Abstract
Rationale: Obstructive sleep apnea is a risk factor for dyslipidemia and atherosclerosis, which have been attributed to chronic intermittent hypoxia (CIH). Intermittent hypoxia inhibits a key enzyme of lipoprotein clearance, lipoprotein lipase, and up-regulates a lipoprotein lipase inhibitor, angiopoietin-like 4 (Angptl4), in adipose tissue. The effects and mechanisms of Angptl4 up-regulation in sleep apnea are unknown.
Objectives: To examine whether CIH induces dyslipidemia and atherosclerosis by increasing adipose Angptl4 via hypoxia-inducible factor-1 (HIF-1).
Methods: ApoE−/− mice were exposed to intermittent hypoxia or air for 4 weeks while being treated with Angptl4-neutralizing antibody or vehicle.
Measurements and Main Results: In vehicle-treated mice, hypoxia increased adipose Angptl4 levels, inhibited adipose lipoprotein lipase, increased fasting levels of plasma triglycerides and very low density lipoprotein cholesterol, and increased the size of atherosclerotic plaques. The effects of CIH were abolished by the antibody. Hypoxia-induced increases in plasma fasting triglycerides and adipose Angptl4 were not observed in mice with germline heterozygosity for a HIF-1α knockout allele. Transgenic overexpression of HIF-1α in adipose tissue led to dyslipidemia and increased levels of adipose Angptl4. In cultured adipocytes, constitutive expression of HIF-1α increased Angptl4 levels, which was abolished by siRNA. Finally, in obese patients undergoing bariatric surgery, the severity of nocturnal hypoxemia predicted Angptl4 levels in subcutaneous adipose tissue.
Conclusions: HIF-1–mediated increase in adipose Angptl4 and the ensuing lipoprotein lipase inactivation may contribute to atherosclerosis in patients with sleep apnea.
Keywords: sleep apnea, lipoprotein clearance, lipoprotein lipase, adipose tissue, hypoxia-inducible factor-1
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic intermittent hypoxia, a hallmark manifestation of obstructive sleep apnea, induces dyslipidemia and atherosclerosis, but the responsible mechanisms are not sufficiently understood.
What This Study Adds to the Field
We have shown that (1) depletion of adipose angiopoietin-like 4, an inhibitor of lipoprotein lipase, abolishes intermittent hypoxia–induced dyslipidemia and atherosclerosis in mice; (2) adipose angiopoietin-like 4 is regulated by hypoxia-inducible factor-1; and (3) angiopoietin-like 4 mRNA levels in subcutaneous adipose tissue correlate with the severity of nocturnal hypoxemia in patients with sleep apnea.
Obstructive sleep apnea (OSA) is a highly prevalent disease characterized by chronic intermittent hypoxia (CIH) (1). OSA increases all-cause and cardiovascular mortality (2–5). OSA is an independent risk factor for atherosclerosis (6), and the severity of atherosclerosis correlates with the severity of nocturnal oxyhemoglobin desaturations (7, 8). The mechanisms by which CIH accelerates atherosclerotic disease are unclear but may include hypertension, dyslipidemia, insulin resistance, systemic inflammation, and oxidative stress (6). In rodent models, CIH accelerates the progression of atherosclerosis in C57BL/6J mice on a high-cholesterol diet (9, 10) and in ApoE-deficient mice (11, 12) by inducing dyslipidemia (10) and systemic inflammation (11, 13–15).
Dyslipidemia is one of the best-characterized risk factors for atherosclerotic cardiovascular disease. We have previously shown that, in patients with OSA, high fasting levels of very low-density lipoprotein (VLDL) are associated with severe nocturnal oxyhemoglobin desaturations (10). A recent study demonstrated that OSA inhibits clearance of triglyceride-rich lipoproteins, a defect that is improved by treatment with continuous positive airway pressure (16). Impaired clearance of triglyceride-rich lipoproteins, which include dietary chylomicrons and liver-synthesized VLDL, leads to excessive formation of atherogenic remnant lipoproteins, thereby accelerating progression of atherosclerosis (17) and conferring increased risk of myocardial infarction, ischemic heart disease, stroke, and death (17–19). Mice exposed to CIH exhibit impaired triglyceride-rich lipoprotein clearance and decreased activity of a key enzyme of lipoprotein clearance, lipoprotein lipase (LPL), in adipose tissue (20). Furthermore, LPL inhibition was associated with increased adipose levels of a potent LPL inhibitor, angiopoietin-like 4 (Angptl4) (20). Angptl4 is transcriptionally regulated by hypoxia-inducible factor (HIF)-1 in several cell types (21–23), but the role of HIF-1 in Angptl4 regulation in adipose tissue is not known. We hypothesized that 1) CIH accelerates the progression of atherosclerosis by inducing atherogenic hyperlipidemia via adipose Angptl4 and that CIH-induced dyslipidemia and atherosclerosis would be prevented by Angptl4 depletion and 2) that adipose Angptl4 is regulated by HIF-1. We tested our hypotheses by treating CIH-exposed, ApoE-deficient mice with Angptl4-neutralizing antibody (Ab) and by examining adipose Angptl4 levels in mice with partial deficiency of an O2–regulated α subunit of HIF-1, in HIF-1α overexpressing mice, and in adipocyte cell cultures. To examine the relevance of our hypotheses for human disease, we measured Angptl4 mRNA levels in adipose tissue of patients with OSA. Some of the results of these studies have been previously reported in the form of an abstract (24).
Methods
In the first series of experiments, ApoE−/− mice were exposed to CIH (FiO2 cycling from 21 to 6.5%, 60 times/h, 9:00 a.m. to 9:00 p.m.) or intermittent air (IA) for 4 weeks (12) while being treated with Angptl4-neutralizing Ab (Lexicon Pharmaceuticals, Inc., The Woodlands, TX) at 30 mg/kg or vehicle as previously described (25). Upon completion of the exposure, Angptl4 gene expression, LPL activity, the fasting plasma lipoprotein profile, and atherosclerosis in the aortic origin and in en face preparation of the entire aorta were measured (12, 20). In the second series of experiments, mice that were heterozygous for a germline null (knockout) allele and therefore present with partial global deficiency of HIF-1α (Hif1a+/− mice) (26) and their wild-type (WT) littermates on an outbred C57BL/6J X 129 genetic background were exposed to CIH and IA for 4 weeks, and fasting plasma lipid profile and adipose Angptl4 were measured. In the third series of the experiments, transgenic (Tg) mice expressing a constitutively active form of human HIF-1α with a deleted oxygen degradation domain (HIF-1αΔODD) under the control of the aP2 promoter and their WT littermates on the FVB background (27) were killed at normoxic conditions, and the fasting plasma lipid profile and adipose Angptl4 were measured. For surgical procedures, anesthesia was maintained with 2% isoflurane. The study was approved by the Johns Hopkins University Animal Use and Care Committee and complied with the American Physiological Society Guidelines for Animal Studies.
3T3-L1 cells were differentiated, transfected with HIF-1α siRNA or nontarget siRNA, and exposed to a prolyl hydroxylase inhibitor dimethyloxaloylglycine (DMOG) or vehicle for 24 hours. Angptl4 and HIF-1α mRNA and protein levels were measured.
Twenty-one patients (all women) without significant comorbidities were retrospectively recruited from the Johns Hopkins Bayview Medical Center (JHBMC) Bariatric Surgery Clinic. The protocol was approved by the Western Institutional Review Board. Sleep studies were performed. Angpl4 mRNA was measured in subcutaneous and visceral adipose tissues obtained during bariatric surgery. Angpl4 levels were correlated with indices of sleep-disordered breathing, including the apnea-hypopnea index (AHI) and the average fall in oxyhemoglobin saturation (ΔSpO2) during apneic/hypopneic episodes, and with fasting serum triglyceride and total cholesterol levels.
All values are reported as means ± SEM after confirming that all continuous variables were normally distributed using the Kolmogorov-Smirnov test. Statistical significance for all comparisons was determined by two-way ANOVA with Bonferroni post hoc correction for multiple comparisons. Statistical significance of correlations was ascertained with Pearson and Spearman tests. All tests were two-sided, and the significance level was established at P < 0.05. Methods are described in detail in the online supplement.
Results
Angptl4-Neutralizing Antibodies Reverse Effects of CIH in ApoE−/− Mice
We performed our experiment in four groups of ApoE−/− mice: animals exposed to CIH and treated with Angptl4 Ab (CIH-Ab); mice exposed to CIH and treated with vehicle (saline); mice exposed to intermittent air (IA), treated with Angptl4 Ab (IA-Ab), and weight matched to the CIH-Ab group; and mice exposed to IA, treated with vehicle, and weight matched to the CIH-vehicle group. There was no difference in body weight, food intake, liver weight, and epididymal fat weight between the groups. CIH induced a significant increase in systolic blood pressure, whereas Ab had no effect (Table 1).
TABLE 1.
FOOD INTAKE, BODY WEIGHT, AND BLOOD PRESSURE IN APOE−/− MICE EXPOSED TO INTERMITTENT AIR OR CHRONIC INTERMITTENT HYPOXIA TREATED WITH ANGPTL-4 ANTIBODY VERSUS VEHICLE FOR 4 WEEKS
| Characteristics | IA-Vehicle | CIH-Vehicle | IA-Ab | CIH-Ab |
|---|---|---|---|---|
| N |
15 |
16 |
16 |
17 |
| Mean food intake, g/d |
2.63 ± 0.04 |
2.68 ± 0.03 |
2.57 ± 0.02 |
2.75 ± 0.03 |
| Body weight, g |
|
|
|
|
| Day 0 |
25.5 ± 0.5 |
26.3 ± 0.4 |
26.0 ± 0.5 |
26.0 ± 0.4 |
| Day 28 |
25.5 ± 0.4 |
25.7 ± 0.5 |
26.0 ± 0.4 |
26.1 ± 0.4 |
| Liver weight |
|
|
|
|
| g |
1.15 ± 0.033 |
0.99 ± 0.023 |
1.11 ± 0.024 |
1.13 ± 0.026 |
| % of body weight |
4.49 ± 0.10 |
3.88 ± 0.05 |
4.14 ± 0.05 |
4.30 ± 0.07 |
| Epididymal fat |
|
|
|
|
| g |
0.13 ± 0.01 |
0.15 ± 0.01 |
0.16 ± 0.01 |
0.17 ± 0.02 |
| % of body weight |
0.52 ± 0.05 |
0.60 ± 0.05 |
0.60 ± 0.03 |
0.66 ± 0.05 |
| Systolic blood pressure, mm Hg |
114 ± 5 |
123 ± 5* |
115 ± 2 |
123 ± 5* |
| Diastolic blood pressure, mm Hg | 82 ± 4 | 85 ± 3 | 85 ± 2 | 87 ± 2 |
Definition of abbreviations: Ab = antibody; CIH = chronic intermittent hypoxia; IA = intermittent air.
P < 0.05 versus IA. Remaining comparisons were not significant.
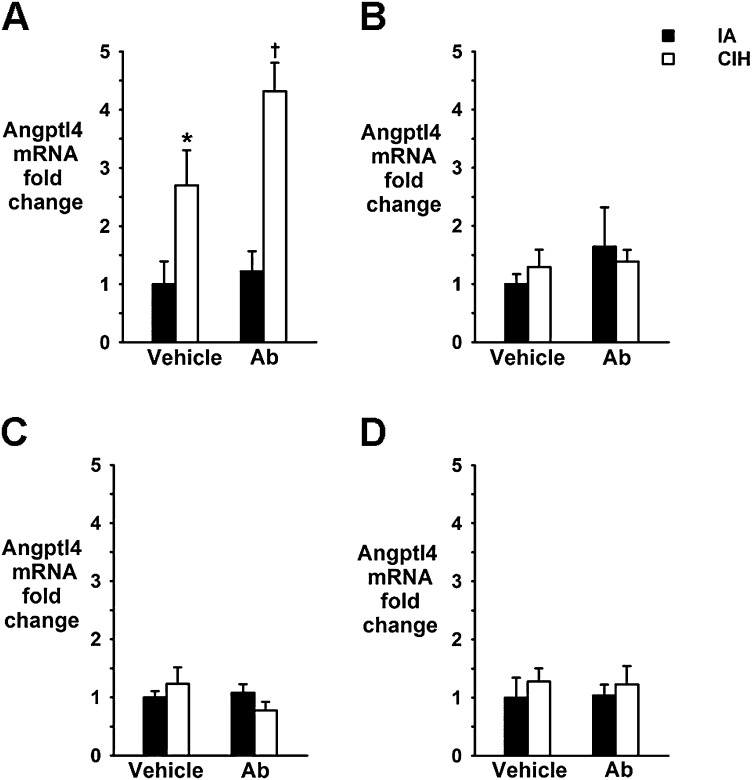
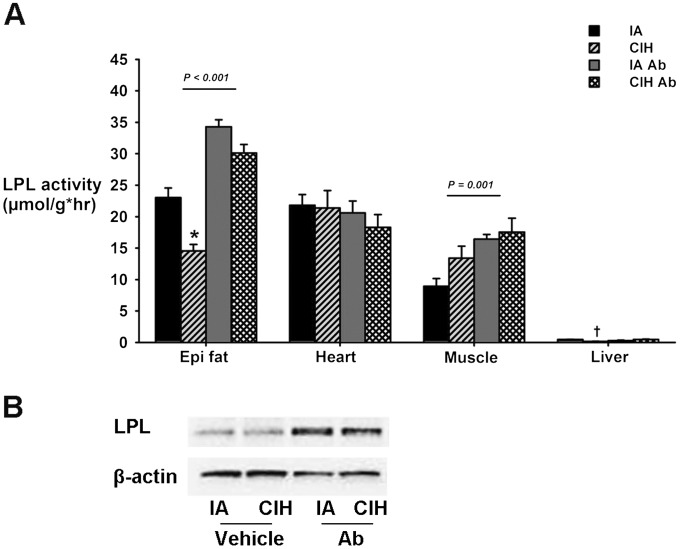
CIH caused a 2- to 4.5-fold increase in Angptl4 mRNA levels in epididymal fat but not in the heart, skeletal muscle (quadriceps), or the liver (Figure 1), which was consistent with our previous data in WT mice (20). CIH induced a significant decrease in adipose LPL activity (Figure 2A), which was abolished by Ab. Neither CIH nor Ab affected LPL activity in heart tissue. In muscle, CIH had no effect, whereas Ab significantly increased LPL activity. As expected, LPL activity was low at baseline in the liver (28). It was further decreased by CIH, and the decrease was abolished by Ab. CIH did not alter adipose LPL protein levels, whereas Ab increased it (Figure 2B; n = 6 per group; representative samples shown).
Figure 1.
Effect of chronic intermittent hypoxia (CIH) on angiopoietin-like 4 (Angptl4) mRNA in epididymal fat (A), heart (B), liver (C), or skeletal muscle (D) of ApoE−/− mice. Ab = Angptl4-neutralizing antibodies; Epi fat = epididymal fat; IA = intermittent air. *P < 0.05 for CIH-vehicle versus IA-vehicle. †P < 0.01 for CIH-Ab versus IA-Ab.
Figure 2.
Effect of chronic intermittent hypoxia (CIH) and Angptl4-neutralizing antibodies (Ab) on lipoprotein lipase (LPL) activity in epididymal fat, heart, skeletal muscle, and liver (A) and on LPL protein levels (B) in epididymal fat (n = 6 per group; representative samples shown) in ApoE−/− mice. IA = intermittent air; *P < 0.001 for CIH-vehicle versus IA-vehicle. †P < 0.01 for CIH-vehicle versus IA-vehicle.
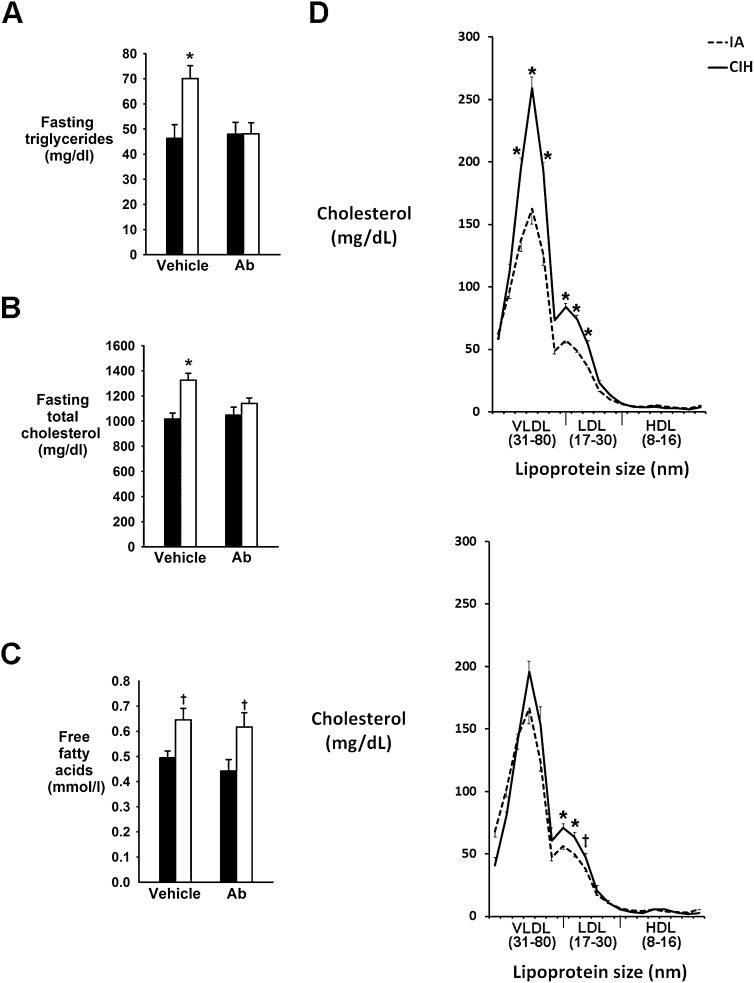
We next determined whether Angptl4 depletion attenuates CIH-induced hyperlipidemia. CIH induced a greater than 50% increase in fasting triglycerides (Figure 3A) and a significant increase in total cholesterol (Figure 3B), and both changes were abolished by Angptl4 Ab. CIH increased free fatty acid (FFA) levels, but Ab had no effect (Figure 3C). CIH greatly increased VLDL and LDL cholesterol levels (P < 0.001) (Figure 3D, top panel), whereas HDL cholesterol was unchanged. Angptl4-neutralizing Ab abolished CIH-induced increases in fasting VLDL cholesterol (Figure 3D, bottom panel), whereas the increase in LDL cholesterol was partially attenuated (from a 46% increase in vehicle-treated mice to a 22% increase in Ab-treated mice; P < 0.05).
Figure 3.
Effect of chronic intermittent hypoxia (CIH) and angiopoietin-like 4 (Angptl4) antibodies (Ab) on serum fasting levels of triglycerides (A), total cholesterol (B), and free fatty acids (C), on the fasting cholesterol profile (D; upper panel: vehicle treated; lower panel: Ab treated) in ApoE−/− mice. IA = intermittent air. *P < 0.001 versus IA groups. †P < 0.01 versus IA groups.
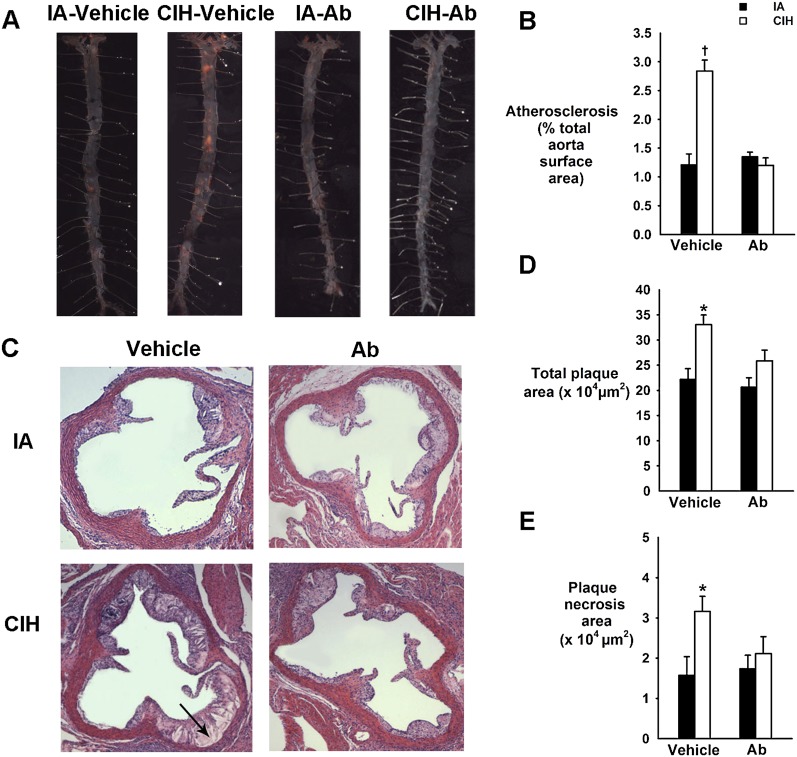
CIH increased the size of atherosclerotic lesions in en face preparations of the entire aorta (2-fold increase; P < 0.001) (Figures 4A and 4B) and in cross sections of the aortic root (Figures 4C and 4D) and augmented plaque necrosis (Figures 4C and 4E). Angptl4 Ab prevented CIH-induced progression of atherosclerosis (Figure 4).
Figure 4.
Effect of chronic intermittent hypoxia (CIH) and angiopoietin-like 4 (Angptl4) antibodies (Ab) on the atherosclerotic plaque size in en face preparations of the entire aorta (A and B) and in cross-sections of the aortic root of ApoE−/− mice (C–E). (A) Representative images of the entire aorta with atherosclerotic lesions stained in red. Sudan IV; original magnification: ×10. (B) Percentage of the total aortic surface covered by the atherosclerotic lesions. (C) Representative cross sections of the aortic root. HE staining. Original magnification: ×100. The arrow points to plaque necrosis. (D) Total plaque cross-sectional area (μm2). (E) Plaque necrosis area (μm2). IA = intermittent air. *P < 0.05 for CIH-vehicle versus remaining groups. †P < 0.001 for CIH-vehicle versus remaining groups.
Adipose Angptl4 Is Regulated by HIF-1
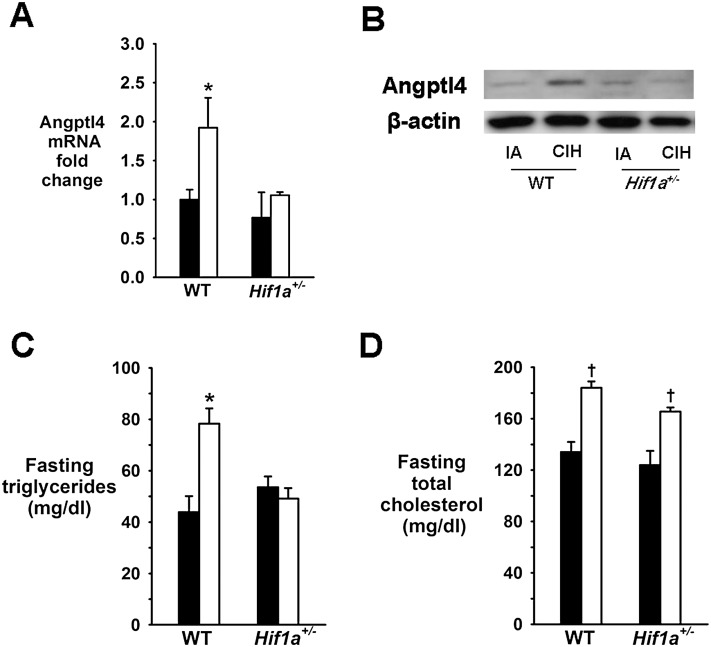
In ApoE−/− mice, CIH increased protein levels of HIF-1α and mRNA levels of a canonical HIF-1–regulated gene, vascular endothelial growth factor (VEGF)-A, in epididymal fat (see Figure E1 in the online supplement). Therefore, we examined relationships between adipose Angptl4 and HIF-1 in different Tg mouse strains and in cell culture. First, we studied mice with global partial HIF-1α deficiency (Hif1a+/−). CIH induced similar weight loss in Hif1a+/− mice and their WT littermates (Table E1). CIH mice showed a slight increase in omental fat pads, but there was no difference between Hif1a+/− and WT mice. CIH increased Angptl4 mRNA and protein levels in epididymal fat of WT mice, and this increase was abolished by partial HIF-1α deficiency (Figures 5A and 5B). In WT mice, CIH raised fasting serum triglyceride and total cholesterol levels (Figures 5C and 5D). HIF-1α deficiency abolished the increase in triglyceride levels but not in cholesterol levels.
Figure 5.
Effect of chronic intermittent hypoxia (CIH) on angiopoietin-like 4 (Angptl4) mRNA levels in epididymal fat (A), Angptl4 protein levels in epididymal fat (representative samples) (B), fasting serum triglycerides (C), and fasting serum total cholesterol in Hif1a+/− mice and their wild-type (WT) littermates (D). IA = intermittent air. *P < 0.05 for the difference between CIH and IA. †P < 0.01 for the difference between CIH and IA.
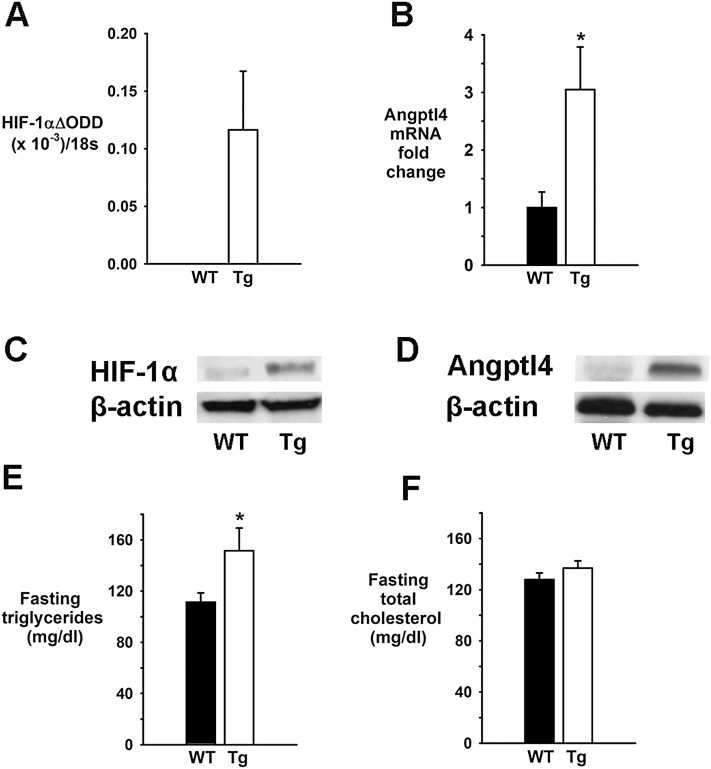
We have also used mice overexpressing HIF-1α in adipose tissue. Under normoxic conditions, HIF-1αΔODD Tg mice and WT littermates had identical body weights and similar sizes of white adipose tissue pads (see Table E1). Tg mice exhibited overexpression of HIF-1α in subcutaneous and omental white adipose tissue, whereas epididymal fat was less affected (27). Tg mice showed higher mRNA and protein levels of HIF-1α and Angptl4 in omental fat and higher fasting serum triglyceride levels compared with WT mice, whereas total cholesterol levels were the same (Figure 6).
Figure 6.
Effect of hypoxia-inducible factor (HIF)-1α overexpression in adipose tissue (a HIF-1α ΔODD mutation) of transgenic (Tg) mice on HIF-1α ΔODD mRNA in omental fat (A), angiopoietin-like 4 (Angptl4) mRNA in omental fat (B), protein levels of HIF-1α (C) and Angptl4 (D) (representative samples) in omental fat, fasting serum triglyceride levels (E), and fasting serum total cholesterol levels (F). *P < 0.05 for the difference between Tg and wild-type (WT) mice.
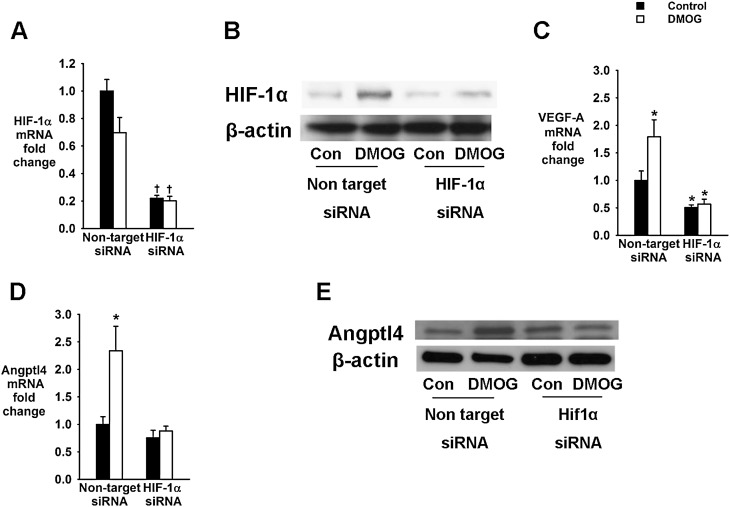
In cell culture experiments, we exposed 3T3-L1 cells to the nonselective prolyl hydroxylase inhibitor DMOG or vehicle for 24 hours. DMOG induces constitutive expression of HIF-1α and 2α proteins by inhibiting O2–dependent degradation (29, 30). To examine the role of HIF-1α in Angptl4 regulation, DMOG exposure was performed in the presence and absence of HIF-1α siRNA, which depleted HIF-1α mRNA (Figure 7A). As expected, DMOG increased HIF-1α protein levels and expression of VEGF-A mRNA (Figures 7B and 7C). DMOG also significantly increased mRNA and protein levels of Angptl4 (Figures 7D and 7E). All effects of DMOG on HIF-1α, VEGF-A, and Angptl4 expression were abolished by transfection of cells with HIF-1α siRNA.
Figure 7.
Effects of hypoxia-inducible factor (HIF)-1α protein stabilization with a prolyl hydroxylase inhibitor dimethyloxaloylglycine (DMOG) in 3T3-L1 adipocytes transfected with HIF-1α or nontarget small interfering RNA (siRNA). (A) HIF-1α mRNA levels. (B) HIF-1 protein levels (representative bands). (C) Vascular endothelial growth factor (VEGF)-A mRNA levels. (D) Angiopoietin-like 4 (Angptl4) mRNA levels. (E) Angptl4 protein levels (representative bands). *P < 0.05 for the difference with control cells transfected with nontarget siRNA. †P < 0.001 for the difference with control cells transfected with nontarget siRNA. Con = control.
Adipose Angptl4 Levels in Patients with OSA Correlate with Nocturnal Hypoxemia
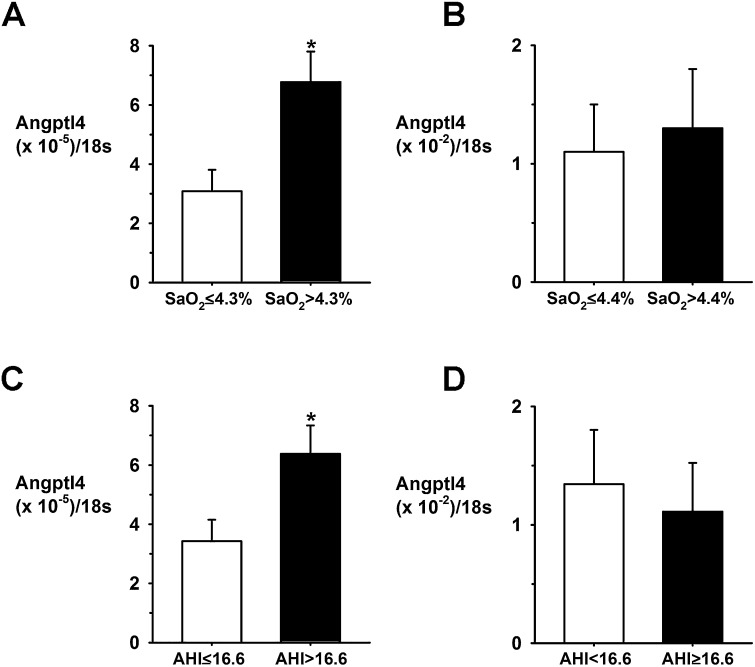
We took advantage of the availability of sleep studies as well as adipose tissue biopsies in obese subjects who underwent bariatric surgery (Table E2). Their body mass index (BMI) varied between 41 and 63 kg/m2, and the severity of OSA varied from an AHI of 0 to 71.3 events/h. Angptl4 mRNA levels in subcutaneous adipose tissue significantly correlated with the severity of nocturnal oxyhemoglobin desaturation (P < 0.05) (Fig. E2) but did not correlate with the BMI or AHI (not shown). Angptl4 mRNA levels in visceral fat and Angptl4 serum levels were not associated with indexes of sleep apnea (Figure E2 and Table E2). When our patient population was dichotomized according to median splits of nocturnal oxyhemoglobin desaturation and the AHI, patients with ΔSpO2 > 4.3% had 2-fold higher Angptl4mRNA levels in subcutaneous adipose tissue than those with ΔSpO2 ≤ 4.3%, and patients with the AHI > 16.6/h had a 1.8-fold higher Angptl4 mRNA levels than those with the AHI ≤ 16.6 hours (Figure 8). There was no difference in the BMI or Angptl4 expression in visceral adipose tissue between the groups. Fasting serum triglyceride levels correlated with Angptl4 expression in visceral adipose tissue (Pearson r = 0.496, P = 0.022; Spearman r = 0.4327, P = 0.057) but not in subcutaneous fat (Fig. E2). There was no significant relationship between fasting serum total cholesterol and adipose Angptl4.
Figure 8.
Adipose Angptl4 mRNA levels in obese patients grouped according to median splits of the average fall in oxyhemoglobin saturation (ΔSpO2) during apneic/hypopneic episodes and the apnea-hypopnea index (AHI). (A) Angiopoietin-like 4 (Angptl4) mRNA levels in subcutaneous adipose tissue in patients with ΔSpO2 ≤ 4.3% (n = 8) and > 4.3% (n = 7). (B) Angptl4 mRNA levels in visceral adipose tissue in patients with ΔSpO2 ≤ 4.4% (n = 11) and > 4.4% (n = 10). (C) Angptl4 mRNA levels in subcutaneous adipose tissue in patients with AHI ≤ 16.6/h (n = 8) and > 16.6/h (n = 7). (D) Angptl4 mRNA levels in visceral adipose tissue in patients with the AHI < 16.6/h (n = 11) and ≥ 16.6/h (n = 10). *P < 0.05.
Discussion
We have previously shown that CIH induces atherosclerosis and dyslipidemia in mice (10, 12, 20). The purpose of this study was to examine the role of the LPL inhibitor Angptl4 in the pathogenesis of CIH-induced atherosclerosis. The main novel finding of the study was that Angptl4 depletion with neutralizing Ab prevented CIH-induced progression of atherosclerosis in ApoE−/− mice. Angptl4 depletion also abolished CIH-induced decreases in adipose LPL activity and increases in plasma VLDL cholesterol and triglyceride levels. In addition, we explored the role of HIF-1 in up-regulation of adipose Angptl4 and found that both CIH and HIF-1α overexpression increased adipose Angptl4 in a similar manner and that this increase was abolished by HIF-1α deficiency. Finally, we showed that in patients with OSA, Angptl4 mRNA levels in subcutaneous adipose tissue correlated with the severity of nocturnal oxyhemoglobin desaturation.
CIH and Angptl4
Severe hypoxia up-regulates Angptl4 in cultured adipocytes, cardiomyocytes, and endothelial cells (21, 22, 31). Constitutive expression of active HIF-1α in endothelial cells and cardiomyocytes results in robust up-regulation of Angptl4 mRNA (21, 22). Functional HIF-1 binding sites were identified in the human Angptl4 gene (23). HIF-1 is a heterodimer, which consists of a constitutively expressed β subunit and an O2–regulated α subunit (32). HIF-1α activation by sustained hypoxia occurs due to inhibition of O2–dependent prolyl hydroxylation (29). In contrast, HIF-1α activation by CIH occurs not only through decreased degradation but also via increased HIF-1α biosynthesis (33). CIH increases generation of reactive oxygen species through NADPH oxidase, which increases HIF-1α biosynthesis via mammalian target of rapamycin (34). CIH induces severe hypoxia and oxidative stress in white adipose tissue (35), which may be sufficient to up-regulate HIF-1α levels and induce Angptl4 expression.
The role of HIF-1 is supported by several lines of evidence. First, up-regulation of adipose Angptl4 during CIH was abolished in mice with partial global HIF-1α deficiency (Figure 5). Global HIF-1α deficiency may affect Angptl4 levels systemically because it attenuates hypoxic sensitivity of the carotid bodies with loss of downstream sympathetic nervous system (SNS) responses to CIH (36). The SNS is a major regulator of adipose tissue lipolysis (37). CIH increases circulating FFA levels (12) (Figure 3C). FFAs induce Angptl4 gene expression (38). However, very modest elevations of FFAs in CIH may not be sufficient to induce dramatic increases in adipose Angptl4 (compare Figures 1A and 3C). Therefore, it is more likely that global HIF-1α deficiency abolishes Angptl4 up-regulation not through suppression of SNS responses to CIH but directly at the level of adipocytes. Second, in support of the direct effect of HIF-1, mice expressing a constitutively active form of HIF-1α in adipose tissue showed increased Angptl4 levels (Figure 6). Third, activation of HIF-1α in adipocyte cell culture resulted in increased Angptl4 expression, which was abolished by HIF-1α siRNA (Figure 7). Fourth, HIF-1α overexpression in adipose tissue increased levels of serum triglycerides in parallel with up-regulation of adipose Angptl4 (Figure 6), whereas HIF-1α deficiency abolished CIH-induced increases in serum triglycerides and adipose Angptl4, supporting the role of HIF-1α in Angptl4-mediated dyslipidemia (Figure 5). Based on all of the above, we hypothesize that increased HIF-1α levels mediate Angptl4 up-regulation in adipose tissue during CIH. The lack of an increase in Angptl4 levels in liver, skeletal muscle, and heart may be attributable to relatively moderate levels of tissue hypoxia and/or oxidative stress (35), which were not sufficient to stabilize HIF-1α, and low levels of Angptl4 expression compared with adipose tissue (38, 39).
Angptl4 and LPL Activity during CIH
Angptl4 inhibits LPL by converting active dimers to inactive monomers (40). Neutralizing Ab recognize a LPL binding domain of Angptl4, thereby preventing LPL inactivation (25). Our data demonstrate that up-regulation of Angptl4 in adipose tissue during CIH causes inhibition of adipose LPL. LPL protein levels were not altered by CIH, which is consistent with the posttranslational mode of Angptl4 action (39, 40). Angptl4 Ab reversed adipose LPL inactivation and increased LPL protein level (Figure 2), which can be attributed to increased levels of LPL dimers. The latter have higher affinity for cell surface proteoglycans than monomers and therefore are more stable (40). Neither CIH nor Ab affected LPL activity in skeletal muscle or the heart, suggesting that the effect of adipose Angptl4 is local. In addition, LPL is regulated by multiple mechanisms (28), and it is conceivable that CIH affects LPL in skeletal and cardiomyocytes via other pathways counterbalancing any impact of Angptl4. Overall, our data suggest that CIH inhibits adipose LPL via Angptl4.
Angptl4 and Dyslipidemia during CIH
Our previous and present work showed that CIH increases plasma levels of triglyceride-rich lipoproteins by delaying lipoprotein clearance (20). LPL plays a pivotal role in the clearance of VLDL and chylomicrons (28). The current study revealed that depletion of Angptl4 abolished CIH-induced increases in plasma VLDL, suggesting that Angptl4-mediated inactivation of LPL is the principal mechanism of VLDL dyslipidemia during CIH. Angpl4 Ab also attenuated the CIH-induced increase in LDL cholesterol and the incomplete inhibition indicates that other factors may also contribute to CIH-induced LDL hyperlipidemia. Indeed, hepatic VLDL secretion may also be increased by CIH via up-regulation of hepatic stearoyl coenzyme A desaturase (9, 10). Thus, we found that Angptl4-mediated suppression of adipose LPL plays a pivotal role in triglyceride-rich lipoprotein hyperlipidemia during CIH.
Angptl4 and CIH-induced Atherosclerosis
Multiple mechanisms may contribute to accelerated atherosclerosis during CIH, including proatherogenic dyslipidemia, hypertension, vascular inflammation, and oxidative stress (9–14). Shear stress of elevated blood pressure damages the endothelium allowing macrophage infiltration of the arterial intima. Macrophages take up oxidized LDL, becoming foam cells that form the atherosclerotic lesions. Macrophage foaming occurs due to HIF-1α–dependent accumulation of sterols and triglycerides and decreased cholesterol efflux (41). The main finding of our study is that Angptl4-mediated inactivation of adipose LPL and resulting hyperlipidemia is the key mechanism in the progression of atherosclerosis during CIH. In fact, Angptl4 deficiency may confer a partial protection against atherosclerosis in ApoE-deficient mice at baseline (42). Other mechanisms of atherogenesis triggered by CIH were not sufficient to induce the progression of atherosclerosis when Angptl4 was depleted. Thus, the progression of atherosclerosis during CIH occurs due to inefficient clearance of atherogenic, triglyceride-rich lipoproteins.
Clinical Implications
An E40K loss-of-function variant of the ANGPTL4 gene in humans was associated with substantially reduced plasma triglyceride levels, increased plasma HDL levels, and reduced risk of stroke, peripheral vascular disease, and carotid atherosclerosis (39, 43, 44). Human OSA is associated with non-HDL hyperlipidemia and triglyceridemia and atherosclerosis (6). OSA has been associated with increased postprandial hyperlipidemia (16), which is consistent with LPL inhibition. In addition, circulating LPL concentrations in patients with OSA are decreased in proportion to the severity of the disease (45). Our human data revealed that Angptl4 levels in subcutaneous adipose tissue directly correlated with nocturnal hypoxemia in patients with OSA and that subcutaneous adipose Angptl4 levels were increased in patients with more severe OSA, independent of BMI (Figure 8). Taken together with our murine data, these data suggest that up-regulation of adipose Angptl4 may play a role in the progression of atherosclerosis in OSA.
Limitations of the Study
Our study had several limitations. First, murine CIH mimics oxyhemoglobin desaturation in severe OSA and induces sleep fragmentation but does not model other features of OSA, such as snoring, hypercapnea, and transpulmonary pressure swings. Second, it remains unknown why CIH up-regulates Angptl4 exclusively in adipose tissue, although the severity of tissue hypoxia and oxidative stress with ensuing activation of HIF-1α may play a role. Third, Angptl4 Ab reversed CIH-induced hypertriglyceridemia and hypercholesterolemia in ApoE−/− mice, whereas HIF-1α knockout and overexpression affected only plasma triglyceride levels in HIF-1a+/− and HIF-1aΔODD mice. This phenomenon is likely explained by differences in the lipoprotein profile, with the most of cholesterol confined to the VLDL fraction in ApoE−/− mice and to the HDL fractions in other strains. Fourth, we did not observe correlations between visceral adipose Angptl4 levels and the AHI or nocturnal hypoxemia in patients with OSA. Differences between our human and mouse data may be related to the fact that human data were obtained in severely obese individuals, whereas mouse experiments were performed in lean mice. Obesity per se leads to adipose tissue hypoxia and up-regulation of HIF-1α in the absence of OSA or IH (46–48). In addition, factors other than hypoxia factors could influence Angptl4 levels in visceral fat (e.g., high levels of FFA compared with subcutaneous fat) (38, 49). Fifth, we lacked data on the severity of atherosclerosis in our human subjects. However, an association between OSA and atherosclerosis (7, 8) and attenuation of markers of atherosclerosis after continuous positive airway pressure (50) have been previously reported.
Conclusions
CIH causes atherosclerosis via HIF-1–mediated up-regulation of adipose Angptl4, which inactivates adipose LPL and inhibits clearance of triglyceride-rich lipoproteins in mice. Intermittent nocturnal hypoxemia is associated with increased Angptl4 mRNA levels in subcutaneous adipose tissue in obese humans, but the role of adipose Angptl4 in the progression of atherosclerosis in patients with OSA is yet to be confirmed.
Acknowledgments
Acknowledgment
The authors thank Prof. Paul Trayhurn, University of Liverpool, Liverpool, UK, for providing rabbit polyclonal antibodies against Angptl4; Dr. Carol Sztalryd and Ms. Nicole M. Glynn-Cunningham, M.S., University of Maryland, for invaluable assistance with lipoprotein lipase activity measurements; Dr. Kimberley E. Steele and Dr. Michael A. Schweitzer, Johns Hopkins Bayview Medical Center, for obtaining intraoperative biopsies of subcutaneous fat during bariatric surgery; and Dr. Susheel Patil and Ms. Michelle Guzman for bariatric surgery database management.
Footnotes
This study was supported by the Mid-Atlantic Nutrition Obesity Research Center (NORC) of Maryland (National Institutes of Health grant P30 DK072488) and by the Johns Hopkins Bayview Clinical Research Unit (National Institutes of Health grant M01-RR02719); by National Institutes of Health grants HL080105 (V.Y.P.), HL084945 (A.R.S. and V.Y.P.), and HL109475 (J.C.J.); and by American Heart Association grants 10GRNT3360001(V.Y.P.) and 12POST118200001 (M.-K.S.).
Author Contributions: Conception and design, acquisition of data, or analysis and interpretation of data: L.F.D., Q.Y., K.L.H., M.K.S., S.B.-F., J.G., T.E.S., J.C.J., A.C.M., G.O., A.R.S., N.H., P.E.S., G.L.S., D.R.P., V.Y.P. Drafting the article or revising it critically for important intellectual content: L.F.D., Q.Y., G.L.S., D.R.P., V.Y.P. Final approval of the version to be published: all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201209-1688OC on January 17, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 4.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 6.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140:534–542. doi: 10.1378/chest.10-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 8.Baguet JP, Hammer L, Levy P, Pierre H, Launois S, Mallion JM, Pepin JL. The severity of oxygen desaturation is predictive of carotid wall thickening and plaque occurrence. Chest. 2005;128:3407–3412. doi: 10.1378/chest.128.5.3407. [DOI] [PubMed] [Google Scholar]
- 9.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, Bhanot S, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res. 2008;103:1173–1180. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnaud C, Poulain L, Levy P, Dematteis M. Inflammation contributes to the atherogenic role of intermittent hypoxia in apolipoprotein-E knock out mice. Atherosclerosis. 2011;219:425–431. doi: 10.1016/j.atherosclerosis.2011.07.122. [DOI] [PubMed] [Google Scholar]
- 12.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnaud C, Beguin PC, Lantuejoul S, Pepin JL, Guillermet C, Pelli G, Burger F, Buatois V, Ribuot C, Baguet JP, et al. The inflammatory preatherosclerotic remodeling induced by intermittent hypoxia is attenuated by RANTES/CCL5 inhibition. Am J Respir Crit Care Med. 2011;184:724–731. doi: 10.1164/rccm.201012-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li RC, Haribabu B, Mathis SP, Kim J, Gozal D. Leukotriene B4 receptor-1 mediates intermittent hypoxia-induced atherogenesis. Am J Respir Crit Care Med. 2011;184:124–131. doi: 10.1164/rccm.201012-2039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang G, Song D, Ye X, Mao SZ, Liu G, Liu SF. chronic intermittent hypoxia exposure induces atherosclerosis in ApoE knockout mice: role of NF-kappaB p50. Am J Pathol. 2012;181:1530–1539. doi: 10.1016/j.ajpath.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med. 2011;184:355–361. doi: 10.1164/rccm.201102-0316OC. [DOI] [PubMed] [Google Scholar]
- 17.Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012;220:22–33. doi: 10.1016/j.atherosclerosis.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, Nordestgaard BG. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142–2152. doi: 10.1001/jama.2008.621. [DOI] [PubMed] [Google Scholar]
- 19.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 20.Drager LF, Li J, Shin MK, Reinke C, Aggarwal NR, Jun JC, Bevans-Fonti S, Sztalryd C, O'Byrne SM, Kroupa O, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783–790. doi: 10.1093/eurheartj/ehr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 22.Belanger AJ, Lu H, Date T, Liu LX, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1alpha. J Mol Cell Cardiol. 2002;34:765–774. doi: 10.1006/jmcc.2002.2021. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Wong CC, Wei H, Gilkes DM, Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B, Winnard PT, Jr, et al. HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs. Oncogene. 2012;31:1757–1770. doi: 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Drager LF, Yao Q, Hernandez KL, Shin M-K, Gay J, Sussan TE, Jun JC, Myers AC, Powell DR, Polotsky VY. Chronic intermittent hypoxia (CIH) induces atherosclerosis via angiopoietin-like proten 4 (Angptl4) Am J Respir Crit Care Med. 2012;185:A1050. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, Hansen G, Machajewski D, Platt KA, Sands AT, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci USA. 2007;104:11766–11771. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 29.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 30.Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Gunzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci USA. 2005;102:10212–10217. doi: 10.1073/pnas.0504520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang B, Wood IS, Trayhurn P. PCR arrays identify metallothionein-3 as a highly hypoxia-inducible gene in human adipocytes. Biochem Biophys Res Commun. 2008;368:88–93. doi: 10.1016/j.bbrc.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza GL. Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol. 2006;91:803–806. doi: 10.1113/expphysiol.2006.033498. [DOI] [PubMed] [Google Scholar]
- 33.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol. 2011;111:881–890. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1{alpha} deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaworski K, Sarkadi-Nagy E, Duncan RE, Ahmadian M, Sul HS. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1–G4. doi: 10.1152/ajpgi.00554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, Muller M, Kersten S. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106:1712–1721. doi: 10.1161/CIRCRESAHA.110.217380. [DOI] [PubMed] [Google Scholar]
- 39.Mattijssen F, Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochim Biophys Acta. 2012;1821:782–789. doi: 10.1016/j.bbalip.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, Gassmann M, Gardner LB, Fisher EA. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109:1141–1152. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adachi H, Fujiwara Y, Kondo T, Nishikawa T, Ogawa R, Matsumura T, Ishii N, Nagai R, Miyata K, Tabata M, et al. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochem Biophys Res Commun. 2009;379:806–811. doi: 10.1016/j.bbrc.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet. 2007;39:513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smart-Halajko MC, Kelley-Hedgepeth A, Montefusco MC, Cooper JA, Kopin A, McCaffrey JM, Balasubramanyam A, Pownall HJ, Nathan DM, Peter I, et al. ANGPTL4 variants E40K and T266M are associated with lower fasting triglyceride levels in non-Hispanic white Americans from the Look AHEAD Clinical Trial. BMC Med Genet. 2011;12:89. doi: 10.1186/1471-2350-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iesato K, Tatsumi K, Saibara T, Nakamura A, Terada J, Tada Y, Sakao S, Tanabe N, Takiguchi Y, Kuriyama T. Decreased lipoprotein lipase in obstructive sleep apnea syndrome. Circ J. 2007;71:1293–1298. doi: 10.1253/circj.71.1293. [DOI] [PubMed] [Google Scholar]
- 46.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, Aja S, Bhanot S, Polotsky VY. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1alpha. PLoS ONE. 2012;7:e46562. doi: 10.1371/journal.pone.0046562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali AH, Koutsari C, Mundi M, Stegall MD, Heimbach JK, Taler SJ, Nygren J, Thorell A, Bogachus LD, Turcotte LP, et al. Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes. 2011;60:2300–2307. doi: 10.2337/db11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Effects of CPAP on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]