Abstract
Rationale: Chronic obstructive pulmonary disease (COPD) guidelines make no recommendations for allergy diagnosis or treatment.
Objectives: To determine whether an allergic phenotype contributes to respiratory symptoms and exacerbations in patients with COPD.
Methods: Two separate cohorts were analyzed: National Health and Nutrition Survey III (NHANES III) and the COPD and domestic endotoxin (CODE) cohort. Subjects from NHANES III with COPD (n = 1,381) defined as age > 40 years, history of smoking, FEV1/FVC < 0.70, and no diagnosis of asthma were identified. The presence of an allergic phenotype (n = 296) was defined as self-reported doctor diagnosed hay fever or allergic upper respiratory symptoms. In CODE, former smokers with COPD (n = 77) were evaluated for allergic sensitization defined as a detectable specific IgE to perennial allergens. Bivariate and multivariate models were used to determine whether an allergic phenotype was associated with respiratory symptoms and exacerbations.
Measurements and Main Results: In NHANES III, multivariate analysis revealed that individuals with allergic phenotype were more likely to wheeze (odds ratio [OR], 2.1; P < 0.01), to have chronic cough (OR, 1.9; P = 0.01) and chronic phlegm (OR, 1.5; P < 0.05), and to have increased risk of COPD exacerbation requiring an acute doctor visit (OR, 1.7; P = 0.04). In the CODE cohort, multivariate analysis revealed that sensitized subjects reported more wheeze (OR, 5.91; P < 0.01), more nighttime awakening due to cough (OR, 4.20; P = 0.03), increased risk of COPD exacerbations requiring treatment with antibiotics (OR, 3.79; P = 0.02), and acute health visits (OR, 11.05; P < 0.01). An increasing number of sensitizations was associated with a higher risk for adverse health outcomes.
Conclusions: Among individuals with COPD, evidence of an allergic phenotype is associated with increased respiratory symptoms and risk of COPD exacerbations.
Keywords: atopy, allergic sensitization, allergy, chronic obstructive pulmonary disease
At a Glance Commentary
Scientific Knowledge on the Subject
International chronic obstructive pulmonary disease (COPD) guidelines such as the Global Initiative for Chronic Obstructive Lung Disease (GOLD) make no recommendations for allergy diagnosis or treatment. This absence of recommendations may be appropriate given the lack of available evidence, but not because allergy has been shown to be irrelevant in the management of patients with COPD. Allergic sensitization has been shown to be a significant predictor of a faster rate of lung function decline and allergen exposure in sensitized individuals may provoke neutrophilic bronchial inflammation, similar to the inflammation seen in airways of patients with COPD. However, whether allergy worsens respiratory symptoms in patients with COPD remains unknown.
What This Study Adds to the Field
The present study addresses a significant gap in the current evidence base by investigating whether an allergic phenotype worsens respiratory symptoms in patients with COPD. We used two complementary cohorts of subjects with COPD. The National Health and Nutrition Survey III assessed clinical allergic symptoms and is a nationally representative sample, allowing for broad generalizability. The second cohort is a COPD-specific, which included comprehensive COPD characterization, and specific IgE testing for assessment of sensitization. Our results showed that an allergic phenotype was linked to an increase in COPD symptoms and exacerbations.
Chronic obstructive pulmonary disease (COPD), a chronic disease of the airways, is caused by tobacco smoke and other air pollutant exposures (1). In the United States, COPD affects 14% of the adult population and is the third leading cause of death (2). Annually, COPD is responsible for 726,000 hospitalizations and over $32 billion in estimated cost, reflecting a large public health burden (3). Patients with COPD suffer significant morbidity, including respiratory symptoms that adversely affect quality of life and limit activity (4). Given the significant morbidity, understanding the different factors that contribute to symptom burden in COPD is an important focus of research.
Allergic sensitization, assessed by allergen-specific IgE or skin prick testing, is a known risk factor for asthma (5, 6), and exposure to specific allergens in sensitized patients with asthma is known to worsen pulmonary symptoms (7, 8). National and international guidelines recommend assessment of allergic sensitization and environmental exposures in patients with asthma (9). In contrast, allergic sensitization may not be commonly assessed in patients with COPD. International COPD guidelines, such as the Global Initiative for Chronic Obstructive Lung Disease (GOLD), make no recommendations for allergy diagnosis or treatment (4). This absence of recommendations may be appropriate given the lack of available evidence but not because allergy has been shown to be irrelevant in the management of patients with COPD. Allergic sensitization defined by skin prick testing was found to be a significant predictor of a faster rate of lung function decline, a hallmark feature of COPD, in a general population cohort (10) and among smokers (11). Furthermore, several studies show that allergen exposure in sensitized individuals may provoke neutrophilic bronchial inflammation similar to the inflammation seen in airways of patients with COPD (12). Whether allergy worsens respiratory symptoms in patients with COPD remains unknown.
The purpose of this study was to assess whether the presence of allergic disease is associated with worse respiratory health in adults with COPD. We used two complementary cohorts of subjects with COPD. The National Health and Nutrition Survey III (NHANES) assessed clinical allergic symptoms and is a nationally representative sample, allowing for broad generalizability. The second cohort is a COPD-specific cohort recruited as part of the COPD and domestic endotoxin (CODE) study, which included comprehensive COPD characterization and specific IgE testing for assessment of sensitization.
Methods
NHANES Study Population
The NHANES III study was approved by the NCHS Research Ethics Review Board, and consent was obtained. NHANES methodology is previously published (13). Spirometry was performed according to American Thoracic Society recommendations (14). Predicted FEV1 was based on sex, age, and height (15). Participants were determined to have COPD (n = 1,381) if they were older than 40 years of age, smoked more than 100 cigarettes, and had an FEV1/FVC < 0.70 and no asthma diagnosis (16).
Allergic phenotype was defined as doctor diagnosed hay fever or allergic symptoms defined by a positive response to at least one symptom of stuffy, itchy, or runny nose or watery, itchy eyes brought on by house dust, animals, or pollen in the past 12 months.
Participants were characterized based on self-reported race and ethnicity and years of education. Calculation of pack-years of smoking is based on previously published formulas (17).
Respiratory outcomes.
Health care utilization was defined as any participant report of hospitalization or visit to a doctor or emergency department (ED) for wheezing or whistling in the chest in the past 12 months.
Chronic cough and phlegm were considered present if reported on most days for at least 3 months. Wheeze and pneumonia were considered present if there was report of any episodes in the last 12 months.
CODE Study Population
Participants with physician-diagnosed COPD were recruited from the Baltimore area. Inclusion criteria included age ≥ 40 years, 10 or more pack-years smoking, and COPD defined by a postbronchodilator FEV1 (% predicted) between 30 and 80% and FEV1/FVC < 70%. Additional criteria are provided in the online supplement. The Johns Hopkins Medical Institution Review Board approved the study protocol, and all participants provided written informed consent.
Serum was analyzed for specific IgE by ImmunoCAP (Phadia; ThermoFisher, Portage, MI) for mouse, cockroach, cat, dog, and dust mite allergens. A participant was considered atopic if at least one test was positive. An immunocap test was considered positive if detectable (>0.1 kUA/L). Subjects were further categorized by number of positive sensitizations (0, 1–2, or ≥3 sensitizations).
Respiratory outcomes.
Respiratory health was determined through the modified ATS-DLD questionnaire. Chronic cough and sputum were defined as present if reported for three or more consecutive months. Wheeze was considered present if the participant experienced dyspnea from wheezing or whistling in the chest. If a participant reported having been awakened from sleep by cough/dyspnea in the past 12 months, they were classified as having nocturnal symptoms.
Exacerbations were determined by treatment with antibiotics or oral corticosteroids in the past year. Health care utilization was assessed (yes/no) by the participant reporting an ED visit or hospitalization for respiratory symptoms in the past year.
Statistical Analysis
Summary statistics were analyzed using Spearman correlations, χ2 tests for proportions, and t tests for continuous data. For the NHANES analyses, sampling weights and strata were used (13).
Bivariate and multivariate analysis models were run using linear and logistic regressions. Multivariate regression models were used to adjust for potential confounders including age, gender, race, education, smoking pack-years, and lung function (FEV1% predicted). Multivariate models including subjects reporting a diagnosis of asthma (n = 198 for a total sample size of 1,579) were conducted with asthma as a confounding variable for sensitivity analyses. Interaction terms were used to determine whether the association between allergic phenotype and health outcomes differed by asthma status and subsequent stratified analyses were conducted. A Cuzick test for trend was performed to determine whether categories of sensitization (0, 1–2, or ≥3) were associated with health outcomes (18).
Analyses were conducting using StataSE 11.0 (StataCorp, College Station, TX). Statistical significance was defined as a two-tailed P value less than 0.05. Additional details are provided in the online supplement.
Results
NHANES
Of the subjects with COPD (n = 1,381), the mean age was 60 years, with 49 pack-years of smoking and mean FEV1% predicted of 76%. Twenty-five percent of subjects with COPD had an allergic phenotype (as compared with 30.3% of the overall NHANES population). Men were less likely to be allergic, but subjects with and without allergy were otherwise similar by age, education, pack-years of tobacco smoking, and lung function (Table 1). Reported use of antihistamine was low but was higher in those with an allergic phenotype compared with those without (5.6 vs. 2.2%; P = 0.01). Of subjects with an allergic phenotype, 29% had doctor-diagnosed hay fever, and 88% had allergic upper respiratory symptoms. Of those with upper respiratory symptoms, 82% reported upper respiratory symptoms to pollen, 27% to house dust, and 9% to animals.
TABLE 1.
NATIONAL HEALTH AND NUTRITION SURVEY PARTICIPANT CHARACTERISTICS
| Participant Characteristics | Allergic (n = 296) | Nonallergic (n = 1,085) | P Value |
|---|---|---|---|
| Ethnicity, % |
|
|
0.11 |
| White |
99 |
96 |
|
| Black |
1 |
2 |
|
| Other |
0 |
2 |
|
| Sex, % male |
53 |
67 |
<0.05 |
| Highest education, % |
|
|
0.69 |
| Elementary school |
17 |
19 |
|
| High school |
52 |
53 |
|
| Beyond high school |
31 |
28 |
|
| Age, yr |
66 (12)* |
67 (12)* |
0.06 |
| Pack-years |
47 (34)* |
46 (38)* |
0.90 |
| FEV1, % predicted |
77 (22)* |
76 (21)* |
0.60 |
| GOLD stage |
|
|
0.23 |
| I |
46.2 |
50.1 |
|
| II |
40.5 |
39 |
|
| III |
12.5 |
9 |
|
| IV |
0.8 |
1.9 |
|
| Current smoker, % |
45 |
45 |
1 |
| Years since quit if not current smoker |
16.7 (13.1)* |
17.4 (14.1)* |
0.72 |
| Doctor-diagnosed chronic bronchitis, % |
11 |
9.7 |
0.56 |
| Doctor-diagnosed emphysema, % |
10 |
10.4 |
0.92 |
| Medications in the last month, % | |||
| Antihistamines |
5.6 |
2.2 |
0.01 |
| Nasal steroids |
0.5 |
0.5 |
0.96 |
| Oral steroids | 1.7 | 1.2 | 0.53 |
Definition of abbreviation: GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Mean (SD).
In bivariate analysis, those with an allergic phenotype were more likely to report chronic cough (odds ratio [OR], 1.84; P = 0.02) and wheezing (OR, 1.92; P < 0.01) compared with those without allergy (Table 2). Subjects with an allergic phenotype were also more likely to go to a doctor’s office or ED for wheezing (OR, 1.7; P = 0.05). There was no association between allergic phenotype and chronic phlegm, pneumonia, or hospitalizations.
TABLE 2.
ASSOCIATION OF ALLERGIC PHENOTYPE AND CHRONIC OBSTRUCTIVE PULMONARY DISEASE OUTCOMES USING NATIONAL HEALTH AND NUTRITION SURVEY III
| Bivariate Analysis |
Multivariate Analysis* |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Chronic cough |
1.84 |
1.1–3.0 |
0.02 |
1.9 |
1.2–3.1 |
0.01 |
| Chronic phlegm |
1.43 |
1.0–2.1 |
0.08 |
1.5 |
1.0–2.3 |
<0.05 |
| Wheeze |
1.92 |
1.4–2.6 |
<0.01 |
2.1 |
1.4–3.2 |
<0.01 |
| Pneumonia |
0.8 |
0.3–2.1 |
0.68 |
0.9 |
0.3–2.4 |
0.8 |
| Hospitalization |
0.92 |
0.5–1.5 |
0.74 |
1.1 |
0.6–1.8 |
0.9 |
| Doctor/ED visit | 1.7 | 1.0–2.9 | 0.05 | 1.7 | 1.0–2.8 | 0.04 |
Definition of abbreviations: CI = confidence interval; ED = emergency department; OR = odds ratio.
Multivariate regression models were used to adjust for potential confounders including age, gender, race, education, smoking pack-years, and lung function (FEV1% predicted).
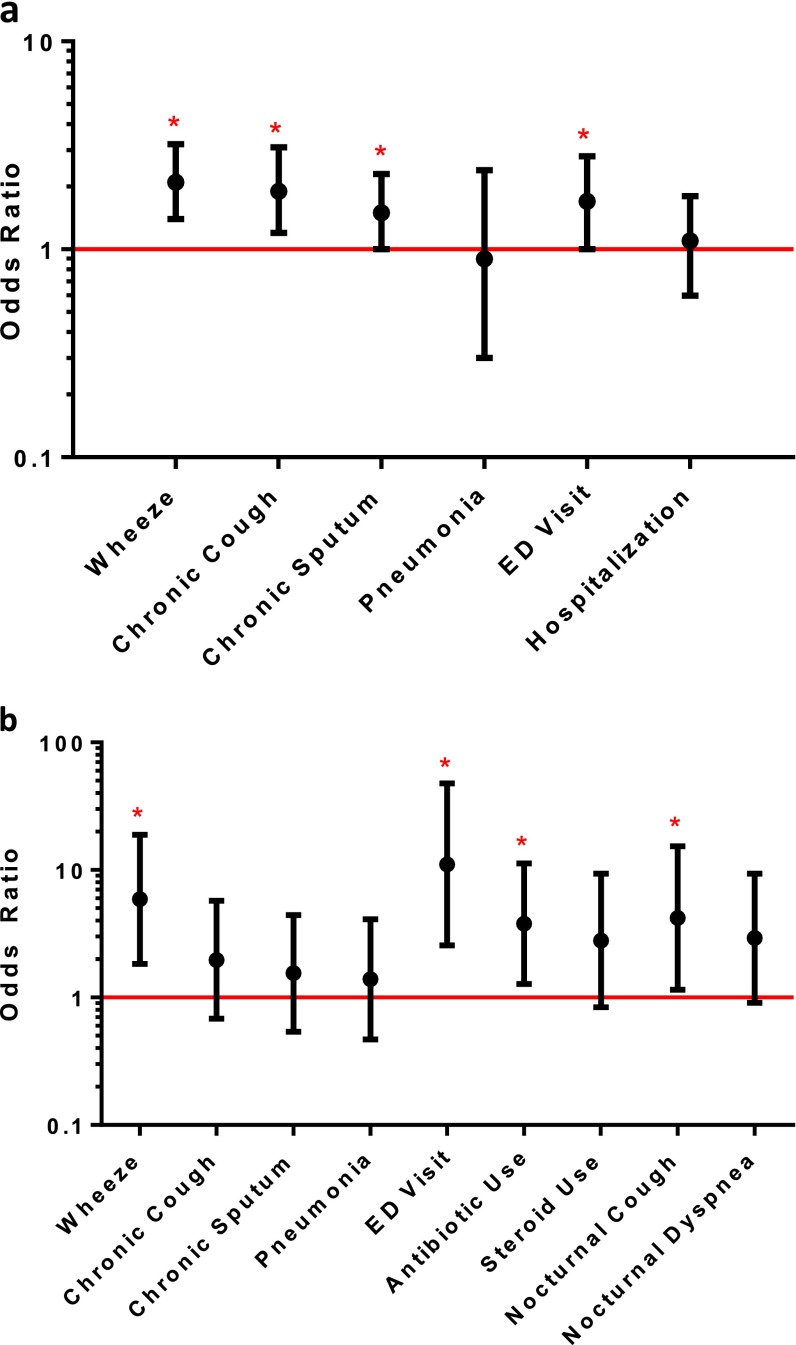
After adjustment for potential confounders (Table 2, Figure 1a), individuals with an allergic phenotype continued to be more likely to report wheeze (OR, 2.1; P < 0.01) or chronic cough (OR, 1.9; P = 0.01) compared with those without evidence of an allergic phenotype. Subjects with an allergic phenotype were also still more likely to report a doctor’s or ED visit for wheezing (OR, 1.7; P = 0.04). In multivariate models, an allergic phenotype was significantly associated with chronic phlegm production (OR, 1.5; P < 0.05). There continued to be no significant association with pneumonia or hospitalizations. Including subjects with reported diagnosis of asthma did not substantially change our results; however, among individuals reporting doctor-diagnosed asthma, those that were also allergic had an increased risk of pneumonia (OR, 37; P = 0.05) (see Table E1 in the online supplement).
Figure 1.
(a) Adjusted association of allergic phenotype and chronic obstructive pulmonary disease (COPD) outcomes using the National Health and Nutrition Survey III (NHANES III) population. Odds ratio represented with black dots. Bars represent 95% confidence interval. *P < 0.05. Multivariate regression models were used to adjust for potential confounders including age, gender, race, education, smoking pack-years, and lung function (FEV1% predicted). (b) Adjusted association of allergic phenotype and COPD outcomes using the COPD and domestic endotoxin population. Odds ratio represented with black dots. Bars represent 95% confidence interval. *P < 0.05. Multivariate regression models were used to adjust for potential confounders including age, gender, race, education, smoking pack-years, and lung function (FEV1% predicted). ED = emergency department.
CODE
Participants (n = 77) had a mean (± SD) age of 69.4 ± 7.0 years and 57.9 ± 28.8 pack-years of smoking. All participants had moderate or severe COPD with a mean baseline postbronchodilator FEV1 % predicted of 52.3% (Table 3). Thirty percent of individuals had evidence of allergic sensitization. Of those with evidence of allergic sensitization, 17 tested positive to cockroach, 16 to house dust mite, 7 to dog, 6 to cat, and 1 to mouse allergen. Fourteen percent of subjects had one or two sensitizations, and 16% had three or more sensitizations.
TABLE 3.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE AND DOMESTIC ENDOTOXIN PARTICIPANT CHARACTERISTICS
| Allergic (n = 23) | Nonallergic (n = 54) | P Value | |
|---|---|---|---|
| Race, % |
|
|
0.71 |
| White |
83 |
89 |
|
| Black |
13 |
9 |
|
| Other |
4 |
2 |
|
| Male sex, % |
57 |
63 |
0.60 |
| Education, % |
|
|
0.63 |
| Some high school |
22 |
19 |
|
| High school graduate |
9 |
24 |
|
| Some college |
39 |
30 |
|
| College graduate |
13 |
13 |
|
| At least some graduate school |
17 |
15 |
|
| Age, yr |
69.7 (7.1)* |
69.3 (7.1)* |
0.86 |
| Pack-years |
60.7 (31.0)* |
56.8 (28.4)* |
0.61 |
| FEV1, % predicted |
51.9 (17.3)* |
52.4 (16.1)* |
0.45 |
| GOLD stage, % |
|
|
0.59 |
| II |
52 |
41 |
|
| III |
39 |
44 |
|
| IV |
9 |
15 |
|
| Years since quit smoking |
13.4 (8.0)* |
13.0 (9.7)* |
0.33 |
| Doctor diagnosed COPD, % |
85 |
91 |
0.47 |
| Doctor diagnosed chronic bronchitis, % |
35 |
39 |
0.73 |
| Doctor diagnosed emphysema, % |
70 |
57 |
0.32 |
| Medications, % |
|
|
|
| LABA |
4 |
8 |
0.61 |
| ICS |
5 |
19 |
0.12 |
| ICS/LABA combination |
65 |
44 |
0.10 |
| Long-acting muscarinic antagonist |
43 |
37 |
0.60 |
| Theophylline |
9 |
2 |
0.16 |
| Nasal steroids |
9 |
6 |
0.61 |
| Leukotriene modifiers |
9 |
8 |
0.88 |
| Antihistamine | 9 | 2 | 0.16 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroids; LABA = long-acting beta agonist.
Mean (SD).
Overall, sensitized individuals were more likely to report respiratory symptoms and had an increased risk of COPD exacerbations requiring antibiotics or an ED visit or hospitalization compared with nonsensitized individuals (Table 4, Figure 1b). Specifically, in bivariate analysis, subjects with allergic sensitization were more likely to report wheeze (OR, 4.08; P < 0.01) and nighttime awakening due to cough (OR, 3.58; P = 0.03). In addition, sensitized individuals, compared with nonsensitized individuals, had an increased risk of COPD exacerbations requiring treatment with antibiotics (OR, 3.29; P = 0.02) or an ED visit or hospitalization (OR, 6.15; P < 0.01).
TABLE 4.
ASSOCIATION OF ALLERGIC PHENOTYPE AND CHRONIC OBSTRUCTIVE PULMONARY DISEASE OUTCOMES USING THE CHRONIC OBSTRUCTIVE PULMONARY DISEASE AND DOMESTIC ENDOTOXIN POPULATION
| Bivariate Analysis |
Multivariate Analysis* |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Chronic cough |
1.75 |
0.64–4.74 |
0.28 |
1.97 |
0.68–5.73 |
0.21 |
| Chronic phlegm |
1.56 |
0.58–4.18 |
0.38 |
1.55 |
0.54–4.43 |
0.41 |
| Nocturnal cough |
3.58 |
1.11–11.53 |
0.03 |
4.20 |
1.15–15.35 |
0.03 |
| Nocturnal dyspnea |
2.51 |
0.86–7.31 |
0.09 |
2.92 |
0.91–9.34 |
0.07 |
| Wheeze |
4.08 |
1.45–11.45 |
<0.01 |
5.91 |
1.84–18.94 |
<0.01 |
| Antibiotic use |
3.29 |
1.19–9.11 |
0.02 |
3.79 |
1.28–11.28 |
0.02 |
| Steroid use |
2.04 |
0.69–6.03 |
0.20 |
2.80 |
0.84–9.36 |
0.10 |
| Pneumonia |
1.28 |
0.48–3.44 |
0.62 |
1.39 |
0.47–4.10 |
0.55 |
| ED visit and/or hospitalization | 6.15 | 1.88–20.09 | <0.01 | 11.05 | 2.56–47.80 | <0.01 |
Definition of abbreviations: CI = confidence interval; ED = emergency department; OR = odds ratio.
Multivariate regression models were used to adjust for potential confounders including age, gender, race, education, smoking pack-years, and lung function (FEV1% predicted).
Results were not substantively different after adjustment for potential confounders in multivariate analysis. Subjects with allergic sensitization were more likely to report wheeze (OR, 5.91; P < 0.01) and nighttime awakening due to cough (OR, 4.20; P = 0.03). Similarly, they also continued to have an increased risk of COPD exacerbations requiring treatment with antibiotics (OR, 3.79; P = 0.02) or an ED visit or hospitalization (OR, 11.05; P < 0.01). There was no significant association with chronic cough, chronic phlegm, or pneumonia.
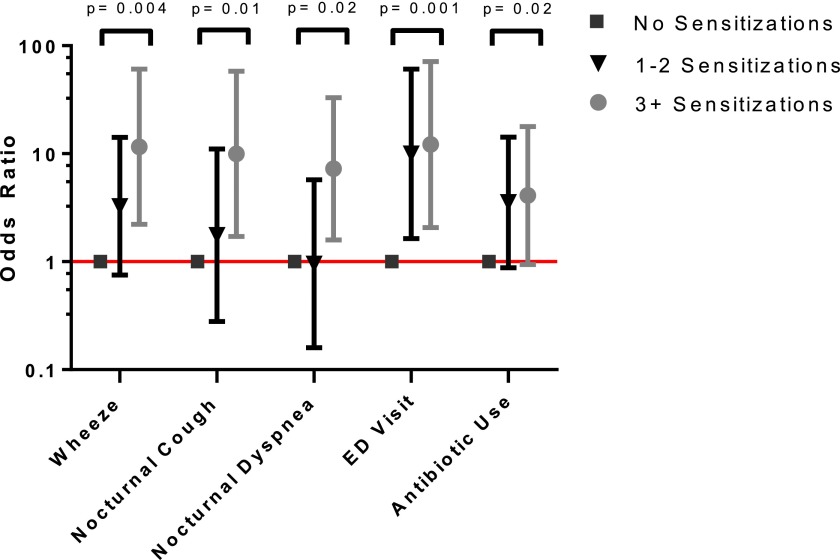
An increasing number of sensitizations was associated with a higher risk for certain adverse health outcomes (Figure 2 and Table E2; trend test P < 0.05), including wheeze (OR, 3.3; 95% confidence interval [CI], 0.75–14.1 for one or two sensitization and OR, 11.6; 95% CI, 2.2–60.8 for three or more sensitizations), nocturnal cough (OR, 1.75; 95% CI, 0.28–11.1 for one or two sensitizations and OR, 10.0; 95% CI, 1.7–58.1 for three or more sensitizations), nocturnal dyspnea (OR, 0.95; 95% CI, 0.16–5.7 for one or two sensitizations and OR, 7.3; 95% CI, 1.6–33.1 for three or more sensitizations), ED visits (OR, 10.0; 95% CI, 1.6–60.7 for one or two sensitizations and OR 12.2; 95% CI, 2.1–71.4 for three or more sensitizations), and COPD exacerbation requiring antibiotic use (OR, 3.5; 95% CI, 0.88–14.26 for one or two sensitizations and OR, 4.1; 95% CI, 0.94–17.8 for three or more sensitizations).
Figure 2.
An increased number of allergic sensitizations is associated with worse outcomes in the COPD and domestic endotoxin population. P value determined by Cuzick’s test for trend.
Discussion
We used two independent populations to show that individuals with COPD with evidence of an allergic phenotype are more likely to have lower respiratory symptoms and COPD exacerbations requiring medical treatment compared with those without evidence of allergic disease. We used two separate definitions of allergic phenotype, including self-report of allergic upper airway symptoms and IgE sensitization to perennial allergens. We asked our questions in a large nationally representative sample and in a well-characterized, COPD-specific regional cohort with comprehensive COPD phenotyping. By using results from both cohorts, we showed that the findings were not dependent on a single definition of disease or confined to one study group. COPD guidelines prioritize control of symptoms and prevention of exacerbations, but there is no guidance on management of allergic disease. A substantial proportion of individuals with COPD have an allergic phenotype: in our studied cohorts, the prevalence was 25 to 30%. A better understanding of the effect of allergic disease, and especially the role of targeted treatments, could lead to improvements in the management and outcomes of patients with COPD.
The mechanism through which allergy may affect respiratory health in smoking-induced COPD is not clearly understood. Several studies support the role of allergen exposure in bronchial inflammation and have shown that allergen exposure affects cytokine and antiinflammatory production on a cellular level. In particular, tumor necrosis factor α, IL-6, and IL-8 have been shown to be up-regulated in cell culture of airway epithelium after exposure to allergen (19). A recent study comparing inflammatory markers in allergic versus nonallergic patients with COPD showed a higher serum level of IL-8 in patients with COPD who were allergic to mites, indicating that IL-8–related neutrophilic airway inflammation in COPD may be up-regulated by mite allergy (20). This finding supports the hypothesis that increased airway inflammation is a mechanism through which allergy may worsen symptoms in COPD.
Despite the possible role that the presence of an allergic phenotype might play in COPD, the association of allergy with increased respiratory symptoms in patients with COPD has not been adequately investigated. In the NHANES analysis, report of hay fever or allergic upper respiratory symptoms was associated with increased wheeze, chronic cough, chronic phlegm, and respiratory exacerbations leading to an acute visit to the doctor’s office or ED. Similarly, results from the CODE study showed that allergic sensitization to perennial allergens was associated with increased wheeze, nocturnal cough, and COPD exacerbations leading to antibiotic use or to ED visit and/or hospitalization. An increasing number of sensitizations was associated with a higher risk for adverse health outcomes. In contrast to the NHANES analysis, sensitization to perennial allergens in the CODE study was not statistically significantly linked to chronic cough or phlegm, although analysis may have been limited by smaller sample size.
Wheeze and cough, both manifestations of lower airway disease, are common respiratory symptoms in patients with COPD and negatively affect quality of life (21). Similarly, COPD exacerbations are a significant contributor to poor quality of life, disease progression, and economic burden (22). Our analysis demonstrates that allergic disease may contribute to these adverse COPD outcomes in a cohort of patients whose symptoms are often ascribed solely to smoking-induced disease. Given the cross-sectional study design and the small percentage of subjects reporting use of antiallergic medications, it remains unknown if the treatment of upper airway allergic symptoms or allergen avoidance strategies improves outcomes in patients with COPD and allergic sensitization.
There is some prior evidence that an allergic phenotype is associated with worse prognosis in people with obstructive airways disease. In children with asthma, the presence of allergen sensitization has been associated with more severe disease (23). Furthermore, atopic adults with asthma who are repeatedly exposed to allergen may demonstrate greater lung function decline than those who are not repeatedly exposed (24). The Normative Aging Study, a general population cohort, evaluated skin test reactivity and prospectively followed lung function over 3.5 years. In subjects with COPD, skin test reactivity was a significant predictor of annual rates of decline of FEV1 and FEV1/FVC ratio (10). Our data do not show differences in lung function between those with and without an allergic phenotype, and because our analyses are cross-sectional we cannot confirm effects of allergic phenotype on longitudinal changes in lung function.
Our study has notable strengths and limitations. The association of an allergic phenotype with worse COPD outcomes in two separate cohorts strengthens the validity of our findings. Within NHANES, postbronchodilator spirometry was not available, so we used prebronchodilator spirometry to define obstruction, an approach that is common in analyses of the NHANES dataset (25). This may lead to misclassification by including subjects with reversible airways obstruction or asthma. To minimize misclassification we excluded subjects with self-reported doctor diagnosed asthma and limited subjects to smokers greater than 40 years of age. This potential misclassification was limited in the CODE study, where patients were recruited and comprehensively phenotyped for participation in a COPD-specific study. CODE study participants had evidence of moderate to severe disease and evidence of airflow obstruction after administration of bronchodilator and were similarly excluded if they had a concomitant diagnosis of asthma. Even if patients with underlying asthma were inadvertently included in the analyses, there is increasing recognition that bronchodilator reversibility and asthma coexist in a substantial proportion of subjects with COPD (26, 27). Even when including subjects from NHANES with doctor-diagnosed asthma, the role of the allergic phenotype on health outcomes was not substantially altered. Whether the contribution of allergic disease to worse COPD outcomes is a result of a direct interplay between allergic inflammation and the inflammatory process of COPD or whether allergic disease is a marker of subjects with unrecognized concomitant asthma remains unclear (28). Regardless, our samples represent real-life populations of smokers who do not carry a diagnosis of asthma but who may benefit from further investigation of an allergic contribution to their respiratory symptoms.
Although skin testing was performed as a part of NHANES III, it was only performed on a small proportion (13%) of our cohort with COPD and therefore represents less than 1% of the NHANES population overall. Using skin testing within our definition of allergic phenotype in NHANES would have decreased the value of using such a large nationally representative database with its inherent limitations of a less well-characterized population for COPD-specific outcomes. We overcame this limitation by examining specific sensitization in the CODE cohort to corroborate the NHANES findings. Although allergic phenotype was defined through two different methods, we came to very similar conclusions in each of the analyses, adding validity to the importance of an allergic phenotype in COPD outcomes. Further study is needed to determine whether specific sensitizations and exposures are more likely to contribute to respiratory symptoms. Respiratory symptoms and exacerbations were based on self-report and not validated by review of medical records. In addition, although allergic sensitization in adults is generally believed to be stable over time, this study is cross-sectional, and therefore we cannot conclude causality.
In conclusion, our study demonstrates that the presence of an allergic phenotype is associated with increased risk of lower respiratory symptoms and respiratory exacerbations among individuals with COPD. Our findings are robust in that the associations are evident in two independent cohorts, one of which is representative of the United States population, with an allergic phenotype determined by clinical symptoms or allergic sensitization. Given that allergic symptoms can cause morbidity in anyone, regardless of whether or not they have COPD, we urge clinicians to remember to not ignore allergic symptoms in their patients with COPD. Furthermore, because our observations suggest that active allergic disease may worsen COPD, studies are needed to determine whether or not pharmacologic treatment of allergic disease or allergen avoidance strategies may lead to improvement in respiratory health in this important population.
Footnotes
This work was supported by National Institute of Environmental Health Science grants ES01578, ES003819, ES015903, ES018176, and ES016819 and by USEPA grant RD83451001.
Author Contributions: D.B.J., E.C.M., M.C.M., P.N.B., G.B.D., and N.N.H. provided substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. A.B., E.P., S.P.-L., and J.C.-B. contributed to data analysis and interpretation of data. All authors contributed to revising the manuscript critically for important intellectual content and provided final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201211-2103OC on May 8, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Romieu I, Silverman EK, Balmes JR Committee on Nonsmoking COPD, Environmental and Occupational Health Assembly. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 2.Heron M, Tejada-Vera B. Deaths: leading causes for 2005. Natl Vital Stat Rep. 2009;58:1–97. [PubMed] [Google Scholar]
- 3.Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2007;4:502–506. doi: 10.1513/pats.200701-001FM. [DOI] [PubMed] [Google Scholar]
- 4.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH, Wood RA American Academy of Pediatrics Section On Allergy And Immunology. Allergy testing in childhood: using allergen-specific IgE tests. Pediatrics. 2012;129:193–197. doi: 10.1542/peds.2011-2382. [DOI] [PubMed] [Google Scholar]
- 6.Lanphear BP, Kahn RS, Berger O, Auinger P, Bortnick SM, Nahhas RW. Contribution of residential exposures to asthma in us children and adolescents. Pediatrics. 2001;107:E98. doi: 10.1542/peds.107.6.e98. [DOI] [PubMed] [Google Scholar]
- 7.Torjusen EN, Diette GB, Breysse PN, Curtin-Brosnan J, Aloe C, Matsui EC.Dose–response relationships between mouse allergen exposure and asthma morbidity among urban children and adolescents Indoor AirIn press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulet LP, Cartier A, Thomson NC, Roberts RS, Dolovich J, Hargreave FE. Asthma and increases in nonallergic bronchial responsiveness from seasonal pollen exposure. J Allergy Clin Immunol. 1983;71:399–406. doi: 10.1016/0091-6749(83)90069-6. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health, National Heart, Lung, and Blood Institute, National Asthma Education and Prevention ProgramExpert Panel Report 3: guidelines for the diagnosis and management of asthma. NIH publication no. 07-4051 [accessed 2012 Nov 1]. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/index.htm
- 10.Gottlieb DJ, Sparrow D, O'Connor GT, Weiss ST. Skin test reactivity to common aeroallergens and decline of lung function: The Normative Aging Study. Am J Respir Crit Care Med. 1996;153:561–566. doi: 10.1164/ajrccm.153.2.8564098. [DOI] [PubMed] [Google Scholar]
- 11.Frew AJ, Kennedy SM, Chan-Yeung M. Methacholine responsiveness, smoking, and atopy as risk factors for accelerated FEV1 decline in male working populations. Am Rev Respir Dis. 1992;146:878–883. doi: 10.1164/ajrccm/146.4.878. [DOI] [PubMed] [Google Scholar]
- 12.Jeffery PK, Haahtela T. Allergic rhinitis and asthma: inflammation in a one-airway condition. BMC Pulm Med. 2006;6:S5. doi: 10.1186/1471-2466-6-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;32:1–407. [PubMed] [Google Scholar]
- 14.Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 16.Mannino DM, Valvi D, Mullerova H, Tal-Singer R. Fibrinogen, COPD and mortality in a nationally representative US cohort. COPD. 2012;9:359–366. doi: 10.3109/15412555.2012.668249. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Post WS, Dalal D, Blasco-Colmenares E, Tomaselli GF, Guallar E. Coffee, alcohol, smoking, physical activity and QT interval duration: results from the Third National Health and Nutrition Examination Survey. PLoS ONE. 2011;6:e17584. doi: 10.1371/journal.pone.0017584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 19.Vroling AB, Duinsbergen D, Fokkens WJ, van Drunen CM. Allergen induced gene expression of airway epithelial cells shows a possible role for TNF-alpha. Allergy. 2007;62:1310–1319. doi: 10.1111/j.1398-9995.2007.01495.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai JJ, Liao EC, Hsu JY, Lee WJ, Lai YK. The differences of eosinophil- and neutrophil-related inflammation in elderly allergic and non-allergic chronic obstructive pulmonary disease. J Asthma. 2010;47:1040–1044. doi: 10.1080/02770903.2010.491145. [DOI] [PubMed] [Google Scholar]
- 21.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 22.Halpin DM, Decramer M, Celli B, Kesten S, Liu D, Tashkin DP. Exacerbation frequency and course of COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:653–661. doi: 10.2147/COPD.S34186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Visness CM, Calatroni A, Gergen PJ, Mitchell HE, Sampson HA. Effect of environmental allergen sensitization on asthma morbidity in inner-city asthmatic children. Clin Exp Allergy. 2009;39:1381–1389. doi: 10.1111/j.1365-2222.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Giampaolo L, Cavallucci E, Braga M, Renzetti A, Schiavone C, Quecchia C, Petrarca C, Di Gioacchino M. The persistence of allergen exposure favors pulmonary function decline in workers with allergic occupational asthma. Int Arch Occup Environ Health. 2012;85:181–188. doi: 10.1007/s00420-011-0653-4. [DOI] [PubMed] [Google Scholar]
- 25.Yentes JM, Sayles H, Meza J, Mannino DM, Rennard SI, Stergiou N. Walking abnormalities are associated with COPD: an investigation of the NHANES III dataset. Respir Med. 2011;105:80–87. doi: 10.1016/j.rmed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Shaya FT, Maneval MS, Gbarayor CM, Sohn K, Dalal AA, Du D, Scharf SM. Burden of COPD, asthma, and concomitant COPD and asthma among adults: racial disparities in a medicaid population. Chest. 2009;136:405–411. doi: 10.1378/chest.08-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tashkin DP, Celli B, Decramer M, Liu D, Burkhart D, Cassino C, Kesten S. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31:742–750. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 28.Hardin M, Silverman EK, Barr RG, Hansel NN, Schroeder JD, Make BJ, Crapo JD, Hersh CP. COPDGene Investigators. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]