Abstract
Rationale: Neutrophilic inflammation is an important pathologic feature of chronic obstructive pulmonary disease (COPD) and infectious exacerbations of COPD. Serum amyloid A (SAA) promotes neutrophilic inflammation by its interaction with lung mucosal ALX/FPR2 receptors. However, little is known about how this endogenous mediator regulates IL-17A immunity.
Objectives: To determine whether SAA causes neutrophilic inflammation by IL-17A–dependent mechanisms.
Methods: The relationship between SAA and neutrophils was investigated in lung sections from patients with COPD and a chronic mouse model of SAA exposure. A neutralizing antibody to IL-17A was used to block SAA responses in vivo, and a cell-sorting strategy was used to identify cellular sources.
Measurements and Main Results: SAA mRNA expression was positively associated with tissue neutrophils in COPD (P < 0.05). SAA predominately promoted expression of the TH17 polarizing cytokine IL-6, which was opposed by 15-epi-lipoxin A4, a counter-regulatory mediator, and ALX/FPR2 ligand. SAA-induced inflammation was markedly reduced by a neutralizing antibody to IL-17A in vivo. Cellular sources of IL-17A induced by SAA include CD4+ T cells, γδ T cells, and an Epcam+CD45− population enriched for epithelial cells. SAA promotes expression of IL-17A in γδ T cells and this innate cell proportionally expressed higher levels of IL-17A transcript than CD4+ T cells or epithelial cells.
Conclusions: The SAA–IL-17A axis represents an important innate defense network that may underlie persistent neutrophilic airway inflammation in COPD and modulating the ALX/FPR2 receptor represents a novel approach to targeting aberrant IL-17A–mediated lung immunity.
Keywords: inflammation, neutrophils, chronic obstructive pulmonary disease, innate immunity
At a Glance Commentary
Scientific Knowledge on the Subject
Serum amyloid A (SAA) and IL-17A are present in the submucosa of chronic obstructive pulmonary disease (COPD) airways. However, it is not known how SAA regulates neutrophilic inflammation and whether IL-17A is pivotal in this process.
What This Study Adds to the Field
This study demonstrates a direct relationship between neutrophils and SAA levels in COPD airways and that neutralizing IL-17A prevents neutrophilic inflammation caused by SAA challenge in vivo. In addition, SAA increased the TH17 regulating cytokine IL-6 and IL-17A expression by ALX-FPR2–dependent mechanisms, and proportionally γδ T cells expressed the highest levels of IL-17A transcript relative to CD4+ T cells and Epcam+CD45− cells.
Chronic lung diseases, such as chronic obstructive pulmonary disease (COPD) and severe asthma, are characterized by an exaggerated inflammatory profile involving accumulation of neutrophils with disease progression (1). Neutrophilic inflammation increases with COPD severity despite escalating use of glucocorticosteroids (2, 3), which contributes to excessive proteinase release and host tissue damage (4). Respiratory infections also trigger acute exacerbations of COPD (AECOPD), where airway neutrophilia increases with severity (5). AECOPDs have a major impact because they lead to impaired health-related quality of life (6) and a more rapid decline in lung function (7). We have previously shown that circulating serum amyloid A (SAA) levels acutely rise during AECOPD, where its levels were predictive of event severity (8). Furthermore, we have demonstrated elevated SAA immunoreactivity in the submucosa of COPD lung sections in close proximity to the basal epithelium and show a positive correlation between secreted SAA and the neutrophil activation marker, neutrophil elastase (9). SAA promotes expression of inflammatory mediators and neutrophil chemotaxis and survival by the ALX-FPR2 receptor in a manner that is opposed by the endogenous proresolving lipid mediator lipoxin A4 (LXA4) (9–12).
SAA is also a potent endogenous ligand that stimulates expression of TH17 polarizing mediators (13, 14), and influences in vitro TH17 differentiation of CD4+ T cells (15). IL-17A promotes inflammation by coordinating granulopoiesis and neutrophil mobilization through its regulation of leukocyte growth factors and cytokines. IL-17A is particularly central to lung immunity because innate host defenses to respiratory pathogens are compromised in mice lacking this cytokine or its receptor (IL-17RA), leading to reduced neutrophil recruitment and increased bacterial burden (16, 17). An increase in IL-17A+ immunoreactive cells has been identified in the submucosa of patients with COPD (18). IL-17A is also elevated in a chronic model of cigarette smoke exposure, where mice lacking IL-17RA were protected from developing emphysema (19). In asthma, IL-17A expressing CD4+ T cells are present in tissue biopsies and levels of IL-17A are elevated in severe asthma where neutrophils are more prominent (20, 21). Although IL-17A is implicated in airway neutrophilia associated with chronic lung diseases, such as COPD and severe asthma (reviewed in [22]), there is a poor understanding of disease-related endogenous mediators that can regulate this cytokine network.
In this study, we demonstrate a direct relationship between SAA and neutrophils in COPD lung tissue and that blocking IL-17A activity in vivo with a neutralizing antibody significantly reduced neutrophil recruitment in response to SAA challenge. Furthermore, we identify CD4+ T cells, γδ T cells, and Epcam+CD45− cells as cellular sources of IL-17A in the lung, where γδ T cells proportionally express higher levels of IL-17A transcript relative to CD4+ T cells. These data demonstrate that SAA represents a potent endogenous innate molecule that drives neutrophilic airway inflammation by ALX-FPR2–dependent regulation of IL-17A.
Methods
Mice and Animal Ethics Statement
The experiments described in this manuscript were approved by the Animal Experimentation Ethics Committee of The University of Melbourne and performed according to the guidelines of the National Health and Medical Research Council of Australia. Full details are provided in the online supplement.
Reagents and SAA Administration
Recombinant human Apo SAA (Peprotech, Rocky Hill, NJ), anti–IL-17, and rat IgG2A (R&D Systems, Inc, Minneapolis, MN) were reconstituted in 0.01 M phosphate-buffered saline and stored at −80°C according to the manufacturer’s instructions. SAA was confirmed to be endotoxin free by neutralizing the SAA preparation with polymyxin B, which did not alter neutrophilic responses (see Figure E1 in the online supplement). Mice were challenged intranasally as previously described (23) and as detailed in the online supplement.
Immunohistochemistry and Granulocyte Staining
Immunohistochemical analysis was performed as previously described, where the SAA tissue scoring has previously been determined (9). Briefly, lung tissue from resection surgery for treatment of a solitary peripheral carcinoma was collected from 13 subjects with Global Initiative for Chronic Obstructive Lung Disease stage I–II COPD (mean age, 70 ± 5 yr; mean smoking history, 49 ± 25 packs per year) and from 4 subjects with no airflow obstruction (mean age, 64 ± 8) that were used as control subjects in this study. Tissue blocks of subpleural parenchyma avoiding areas involved by tumor and mouse lungs were fixed in 10% neutral buffered formalin, embedded in paraffin, and 5-μm sections were prepared. Mouse lung sections were stained with hematoxylin and eosin to visualize cell infiltrate. Detection of neutrophils and granulocytes was performed using the Naphthol AS-D Chloroacetate (Specific Esterase) Kit (Sigma Aldrich, St. Louis, MO) according to the manufacturer’s instructions. Areas containing muscle and vessels were excluded and esterase-positive neutrophils were blinded counted at ×200 magnification, with at least 20 fields being captured per sample for analysis using ImagePro software.
Cell Culture
Human lung type II alveolar A549 epithelial cells, which do not express ALX-FPR2, were transfected to constitutively express full-length recombinant human ALX-FPR2 receptors as previously described (24). Full details of all other cell lines are provided in the online supplement.
Bronchoalveolar Lavage and Preparation of Single–Lung-Cell Suspensions
Mice were culled by an intraperitoneal overdose of ketamine-xylazine (Parnell Laboratories, Australia). Bronchoalveolar lavage (BAL) was performed by tracheotomy and total and differential BAL cell counts determined as previously described (3). Single-cell suspensions were prepared as detailed in the online supplement.
Quantitative Real-Time Polymerase Chain Reaction
For extraction of RNA from human lung tissue sections, the RNeasy FFPE kit was used in accordance to the manufacturer’s instructions (Qiagen, GmbH, Hilden). All threshold cycle values (Ct) were normalized to control (glyceraldehyde phosphate dehydrogenase for human tissue sections and 18S rRNA for mouse tissue and cells) and the relative fold change determined by the ΔΔCt value (25). Full details are provided in the online supplement.
Antibodies and Flow Cytometry
A strict gating strategy was used to determine different immune cell populations as fully detailed in the online supplement.
Cytokine Measurements and Enzyme-linked Immunospot Assay
The CBA Flex Systems Kit (BD Biosciences, San Jose, CA) was used to measure cytokines in BAL fluid (BALF). Secreted IL-17A in the supernatant of A549 cells was determined using the ELISA kit (eBiosciences, San Diego, CA) in accordance with the manufacturer’s instructions. An enzyme-linked immunospot (ELISPOT) assay was used to detect IL-17A–secreting cell populations as detailed in the online supplement.
Statistical Analysis
Full details are provided in the online supplement.
Results
SAA in COPD Lung Is Associated with Neutrophil Accumulation
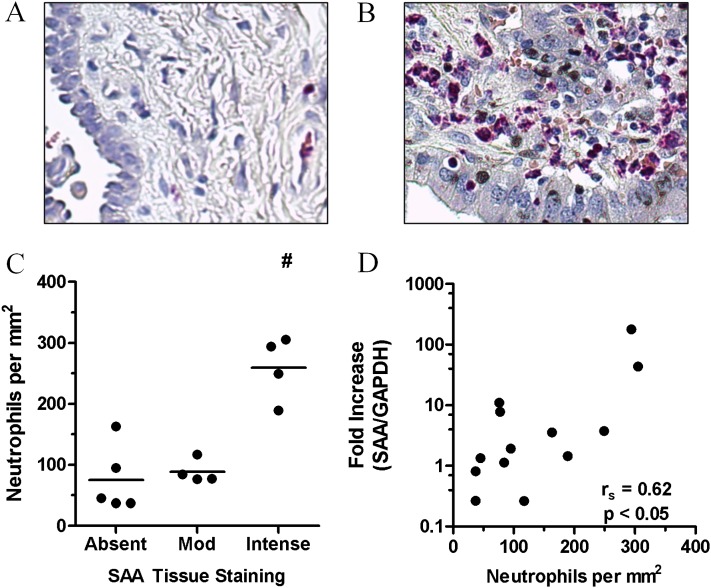
The relationship between SAA expression and airway neutrophil accumulation in stable COPD was investigated by quantifying esterase-positive neutrophils in serial sections, where SAA expression was determined by quantitative polymerase chain reaction (QPCR) and compared with matching tissue sections previously stained for SAA by immunohistochemistry (9). Representative images of esterase-positive staining neutrophils in lung sections from a control subject (Figure 1A) and a patient with COPD (Figure 1B) with elevated neutrophil numbers are shown. In these sections, the number of neutrophils significantly increased (P < 0.05) with the intensity of SAA staining (Figure 1C). In addition, there was a positive correlation (r = 0.62; P < 0.05) between SAA mRNA expression and the numbers of neutrophils in matching serial lung sections (Figure 1D).
Figure 1.
The relationship between tissue neutrophils and serum amyloid A (SAA) levels in chronic obstructive pulmonary disease (COPD) lung. Representative tissue staining for the granulocyte marker esterase in (A) control and (B) COPD lung sections. (C) Esterase-positive tissue neutrophils were quantified and grouped according to the degree of SAA tissue staining, demonstrating a significant increase in neutrophils numbers in lung sections that displayed intense SAA tissue staining. (D) Correlation between SAA expression as determined by quantitative polymerase chain reaction and the number of neutrophils in serial COPD lung sections. n = 13 individual patient samples, #P < 0.05, Spearman correlation. GADPH = glyceraldehyde phosphate dehydrogenase.
Chronic Administration of SAA Induced the Accumulation of Neutrophils In Vivo
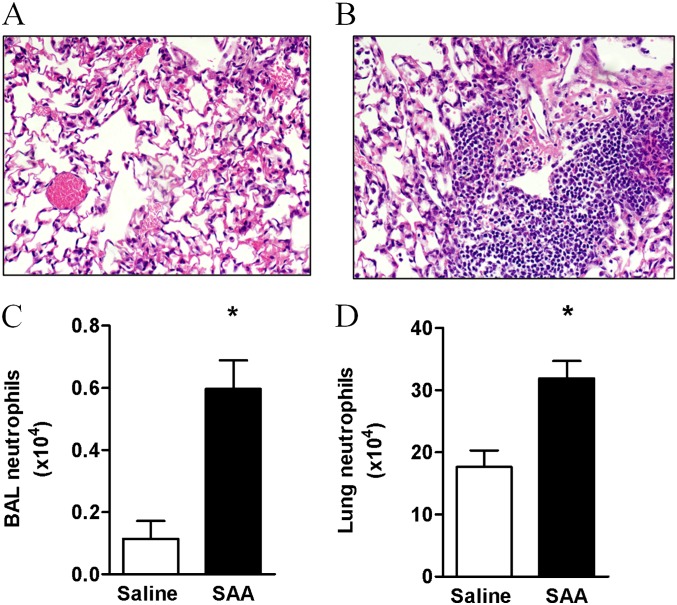
To further explore the direct relationship between SAA and lung neutrophil numbers in the chronic setting, a mouse model of repeated SAA challenge was established. Mice were exposed to intranasal SAA or saline once a week for 5 weeks and immune cells were characterized by fluorescence-activated cell sorter analysis and histology. Hematoxylin and eosin staining revealed minimal cell infiltrate in the lung of mice treated with saline (Figure 2A) compared with SAA where there was a large influx of immune cells into the mouse lung (Figure 2B). Further analysis by fluorescence-activated cell sorter revealed that neutrophil numbers were significantly increased in the BALF (saline 0.11 ± 0.06 vs. SAA 0.60 ± 0.09 × 104) (Figure 2C) and lung tissue (saline 17.62 ± 0.27 vs. SAA 31.86 ± 0.28 × 104) (Figure 2D) in response to SAA.
Figure 2.
Chronic administration of serum amyloid A (SAA) promotes neutrophil accumulation in the mouse lung. Mice were challenged with SAA (2 μg) or saline once a week for 5 weeks and lung tissue and bronchoalveolar lavage (BAL) fluid were harvested 24 hours after the final intranasal treatment of SAA. Representative hematoxylin and eosin staining of sections from (A) saline- or (B) SAA-treated mice. The hematoxylin and eosin staining demonstrates increased immune cell infiltrate in the lung sections after SAA treatment. The number of neutrophils was determined by fluorescence-activated cell sorter and pregated according to a single cell; viable (PI exclusion), large-granular cell gate and neutrophils were determined to be CD11C− and Ly6G+ (Gr-1) in the (C) BAL and (D) lung. n = 3–4 mice, *P < 0.001.
SAA Promotes the IL-17A Cytokine Network via the ALX-FPR2 Receptor
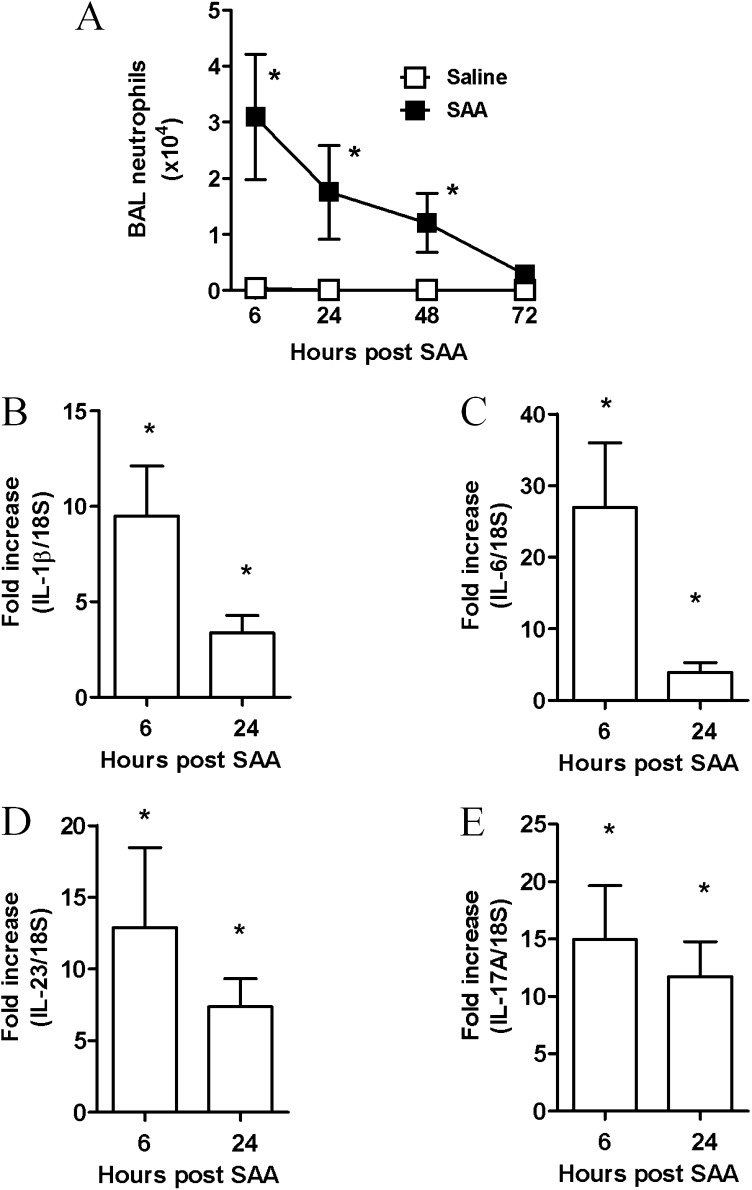
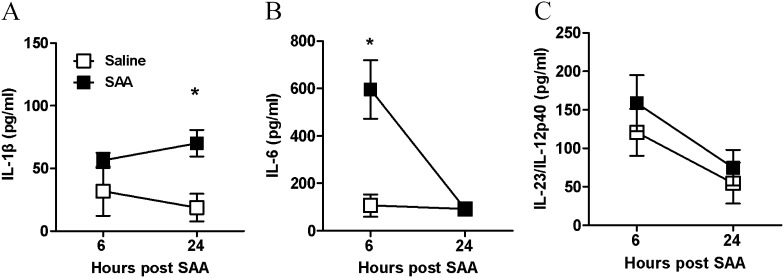
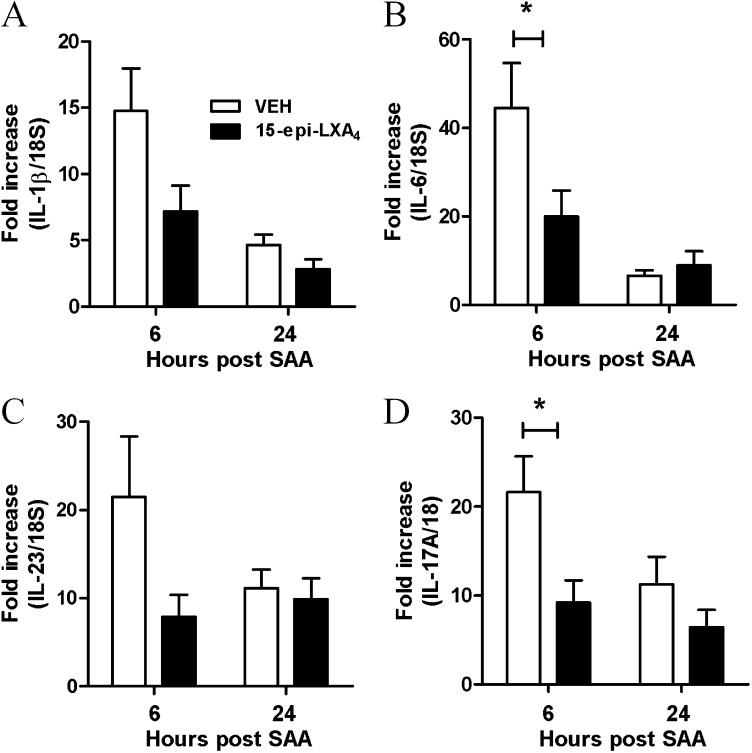
Acute SAA challenge led to the rapid (6–48 h) and significant increase in BAL neutrophils (P < 0.05) (Figure 3A). A significant increase in dendritic and macrophage cell numbers at the later 72-hour time point was observed in mice exposed to SAA (see Table E1).There was a rapid induction of known TH17 polarizing mediators peaking at 6 hours with a 27-fold increase in IL-6 (Figure 3C), 10-fold increase in IL-1β (Figure 3B), and 13-fold increase in IL-23 (Figure 3D), with levels declining at the later 24-hour time point. IL-17A expression in response to SAA also peaked at 6 hours (15-fold) (Figure 3E) and displayed a similar expression profile to TH17 polarizing mediators. SAA did not induce transforming growth factor-β lung expression above control values (data not shown). BAL samples were also retained and secreted levels of IL-1β, IL-6, and IL-23/IL-12p40 protein were determined by flow cytometry using the CBA Flex Systems Kit. No difference in secreted IL-1β was observed at 6 hours, although a significant increase in IL-1β was observed at the later 24-hour time point (saline 18.7 ± 11.0 vs. SAA 70.1 ± 10.5 pg/ml) (Figure 4A). IL-6 was markedly increased compared with saline-treated mice (saline 106.32 ± 46.82 vs. SAA 595.32 ± 123.47 pg/ml) (Figure 4B). No significant difference in secreted IL-23/IL-12p40 was observed in response to SAA (Figure 4C). To determine whether SAA-induced IL-17A and the TH17 cytokine network were dependent on the ALX-FPR2 receptor, mice were treated with 15-epi-LXA4 and QPCR was used to determine the expression of key IL-17A–related genes in the lung. Under conditions that significantly reduce SAA-induced airway neutrophil recruitment (9), 15-epi-LXA4 reduced the expression of IL-1β and IL-23 in the lung (Figures 5A and 5C). A significant two-fold reduction in peak mRNA levels of IL-6 and IL-17A was also observed with 15-epi-LXA4 (Figures 5B and 5D).
Figure 3.
Serum amyloid A (SAA)–mediated bronchoalveolar lavage (BAL) neutrophil recruitment is associated with expression of IL-17–related cytokine members in the lung. Mice were given a single dose of saline or SAA (2 μg) intranasally. BAL was performed and lungs were harvested at the indicated time points. (A) Total neutrophil numbers in the BAL fluid ± SEM, *P < 0.05. The mean ± SEM gene expression from three independent experiments (n = 5–8 mice per experimental time point) are presented. (B–E) Gene expression of IL-17A regulating cytokines, IL-6, IL-1β, IL-23, and IL-17A as determined by quantitative polymerase chain reaction, normalized to 18S and relative to naive mice. *P < 0.05.
Figure 4.
Serum amyloid A (SAA) predominantly promotes protein secretion of the IL-17A–regulating cytokine, IL-6. Mice were treated intranasally with saline or SAA (2 μg) and bronchoalveolar lavage fluid collected at the indicated time points (hours). Bronchoalveolar lavage fluid concentrations of IL-17A–inducing cytokines (A) IL-1β, (B) IL-6, and (C) IL-23 after SAA treatment. n = 3–8 mice per group, *P < 0.05.
Figure 5.
Serum amyloid A (SAA)–mediated expression of IL-17A and its related cytokines is dependent on ALX-FPR2. Mice were simultaneously challenged with SAA (2 μg) and 15-epi-LXA4 (4 μg) or vehicle by intranasal administration and gene expression of (A) IL-1β, (B) IL-6, (C) IL-23, and (D) IL-17A was determined in lung tissue at 6 or 24 hours. Quantitative polymerase chain reaction was used to determine gene expression relative to 18S. n = 4–5 mice per group, *P < 0.001.
Blocking IL-17A Inhibits SAA-induced Neutrophil Recruitment
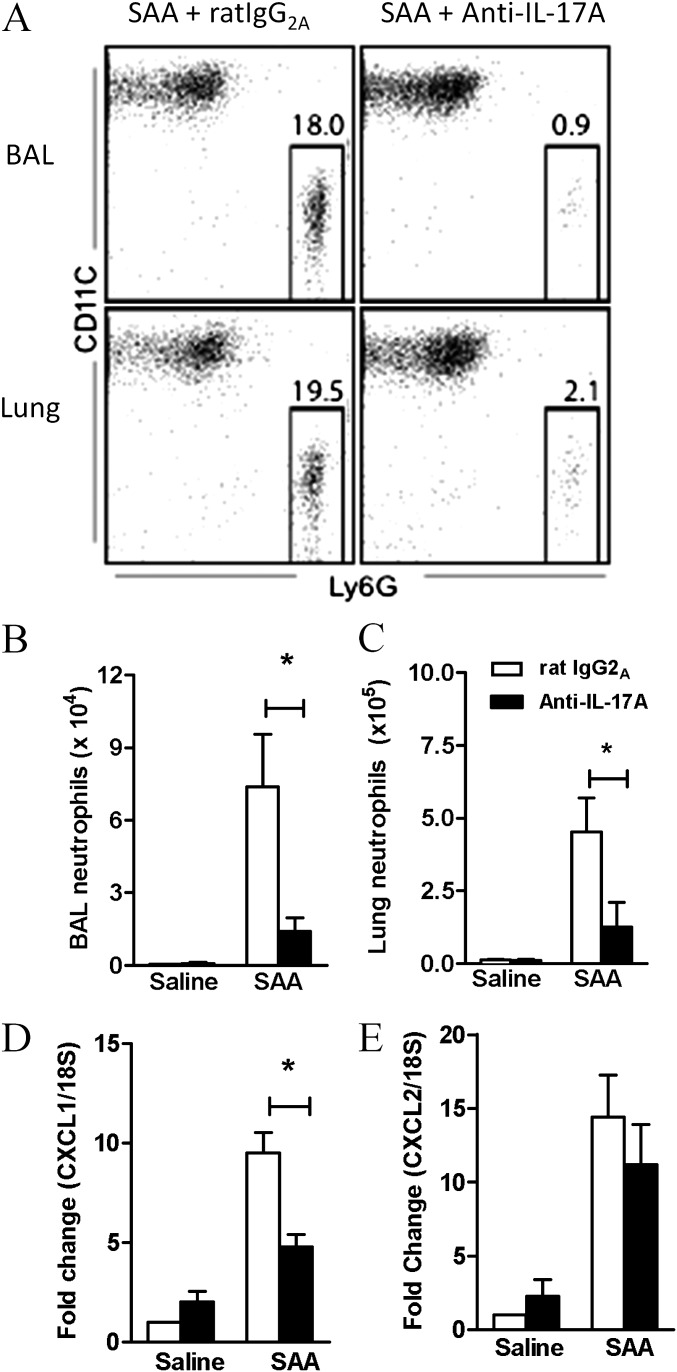
To determine the significance of the IL-17A pathway in SAA-mediated airway neutrophilia, IL-17A was neutralized using a monoclonal antibody. The proportion of neutrophils recruited by SAA was quantified by fluorescence-activated cell sorter in the BAL and lung compartments, where there was a dramatic decrease in mice pretreated with anti–IL-17A antibody (Figure 6A). The total neutrophil numbers recruited by SAA challenge were also significantly decreased in mice that were pretreated with anti–IL-17A in the BAL (IgG2A 7.37 ± 0.55 vs. anti–IL-17A 1.42 ± 0.55 × 104) (Figure 6B) and the lung compartment (IgG2A 4.52 ± 1.18 vs. anti–IL-17A 1.27 ± 0.08 × 105) (Figure 6C). Neutralizing IL-17A also reduced expression of the neutrophil chemokine CXCL1 (Figure 6D) by 50%, whereas CXCL2 was not significantly reduced (Figure 6E). These data suggest that IL-17A and CXCL1 play dominant roles in neutrophilic recruitment initiated by SAA.
Figure 6.
Blocking IL-17A inhibits serum amyloid A (SAA)–induced neutrophil recruitment. Mice were pretreated with anti–IL-17A or rat IgG2A (isotype) antibody (50 μg) intranasally 1 hour before saline or SAA (2 μg) administration. (A) Representative fluorescence-activated cell sorter plot of each treatment group 24 hours after SAA administration. (B) Total number of neutrophils in the bronchoalveolar lavage (BAL) and (C) lung of treated mice. Pooled data from two independent experiments. n = 5–8 mice per group. Gene expression of the neutrophilic mediators (D) CXCL1 and (E) CXCL2 in the lung 6 hours after SAA administration. n = 4–5 mice, *P < 0.05.
SAA-induced IL-17A Expression in CD4+ T cells, γδ T Cells, and Epithelial Cells
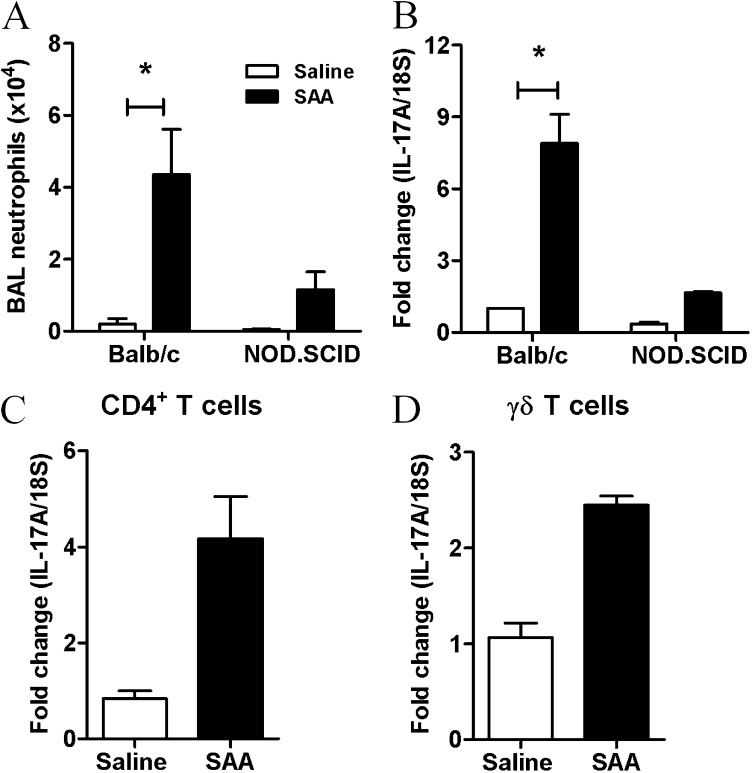
Balb/c.NOD.SCID mice phenotypically characterized by the absence of functional B and T cells were used to determine whether the expression of IL-17A was completely dependent on T cells. The recruitment of neutrophils was reduced in Balb/c.NOD.SCID relative to the control Balb/c mice in response to SAA challenge (Balb/c 4.37 ± 1.24 vs. NOD.SCID 1.16 ± 0.49 × 104) (Figure 7A). This was associated with a similar reduction in the mRNA levels of IL-17A in the lungs of SAA-treated NOD.SCID mice (Figure 7B). Although these data identify T cells as the predominant source of IL-17A in this model, the capacity to respond to SAA challenge in NOD.SCID mice implicates alternative additional sources. To investigate this further, cell populations were sorted from the lungs of mice treated with saline or SAA for 24 hours including macrophages, NK-NKT cells, CD4+T/TH17 cells, epithelial cells, and γδ T cells. The levels of IL-17A were subsequently determined in these isolated cell populations by QPCR. Using this approach, an increase in IL-17A expression was observed in CD4+ T cells (Figure 7C) and in γδ T cells (Figure 7D) in response to SAA challenge in vivo, whereas IL-17A transcript was not detected in sorted macrophages and NK-NKT cells.
Figure 7.
Serum amyloid A (SAA)–induced IL-17A from multiple cellular sources. (A) Total bronchoalveolar lavage (BAL) neutrophils as determined by fluorescence-activated cell sorter in Balb/c and NOD.SCID mice treated with saline or SAA. (B) IL-17A gene expression in NOD.SCID or control mice 24 hours after intranasal SAA (2 μg) administration was determined by quantitative polymerase chain reaction. *P < 0.05. IL-17A gene expression from (C) CD4+T and (D) γδ T cells.
The mean ΔCt value (IL-17A minus housekeeping 18S) derived from Figure 7 for the sorted γδ T cells was 19 cycles, whereas the mean ΔCt value for the sorted CD4+ T cells was 23.8 cycles. Because there is a doubling of PCR product per cycle, γδ T cells synthesize approximately 32-fold more IL-17A transcript then CD4+ T cells. Proportionally, there is approximately 20-fold more CD4+ T cells than γδ T cells per milligram of lung tissue (see Table E1, 24-h data), which is consistent with γδ T cells being a major contributor to IL-17A in SAA-treated lungs, in addition to CD4+ T cells. IL-17A transcript was detected in Epcam+CD45− cells isolated from SAA-treated mice (see Figure E2A). A pan hematopoietic marker (CD45) was used to exclude any immune cell from this cell fraction enriched for epithelial cells. The mean ΔCt value for sorted Epcam+ cells was 28 cycles, which indicates that this cell is a minor contributor to IL-17A expression in SAA-treated lungs. Consistent with this finding, A549 epithelial cells expressing ALX-FPR2 secreted detectable levels of IL-17A in the supernatant in response to SAA treatment over 24 hours (72.2 ± 26.2 pg/ml) (see Figure E2B).
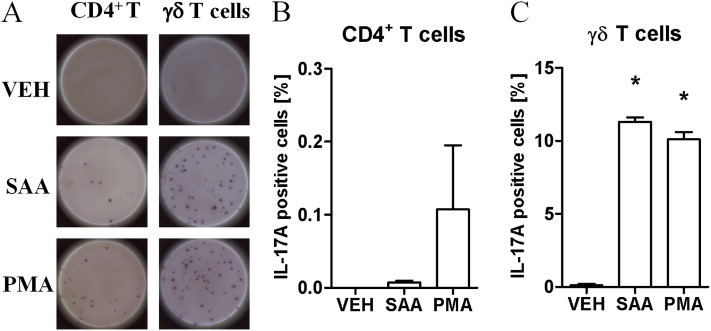
To determine the relative contribution of the classical CD4+ TH17 response versus production from γδ T cells, the T-cell populations were subjected an ELISPOT assay that detects secreted IL-17A. Isolated cell populations from SAA-treated mice were seeded and then restimulated with either vehicle, SAA, or phorbol myristate acetate–ionomycin as a positive control for 24 hours, where individual positive colonies represent a single IL-17A–secreting cell (Figure 8A). Vehicle-treated CD4+ T cells did not generate newly secreted IL-17A, and the percentage of CD4+ T cells modestly increased with SAA (0.007 ± 0.003%) and phorbol myristate acetate restimulation (0.11 ± 0.09%) (Figure 8B). In contrast, the frequency of γδ T cells that secreted IL-17A markedly increased in response to SAA restimulation (vehicle 0.10 ± 0.002% vs. SAA 11.30 ± 0.30%) (Figure 8C), which was as potent as restimulation with phorbol myristate acetate–ionomycin (10.1 ± 0.5%). A mean of 56.50 ± 1.50 IL-17A–positive colonies were counted per 500 γδ T cells and 14.33 ± 5.45 per 100,000 CD4+ T cells. Because the numbers of CD4+ T cells and γδ T cells were not significantly different in the lungs of saline or SAA-challenged mice (see Table E1), increased expression of IL-17A is consistent with polarization of lung T-cell populations. Furthermore, the data demonstrate that SAA primed γδ T cells can secrete IL-17A on restimulation independently of crosstalk between resident lung cell populations, such as dendritic cells, macrophages, and the epithelium.
Figure 8.
Enzyme-linked immunospot assay of the cellular source of IL-17A in response to serum amyloid A (SAA). Mice were intranasally treated with SAA and γδT (CD45+, γδTCR+), and CD4+T (TCR+, CD4+ TCR) cells were sorted. All cell populations were pregated according to single, viable (PI exclusion) cells. A total of 100,000 CD4+T or 500 γδT cells were stimulated with media alone (Veh), saline, SAA (1 μg/ml), or phorbol myristate acetate–ionomycin (PMA) for 24 hours. (A) Representative well of each treatment group shown. Percentage of IL-17A–producing (B) CD4+T and (C) γδT as determined by the number of positive spots divided by the total number of cells per well. Each sample was pooled from 8–10 mice. n = 2–3 wells per treatment, *P < 0.05.
Discussion
In this study, we have demonstrated that SAA promotes neutrophilic inflammation by an IL-17A–dependent mechanism. Furthermore, in vivo lung expression of IL-17A was potently suppressed by the alternate ALX-FPR2 ligand, 15-epi-LXA4, which interacts with this receptor to oppose SAA-induced inflammatory mediator release and airway neutrophil recruitment (9–12). We have previously shown basolateral epithelial expression of ALX-FPR2 in COPD lung biopsies (9), which is in close proximity to submucosal expression of SAA (9) and IL-17A (18). ALX-FPR2 belongs to a family of G protein coupled receptors originally characterized by their ability to bind N-formyl peptides and is unique in that it is the only FPR member to bind to the eicosanoid, LXA4 (26). We have previously shown that the early recruitment of neutrophils (at 6 h) after in vivo SAA challenge was inhibited by 15-epi-LXA4 (9). This early time point suggests that 15-epi-LXA4 can directly oppose neutrophil migration in response to SAA independently of neutrophil survival and efferocytosis. In addition to stimulating neutrophil chemokines, SAA is known to act as a direct chemotactic factor for neutrophils by ALX-FPR2; however, our data suggest that direct chemotaxis is only a minor contributor in SAA-treated mice because neutralizing IL-17A potently suppressed neutrophil airway recruitment. Consistent with this, NOD.SCID mice that have functional neutrophils displayed a 75% reduction in BAL neutrophilia in response to SAA.
In addition to ALX-FPR2, Toll-like receptor-2 (TLR2) has been described as a functional receptor for SAA on macrophage populations (27) and γδ T cells express a suite of pathogen recognition receptors including TLR2 (28). Our findings identify an important role for ALX-FPR2, because 15-epi-LXA4 does not interact with TLR2 or other known SAA receptors. The study by Ather and coworkers (13) identifies a role for TLR2-mediated activation of the inflammasome in response to SAA. In this study, they also examine in vivo responses to SAA in TLR2 knockout mice, where they show a 75% reduction in neutrophil recruitment and implicate ALX-FPR2 as the alternative receptor that accounts for the remaining 25% response. It is likely that there will be heterologous communication between TLR2 and ALX-FPR2 to maximize responses to SAA in vivo. In support of this, TLR2 has been shown to regulate the expression of mFPR2, because stimulation of TLR2 increased mFPR2 levels in a manner that was opposed by short interfering RNA to TLR2 (29, 30). The modest induction of secreted IL-1β relative to IL-6 suggests a minor role for the inflammasome pathway in the nonallergic lung setting, which is consistent with inflammasome-deficient (Nrlp3 knockout) mice displaying normal airway neutrophil recruitment in response to SAA (13).
Because ALX-FPR2 signaling can promote opposing biologic actions contingent on the type of ligand that interacts with this receptor, the relative balance of opposing ligands is likely to be central to controlling the intensity and resolution of inflammation in chronic airways disease. For example, ALX-FPR2 regulates resolution of allergic airways inflammation by reducing IL-17A production in response to exogenous 15-epi-LXA4 treatment (31). We have previously demonstrated that SAA is disproportionally expressed relative to ALX-FPR2 agonist LXA4 during AECOPD (9). We have also demonstrated that induction of the neutrophil chemokines CXCL1 and CXCL2 was dependent on the ALX-FPR2 receptor because exogenous 15-epi-LXA4 functionally antagonized this inflammatory response in vivo (9). In addition, A549 epithelial cells deficient in ALX-FPR2 signaling only stimulated inflammatory mediator release (monocyte chemoattractant protein-1, granulocyte-macrophage colony–stimulating factor, IL-8) in response to exogenous SAA when this receptor complex was genetically introduced in this cell line (9). Here, we demonstrate for the first time a direct relationship between SAA and neutrophil numbers in COPD lung sections, which identifies this endogenous innate molecule as an important candidate mediator of persistent neutrophilic inflammation in COPD. Furthermore, chronic SAA challenge in mice caused the accumulation of airway neutrophils that was reflective of the accumulation observed in diseased airways.
We found that T cells represent a major regulator of SAA-mediated neutrophilic inflammation because NOD.SCID mice deficient in functional T cells displayed a 75% reduction in BAL neutrophils. A 4.5-fold increase in IL-17A expression in NOD.SCID mice in response to SAA also suggests that alternative non–T-cell sources of IL-17A are being generated. A cell-sorting strategy to isolate lung macrophages, neutrophils, NK-NKT, and Epcam+ CD45− cells from SAA-treated mice identified IL-17A expression in Epcam+ cells, to strongly suggest epithelial expression. Furthermore, A549 cells expressing ALX-FPR2 secreted IL-17A in response to SAA in vitro. However, because Epcam expression has been observed in murine T cells and thymocytes, and because A549 cells are a transformed cell line, further work is needed to confirm epithelial expression in primary epithelial cells, which is beyond the scope of this study. In addition, sorted CD4+ T cells isolated from the lungs of SAA-challenged mice expressed increased IL-17A transcript levels. Ex vivo restimulation with SAA demonstrated a very low frequency (0.007%) of CD4+ IL-17A–secreting T cells. Our finding that isolated CD4+ T cells respond poorly to restimulation with SAA is consistent with the expression of IL-17A being dependent on coculture with other innate immune cells capable of inducing IL-17A–related genes, such as IL-6 and IL-23 (15). Levels of secreted IL-6 in response to SAA were significantly higher than IL-1β or IL-23 in vivo, and no increase in transforming growth factor-β lung expression was detected. Using 15-epi-LXA4, we demonstrate that increased IL-6 expression in response to SAA challenge is dependent on the ALX-FPR2 receptor complex. Although IL-6 is known to promote expression of SAA from hepatic and epithelial cells (32, 33), we propose a novel positive feedback mechanism, whereby SAA initiates expression of IL-6 from airway epithelial cells and macrophages, and this promotes polarization of CD4+ T cell populations to express IL-17A.
Our study is the first to identify γδ T cells as a major producer of IL-17A in response to SAA. Furthermore, the secretion of IL-17A by isolated γδ T cells in the absence of coculture with dendritic cells or macrophages demonstrates a direct mechanism by which γδ T cells can produce IL-17A independently of the classic TΗ17 paradigm. It has been shown that γδ T cells can selectively expand in response to pathogen products in a manner that is amplified by IL-23 (28). Here, we did not observe an expansion of γδ T cell numbers in the airways after SAA challenge, which is consistent with the observation that γδ T cells numbers are not elevated in COPD (34). Although the role of γδ T cells in COPD pathogenesis remains poorly defined, direct interactions between the innate endogenous mediator SAA and γδ T cells represents a novel mechanism by which neutrophilic recruitment is sustained in chronic lung diseases, such as COPD. Furthermore, therapeutically targeting the ALX-FPR2 receptor using the proresolving mediator 15-epi-LXA4 represents a novel approach to dampening IL-17A–dependent immunity responsible for excessive neutrophilic inflammation in an environment where SAA is abundantly present.
Footnotes
Supported in part by National Health Medical Research Council of Australia and National Institutes of Health grants HL068669 and GM095467.
Author Contributions: D.A. and S.B. designed the study, performed the experiments and data analysis, and prepared the manuscript. H.J.S., M.U., M.T., L.D., and R.V. performed the experiments and data analysis. L.B.I., B.D.L., and G.P.A. designed the study and provided critical revision of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201211-2139OC on April 29, 2013
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 2.Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1635–1639. doi: 10.1164/ajrccm.160.5.9811058. [DOI] [PubMed] [Google Scholar]
- 3.Vlahos R, Wark PA, Anderson GP, Bozinovski S. Glucocorticosteroids differentially regulate MMP-9 and neutrophil elastase in COPD. PLoS ONE. 2012;7:e33277. doi: 10.1371/journal.pone.0033277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taggart CC, Greene CM, Carroll TP, O’Neill SJ, McElvaney NG. Elastolytic proteases: inflammation resolution and dysregulation in chronic infective lung disease. Am J Respir Crit Care Med. 2005;171:1070–1076. doi: 10.1164/rccm.200407-881PP. [DOI] [PubMed] [Google Scholar]
- 5.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:446–452. doi: 10.1164/rccm.200408-1054OC. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozinovski S, Hutchinson A, Thompson M, Macgregor L, Black J, Giannakis E, Karlsson AS, Silvestrini R, Smallwood D, Vlahos R, et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:269–278. doi: 10.1164/rccm.200705-678OC. [DOI] [PubMed] [Google Scholar]
- 9.Bozinovski S, Uddin M, Vlahos R, Thompson M, McQualter JL, Merritt AS, Wark PA, Hutchinson A, Irving LB, Levy BD, et al. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Kebir D, József L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 11.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 12.Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, Wang JM. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ather JL, Ckless K, Martin R, Foley KL, Suratt BT, Boyson JE, Fitzgerald KA, Flavell RA, Eisenbarth SC, Poynter ME. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187:64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He R, Shepard LW, Chen J, Pan ZK, Ye RD. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol. 2006;177:4072–4079. doi: 10.4049/jimmunol.177.6.4072. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D’Anna SE, Zanini A, Brun P, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS ONE. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, Laviolette M, Boulet LP, Hamid Q. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 22.Alcorn JF, Crowe CR, Kolls JKT. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 23.Bozinovski S, Jones JE, Vlahos R, Hamilton JA, Anderson GP. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem. 2002;277:42808–42814. doi: 10.1074/jbc.M207840200. [DOI] [PubMed] [Google Scholar]
- 24.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bozinovski S, Seow HJ, Crack PJ, Anderson GP, Vlahos R. Glutathione peroxidase-1 primes pro-inflammatory cytokine production after LPS challenge in vivo. PLoS ONE. 2012;7:e33172. doi: 10.1371/journal.pone.0033172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol. 2008;181:22–26. doi: 10.4049/jimmunol.181.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, Lockett S, Dunlop NM, Wang JM. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem. 2006;281:3651–3659. doi: 10.1074/jbc.M508125200. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Zhang L, Huang J, Gong W, Dunlop NM, Wang JM. Cooperation between NOD2 and Toll-like receptor 2 ligands in the up-regulation of mouse mFPR2, a G-protein-coupled Abeta42 peptide receptor, in microglial cells. J Leukoc Biol. 2008;83:1467–1475. doi: 10.1189/jlb.0907607. [DOI] [PubMed] [Google Scholar]
- 31.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorn CF, Lu Z-Y, Whitehead AS. Regulation of the human acute phase serum amyloid A genes by tumour necrosis factor-alpha, interleukin-6 and glucocorticoids in hepatic and epithelial cell lines. Scand J Immunol. 2004;59:152–158. doi: 10.1111/j.0300-9475.2004.01369.x. [DOI] [PubMed] [Google Scholar]
- 33.Thorn CF, Whitehead AS. Differential glucocorticoid enhancement of the cytokine-driven transcriptional activation of the human acute phase serum amyloid A genes, SAA1 and SAA2. J Immunol. 2002;169:399–406. doi: 10.4049/jimmunol.169.1.399. [DOI] [PubMed] [Google Scholar]
- 34.Pons J, Sauleda J, Ferrer JM, Barceló B, Fuster A, Regueiro V, Julià MR, Agustí AG. Blunted γ δ T-lymphocyte response in chronic obstructive pulmonary disease. Eur Respir J. 2005;25:441–446. doi: 10.1183/09031936.05.00069304. [DOI] [PubMed] [Google Scholar]