exposure assignments and highlights the need for improved monitoring and additional research in this high-risk population.
Our study is also subject to the limitations inherent to our air pollution estimates, because we did not have the ability to measure air pollution by personal air sampling. Although more accurate methods for measuring pollution are available by directly monitoring pollutants at a residence or school (17, 32), it is often infeasible to sample continuously throughout the year by these methods. Children spend extended time away from the home residence and may be exposed to different levels of air pollution at daycare or school settings. Most children would be expected to spend most time at or near home, especially in early childhood, and air pollution estimates at their residential addresses will most accurately capture their overall exposure. There is a well-established variation in the risk of asthma by birth month (49). Although outside the scope of this study, other important seasonally varying asthma risk factors such as respiratory viral infections and aeroallergen exposures should be included in subsequent analyses. Future work will need to more closely investigate birth timing with differing aspects of air quality and susceptibility, including their potential interactions. Our use of yearly averages overcame the potential for confounding by other temporally varying risk factors but precluded a more granular evaluation of whether infants born during seasons of high pollution are particularly vulnerable to the effect of air pollution. Although we would have liked to stratify by atopic or nonatopic asthma, we did not have skin-prick testing results for everyone and could only use total IgE as a proxy. Finally, we cannot exclude the possibility of unmeasured confounders. For example, we did not have the ability to adequately measure indoor or in utero air pollution, which are believed to be linked to asthma (36, 50).
In this study we attempted to establish a causal association between early-life air pollution exposures and childhood asthma in U.S. minorities. We conclude that exposures to traffic-related air pollutants during the first year and first 3 years of life are associated with childhood asthma. Regional differences throughout the country suggest that risk heterogeneity may exist. Finally, asthma risk appears to exist even though all regions achieved the current EPA annual air quality standards for NO2. In future analyses, we plan to leverage the genetic data from these studies to assess gene–environment interactions and genetic ancestry to further understand this relationship.
Acknowledgments
Acknowledgment
The authors acknowledge the families and patients for their participation and thank the numerous health care providers and community clinics for their support and participation in GALA II and SAGE II. In particular, the authors thank the study coordinator Sandra Salazar; the recruiters who obtained the data: Duanny Alva, M.D., Gaby Ayala-Rodriguez, Ulysses Burley, Lisa Caine, Elizabeth Castellanos, Jaime Colon, Denise DeJesus, Iliana Flexas, Blanca Lopez, Brenda Lopez, M.D., Louis Martos, Vivian Medina, Juana Olivo, Mario Peralta, Esther Pomares, M.D., Jihan Quraishi, Johanna Rodriguez, Shahdad Saeedi, Dean Soto, Ana Taveras, Emmanuel Viera; lab manager Celeste Eng; and John Neuhaus for statistical advice.
Footnotes
Supported in part by National Institutes of Health grants R01-ES015794, U19-AI077439, R01-HL088133, R01-HL078885, R25-CA113710, T32-GM007546, R01-HL004464, and R01-HL104608 and the National Institute on Minority Health and Health Disparities under award no. P60MD006902 to K.B.-D. and E.G.B.; National Heart, Lung, and Blood Institute grant K23-HL093023 to R.K.; National Center for Research Resources grant M01-RR00188 to H.J.F.; the Flight Attendant Medical Research Institute, RWJF Amos Medical Faculty Development Award, the Sandler Foundation, and the American Asthma Foundation to E.G.B.; and by an Ernest S. Bazley grant to P.C.A. J.M.G. was supported by career development awards from the NCATS (KL2 TR000143) and NHLBI (K23 HL111636), as well as the Hewett Fellowship.
Author Contributions: K.K.N. was responsible for analyzing the data, with supervision and input from J.M.G., L.A.R., S.S.O., S.S., F.L., J.R.B., and E.G.B. K.K.N. wrote the first version of this manuscript. S.S. provided statistical guidance for all analyses. E.A.N. cleaned and recoded all residential history addresses. F.L. calculated all pollution measurements and contributed to the interpretation of these measurements. H.J.F., D.S., R.K., L.N.B., E.B.-B., A.D., M.A.L., K.M., W.R.-C., P.C.A., S.T., J.R.R.-S., and E.G.B. planned and supervised the collection of data from the various recruitment regions in the initial cohort. All authors contributed to interpretation of results, and provided revisions and approval of the final manuscript.
This article has an online data supplement, which is accessible from this issue’s table of contents online at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201302-0264OC on June 10, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wang LY, Zhong Y, Wheeler L. Direct and indirect costs of asthma in school-age children. Prev Chronic Dis. 2005;2:A11. [PMC free article] [PubMed] [Google Scholar]
- 2.American Lung Association, Epidemiology & Statistics Unit, Research and Health Education Division. Trends in asthma morbidity and mortality. Washington, DC: American Lung Association; 2012.
- 3.American Lung Association, Epidemiology & Statistics Unit, Research and Program Services Division. Trends in asthma morbidity and mortality, July 2011. Washington, DC: American Lung Association; 2011.
- 4.Prüss-Üstün A, Corvalán A.Preventing disease through healthy environments: towards an estimate of the environmental burden of disease. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 5.Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, Priftis KN. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environ Res. 2011;111:418–424. doi: 10.1016/j.envres.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Erbas B, Kelly AM, Physick B, Code C, Edwards M. Air pollution and childhood asthma emergency hospital admissions: estimating intra-city regional variations. Int J Environ Health Res. 2005;15:11–20. doi: 10.1080/09603120400018717. [DOI] [PubMed] [Google Scholar]
- 7.Nastos PT, Paliatsos AG, Anthracopoulos MB, Roma ES, Priftis KN. Outdoor particulate matter and childhood asthma admissions in Athens, Greece: a time-series study. Environ Health. 2010;9:45. doi: 10.1186/1476-069X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YL, Wang WH, Lu CW, Lin YH, Hwang BF. Effects of ambient air pollution on pulmonary function among schoolchildren. Int J Hyg Environ Health. 2011;214:369–375. doi: 10.1016/j.ijheh.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Barraza-Villarreal A, Sunyer J, Hernandez-Cadena L, Escamilla-Nuñez MC, Sienra-Monge JJ, Ramírez-Aguilar M, Cortez-Lugo M, Holguin F, Diaz-Sánchez D, Olin AC, et al. Air pollution, airway inflammation, and lung function in a cohort study of Mexico City schoolchildren. Environ Health Perspect. 2008;116:832–838. doi: 10.1289/ehp.10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, Künzli N. Chronic exposure to ambient ozone and lung function in young adults. Epidemiology. 2005;16:751–759. doi: 10.1097/01.ede.0000183166.68809.b0. [DOI] [PubMed] [Google Scholar]
- 11.Schultz ES, Gruzieva O, Bellander T, Bottai M, Hallberg J, Kull I, Svartengren M, Melén E, Pershagen G. Traffic-related air pollution and lung function in children at 8 years of age: a birth cohort study. Am J Respir Crit Care Med. 2012;186:1286–1291. doi: 10.1164/rccm.201206-1045OC. [DOI] [PubMed] [Google Scholar]
- 12.Jacquemin B, Kauffmann F, Pin I, Le Moual N, Bousquet J, Gormand F, Just J, Nadif R, Pison C, Vervloet D, et al. Epidemiological study on the Genetics and Environment of Asthma (EGEA) Air pollution and asthma control in the epidemiological study on the genetics and environment of asthma. J Epidemiol Community Health. 2012;66:796–802. doi: 10.1136/jech.2010.130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández-Cadena L, Holguin F, Barraza-Villarreal A, Del Río-Navarro BE, Sienra-Monge JJ, Romieu I. Increased levels of outdoor air pollutants are associated with reduced bronchodilation in children with asthma. Chest. 2009;136:1529–1536. doi: 10.1378/chest.08-1463. [DOI] [PubMed] [Google Scholar]
- 14.Anderson HR, Favarato G, Atkinson RW. Long-term exposure to outdoor air pollution and the prevalence of asthma: meta-analysis of multi-community prevalence studies. Air Qual Atmos Health. 2013;6:57–68. [Google Scholar]
- 15.Akinbami LJ, Lynch CD, Parker JD, Woodruff TJ. The association between childhood asthma prevalence and monitored air pollutants in metropolitan areas, United States, 2001–2004. Environ Res. 2010;110:294–301. doi: 10.1016/j.envres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Gasana J, Dillikar D, Mendy A, Forno E, Ramos Vieira E. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res. 2012;117:36–45. doi: 10.1016/j.envres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Gauderman WJ, Avol E, Lurmann F, Kuenzli N, Gilliland F, Peters J, McConnell R. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology. 2005;16:737–743. doi: 10.1097/01.ede.0000181308.51440.75. [DOI] [PubMed] [Google Scholar]
- 18.Lindgren A, Stroh E, Montnémery P, Nihlén U, Jakobsson K, Axmon A. Traffic-related air pollution associated with prevalence of asthma and COPD/chronic bronchitis: a cross-sectional study in southern Sweden. Int J Health Geogr. 2009;8:2. doi: 10.1186/1476-072X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang BF, Lee YL, Lin YC, Jaakkola JJ, Guo YL. Traffic related air pollution as a determinant of asthma among Taiwanese school children. Thorax. 2005;60:467–473. doi: 10.1136/thx.2004.033977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Portnov BA, Reiser B, Karkabi K, Cohen-Kastel O, Dubnov J. High prevalence of childhood asthma in northern Israel is linked to air pollution by particulate matter: evidence from GIS analysis and Bayesian model averaging. Int J Environ Health Res. 2012;22:249–269. doi: 10.1080/09603123.2011.634387. [DOI] [PubMed] [Google Scholar]
- 21.Rios JL, Boechat JL, Sant’Anna CC, França AT. Atmospheric pollution and the prevalence of asthma: study among schoolchildren of 2 areas in Rio de Janeiro, Brazil. Ann Allergy Asthma Immunol. 2004;92:629–634. doi: 10.1016/S1081-1206(10)61428-7. [DOI] [PubMed] [Google Scholar]
- 22.Studnicka M, Hackl E, Pischinger J, Fangmeyer C, Haschke N, Kühr J, Urbanek R, Neumann M, Frischer T. Traffic-related NO2 and the prevalence of asthma and respiratory symptoms in seven year olds. Eur Respir J. 1997;10:2275–2278. doi: 10.1183/09031936.97.10102275. [DOI] [PubMed] [Google Scholar]
- 23.Wang TN, Ko YC, Chao YY, Huang CC, Lin RS. Association between indoor and outdoor air pollution and adolescent asthma from 1995 to 1996 in Taiwan. Environ Res. 1999;81:239–247. doi: 10.1006/enrs.1999.3985. [DOI] [PubMed] [Google Scholar]
- 24.Anderson HR, Favarato G, Atkinson RW. Long-term exposure to air pollution and the incidence of asthma: meta-analysis of cohort studies. Air Qual Atmos Health. 2013:47–56. [Google Scholar]
- 25.Gowers AM, Cullinan P, Ayres JG, Anderson HR, Strachan DP, Holgate ST, Mills IC, Maynard RL. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence: a review. Respirology. 2012;17:887–898. doi: 10.1111/j.1440-1843.2012.02195.x. [DOI] [PubMed] [Google Scholar]
- 26.Mott L. The disproportionate impact of environmental health threats on children of color. Environ Health Perspect. 1995;103:33–35. doi: 10.1289/ehp.95103s633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X.Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010 [NCHS Data Brief]. Hyattsville, MD: National Center for Health Statistics; 2012, pp. 1–8. [PubMed] [Google Scholar]
- 28.Metzger R, Delgado JL, Herrell R. Environmental health and Hispanic children. Environ Health Perspect. 1995;103:25–32. doi: 10.1289/ehp.95103s625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miranda ML, Edwards SE, Keating MH, Paul CJ. Making the environmental justice grade: the relative burden of air pollution exposure in the United States. Int J Environ Res Public Health. 2011;8:1755–1771. doi: 10.3390/ijerph8061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yabuhara A, Macaubas C, Prescott SL, Venaille TJ, Holt BJ, Habre W, Sly PD, Holt PG. TH2-polarized immunological memory to inhalant allergens in atopics is established during infancy and early childhood. Clin Exp Allergy. 1997;27:1261–1269. [PubMed] [Google Scholar]
- 31.Ho WC, Hartley WR, Myers L, Lin MH, Lin YS, Lien CH, Lin RS. Air pollution, weather, and associated risk factors related to asthma prevalence and attack rate. Environ Res. 2007;104:402–409. doi: 10.1016/j.envres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 32.van Vliet P, Knape M, de Hartog J, Janssen N, Harssema H, Brunekreef B. Motor vehicle exhaust and chronic respiratory symptoms in children living near freeways. Environ Res. 1997;74:122–132. doi: 10.1006/enrs.1997.3757. [DOI] [PubMed] [Google Scholar]
- 33.McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Künzli N, Gauderman J, Avol E, Thomas D, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect. 2006;114:766–772. doi: 10.1289/ehp.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gehring U, Cyrys J, Sedlmeir G, Brunekreef B, Bellander T, Fischer P, Bauer CP, Reinhardt D, Wichmann HE, Heinrich J. Traffic-related air pollution and respiratory health during the first 2 yrs of life. Eur Respir J. 2002;19:690–698. doi: 10.1183/09031936.02.01182001. [DOI] [PubMed] [Google Scholar]
- 35.Sanz ML, Prieto I, García BE, Oehling A. Diagnostic reliability considerations of specific IgE determination. J Investig Allergol Clin Immunol. 1996;6:152–161. [PubMed] [Google Scholar]
- 36.Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environ Health Perspect. 2010;118:284–290. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, Oldenwening M, Smit HA, Brunekreef B. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010;181:596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 38.Oftedal B, Nystad W, Brunekreef B, Nafstad P. Long-term traffic-related exposures and asthma onset in schoolchildren in Oslo, Norway. Environ Health Perspect. 2009;117:839–844. doi: 10.1289/ehp.11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordian ME, Haneuse S, Wakefield J. An investigation of the association between traffic exposure and the diagnosis of asthma in children. J Expo Sci Environ Epidemiol. 2006;16:49–55. doi: 10.1038/sj.jea.7500436. [DOI] [PubMed] [Google Scholar]
- 40.Baïz N, Slama R, Béné MC, Charles MA, Kolopp-Sarda MN, Magnan A, Thiebaugeorges O, Faure G, Annesi-Maesano I. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations: the EDEN study cohort. BMC Pregnancy Childbirth. 2011;11:87. doi: 10.1186/1471-2393-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179:572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, Tager I.Ambient air pollution impairs regulatory T-cell function in asthma J Allergy Clin Immunol 2010126845–852., e810. [DOI] [PubMed] [Google Scholar]
- 43.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60:612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008;31:179–197. doi: 10.1183/09031936.00128106. [DOI] [PubMed] [Google Scholar]
- 45.London SJ. Gene–air pollution interactions in asthma. Proc Am Thorac Soc. 2007;4:217–220. doi: 10.1513/pats.200701-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerkhof M, Postma DS, Brunekreef B, Reijmerink NE, Wijga AH, de Jongste JC, Gehring U, Koppelman GH. Toll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthma. Thorax. 2010;65:690–697. doi: 10.1136/thx.2009.119636. [DOI] [PubMed] [Google Scholar]
- 47.Lee YL, Lin YC, Lee YC, Wang JY, Hsiue TR, Guo YL. Glutathione S-transferase P1 gene polymorphism and air pollution as interactive risk factors for childhood asthma. Clin Exp Allergy. 2004;34:1707–1713. doi: 10.1111/j.1365-2222.2004.02099.x. [DOI] [PubMed] [Google Scholar]
- 48.Islam T, Berhane K, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax. 2009;64:197–202. doi: 10.1136/thx.2008.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aberg N. Birth season variation in asthma and allergic rhinitis. Clin Exp Allergy. 1989;19:643–648. doi: 10.1111/j.1365-2222.1989.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 50.Heinrich J. Influence of indoor factors in dwellings on the development of childhood asthma. Int J Hyg Environ Health. 2011;214:1–25. doi: 10.1016/j.ijheh.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 51.United States Environmental Protection Agency. National Ambient Air Quality Standards [accessed 2013 Jan 8]. Available from: http://www.epa.gov/air/criteria.html#2
- 52.World Health Organization. Air quality and health, fact sheet No. 313 [updated 2011 Sep; accessed 2013 Jan 8]. Available from: http://www.who.int/mediacentre/factsheets/fs313/en/index.html
- 53.California Environmental Protection Agency Air Resources Board. California Ambient Air Quality Standards (CAAQS) [accessed 2013 Jan 8]. Available from: http://www.arb.ca.gov/research/aaqs/caaqs/caaqs.htm