Abstract
Rationale: Lung clearance index (LCI), measured by multiple breath washout (MBW), is a noninvasive measure of ventilation inhomogeneity that holds promise as an objective physiologic endpoint for clinical trials in infants and preschool children with cystic fibrosis (CF).
Objectives: To study the feasibility of using LCI to assess treatment effect outcomes in CF trials of infants and preschoolers.
Methods: The Infant Study of Inhaled Saline trial was a multicenter, randomized, controlled trial of hypertonic (7%) versus isotonic (0.9%) saline inhaled twice daily for 48 weeks in children with CF under 6 years of age. LCI measurements were performed in a single-center pilot substudy at baseline and 48 weeks using a respiratory mass spectrometer and sulfur hexafluoride as the tracer gas. LCI measurements were standardized using published normative data (zLCI) to account for height-related changes in LCI during early childhood. A generalized estimating equation model with an interaction between treatment group and test occasion was used to estimate a treatment effect.
Measurements and Main Results: A total of 27 participants were randomized; 25 participants, aged (median [range]) 2.6 (0.34–4.95) years, had acceptable baseline and follow-up LCI measures. On average, LCI decreased in the hypertonic saline group (n = 12) by 1.19 z-scores units (95% confidence interval [CI] = −2.46 to 0.06), and remained stable in the isotonic saline group (n = 13) at 0.81 (95% CI = −0.40 to 2.02). A significant treatment effect was observed for zLCI (2.01; 95% CI = 0.26 to 3.76; P = 0.025).
Conclusions: MBW testing is feasible in an interventional study in infants and preschool children with CF. These pilot findings support the development of MBW and LCI as an objective outcome measure in interventional trials in young children with CF, and provide estimates for sample size calculations for future studies.
Clinical trial registered with www.clinicaltrials.gov (NCT00709280).
Keywords: cystic fibrosis, lung clearance index, infant, hypertonic saline, preschool child
At a Glance Commentary
Scientific Knowledge on the Subject
Lung clearance index (LCI) is a very sensitive marker of early lung disease in cystic fibrosis (CF) and has the advantage of high feasibility in infants and very young children.
What This Study Adds to the Field
This is the first study to assess LCI as an outcome measure of treatment response in a clinical trial of infants and preschoolers with CF. The superior feasibility of LCI supports its potential use as an outcome measure for early interventions.
Cystic fibrosis (CF) is characterized by early lung disease, often with onset before symptoms. Persistent airway inflammation and infection ultimately lead to irreversible structural damage and bronchiectasis (1–3). Lung clearance index (LCI), a measure of ventilation inhomogeneity derived from the multiple breath washout (MBW) test, holds promise for the early detection of lung disease in CF (4). It has been shown to be more predictive of later lung function abnormalities than FEV1 (5) and to correlate with structural changes (6, 7). Furthermore, LCI has been shown to detect treatment responses to interventions in children with CF between the ages of 6 and 18 years with baseline spirometry in the normal range (FEV1 ≥ 80%) (8, 9).
Hypertonic saline (HS) has become part of clinical care in patients with CF who are 6 years of age and older, based on evidence that it is effective in reducing exacerbations (10, 11). As the proposed mechanism of action for this agent is to improve mucociliary clearance (12), HS is a potential candidate for effective early intervention before fixed structural damage of the airways has occurred. The Infant Study of Inhaled Saline (ISIS), which compared 7% HS to isotonic saline (IS) in children with CF under 6 years of age, showed no between-group differences in rates of pulmonary exacerbation (13). Based on previous work (8, 9) demonstrating the ability of LCI as an objective outcome measure to detect a treatment effect of HS in children over 6 years of age, we performed a pilot substudy at the Toronto site to assess the potential ability of LCI to assess the treatment effect of HS in infants and preschool children with CF, and derive sample sizes for future clinical trials in this age range.
Some of these results have been previously reported in the form of an abstract (14).
Methods
Details of the ISIS study have been reported previously (13). In brief, infant (>4 mo) and preschool children (<6 yr) with CF who demonstrated tolerability to HS were recruited to a randomized multicenter study of treatment with HS or IS given twice daily after administration of a bronchodilator. Infants (<16 mo) underwent infant pulmonary function testing (PFT).
All participants in the ISIS study at the Hospital for Sick Children (Toronto, ON, Canada) consented to participate in this single-center MBW substudy approved by the local research ethics board (Hospital for Sick Children Research Ethics Board file no. 1000012484). Anthropometrics were measured using a standardized scale and stadiometer. Height was measured standing in all preschool children and supine in all infants. MBW measurements were made at enrollment and at the 48-week final study visit. In infants (<16 mo), measurements were performed during quiet sleep in a supine position while under sedation with chloral hydrate (80–100 mg/kg). MBW measurement was performed before the other tests (plethysmography and raised-volume rapid thoracic compression). All infant testing was performed using facemask (Silkomed, Rendell Baker Masks sizes 2 and 3; Rusch Canada Inc., Benson Medical Industries, Markham, ON, Canada) sealed with therapeutic putty (Air Putty; Sammons Preston Canada Inc., Mississauga, ON, Canada). In children under 16 months of age, measurements were performed while awake in the seated position wearing a facemask, sealed with therapeutic putty, watching a video, and quietly breathing. Baseline and follow-up measurements were performed with the same protocol (e.g., if sedated at baseline and tested using the infant protocol, follow-up visit was also performed under sedation using the infant protocol).
A mass spectrometer–based setup (AMIS 2000; Innovision A/S, Odense, Denmark) and technique was used to perform MBW testing with a sulfur hexafluoride (SF6)/helium gas mixture as previously described (4). Briefly, participants breathed in a dry gas mixture containing 4% SF6, 4% helium, 21% oxygen (balance nitrogen) via an open circuit bias flow system through a mask and an attached pneumotachograph (Hans Rudolph, Shawnee, KS) until equilibrium was reached. Once the end-expiratory inert tracer gas (SF6) had stabilized at 4%, the gas source was removed and the subject breathed room air until [SF6] reaching 1/40th of the starting concentration. End of test was considered to be the first of three breaths where the end-tidal concentration of SF6 was below target concentration (15). Tests were performed in triplicate or until the operator was satisfied that two technically acceptable trials had been obtained. The average LCI for each test occasion was calculated for each subject.
Statistical Analysis
Height, weight, and body mass index measurements were converted to centiles using the World Health Organization growth charts for infants (16) and Centers for Disease Control and Prevention growth charts for preschool children (17). LCI was standardized to a z score (zLCI) using a reference population to account for the height-related changes in LCI during early childhood (18). Comparisons of treatment groups at baseline were made using nonparametric tests (Fischer’s exact test, Mann-Whitney test). The treatment effect (i.e., change in LCI) of 7% HS relative to IS was determined using a generalized estimating equation model to account for the repeated measurements, with an interaction term between test occasion and treatment. Treatment effect analyses were repeated using zLCI as the outcome. The final models were also adjusted for baseline LCI and age-adjusted centile for height, weight, and body mass index. Regression to the mean was assessed by comparing the change in LCI against the baseline LCI (19). Analyses were also stratified for infants and preschool children.
Results
A total of 44 patients with CF were approached to participate in the main ISIS trial at the Hospital for Sick Children in Toronto. Of the 28 patients that provided consent, 1 patient was intolerant to the test dose of HS, and thus was not randomized. A total of 27 participants were randomized: 13 participants to HS and 14 participants to the IS. Two preschool children did not tolerate the facemask at baseline MBW testing; therefore, results are presented for 25 subjects (93%), all of whom completed both baseline and follow-up measurements: 12 HS (four infant, eight preschool children) and 13 IS (six infant, seven preschool children). For the 25 subjects who had MBW measurements at baseline, patients attempted the measurement an average of four times (range, two to five) to achieve an average of three analyzable tests per occasion (range, two to five), and took an additional 15–20 minutes to perform. Baseline characteristics of the study participants are presented in (Table 1). The baseline characteristics of the Toronto study participants were similar to those of the overall study population (data not shown). Success rates for baseline testing for infant pulmonary function parameters in the 10 infants were 10/10 (100%) for plethysmographic lung volumes and 7/10 (70%) for raised-volume parameters (FEV0.5), similar to the overall main ISIS study population. Given the small numbers of subjects who performed these measures, comparison with LCI outcomes are not possible.
TABLE 1.
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
| Hypertonic Saline (n = 12) | Isotonic Saline (n = 13) | P Value* | |
|---|---|---|---|
| Age, median (IQR) |
2.57 (1.01 to 4.50) |
2.96 (1.15 to 3.75) |
0.826 |
| Boys, n (%) |
8 (53.3) |
7 (46.7) |
0.404 |
| Height-for-age centile, median (IQR) |
22.29 (9.75 to 36.50) |
33.25 (29.37 to 62.0) |
0.092 |
| Weight-for-age centile, median (IQR) |
43.86 (9.64 to 55.68) |
80.7 (52.85 to 85.18) |
0.002 |
| BMI-for-age centile, median (IQR) |
66.93 (18.74 to 81.88) |
76.28 (73.61 to 97.49) |
0.174 |
| LCI, median (IQR) |
7.56 (6.95 to 10.57) |
6.78 (6.58 to 9.40) |
0.301 |
| zLCI, median (IQR) |
1.13 (−0.12 to 4.62) |
−0.17 (−0.45 to 3.62) |
0.253 |
| No. of positive bacterial throat swabs/total (%) | 10/12 (83) | 11/13 (85) | 1.0 |
Definition of abbreviations: BMI = body mass index; IQR = interquartile range; LCI = lung clearance index; zLCI = LCI standardized to a z score.
Height, weight and body mass index were converted to centiles corrected for age using the World Health Organization growth charts for infants (13) and Centers for Disease Control and Prevention growth charts for preschool children (14). zLCI was calculated using equations derived from normative data from methodology reference population (8).
P values were calculated using nonparametric tests to coincide with median values; Fischer’s exact test for categorical variables, Mann-Whitney for continuous variables.
In the main ISIS trial (13), there was no significant difference between treatment groups in the primary study endpoint: rate of pulmonary exacerbations per patient year. The same was observed for this substudy sample (HS 1.4 [95% confidence interval (CI) = 0.9–2.3] vs. IS 1.6 [95% CI = 1.0–2.4]). In the MBW substudy, the IS group had higher weight-for-age centiles compared with the HS group, whereas LCI and zLCI were not different between the two groups at baseline. Using an upper limit of normal, defined by a reference population (15), all 10 infants had LCI values within the normal range, whereas 9 of 15 (60%) preschool children had LCI values above the upper limit of normal (five HS, four IS; Figure 1).
Figure 1.
Lung clearance index (LCI) at baseline. Closed symbols indicate the isotonic saline (IS) group and open symbols indicate the hypertonic saline (HS) group. The upper limit of normal (ULN) was defined using a reference population (18).
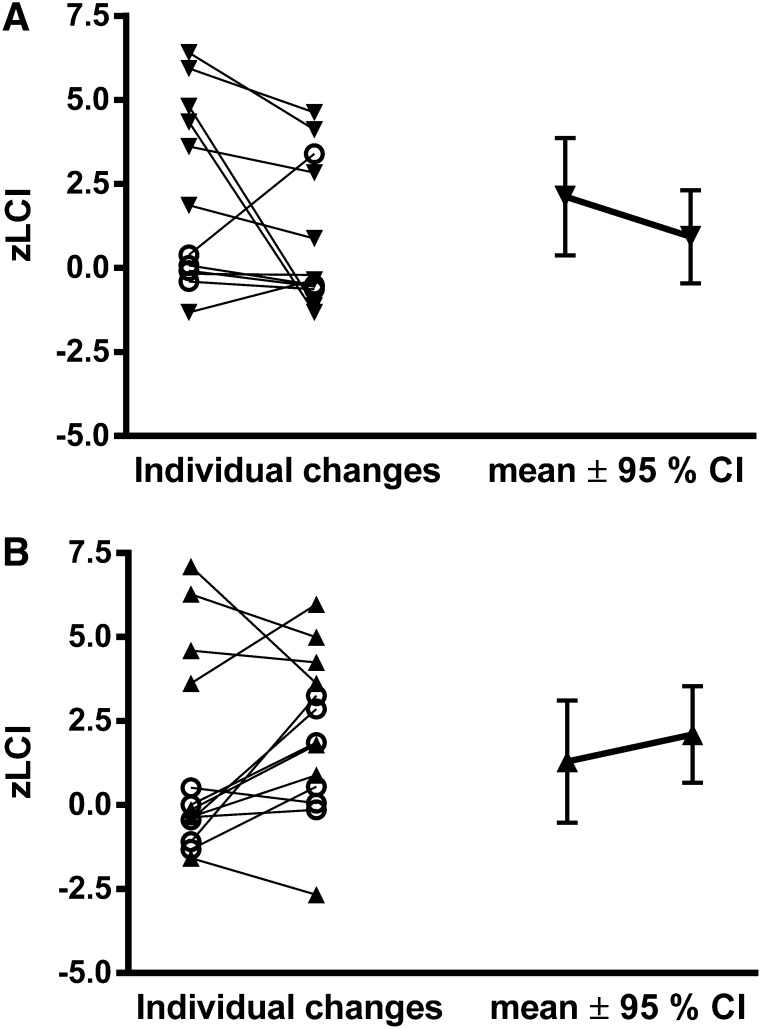
In the HS group, LCI decreased significantly from baseline to the 48-week follow-up by 1.20 units (95% CI = −2.29 to −0.12; P = 0.03; Table 2). Conversely, in the IS group, LCI did not change from baseline to follow-up (Δ = 0.23 units; 95% CI −0.90 to 1.35; P = 0.69; Table 2). Because LCI decreased in the HS group and remained unchanged in the IS group over the treatment period, there was an overall trend toward a treatment effect of 1.43 units (95% CI = −0.13 to 2.99; P = 0.07). The treatment effect was stronger when zLCI was used as the outcome (2.0 [95% CI = 0.25 to 3.76]; P = 0.03; Table 2, Figure 2), where zLCI accounts for the inverse relationship between height and LCI observed in the reference population. Adjustment for baseline LCI and weight-for-age centiles attenuated the treatment effect, but the treatment effect remained in favor of HS (data not shown). Regression to the mean analysis showed that this phenomenon was equally present in both the IS and HS groups.
TABLE 2.
AVERAGE CHANGE IN LUNG CLEARANCE INDEX BETWEEN BASELINE AND FOLLOW-UP FOR EACH TREATMENT GROUP AND THE RELATIVE TREATMENT EFFECT IN THE HYPERTONIC SALINE GROUP COMPARED TO THE ISOTONIC SALINE GROUP, AND AVERAGE CHANGE IN Z-SCORE–STANDARDIZED LUNG CLEARANCE INDEX (15)
| n | Hypertonic Saline | SD | P Value | n | Isotonic Saline | SD | P Value | Treatment Effect | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| LCI |
|
|
|
|
|
|
|
|
|
|
| Overall |
12 |
−1.20 (−2.29 to −0.12) |
2.0 |
0.030 |
13 |
0.23 (−0.90 to 1.35) |
2.1 |
0.693 |
1.43 (−0.13 to 2.99) |
0.073 |
| Infant |
4 |
0.19 (−0.92 to 1.30) |
1.2 |
0.737 |
6 |
1.03 (0.11 to 1.95) |
1.2 |
0.028 |
0.84 (−0.60 to 2.28) |
0.251 |
| Preschool |
8 |
−1.90 (−3.19 to −0.60) |
1.9 |
0.004 |
7 |
−0.47 (−2.27 to 1.34) |
2.5 |
0.613 |
1.43 (−0.79 to 3.65) |
0.206 |
| zLCI |
|
|
|
|
|
|
|
|
|
|
| Overall |
12 |
−1.19 (−2.46 to 0.06) |
2.5 |
0.063 |
13 |
0.81 (−0.40 to 2.02) |
2.1 |
0.191 |
2.01 (0.26 to 3.76) |
0.025 |
| Infant |
4 |
0.44 (−1.12 to 1.99) |
1.7 |
0.583 |
6 |
1.86 (0.59 to 3.13) |
1.8 |
0.004 |
1.42 (−0.59 to 3.44) |
0.165 |
| Preschool | 8 | −2.02 (−3.53 to −0.51) | 2.5 | 0.009 | 7 | −0.09 (−1.70 to 1.52) | 2.1 | 0.912 | 1.93 (−0.28 to 4.14) | 0.087 |
Definition of abbreviations: LCI = lung clearance index; zLCI = LCI standardized to a z score.
Results were stratified for the infant and preschool groups. SD derived from the average change in LCI and zLCI. Results for treatment groups reported as mean and 95% confidence interval for the average change in LCI and zLCI.
Figure 2.
Individual changes in lung clearance index (LCI) expressed as z scores (15) between baseline and follow-up, followed by the average change in LCI in the (A) hypertonic saline group and (B) isotonic saline group. Open circles represent infant subjects and closed triangles represent preschool subjects. CI = confidence interval.
The observed effects of HS and IS on change in LCI over the treatment period differed between infants and preschool children (Table 2, Figure 2). In infants, all 10 of whom had LCIs in the normal range at enrollment, LCI increased (worsened) significantly over the treatment period in the IS group, whereas no change in LCI was observed in the HS group. In preschool children, among whom the majority (9 of 15) had an abnormal LCI at enrollment, LCI decreased significantly in the HS group, whereas no change was observed in the IS group.
The size of this pilot study was limited by the number of ISIS participants enrolled at our center and was not a priori powered to detect a significant treatment effect. To help inform future studies using LCI, Table 3 contains sample size estimates for a range of treatment effects.
TABLE 3.
SAMPLE SIZE ESTIMATES FOR INTERVENTION TRIALS USING LUNG CLEARANCE INDEX AS AN OUTCOME MEASURE
| Treatment Effect | SD | n (α = 0.05) | n (α = 0.01) |
|---|---|---|---|
| 1.0 |
2.0 |
85 |
120 |
| 1.0 |
2.5 |
132 |
186 |
| 1.0 |
3.0 |
190 |
268 |
| 1.5 |
2.0 |
38 |
53 |
| 1.5 |
2.5 |
59 |
83 |
| 1.5 |
3.0 |
85 |
120 |
|
2.0 |
2.0 |
22 |
30 |
| 2.0 |
2.5 |
33 |
47 |
| 2.0 |
3.0 |
48 |
67 |
| 2.5 |
2.0 |
14 |
20 |
| 2.5 |
2.5 |
22 |
30 |
| 2.5 | 3.0 | 31 | 43 |
Treatment effects are based on changes in lung clearance index z scores in a parallel group study, and assume the two treatment groups have the same variability in the outcome measure, assuming an α of 0.05 or 0.01 and a power of 90%. In bold is the treatment effect and SD seen in this trial.
Discussion
This is the first study to assess LCI as an endpoint in an interventional trial in infants and preschool children with CF. Feasibility of repeated measures was high, which is an important consideration for interventional studies in this challenging age group. Although significant improvements in absolute LCI were observed within the HS group, the overall treatment effect did not reach statistical significance in this sample of 25 subjects. However, using z scores to adjust for the known growth (i.e., height) -related decline in LCI during early childhood (18), the treatment effect using zLCI was statistically significant.
These pilot findings suggest that a treatment effect in zLCI can be detected with a relatively small sample size in this age range. They also emphasize the need to adjust LCI for height when interpreting results in young children. Taken together, these findings, along with the high feasibility, support the further evaluation of zLCI as an objective outcome measure for clinical trials in infant and preschool CF studies.
In this study, we were able to obtain a baseline measurement in 25/27 (93%) of the study participants and a follow-up measurement in 100% of these children. This compares favorably to other infant PFTs performed in the ISIS study, in which paired, acceptable-quality test data were achieved in 49/73 (67%) for FEV0.5, 62/73 (85%) for FRC, and 43/73 (59%) for expiratory reserve volume measures attempted at baseline and follow-up. Of note, despite the addition of MBW to our infant testing protocol, our success rates for acquisition of baseline data on lung volumes (100%) and raised-volume parameters (70%) were comparable to the main ISIS study. Unlike other PFTs, MBW tests require minimal cooperation in preschool children, as the test is easily performed during normal tidal breathing. The superior feasibility of LCI supports potential use as a suitable outcome measure for early intervention trials in children with CF. Furthermore, the feasibility of using LCI in preschool children as an outcome measure in multicentered trials may be possible, now that new commercial devices are available (20–22). A recent study suggests that the feasibility of the nitrogen system may be further improved by shortening of washout times in children (23). Before large-scale clinical trials can be initiated in this population, significant further work needs to be done, including the assessment of the suitability of the new system for measurement in infants and longitudinal variability of the measure in preschool children and infants.
Compared with the primary outcome of the main ISIS trial, rate of pulmonary exacerbations, for which no treatment effect was observed, physiologic lung function measures, such as FEV0.5 (13) and LCI, performed in subgroups of the ISIS trial, showed significant differences in treatment effect. Although our subgroup analysis in infants and preschool children is based on limited numbers of patients, important patterns were observed. All 10 infants had normal LCIs at baseline, and treatment with HS appeared to slow or halt progression of abnormalities, whereas LCI increased (worsened) in infants receiving IS. In contrast, in the preschool children, the majority (9/15) of whom had an abnormal LCI at baseline, those randomized to HS had a significant improvement in LCI, whereas those randomized to IS had no change in LCI over the 48-week treatment period. These data support the previously reported finding of higher FEV0.5 in HS- versus IS-treated infants (13). Given these important differences in baseline LCI values and patterns of change over time, future workers on studies involving LCI may consider studying infants and preschool children separately.
The difference in physiologic measures of lung function versus clinical signs and symptoms in infants and preschool children could reflect the silently progressive nature of early CF lung disease. Previous studies have confirmed the presence of early progressive bronchiectasis and infection in infants with CF due to early infection and inflammation (24). Furthermore, objective infant pulmonary function changes seem to occur before recognition of clinically overt symptoms of lower respiratory illness (25), suggesting that measures of lung function that can be easily performed in young children may aid the future design of clinical trials. Future studies in infants should include both LCI measurements as well other infant pulmonary function parameters, such as flow and lung volume measurements, to allow for comparison of sensitivity of the outcome measures to measure response to treatment. Data from this pilot study will help to provide information on the physiologic changes in LCI expected in infants versus preschool children, and will therefore help the design of future studies in infants with CF.
Sample size calculations for prospective studies are based on this limited sample using respiratory mass spectrometry technology, and are presented in Table 3 for parallel group studies. The variability of LCI measures will likely be different depending on the time interval between measurements, the age of the subjects, and the disease state. An understanding of the normal variability in LCI at different time intervals in both health and disease is required to accurately estimate the required sample size for interventional trials. Based on this pilot study, abnormalities in LCI in newborn screened infants were rare. Studies in other populations of newborn screened infants with CF confirm a low prevalence of abnormal LCI (25%) (26) and a low rate of baseline infection on bronchoalveolar lavage (12%) (27). Based on these studies, sample sizes for trials in infants may need to consider maintaining normal LCI to prevent deterioration; in general, these types of studies require much larger sample sizes. Previous work has suggested that early lung ventilation abnormalities in infants with CF diagnosed by newborn screening may be better detected using computed tomography (CT) scans rather than LCI (27). The current study was not designed or powered to compare LCI to repeated CT scans as a measure of airway function. As a clinical trial outcome, repeated measurements of LCI are inherently of lower risk, given that they do not expose the patient to radiation.
This study has a number of limitations. We used a mass spectrometry–based technology in this pilot study; thus, we cannot generalize our findings to recently available commercial systems (28). The sample sizes calculated are based on mass spectrometry technology, and further work needs to be done before generalizing these results to commercially available systems. In addition, measurements were performed at only two time points, which limits our ability to understand the normal variability of the LCI over time. We could not compare LCI to other potential outcomes (i.e., CT), and thus cannot comment on the clinical relevance of the change that we detected. Infants enrolled in this study were diagnosed by newborn screening, and rates of baseline abnormalities in LCI may be higher in infants diagnosed clinically. Results may not, therefore, be generalizable for regions where newborn screening has not been implemented. Further studies comparing LCI to other measures of lung disease, such as infant and preschool PFTs, as well as imaging to quantify structural changes, are needed to determine whether LCI can be used as an outcome measure for trials in early childhood.
In summary, in this pilot study of infants and preschool children with CF, treatment with HS had positive effects on LCI. The high feasibility and positive trends observed in this study support the further development of LCI as an outcome measure in interventional trials in young children with CF. Implementation of new, objective outcome measures, like LCI, are needed for the evaluation of both current and new early intervention strategies for infants and preschool children with CF (29).
Acknowledgments
Acknowledgment
The authors thank Kate Gent and Nancy McDonald for their assistance in coordination of the study and the families who participated in the study.
Footnotes
Supported by the National Institutes of Health, the Canadian Institutes of Health Research, and the Lynn and Arnold Irwin Foundation.
Author Contributions: Conception and design: P.S., M.R., S.D., and F.R.; acquisition of data: P.S., M.B., R.J., and F.R.; analysis and interpretation: P.S., S.S., M.B., R.J., M.R., S.D., L.B., P.G., and F.R.; drafting the manuscript for important intellectual content: P.S., S.S., M.B., R.J., M.R., S.D., L.B., P.G., and F.R.; final approval of the manuscript: P.S., S.S., M.B., R.J., M.R., S.D., L.B., P.G., and F.R.
Originally Published in Press as DOI: 10.1164/rccm.201302-0219OC on June 6, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. Am J Respir Crit Care Med. 1995;151:939–941. doi: 10.1164/ajrccm.151.4.7697269. [DOI] [PubMed] [Google Scholar]
- 2.Long FR, Williams RS, Castile RG. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr. 2004;144:154–161. doi: 10.1016/j.jpeds.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM AREST CF. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67:509–516. doi: 10.1136/thoraxjnl-2011-200912. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson PM, Aurora P, Lindblad A. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J. 2003;22:972–979. doi: 10.1183/09031936.03.00049502. [DOI] [PubMed] [Google Scholar]
- 5.Aurora P, Stanojevic S, Wade A, Oliver C, Kozlowska W, Lum S, Bush A, Price J, Carr SB, Shankar A, et al. London Cystic Fibrosis Collaboration. Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;183:752–758. doi: 10.1164/rccm.200911-1646OC. [DOI] [PubMed] [Google Scholar]
- 6.Ellemunter H, Fuchs SI, Unsinn KM, Freund MC, Waltner-Romen M, Steinkamp G, Gappa M. Sensitivity of lung clearance index and chest computed tomography in early CF lung disease. Respir Med. 2010;104:1834–1842. doi: 10.1016/j.rmed.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Gustafsson PM, De Jong PA, Tiddens HA, Lindblad A. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax. 2008;63:129–134. doi: 10.1136/thx.2007.077784. [DOI] [PubMed] [Google Scholar]
- 8.Amin R, Subbarao P, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax. 2010;65:379–383. doi: 10.1136/thx.2009.125831. [DOI] [PubMed] [Google Scholar]
- 9.Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J. 2011;37:806–812. doi: 10.1183/09031936.00072510. [DOI] [PubMed] [Google Scholar]
- 10.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 11.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, Lubsch L, Hazle L, Sabadosa K, Marshall B Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld M, Ratjen F, Brumback L, Daniel S, Rowbotham R, McNamara S, Johnson R, Kronmal R, Davis SD ISIS Study Group. Inhaled hypertonic saline in infants and children younger than 6 years with cystic fibrosis: the ISIS randomized controlled trial. JAMA. 2012;307:2269–2277. doi: 10.1001/jama.2012.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subbarao P, Stanojevic S, Brown M, Jensen R, McDonald N, Gent K, Davis SD, Rosenfeld M, Gustaffson P, Ratjen F. Effect of hypertonic saline on lung clearance index in infants and preschool children with CF: a pilot study [abstract] Pediatr Pulmonol Suppl. 2012;35:A223. [Google Scholar]
- 15.Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, Gappa M, Thamrin C, Arets HG, Aurora P, Fuchs SI, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J. 2013;41:507–522. doi: 10.1183/09031936.00069712. [DOI] [PubMed] [Google Scholar]
- 16.de Onis M, Onyango AW, Borghi E, Garza C, Yang H WHO Multicentre Growth Reference Study Group. Comparison of the World Health Organization (WHO) child growth standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programmes. Public Health Nutr. 2006;9:942–947. doi: 10.1017/phn20062005. [DOI] [PubMed] [Google Scholar]
- 17.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;(34):1–27. [PubMed] [Google Scholar]
- 18.Lum S, Stocks J, Stanojevic S, Wade A, Robinson P, Gustafsson P, Brown M, Aurora P, Subbarao P, Hoo AF, et al. Age and height dependence of lung clearance index and functional residual capacity. Eur Respir J. 2013;41:1371–1377. doi: 10.1183/09031936.00005512. [DOI] [PubMed] [Google Scholar]
- 19.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs SI, Ellemunter H, Eder J, Mellies U, Grosse-Onnebrink J, Tümmler B, Staab D, Jobst A, Griese M, Ripper J, et al. Feasibility and variability of measuring the lung clearance index in a multi-center setting. Pediatr Pulmonol. 2012;47:649–657. doi: 10.1002/ppul.21610. [DOI] [PubMed] [Google Scholar]
- 21.Singer F, Kieninger E, Abbas C, Yammine S, Fuchs O, Proietti E, Regamey N, Casaulta C, Frey U, Latzin P. Practicability of nitrogen multiple-breath washout measurements in a pediatric cystic fibrosis outpatient setting. Pediatr Pulmonol. 2013;48:739–746. doi: 10.1002/ppul.22651. [DOI] [PubMed] [Google Scholar]
- 22.Jensen R, Stanojevic S, Gibney K, Salazar JG, Gustafsson P, Subbarao P, Ratjen F. Multiple breath nitrogen washout: a feasible alternative to mass spectrometry. PLoS One. 2013;8:e56868. doi: 10.1371/journal.pone.0056868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yammine S, Singer F, Abbas C, Roos M, Latzin P. Multiple-breath washout measurements can be significantly shortened in children. Thorax. 2013;68:586–587. doi: 10.1136/thoraxjnl-2012-202345. [DOI] [PubMed] [Google Scholar]
- 24.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening J Pediatr 2009155623–628.e1 [DOI] [PubMed] [Google Scholar]
- 25.Ranganathan SC, Dezateux C, Bush A, Carr SB, Castle RA, Madge S, Price J, Stroobant J, Wade A, Wallis C, et al. London Collaborative Cystic Fibrosis Group. Airway function in infants newly diagnosed with cystic fibrosis. Lancet. 2001;358:1964–1965. doi: 10.1016/s0140-6736(01)06970-7. [DOI] [PubMed] [Google Scholar]
- 26.Hoo AF, Thia LP, Nguyen TT, Bush A, Chudleigh J, Lum S, Ahmed D, Balfour Lynn I, Carr SB, Chavasse RJ, et al. London Cystic Fibrosis Collaboration. Lung function is abnormal in 3-month-old infants with cystic fibrosis diagnosed by newborn screening. Thorax. 2012;67:874–881. doi: 10.1136/thoraxjnl-2012-201747. [DOI] [PubMed] [Google Scholar]
- 27.Hall GL, Logie KM, Parsons F, Schulzke SM, Nolan G, Murray C, Ranganathan S, Robinson P, Sly PD, Stick SM, et al. AREST CF. Air trapping on chest CT is associated with worse ventilation distribution in infants with cystic fibrosis diagnosed following newborn screening. PLoS ONE. 2011;6:e23932. doi: 10.1371/journal.pone.0023932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer F, Houltz B, Latzin P, Robinson P, Gustafsson P. A realistic validation study of a new nitrogen multiple-breath washout system. PLoS One. 2012;7:e36083. doi: 10.1371/journal.pone.0036083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stick S, Tiddens H, Aurora P, Gustafsson P, Ranganathan S, Robinson P, Rosenfeld M, Sly P, Ratjen F.Early intervention studies in infants and preschool children with cystic fibrosis: are we ready? Eur Respir J 2013;42:527–538 [DOI] [PubMed] [Google Scholar]