Abstract
Asthma and allergic lung disease occur as complex environmental and genetic interactions. Clinical studies of asthma indicate a number of protective dietary factors, such as vitamin E, on asthma risk. However, these studies have had seemingly conflicting outcomes. In this perspective, we discuss opposing regulatory effects of tocopherol isoforms of vitamin E, mechanisms for tocopherol isoform regulation of allergic lung inflammation, association of vitamin E isoforms with outcomes in clinical studies, and how the variation in global prevalence of asthma may be explained, at least in part, by vitamin E isoforms.
Keywords: asthma, α-tocopherol, γ-tocopherol, human, mouse
Asthma and allergic lung disease occur as complex environmental and genetic interactions (1). The World Health Organization has reported that the prevalence of asthma from 1950 to the present has increased in many countries, including countries with high rates of asthma, intermediate rates of asthma, or low rates of asthma (2–4). The marked differences in rates of asthma within countries, in migrating populations, and over relatively short periods of time support an important role of the local environment, such as diet, in asthma inception. Prospective epidemiological studies, observational cross-sectional studies, and some randomized prevention trials have demonstrated the impact of a number of protective dietary factors, such as vitamin E, on asthma risk. However, these studies have had seemingly conflicting outcomes. We need to determine why there are low rates of allergic disease in developing countries and better understand the differences in diet and lifestyle that may really underpin allergic disease. One environmental change over the past 40 years has been an increase in the γ-tocopherol isoform of vitamin E in the diet and in infant formulas (5, 6). We recently demonstrated that γ-tocopherol increases allergic lung inflammation in mice (6–8). In this perspective, we discuss why we have yet to uncover the complex and potentially protective effects of isoforms of vitamin E on asthma in humans and in animal models of lung inflammation. We also review mechanisms for tocopherol isoform regulation of allergic lung inflammation in animals and discuss how the variation in global prevalence of asthma may be explained, at least in part, by country-specific plasma γ-tocopherol differences.
Vitamin E consists of natural isoforms and synthetic racemic isoforms. The eight natural isomers are d-α-, d-β-, d-γ-, d-δ-tocopherol and d-α-, d-β-, d-γ-, d-δ-tocotrienol. Plants synthesize the natural isoforms from tyrosine and chlorophyll (9). Then, these tocols are consumed in the diet from plant lipids. Mammals do not interconvert the tocopherol isoforms. The most abundant isoforms are α-tocopherol and γ-tocopherol, which differ by one methyl group (Figure 1A). There are approximately 10-fold higher tissue concentrations of α-tocopherol than γ-tocopherol due to preferential transfer of α-tocopherol in the liver by α-tocopherol transfer protein and due to a higher rate of production of γ-tocopherol metabolites for excretion (10, 11). The plasma levels of tocopherols correlate with lung tissue levels of tocopherols in humans and mice (7, 8, 12). Other diet components may influence tocopherol absorption. For example, it is reported that dietary l-carnitine enhances absorption of α-tocopherol in rats (13). α-Tocopherol levels are also affected by genetic variants. Mutations in liver α-tocopherol transfer protein result in human α-tocopherol deficiency (14). It is also reported that human plasma levels of α-tocopherol but not γ-tocopherol are increased in male adults and children by the apolipoprotein A5 1131T>C gene polymorphism (15, 16). In mice, apoE4 mice have lower plasma α-tocopherol than apoE3 mice (17).
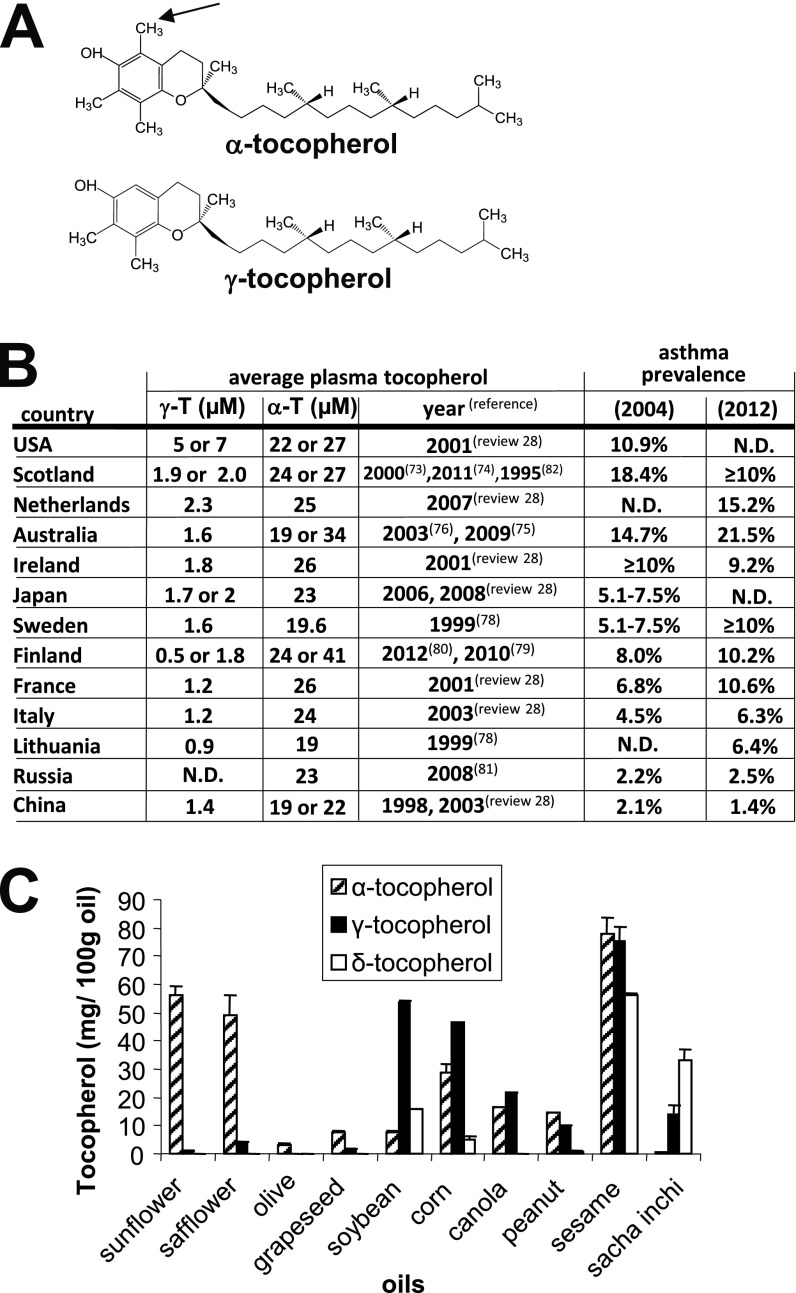
Figure 1.
α-Tocopherol and γ-tocopherol. (A) α-Tocopherol differs from γ-tocopherol by one methyl group (arrow). (B) Plasma γ-tocopherol (γ-T) and plasma α-tocopherol (α-T) in one to two reports per country and publication dates are indicated (28, 73–83). Global asthma prevalence in 2004 (84) and 2012 (85). (C) Tocopherols were extracted from dietary oils (sunflower oil from Spectrum Organic Products, LLC; safflower oil from Spectrum; olive oil from Colavita; soybean oil from Crisco; corn oil from Mazola; grapeseed oil from Kusha, Inc; sesame oil from Lavita; peanut oil from Essentials by Supervalu; canola oil from Crisco; Sacha Inchi from Olivar). Extracted tocopherols were measured by HPLC with an electrochemical detector as previously described (8). ND = not determined.
α-Tocopherol and γ-tocopherol, at equal molar concentrations, have a relatively similar capacity to scavenge reactive oxygen species (ROS) during lipid peroxidation in vitro and in cells (18, 19) and a relatively similar capacity to inhibit activation of protein kinase B (Akt) in cancer cells in vitro (20). Thus, because α-tocopherol is 10-fold higher in tissues than γ-tocopherol, there is 10-fold more total ROS scavenging by α-tocopherol than γ-tocopherol. Besides scavenging ROS, γ-tocopherol, in contrast to α-tocopherol, also reacts with reactive nitrogen species, such as peroxynitrite-forming 5-nitro-γ-tocopherol (21). γ-Tocopherol scavenging of reactive nitrogen species may be beneficial for inflammation with increases in reactive nitrogen species, such as neutrophilic inflammation that is induced by ozone in mice (22). Consistent with this, reports indicate that supplementation with a mixture of tocopherols that are enriched for γ-tocopherol blocks acute endotoxin-stimulated or ozone-stimulated neutrophil inflammation in the rat and human lung (23–25). In another study, γ-tocopherol supplementation reduced antigen induction of rat lung inflammation that was primarily neutrophils (26). It is also reported that nebulized γ-tocopherol reduces neutrophilia in burn and smoke inhalation injury in sheep (27). Therefore, γ-tocopherol may be of benefit for acute neutrophilic inflammation. Nevertheless, most research reports on vitamin E focus on α-tocopherol, which is 10 times more abundant in tissues than γ-tocopherol.
Studies with vitamin E indicate seemingly inconsistent outcomes for allergy and other inflammatory diseases. We have previously provided rationale to explain these conflicting outcomes based on our observation of the differences of levels of vitamin E isoforms present in the study supplements, vehicles, and diets (6, 28, 29). We have additionally demonstrated opposing regulatory functions of α-tocopherol and γ-tocopherol in mice and the mechanisms for anti- and proinflammatory functions of these tocopherols (7, 8, 28, 30, 31).
We have demonstrated that the isoform α-tocopherol is antiinflammatory and blocks airway hyperreactivity and that the isoform γ-tocopherol is proinflammatory and increases airway hyperreactivity during eosinophilic allergic lung inflammation in mice (6–8, 30, 31). In these studies, administration of α-tocopherol or γ-tocopherol to adult mice during allergen challenge effectively raised lung and plasma concentrations of the tocopherol isoform four- to fivefold (8). Moreover, γ-tocopherol elevated lung eosinophil recruitment by 175%, and α-tocopherol reduced lung eosinophil recruitment by 65% (8). Interestingly, γ-tocopherol negated the antiinflammatory benefit of α-tocopherol (8, 28). In these mice, α-tocopherol blocked and γ-tocopherol increased airway hyperresponsiveness (8). Furthermore, α-tocopherol plus γ-tocopherol resulted in an intermediate phenotype for airway responsiveness similar to that of the vehicle control–treated allergic mice, suggesting that these two tocopherols have competing opposing functions (8). The proinflammatory effects of γ-tocopherol in mice were partially reversed by switching supplements from γ-tocopherol to α-tocopherol (7). Thus, we have demonstrated opposing and competing functions of α-tocopherol and γ-tocopherol in vivo. Okamoto and colleagues (32) found that feeding mice α-tocopherol starting 2 weeks before antigen sensitization did not affect IgE levels but did reduce the number of eosinophils in the bronchoalveolar lavage. However, the form and purity of α-tocopherol were not indicated. In addition, Mabalirajan and colleagues (33) reported that oral administration of α-tocopherol in ethanol after antigen sensitization blocked ovalbumin (OVA)-induced lung inflammation and airway hyperresponsiveness. In a report by Suchankova and colleagues (34), purified α-tocopherol was administered in soy oil by gavage, and they found no major effect of α-tocopherol on immune parameters or lung airway responsiveness in mice challenged with OVA. However, the soy oil vehicle used in this study would contain an abundance of γ-tocopherol (Figure 1C), and neither tissue tocopherol levels nor vehicle tocopherol levels were measured. Our interpretation of this study is that high γ-tocopherol in the soy oil negated the effect of the α-tocopherol that was administered. Mice deficient in liver α-tocopherol transfer protein (αTTP) exhibit severe deficiency in tissue α- and γ-tocopherol as well as reduced IgE after OVA challenge to the lung (35). In these mice, it is not known whether severe tocopherol deficiency during mouse development alters leukocyte hematopoiesis or leukocyte responsiveness. Therefore, differences among the reports for tocopherol regulation of eosinophilic lung inflammation likely reflect differences in the intake of tocopherol isoforms, tocopherol isoform plasma concentrations, and time of administration of tocopherols.
We determined a mechanism for the opposing functions for α-tocopherol and γ-tocopherol on leukocyte recruitment in the mouse lung. During allergic inflammation, leukocytes bind to endothelium and are recruited from the blood. We demonstrated in vitro that the migration of leukocytes across endothelial cells is inhibited by pretreatment of the endothelial cells with α-tocopherol and elevated by pretreatment of the endothelial cells with γ-tocopherol (8). Pretreatment of endothelial cells with α-tocopherol plus γ-tocopherol results in an intermediate phenotype similar to the vehicle-treated endothelial cells (8). Thus, α-tocopherol and γ-tocopherol have opposite regulatory functions during leukocyte recruitment and allergic lung inflammation in mice.
The opposing functions of α-tocopherol and γ-tocopherol on endothelial cells during leukocyte migration across endothelial cells can occur through direct regulation of mediators of signal transduction. During allergic inflammation, the endothelial cell adhesion molecules VCAM-1 and ICAM-1 regulate recruitment of leukocytes, and these adhesion molecules signal through protein kinase C α (PKCα) (8, 30). We demonstrated that α-tocopherol inhibits VCAM-1 and ICAM-1 activation of PKCα in endothelial cells and that this is opposed by pretreatment of endothelial cells with γ-tocopherol (8, 30). It is also reported that α-tocopherol inhibits activation of PKCα in other cell systems or cell extracts, but the mechanisms for inhibition were not know (36). We demonstrated that α-tocopherol and γ-tocopherol directly bind to the regulatory domain of PKCα and that γ-tocopherol increases, whereas α-tocopherol decreases, recombinant PKCα activity (31). Thus, γ-tocopherol functions as an agonist and α-tocopherol functions as an antagonist of PKCα (31). In summary, tocopherol isoform regulation of PKCα in endothelial cells regulates leukocyte recruitment, which is critical for allergic lung inflammation and airway hyperresponsiveness.
Clinical studies indicate that higher intake of α-tocopherol may confer a modest protective effect on adult-onset asthma and beneficial effect on lung function (FEV1) or wheeze in studies in Finland and Italy but not in the United States or the Netherlands (37–41). In contrast, a very high dose of an acetate-conjugated d-α-tocopherol (1,500 IU, which is 1,006 mg) to subjects with mild atopic asthma in the United States for 16 weeks resulted in increased plasma α-tocopherol, decreased plasma γ-tocopherol, and improved airway responsiveness to methacholine challenge (42). In a study in England, dietary supplementation with α-tocopherol in soy oil to subjects with asthma had no impact on FEV1, asthma symptom scores, or bronchodilator use, but in our interpretation, the γ-tocopherol in the soy oil may oppose the benefit of the α-tocopherol (43). In a Scottish cohort, reduced maternal intake of vitamin E (likely referring to α-tocopherol) is associated with increased incidence of asthma and wheezing in children up to 5 years old (44, 45). In Devereux’s review of these data and changes in the environment in Scotland (45), it is discussed that from 1967 to 2004 there was a significant increase in vegetable oil intake by the Scottish, and we suggest that this would at least result in an increase in γ-tocopherol, because vegetable oil (soybean oil) is rich in γ-tocopherol (Figure 1C). A metaanalysis of reports on vitamin E and asthma concludes that dietary vitamin E intake is not generally associated with asthma status, although vitamin E was significantly lower among those with severe asthma (46). Our alternative interpretation is that this lack of association could occur if data on dietary intake were combined across studies that included marked variation of vitamin E isoforms that are present in diets, supplements, and supplement vehicles. Most clinical studies on vitamin E include mixed forms of natural and synthetic tocopherols from supplementation or diet. Therefore, differences in outcomes from clinical reports on the associations of vitamin E and asthma may, in part, reflect the opposing regulatory effects of α-tocopherol and γ-tocopherol in the supplements, the tocopherol isoforms in vehicles for the supplements, and the plasma γ-tocopherol levels in the individuals in these countries.
It is reported that there are low plasma α-tocopherol levels in adults or children with asthma (38, 39, 47–50). Because α-tocopherol levels are low in subjects with asthma, and because α-tocopherol can reduce inflammation, then an increase in α-tocopherol in the presence of low γ-tocopherol may be beneficial in combination with other regimens to either prevent or improve control of allergic disease and asthma. Further intervention studies are necessary to examine tocopherol isoform regulation of allergic lung inflammation and asthma. Although we know that α-tocopherol supplementation raises plasma α-tocopherol levels in humans (51) and that plasma and tissue tocopherols correlate (7, 8, 12), information about the clinical impact and the mechanisms whereby tocopherols differentially modulate inflammation will be important in designing interventions to prevent asthma or decrease prevalence of asthma morbidity.
The prevalence rate of asthma is higher in the United States, Netherlands, and Scotland than several European and Asian countries (Figure 1B). Interestingly, countries with the highest prevalence rate for asthma also tend to have high average human plasma levels of γ-tocopherol (Figure 1B). In contrast to the report of 1.4 μM plasma γ-tocopherol in China (Figure 1B), another report indicates an average of 2.4 μM plasma γ-tocopherol for a small group of tin miners in China (52), but it is not known whether tocopherol levels in this group of individuals differ from other areas in China. In the United States, the average human plasma γ-tocopherol levels are two to five times higher than those of many European and Asian countries (Figure 1B), whereas the average human plasma α-tocopherol levels are relatively similar among these countries (28). This fivefold higher level of human plasma γ-tocopherol is similar to the fivefold increase in plasma γ-tocopherol in mice that increased allergic lung inflammation with γ-tocopherol administration (8). The high human plasma γ-tocopherol levels in the United States are consistent with soybean oil, which is high in γ-tocopherol (53, 54) (Figure 1C), as the predominant food oil in the United States (55, 56). It is reported that dietary oils influence plasma tocopherol levels in humans. In studies with soybean oil administration, plasma γ-tocopherol is elevated two- to fivefold in humans and hamsters (57, 58). Also, in a study in which olive oil or soybean oil was administered to preterm human infants starting 24 hours after birth, there was a significant 1.5-fold increase in plasma α-tocopherol after feeding with olive oil as compared with feeding with soybean oil, but unfortunately γ-tocopherol was not reported (59). It is reported that as countries assume western lifestyles, diets change, including increased consumption of soybean oil (60). In contrast to high levels of γ-tocopherol in soybean oil, γ-tocopherol is low in other oils, such as sunflower oil, safflower oil, and olive oil, that are used in several European and Mediterranean countries (Figure 1C) (8). In addition to differences in tocopherol isoforms in diets and human plasma among these countries, there may be other environmental differences among these countries, such as intake of the antiinflammatory omega-3 fatty acid from fish oil consumption (61–63). Dietary unsaturated fatty acids may also modulate asthma (40, 64). There are also differences in asthma prevalence among racial and ethnic groups (65). However, studies examining vitamin E association with clinical outcomes generally adjust for several known confounding factors, such as sex, age, body mass index, race, and smoking. Although there may be other differences regarding the environment and genetics of the people in these countries, the outcomes for tocopherol isoforms and asthma in clinical studies are consistent with the studies demonstrating opposing functions of the tocopherol isoforms on leukocyte recruitment and allergic inflammation in mice (8).
In addition to conflicting outcomes in asthma for vitamin E, there are conflicting outcomes for vitamin E in other inflammatory diseases, including arthritis and cardiovascular disease. For example, it has been reported that human plasma γ-tocopherol is positively associated with osteoarthritis, whereas plasma α-tocopherol is negatively associated with osteoarthritis (66). In contrast, in another report on knee osteoarthritis, vitamin E supplementation (α-tocopherol) did not relieve symptoms, but the authors did not measure patient α-tocopherol or γ-tocopherol levels (67). In coronary heart disease and stroke, studies of tocopherols and heart disease are complex, because different dietary oils not only contain different forms of tocopherols but also contain different lipids that affect heart disease. Nevertheless, plasma γ-tocopherol levels are either not associated with heart disease or are associated with an increase in relative risk for myocardial infarction (68). In contrast, α-tocopherol intake is either not associated with heart disease or is associated with reduced death from heart disease (69–72). Therefore, although the clinical reports on vitamin E association with heart disease are inconsistent, for those reports with an effect on heart disease, γ-tocopherol is associated with an increase in heart disease, whereas α-tocopherol is associated with a decrease in heart disease. In summary, the opposing functions of α-tocopherol and γ-tocopherol in animal models (8) are consistent with the different outcomes for the clinical studies of tocopherol isoforms in heart disease and asthma.
Understanding both the epidemiology and the biology of vitamin E isoforms on asthma inception and control suggest that we should rethink how we study and supplement this nutrient. Analysis of associations of tocopherol isoforms with outcomes using the ratio of concentration of γ-tocopherol/concentration of α-tocopherol does not reflect the magnitude of the tocopherols. For example, a low concentration of γ-tocopherol/low concentration of α-tocopherol may have the same ratio as high γ-tocopherol concentration/high α-tocopherol concentration, but they could have quite different effects. We suggest that vitamin E isoforms should be measured in the supplements, vehicles, and patient plasma. Then, the analysis of opposing functions of tocopherol isoforms should include quartiles of plasma tocopherols with determination of whether there is an association of a tocopherol isoform with the clinical outcome when the concentration of the opposing tocopherol is low and causing the least competing opposing effects. Using this approach, we recently demonstrated in a study with 4,500 individuals (20% with asthma) that plasma γ-tocopherol is inversely associated with lung function (FEV1) and that plasma α-tocopherol is positively associated with lung function (FEV1) in subjects without and with asthma (unpublished observation).
The marked differences in rates of disease across the world, changes over short periods, and changes with migrating populations mean that asthma and allergic diseases are not inevitable consequences of a genetic predisposition. This is profoundly important, as the ability to modify diet and lifestyle means that lower rates of these diseases can be attained without medications or sophisticated medical infrastructures and could be achieved worldwide.
Footnotes
Supported by National Institutes of Health Grant R01 AT004837 (J.M.C.-M.).
Author Contributions: J.M.C.-M. conceived of and wrote the article. H.A.-V. measured the tocopherols in the oils. T.H. discussed and participated in revising the article.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Martinez FD. Genes, environments, development and asthma: a reappraisal. Eur Respir J. 2007;29:179–184. doi: 10.1183/09031936.00087906. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Bousquet PJ, Godard P, Daures JP. The public health implications of asthma. Bull World Health Organ. 2005;83:548–554. [PMC free article] [PubMed] [Google Scholar]
- 3.Vollmer WM, Osborne ML, Buist AS. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am J Respir Crit Care Med. 1998;157:1079–1084. doi: 10.1164/ajrccm.157.4.9704140. [DOI] [PubMed] [Google Scholar]
- 4.Friebele E. The attack of asthma. Environ Health Perspect. 1996;104:22–25. doi: 10.1289/ehp.9610422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uauy R, Hoffman DR, Birch EE, Birch DG, Jameson DM, Tyson J. Safety and efficacy of omega-3 fatty acids in the nutrition of very low birth weight infants: soy oil and marine oil supplementation of formula. J Pediatr. 1994;124:612–620. doi: 10.1016/s0022-3476(05)83144-0. [DOI] [PubMed] [Google Scholar]
- 6.Cook-Mills JM, McCary CA. Isoforms of vitamin E differentially regulate inflammation. Endocr Metab Immune Disord Drug Targets. 2010;10:348–366. doi: 10.2174/1871530311006040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of alpha-tocopherol and gamma-tocopherol’s effects. J Immunol. 2011;186:3674–3685. doi: 10.4049/jimmunol.1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills J. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter SC, Cahoon EB. Enhancing vitamin E in oilseeds: unraveling tocopherol and tocotrienol biosynthesis. Lipids. 2007;42:97–108. doi: 10.1007/s11745-007-3028-6. [DOI] [PubMed] [Google Scholar]
- 10.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled alpha- and gamma-tocopherols demonstrate faster plasma gamma-tocopherol disappearance and greater gamma-metabolite production. Free Radic Biol Med. 2005;38:857–866. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Bella DL, Schock BC, Lim Y, Leonard SW, Berry C, Cross CE, Traber MG. Regulation of the alpha-tocopherol transfer protein in mice: lack of response to dietary vitamin E or oxidative stress. Lipids. 2006;41:105–112. doi: 10.1007/s11745-006-5077-7. [DOI] [PubMed] [Google Scholar]
- 12.Redlich CA, Grauer JN, Van Bennekum AM, Clever SL, Ponn RB, Blaner WS. Characterization of carotenoid, vitamin A, and alpha-tocopheral levels in human lung tissue and pulmonary macrophages. Am J Respir Crit Care Med. 1996;154:1436–1443. doi: 10.1164/ajrccm.154.5.8912761. [DOI] [PubMed] [Google Scholar]
- 13.Zou W, Noh SK, Owen KQ, Koo SI. Dietary L-carnitine enhances the lymphatic absorption of fat and alpha-tocopherol in ovariectomized rats. J Nutr. 2005;135:753–756. doi: 10.1093/jn/135.4.753. [DOI] [PubMed] [Google Scholar]
- 14.Cavalier L, Ouahchi K, Kayden HJ, Di Donato S, Reutenauer L, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency: heterogeneity of mutations and phenotypic variability in a large number of families. Am J Hum Genet. 1998;62:301–310. doi: 10.1086/301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundl I, Guardiola M, Khoschsorur G, Sola R, Vallve JC, Godas G, Masana L, Maritschnegg M, Meinitzer A, Cardinault N, et al. Increased concentrations of circulating vitamin E in carriers of the apolipoprotein A5 gene - 1131T>C variant and associations with plasma lipids and lipid peroxidation. J Lipid Res. 2007;48:2506–2513. doi: 10.1194/jlr.M700285-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Guardiola M, Ribalta J, Gomez-Coronado D, Lasuncion MA, de Oya M, Garces C. The apolipoprotein A5 (ApoA5) gene predisposes Caucasian children to elevated triglycerides and vitamin E (four provinces study) Atherosclerosis. 2010;212:543–547. doi: 10.1016/j.atherosclerosis.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Huebbe P, Jofre-Monseny L, Rimbach G. Alpha-tocopherol transport in the lung is affected by the apoE genotype–studies in transgenic apoE3 and apoE4 mice. IUBMB Life. 2009;61:453–456. doi: 10.1002/iub.177. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida Y, Saito Y, Jones LS, Shigeri Y. Chemical reactivities and physical effects in comparison between tocopherols and tocotrienols: physiological significance and prospects as antioxidants. J Biosci Bioeng. 2007;104:439–445. doi: 10.1263/jbb.104.439. [DOI] [PubMed] [Google Scholar]
- 19.Nishio K, Horie M, Akazawa Y, Shichiri M, Iwahashi H, Hagihara Y, Yoshida Y, Niki E. Attenuation of lipopolysaccharide (LPS)-induced cytotoxicity by tocopherols and tocotrienols. Redox Biology. 2013;1:97–103. doi: 10.1016/j.redox.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang PH, Chuang HC, Chou CC, Wang H, Lee SL, Yang HC, Chiu HC, Kapuriya N, Wang D, Kulp SK, et al. Vitamin E facilitates the inactivation of the kinase Akt by the phosphatase PHLPP1. Sci Signal. 2013;6:ra19. doi: 10.1126/scisignal.2003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel A, Liebner F, Netscher T, Mereiter K, Rosenau T. Vitamin E chemistry. Nitration of non-alpha-tocopherols: products and mechanistic considerations. J Org Chem. 2007;72:6504–6512. doi: 10.1021/jo0706832. [DOI] [PubMed] [Google Scholar]
- 22.Fakhrzadeh L, Laskin JD, Laskin DL. Ozone-induced production of nitric oxide and TNF-alpha and tissue injury are dependent on NF-kappaB p50. Am J Physiol Lung Cell Mol Physiol. 2004;287:L279–L285. doi: 10.1152/ajplung.00348.2003. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez ML, Wagner JG, Aline Kala R, Mills K, Wells HB, Alexis NE, Lay JC, Jiang Q, Zhang H, Zhou H, et al. Vitamin E, γ-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic Biol Med. 2013;60:56–62. doi: 10.1016/j.freeradbiomed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, Peden DB. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic Biol Med. 2008;45:40–49. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner JG, Harkema JR, Jiang Q, Illek B, Ames BN, Peden DB. Gamma-tocopherol attenuates ozone-induced exacerbation of allergic rhinosinusitis in rats. Toxicol Pathol. 2009;37:481–491. doi: 10.1177/0192623309335630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 2008;38:501–511. doi: 10.1111/j.1365-2222.2007.02855.x. [DOI] [PubMed] [Google Scholar]
- 27.Hamahata A, Enkhbaatar P, Kraft ER, Lange M, Leonard SW, Traber MG, Cox RA, Schmalstieg FC, Hawkins HK, Whorton EB, et al. Gamma-tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free Radic Biol Med. 2008;45:425–433. doi: 10.1016/j.freeradbiomed.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15:1607–1638. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cook-Mills JM.Eosinophil-endothelial cell interactions during inflammation. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. Oxford, UK: Elsevier; 2012. pp. 139–153. [Google Scholar]
- 30.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms differentially regulate intercellular adhesion molecule-1 activation of PKCα in human microvascular endothelial cells. PLoS ONE. 2012;7:e41054. doi: 10.1371/journal.pone.0041054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCary CA, Yoon Y, Panagabko C, Cho W, Atkinson J, Cook-Mills JM. Vitamin E isoforms directly bind PKCα and differentially regulate activation of PKCα. Biochem J. 2012;441:189–198. doi: 10.1042/BJ20111318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto N, Murata T, Tamai H, Tanaka H, Nagai H. Effects of alpha tocopherol and probucol supplements on allergen-induced airway inflammation and hyperresponsiveness in a mouse model of allergic asthma. Int Arch Allergy Immunol. 2006;141:172–180. doi: 10.1159/000094896. [DOI] [PubMed] [Google Scholar]
- 33.Mabalirajan U, Aich J, Leishangthem GD, Sharma SK, Dinda AK, Ghosh B. Effects of vitamin E on mitochondrial dysfunction and asthma features in an experimental allergic murine model. J Appl Physiol. 2009;107:1285–1292. doi: 10.1152/japplphysiol.00459.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suchankova J, Voprsalova M, Kottova M, Semecky V, Visnovsky P. Effects of oral alpha-tocopherol on lung response in rat model of allergic asthma. Respirology. 2006;11:414–421. doi: 10.1111/j.1440-1843.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 35.Lim Y, Vasu VT, Valacchi G, Leonard S, Aung HH, Schock BC, Kenyon NJ, Li CS, Traber MG, Cross CE. Severe vitamin E deficiency modulates airway allergic inflammatory responses in the murine asthma model. Free Radic Res. 2008;42:387–396. doi: 10.1080/10715760801976600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahoney CW, Azzi A. Vitamin E inhibits protein kinase C activity. Biochem Biophys Res Commun. 1988;154:694–697. doi: 10.1016/0006-291x(88)90195-7. [DOI] [PubMed] [Google Scholar]
- 37.Weiss ST. Diet as a risk factor for asthma. Ciba Found Symp. 1997;206:244–257. [PubMed] [Google Scholar]
- 38.Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. Am J Respir Crit Care Med. 1995;151:1401–1408. doi: 10.1164/ajrccm.151.5.7735592. [DOI] [PubMed] [Google Scholar]
- 39.Dow L, Tracey M, Villar A, Coggon D, Margetts BM, Campbell MJ, Holgate ST. Does dietary intake of vitamins C and E influence lung function in older people? Am J Respir Crit Care Med. 1996;154:1401–1404. doi: 10.1164/ajrccm.154.5.8912755. [DOI] [PubMed] [Google Scholar]
- 40.Smit HA, Grievink L, Tabak C. Dietary influences on chronic obstructive lung disease and asthma: a review of the epidemiological evidence. Proc Nutr Soc. 1999;58:309–319. doi: 10.1017/s0029665199000427. [DOI] [PubMed] [Google Scholar]
- 41.Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, Heederik D, Kromhout D. Dietary factors and pulmonary function: a cross sectional study in middle aged men from three European countries. Thorax. 1999;54:1021–1026. doi: 10.1136/thx.54.11.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoskins A, Roberts JL, II, Milne G, Choi L, Dworski R. Natural-source d-α-tocopheryl acetate inhibits oxidant stress and modulates atopic asthma in humans in vivo. Allergy. 2012;67:676–682. doi: 10.1111/j.1398-9995.2012.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martindale S, McNeill G, Devereux G, Campbell D, Russell G, Seaton A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med. 2005;171:121–128. doi: 10.1164/rccm.200402-220OC. [DOI] [PubMed] [Google Scholar]
- 45.Devereux G. Early life events in asthma–diet. Pediatr Pulmonol. 2007;42:663–673. doi: 10.1002/ppul.20640. [DOI] [PubMed] [Google Scholar]
- 46.Allen S, Britton JR, Leonardi-Bee JA. Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax. 2009;64:610–619. doi: 10.1136/thx.2008.101469. [DOI] [PubMed] [Google Scholar]
- 47.Kalayci O, Besler T, Kilinc K, Sekerel BE, Saraclar Y. Serum levels of antioxidant vitamins (alpha tocopherol, beta carotene, and ascorbic acid) in children with bronchial asthma. Turk J Pediatr. 2000;42:17–21. [PubMed] [Google Scholar]
- 48.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 49.Schunemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, Klocke RA, Trevisan M. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. 2001;163:1246–1255. doi: 10.1164/ajrccm.163.5.2007135. [DOI] [PubMed] [Google Scholar]
- 50.Al-Abdulla NO, Al Naama LM, Hassan MK. Antioxidant status in acute asthmatic attack in children. J Pak Med Assoc. 2010;60:1023–1027. [PubMed] [Google Scholar]
- 51.Wu D, Han SN, Meydani M, Meydani SN. Effect of concomitant consumption of fish oil and vitamin E on T cell mediated function in the elderly: a randomized double-blind trial. J Am Coll Nutr. 2006;25:300–306. doi: 10.1080/07315724.2006.10719539. [DOI] [PubMed] [Google Scholar]
- 52.Ratnasinghe D, Tangrea JA, Forman MR, Hartman T, Gunter EW, Qiao YL, Yao SX, Barett MJ, Giffen CA, Erozan Y, et al. Serum tocopherols, selenium and lung cancer risk among tin miners in China. Cancer Causes Control. 2000;11:129–135. doi: 10.1023/a:1008977320811. [DOI] [PubMed] [Google Scholar]
- 53.Jiang Q, Christen S, Shigenaga MK, Ames BN. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 54.Talegawkar SA, Johnson EJ, Carithers T, Taylor HA, Jr, Bogle ML, Tucker KL. Total alpha-tocopherol intakes are associated with serum alpha-tocopherol concentrations in African American adults. J Nutr. 2007;137:2297–2303. doi: 10.1093/jn/137.10.2297. [DOI] [PubMed] [Google Scholar]
- 55.Bieri JG, Evarts RP. Tocopherols and fatty acids in American diets. The recommended allowance for vitamin E. J Am Diet Assoc. 1973;62:147–151. [PubMed] [Google Scholar]
- 56.Bieri JG, Evarts RP. Vitamin E adequacy of vegetable oils. J Am Diet Assoc. 1975;66:134–139. [PubMed] [Google Scholar]
- 57.Meydani M, Cohn JS, Macauley JB, McNamara JR, Blumberg JB, Schaefer EJ. Postprandial changes in the plasma concentration of alpha- and gamma-tocopherol in human subjects fed a fat-rich meal supplemented with fat-soluble vitamins. J Nutr. 1989;119:1252–1258. doi: 10.1093/jn/119.9.1252. [DOI] [PubMed] [Google Scholar]
- 58.Sanchez-Moreno C, Dorfman SE, Lichtenstein AH, Martin A. Dietary fat type affects vitamins C and E and biomarkers of oxidative status in peripheral and brain tissues of golden Syrian hamsters. J Nutr. 2004;134:655–660. doi: 10.1093/jn/134.3.655. [DOI] [PubMed] [Google Scholar]
- 59.Gobel Y, Koletzko B, Bohles HJ, Engelsberger I, Forget D, Le Brun A, Peters J, Zimmermann A. Parenteral fat emulsions based on olive and soybean oils: a randomized clinical trial in preterm infants. J Pediatr Gastroenterol Nutr. 2003;37:161–167. doi: 10.1097/00005176-200308000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–1117. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]
- 61.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Weylandt KH, Chiu CY, Gomolka B, Waechter SF, Wiedenmann B. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012;97:73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Junker R, Kratz M, Neufeld M, Erren M, Nofer JR, Schulte H, Nowak-Gottl U, Assmann G, Wahrburg U. Effects of diets containing olive oil, sunflower oil, or rapeseed oil on the hemostatic system. Thromb Haemost. 2001;85:280–286. [PubMed] [Google Scholar]
- 64.McKeever TM, Lewis SA, Cassano PA, Ocke M, Burney P, Britton J, Smit HA. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax. 2008;63:208–214. doi: 10.1136/thx.2007.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zahran HS, Bailey C. Factors associated with asthma prevalence among racial and ethnic groups-united states, 2009–2010 behavioral risk factor surveillance system. J Asthma. 2013;11:11. doi: 10.3109/02770903.2013.794238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jordan JM, De Roos AJ, Renner JB, Luta G, Cohen A, Craft N, Helmick CG, Hochberg MC, Arab L. A case-control study of serum tocopherol levels and the alpha- to gamma-tocopherol ratio in radiographic knee osteoarthritis: the Johnston County Osteoarthritis Project. Am J Epidemiol. 2004;159:968–977. doi: 10.1093/aje/kwh133. [DOI] [PubMed] [Google Scholar]
- 67.Brand C, Snaddon J, Bailey M, Cicuttini F. Vitamin E is ineffective for symptomatic relief of knee osteoarthritis: a six month double blind, randomised, placebo controlled study. Ann Rheum Dis. 2001;60:946–949. doi: 10.1136/ard.60.10.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dietrich M, Traber MG, Jacques PF, Cross CE, Hu Y, Block G. Does gamma-tocopherol play a role in the primary prevention of heart disease and cancer? A review. J Am Coll Nutr. 2006;25:292–299. doi: 10.1080/07315724.2006.10719538. [DOI] [PubMed] [Google Scholar]
- 69.Munteanu A, Zingg JM. Cellular, molecular and clinical aspects of vitamin E on atherosclerosis prevention. Mol Aspects Med. 2007;28:538–590. doi: 10.1016/j.mam.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Siekmeier R, Steffen C, Marz W. Role of oxidants and antioxidants in atherosclerosis: Results of in vitro and in vivo investigations. J Cardiovasc Pharmacol Ther. 2007;12:265–282. doi: 10.1177/1074248407299519. [DOI] [PubMed] [Google Scholar]
- 71.Meydani M. Vitamin E modulation of cardiovascular disease. Ann N Y Acad Sci. 2004;1031:271–279. doi: 10.1196/annals.1331.027. [DOI] [PubMed] [Google Scholar]
- 72.Dutta A, Dutta SK. Vitamin E and its role in the prevention of atherosclerosis and carcinogenesis: a review. J Am Coll Nutr. 2003;22:258–268. doi: 10.1080/07315724.2003.10719302. [DOI] [PubMed] [Google Scholar]
- 73.Boyle SP, Dobson VL, Duthie SJ, Hinselwood DC, Kyle JA, Collins AR. Bioavailability and efficiency of rutin as an antioxidant: a human supplementation study. Eur J Clin Nutr. 2000;54:774–782. doi: 10.1038/sj.ejcn.1601090. [DOI] [PubMed] [Google Scholar]
- 74.Johnstone AM, Lobley GE, Horgan GW, Bremner DM, Fyfe CL, Morrice PC, Duthie GG. Effects of a high-protein, low-carbohydrate v. high-protein, moderate-carbohydrate weight-loss diet on antioxidant status, endothelial markers and plasma indices of the cardiometabolic profile. Br J Nutr. 2011;106:282–291. doi: 10.1017/S0007114511000092. [DOI] [PubMed] [Google Scholar]
- 75.Hodge AM, Simpson JA, Fridman M, Rowley K, English DR, Giles GG, Su Q, O’Dea K. Evaluation of an FFQ for assessment of antioxidant intake using plasma biomarkers in an ethnically diverse population. Public Health Nutr. 2009;12:2438–2447. doi: 10.1017/S1368980009005539. [DOI] [PubMed] [Google Scholar]
- 76.Samman S, Sivarajah G, Man JC, Ahmad ZI, Petocz P, Caterson ID. A mixed fruit and vegetable concentrate increases plasma antioxidant vitamins and folate and lowers plasma homocysteine in men. J Nutr. 2003;133:2188–2193. doi: 10.1093/jn/133.7.2188. [DOI] [PubMed] [Google Scholar]
- 77.Heinonen OP, Albanes D. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The alpha-tocopherol, beta carotene cancer prevention study group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 78.Zieden B, Kaminskas A, Kristenson M, Kucinskiene Z, Vessby B, Olsson AG, Diczfalusy U. Increased plasma 7 beta-hydroxycholesterol concentrations in a population with a high risk for cardiovascular disease. Arterioscler Thromb Vasc Biol. 1999;19:967–971. doi: 10.1161/01.atv.19.4.967. [DOI] [PubMed] [Google Scholar]
- 79.Gylling H, Hallikainen M, Nissinen MJ, Miettinen TA. The effect of a very high daily plant stanol ester intake on serum lipids, carotenoids, and fat-soluble vitamins. Clin Nutr. 2010;29:112–118. doi: 10.1016/j.clnu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Valimaki IA, Vuorimaa T, Ahotupa M, Kekkonen R, Korpela R, Vasankari T. Decreased training volume and increased carbohydrate intake increases oxidized LDL levels. Int J Sports Med. 2012;33:291–296. doi: 10.1055/s-0031-1291223. [DOI] [PubMed] [Google Scholar]
- 81.Safronov ID, Trunov AN, Kokareva ED. Prooxidant-antioxidant factors in the blood of pregnant women with late gestosis of different severity. Bull Exp Biol Med. 2008;146:800–802. doi: 10.1007/s10517-009-0417-2. [DOI] [PubMed] [Google Scholar]
- 82.Ross MA, Crosley LK, Brown KM, Duthie SJ, Collins AC, Arthur JR, Duthie GG. Plasma concentrations of carotenoids and antioxidant vitamins in Scottish males: influences of smoking. Eur J Clin Nutr. 1995;49:861–865. [PubMed] [Google Scholar]
- 83.Wu LS, Sjakste T, Sakalauskas R, Sitkauskiene B, Paramonova N, Gasiuniene E, Jan RL, Wang JY. The burden of allergic asthma in children: a landscape comparison based on data from Lithuanian, Latvian, and Taiwanese populations. Pediatr Neonatol. 2012;53:276–282. doi: 10.1016/j.pedneo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 84.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 85.To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, Boulet LP. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]