Abstract
Rationale: Air trapping and ventilation defects on imaging are characteristics of asthma. Airway wall thickening occurs in asthma and is associated with increased bronchial vascularity and vascular permeability. Vascular endothelial cell products have not been explored as a surrogate to mark structural airway changes in asthma.
Objectives: Determine whether reporters of vascular endothelial cell perturbation correlate with airway imaging metrics in patients with asthma of varying severity.
Methods: Plasma from Severe Asthma Research Program subjects was analyzed by ELISAs for soluble von Willebrand factor mature protein (VWF:Ag) and propeptide (VWFpp), P-selectin, and platelet factor 4. Additional subjects were analyzed over 48 hours after whole-lung antigen challenge. We calculated ventilation defect volume by hyperpolarized helium-3 magnetic resonance imaging and areas of low signal density by multidetector computed tomography (less than −856 Hounsfield units [HU] at functional residual capacity and −950 HU at total lung capacity [TLC]).
Measurements and Main Results: VWFpp and VWFpp/Ag ratio correlated with and predicted greater percentage defect volume on hyperpolarized helium-3 magnetic resonance imaging. P-selectin correlated with and predicted greater area of low density on chest multidetector computed tomography less than −950 HU at TLC. Platelet factor 4 did not correlate. Following whole-lung antigen challenge, variation in VWFpp, VWFpp/Ag, and P-selectin among time-points was less than that among subjects, indicating stability and repeatability of the measurements.
Conclusions: Plasma VWFpp and P-selectin may be useful as surrogates of functional and structural defects that are evident on imaging. The results raise important questions about why VWFpp and P-selectin are associated specifically with different imaging abnormalities.
Keywords: asthma, von Willebrand factor, P-selectin, magnetic resonance imaging, computed tomography
At a Glance Commentary
Scientific Knowledge on the Subject
Increased air trapping and ventilation defects demonstrated by airway and functional techniques are characteristics of asthma, particularly in severe disease. Airway wall thickening also occurs in asthma, caused in part by increased airway wall arterial blood flow (i.e., increased vascular mass, formation of new vessels, and increased vascular permeability leading to edema). However, it is not known if indicators of vascular endothelial cell perturbation can be surrogate markers of changes in airway structure.
What This Study Adds to the Field
Plasma concentration of von Willebrand factor propeptide (VWFpp) and the propeptide/mature protein ratio (VWFpp/Ag), indicators of vascular endothelial cell perturbation, correlate with and predict ventilation defect volume on hyperpolarized helium-3 magnetic resonance imaging. Plasma concentration of soluble P-selectin, an indicator of vascular endothelial cell and platelet activation, correlates with and predicts high percentage area of low density on chest multiple detector computed tomography at TLC in asthma. Thus, VWFpp or VWFpp/Ag and P-selectin are associated, respectively, with different specific imaging abnormalities characteristic of asthma. We present a model of how VWFpp and P-selectin may associate with the two different types of changes.
Air trapping, airflow obstruction, ventilation defects, and thickening of the airway wall (all established characteristics of asthma) can be demonstrated by hyperpolarized helium-3 magnetic resonance imaging (HPHe-MRI) and multidetector computed tomography (MDCT) (1–6). HPHe-MRI depicts inhaled gas distribution and provides 3- to 10-mm spatial resolution where areas with diminished signal are caused by local airway obstruction (Figure 1A) (2, 4–7). MDCT, which provides much higher (0.5–1 mm) spatial resolution and a calibrated scale of attenuation proportional to tissue density, is capable of measuring airway wall thickness and lung parenchymal tissue density (1–3, 6, 8). MDCT is commonly done at both FRC and TLC to measure different components of obstructive disease. Parenchymal density at FRC decreases in asthma because of air trapping (1, 6, 9), whereas decrease in peripheral lung density at TLC because of dilation of alveolar space and destruction of alveolar walls is a hallmark of emphysema (10, 11). In nonsmokers with severe or unstable asthma, however, low-attenuation areas at TLC have also been observed in a diffuse pattern (Figure 1C). This pattern has been attributed in various publications to hyperinflation, airflow limitation, and complexity of terminal airspace geometry (6, 12–17) and remains comparatively understudied in asthma relative to the more common air trapping measure at FRC.
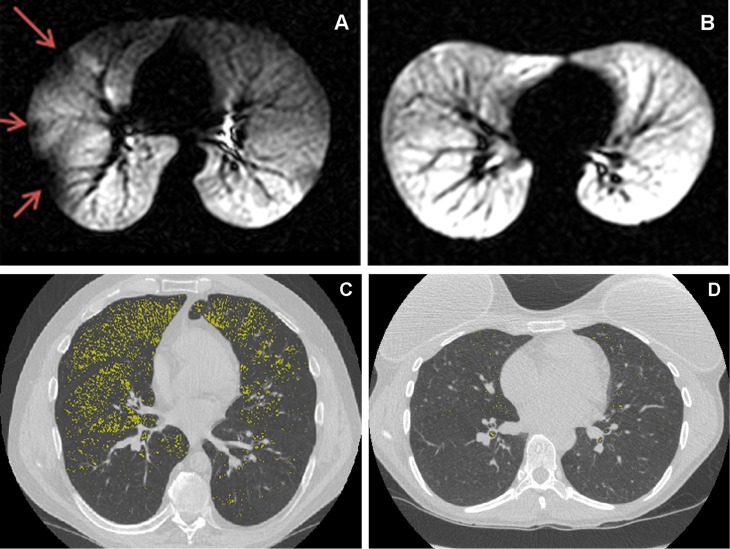
Figure 1.
Hyperpolarized helium-3 magnetic resonance imaging (HPHe-MRI) (A and B) and multidetector computed tomography (MDCT) (C and D) of four Severe Asthma Research Program subjects with asthma illustrating the associations between von Willebrand factor propeptide (VWFpp), VWFpp/VWF mature protein (VWF:Ag), or P-selectin and abnormalities. (A) Subject with nonsevere asthma, P-selectin of 18 ng/ml, VWFpp of 171 U/dl, VWFpp/Ag of 1.65, and defect volume on HPHe-MRI of 11.1%, above the median of 1.8% for all subjects with asthma. Red arrows = defective regions. (B) Subject with severe asthma, P-selectin of 16 ng/ml, VWFpp of 109 U/dl, VWFpp/Ag of 0.68, and no detectable ventilation defect (0.0%) on HPHe-MRI. (C) Subject with severe asthma, P-selectin of 48 ng/ml, VWFpp of 103 U/dl, VWFpp/Ag of 1.10, and area of signal density on MDCT less than −950 HU at TLC of 7.9%, above the median of 1.16% for all subjects with asthma. (D) Subject with nonsevere asthma, P-selectin of 9.4 ng/ml, VWFpp of 107 U/dl, VWFpp/Ag of 1.23, and area of signal density on MDCT less than −950 HU at TLC of 0.25%, below the median of 1.16% for all subjects with asthma. Highlighted in yellow = regions of signal density less than −950 HU at TLC.
Insights into pathophysiology and progression of airway injury in asthma may be gained by linking results of imaging to more readily measured biomarkers. The asthmatic bronchial tree is marked by new blood vessels, increased vessel area and length, and increased vascular permeability, resulting in edema (1, 18–27). Furthermore, the pulmonary endothelium may be activated in asthma (28, 29). We hypothesized that imaging changes and increased vasculature in asthma are accompanied by increases in two products of activated endothelial cells: von Willebrand factor (VWF) and P-selectin. VWF originates from endothelial cells and platelets (30, 31) and is increased in various pulmonary diseases, including acute bronchitis (32), acute lung injury (33), and pulmonary hypertension (34). P-selectin, which also originates from endothelial cells and platelets (35–38), may be elevated under certain circumstances in asthma (37–39) and is increased in other diseases, including hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and acute myocardial infarction (35, 36, 40). VWF is synthesized as pro-VWF and cleaved in the endothelial cell secretory apparatus into N-terminal polypeptide (VWFpp) and C-terminal mature protein (VWF:Ag); VWFpp and VWF:Ag may be secreted constitutively or stored as a complex in Weibel-Palade granules (Figure 2A) (30, 31). P-selectin is a type-I membrane protein sequestered in Weibel-Palade granules. The granules translocate to the endothelial surface in response to inflammatory and thrombogenic mediators, resulting in secretion of VWFpp and VWF:Ag and cell-surface display of P-selectin and its subsequent proteolytic release (Figure 2A) (30, 31, 35, 36, 41–43). Platelet α-granules also contain P-selectin and VWFpp and VWF:Ag; animal studies indicate that platelets are a major source of plasma P-selectin, whereas most VWF originates from endothelial cells (30, 35, 36, 44).
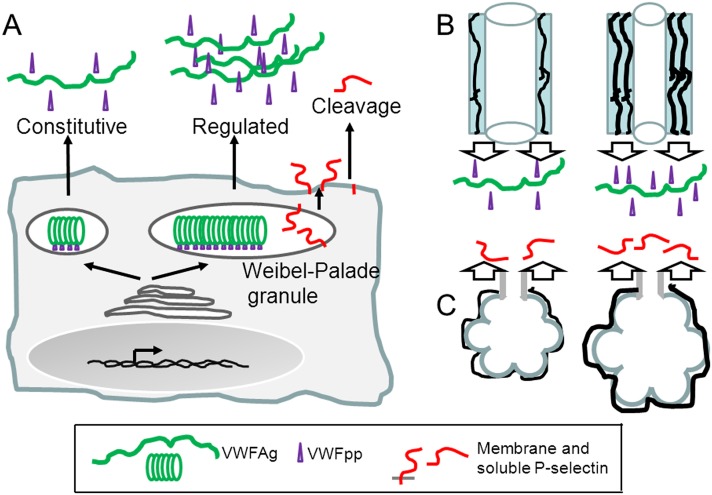
Figure 2.
Secretory pathways of von Willebrand factor (VWF) and P-selectin from endothelial cells and proposed explanation for differential associations of VWF propeptide (VWFpp) and P-selectin with airway changes or defects in asthma. (A) VWF mature protein (VWF:Ag) and VWFpp are constitutively secreted into blood plasma and packaged together as a complex in tubular structures within Weibel-Palade granules, from which they are secreted in a regulated manner. P-selectin is a type I membrane protein sequestered in Weibel-Palade granules with its extracellular domain jutting into the granule. P-selectin becomes displayed on the endothelial cell surface when granules translocate to the surface on endothelial cell activation, and may be released subsequently into blood plasma by proteolysis. Endothelial cells of capillary cells lack Weibel-Palade granules and thus have only constitutive secretion of VWF and P-selectin. (B) Left, airway from subject with normal extent of vascularity and level of VWFpp secretion. Right, airway from subject with increased vascularity and high plasma VWFpp originating from the increased mass of endothelial cells. The increased vascularity reported by high VWFpp is thought to be associated with obstruction and ventilation defects in the larger and more central airways as detected by hyperpolarized helium-3 magnetic resonance imaging. (C) Left, normal terminal airspace and normal level of P-selectin secretion. Right, abnormal terminal airspace structure detected by multidetector computed tomography at TLC is associated with abnormal vasculature containing endothelial cells with increased synthesis and release of P-selectin into plasma. Green = VWF:Ag; purple = VWFpp; red = P-selectin. Based on present data and published information (22, 24, 25, 27, 30, 31, 35, 36, 41–43, 65, 66, 70).
Possible relationships of VWF and P-selectin with abnormalities on HPHe-MRI and MDCT were explored in subjects enrolled in the Severe Asthma Research Program (SARP) (9, 45, 46). VWF:Ag and VWFpp can increase acutely because of regulated release from Weibel-Palade granules in response to various stimuli (30, 31); thus, we also measured the analytes in subjects with nonsevere asthma over a 48-hour period after whole-lung antigen challenge, a model of asthma exacerbation (47) and provocation that is known to increase plasma P-selectin (37). Finally, because the platelet activation that accompanies asthma (48, 49) could in itself result in increased plasma VWF and P-selectin, we measured platelet factor 4 (PF4), a platelet-specific α-granule protein (37). Some of these results have been reported in abstract form (50, 51).
Methods
Subjects and Assessments
The SARP was a HIPAA-compliant prospective study (Table 1) on subjects with severe (based on American Thoracic Society criteria [52]) or nonsevere asthma and normal subjects screened and enrolled (9, 45) at the University of Wisconsin as described (46). Table 2 shows SARP subject data generated as described previously (48). Independent of SARP, subjects with allergic nonsevere asthma were studied after whole-lung antigen challenge (48). SARP and the antigen challenge protocol were approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. Informed written consent was obtained from each subject. P-selectin and PF4 data from the SARP subjects were used previously in a manuscript on platelet activation and eosinophil β1 integrin activation in asthma (48).
TABLE 1.
SEVERE ASTHMA RESEARCH PROGRAM STUDY DESIGN
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Consent and medical history |
x |
|
|
|
|
|
|
|
| Spirometry |
x |
|
|
|
|
|
|
|
| Methacholine challenge |
|
x |
|
|
|
|
|
|
| Allergy skin test |
|
x |
|
|
|
|
|
|
| Blood draw: IgE, leukocyte (including eosinophil and neutrophil) and platelet counts |
|
|
x |
|
|
|
|
|
| Blood draw: plasma preparation |
|
|
|
x |
|
|
x |
x |
| Hyperpolarized helium-3 magnetic resonance imaging |
|
|
|
|
x |
|
|
|
| Multiple detector computed tomography |
|
|
|
|
x |
|
|
|
| Fraction of exhaled nitric oxide |
|
|
|
|
|
x |
|
|
| Sputum induction | x |
Inhaled β agonist was withheld for 4 hours before Visits 1 and 2.
Visit 4 was at least 2 weeks after Visit 1, Visits 5 and 6 were within 4 weeks of Visit 4, Visit 7 was 6 weeks after Visit 4, and Visit 8 was 12 weeks after Visit 4.
TABLE 2.
SEVERE ASTHMA RESEARCH PROGRAM SUBJECT CHARACTERISTICS
| |
Group |
||
|---|---|---|---|
| Variable | Severe Asthma | Nonsevere Asthma | No Asthma |
| N |
21 |
42 |
15 |
| Sex (female, male) |
13, 8 |
19, 23 |
10, 5 |
| Age, yr (mean ± SD) |
42 ± 14*,† |
32 ± 13‡ |
23 ± 6 |
| Race/ethnicity (white, African American, Asian American, Hispanic) |
15, 6, 0, 0‡ |
37, 4, 0, 1 |
12, 0, 1, 2 |
| FEV1, % predicted (mean ± SD) |
71 ± 24§,† |
87 ± 14† |
102 ± 12 |
| FEV1/FVC, % predicted (mean ± SD) |
85 ± 18|| |
89 ± 10† |
99 ± 7 |
| FVC, % predicted (mean ± SD) |
82 ± 17¶,† |
98 ± 12 |
103 ± 10 |
| PC20, mg/ml (median [25th, 75th percentiles]) |
1.8 (0.9, 4.4)|| |
1.7 (0.9, 8.0)† |
50 (50, 50) |
| IgE, IU/ml (median [25th, 75th percentiles]) |
150 (42, 280)† |
120 (42, 300)† |
9 (2, 20) |
| Positive skin tests, number (mean ± SD) |
5 ± 3‡ |
6 ± 3† |
0 ± 0 |
| Blood eosinophils, per μl (mean ± SD) |
190 ± 200 |
260 ± 180‡ |
150 ± 110 |
| Blood neutrophils, per μl (mean ± SD) |
5,000 ± 3,400 |
3,600 ± 1,200 |
3,500 ± 1,200 |
| Platelets, thousands per μl (mean ± SD) |
270 ± 50§ |
230 ± 50‡ |
270 ± 40 |
| Sputum eosinophils, % of WBC (median [25th, 75th percentiles]) |
0.4 (0.0, 6.3) |
0.8 (0.1,1.6) |
0.2 (0.0,1.0) |
| Sputum neutrophils, % of WBC (mean ± SD) |
56 ± 19* |
40 ± 21 |
52 ± 22 |
| FeNO, ppb (median [25th, 75th percentiles]) |
30 (16, 60)|| |
25 (15, 37)|| |
14 (10, 20) |
| n (%) on ICS |
21 (100)*,† |
27 (64)† |
0 (0) |
| n (%) on oral CS |
8 (38) ¶,|| |
2 (5) |
0 (0) |
| ICS dose, μg/d (median [25th, 75th percentiles]) | 1,000 (910, 1,000)¶,† | 250 (0, 500) | 0 (0, 0) |
Definition of abbreviations: CS = corticosteroid; FeNO = fraction of exhaled nitric oxide; ICS = inhaled CS; PC20 = provocative concentration of methacholine producing a 20% fall in FEV1; ppb = part per billion; WBC = white blood cells.
The allergen extracts used for skin testing were Eastern seven-tree mix, grass mix, short ragweed, common weed mix, dog, cat, Alternaria, Cladosporium, Aspergillus mix, Dermatophagoides farinae, Dermatophagoides pteronyssinus, and American cockroach, with histamine as positive control and diluting fluid as negative control, and were from Greer Laboratories (Lenoir, NC).
ICS dose is expressed as fluticasone equivalent, based on the equivalence of 1,200 μg budenoside or 1,260 μg beclomethasone with 880 μg fluticasone (52) (39 subjects were on fluticasone, 7 on budenoside, 1 on beclomethasone, and 1 on fluticasone and budenoside).
Exclusion criteria were current smoker or more than 5 pack-year previous smoking; diagnosis of vocal cord dysfunction, chronic obstructive pulmonary disease, cystic fibrosis, or congestive heart failure (46).
P less than or equal to 0.01 versus nonsevere asthma.
P less than or equal to 0.001 versus no asthma.
P less than or equal to 0.05 versus no asthma.
P less than or equal to 0.05 versus nonsevere asthma.
P less than or equal to 0.01 versus no asthma.
P less than or equal to 0.001 versus nonsevere asthma.
ELISAs
Plasma was collected by a procedure that minimizes platelet activation (48). ELISAs for P-selectin and PF4 were described previously (48). Plasma VWF:Ag and VWFpp concentrations were determined by sandwich ELISAs (53); each sample was tested at 1:100, 1:200, and 1:400 dilutions, and values were averaged.
Imaging
HPHe-MRI and MDCT were performed as described (2) on Visit 5 (Table 1) within 48–72 hours of each other. HPHe-MRI and MDCT scan parameters are summarized in Table 3. Details are in the online supplement.
TABLE 3.
MRI AND MDCT SCAN PARAMETERS
| MRI Parameter | T1-weighted Fast-Spin Echo | Hyperpolarized 3He-MRI |
|---|---|---|
| TR/TE |
∞/8 ms |
8.4/3.1 ms |
| Flip angle |
90° |
7° |
| Bandwidth |
31.25 kHz |
31.25 kHz |
| Acquisition matrix |
128 × 64 |
128 × 128 |
| Imaging time |
7–8 s |
18–24 s |
| Axial plane FOV |
32–38 × 24–29 cm2 |
32–38 × 24–29 cm2 |
| Slice FOV |
13–19 × 1.5 cm slices |
13–19 × 1.5 cm slices |
|
MDCT Parameter |
16 Detectors |
64 Detectors |
| Collimation |
1.25 mm |
1.25 mm |
| Pitch |
1.675 |
0.95 |
| Source current/voltage |
100–300 mA/120 kV(p) |
100–300 mA/120 kV(p) |
| Gantry speed |
0.5 s/rotation |
0.5 s/rotation |
| Matrix |
512 × 512 |
512 × 512 |
| Lung volume |
FRC (end expiration) and TLC (end inspiration) |
FRC (end expiration) and TLC (end inspiration) |
| Reconstruction kernel |
“Standard” and “lung” |
“Standard” and “lung” |
| Reconstructed slice thickness | 0.625 mm (quantitative) and 5 mm (qualitative) | 0.625 mm (quantitative) and 5 mm (qualitative) |
Definition of abbreviations: FOV = field of view; 3He-MRI = helium-3 magnetic resonance imaging; kV(p) = peak kilovolt; MDCT = multidetector computed tomography; MRI = magnetic resonance imaging; TE = time to echo; T1 = longitudinal relaxation time; TR = time of repetition.
Statistics
Mann-Whitney U, Kruskal Wallis, or chi-square tests were used to compare data between or among groups. Spearman rank test was used to analyze correlations. P less than or equal to 0.05 was considered significant. Analyses were performed using Prism (GraphPad, La Jolla, CA) or as described (54). Group data are reported as mean ± SD or median with 25th and 75th percentiles, if the variable was or was not normally distributed, respectively. Receiver operating characteristic (ROC) curve analysis was performed using MedCalc (Ostend, Belgium, http://www.medcalc.org).
Results
Subject Characteristics
ELISAs and HPHe-MRI and/or MDCT were performed on 78 subjects, 21 with severe asthma, 42 with nonsevere asthma, and 15 healthy control subjects (Table 2). Criteria for severe and nonsevere asthma were previously described (45, 46, 52). Each subject’s status was not known when ELISAs and image evaluations were performed.
Mean age was higher in subjects with severe asthma than nonsevere asthma and lowest in normal subjects (Table 2). FEV1 was different among the groups. FEV1/FVC was lower in asthma than in normal subjects. Subjects with severe asthma had lower FVC than subjects with nonsevere asthma, as described in larger studies (45, 46). Serum IgE concentration was higher in subjects with asthma than in normal subjects. Blood eosinophil concentration in subjects with nonsevere asthma was significantly higher than in normal subjects. The proportion of neutrophils in sputum was greater in subjects with severe compared with nonsevere asthma. Fraction of exhaled nitric oxide was higher in subjects with asthma than in normal subjects. Of the severe and nonsevere subjects with asthma, 100% and 64%, respectively, were being treated with inhaled corticosteroids, whereas 38% and 5% also received oral corticosteroids (CS). The daily inhaled corticosteroid dose in subjects with severe asthma was higher than in nonsevere subjects.
Concentrations of the plasma markers and imaging data in subjects with severe asthma, nonsevere asthma, all asthma, and no asthma are shown in Table 4. VWF:Ag was not significantly different among groups, in accord with a previously published study (55). VWF:Ag is known to vary considerably among normal subjects (56, 57). We found similar variation here. No instances were found of increases in VWF:Ag of more than threefold, whereas such increases have been observed in patients with acute bronchitis, lung injury, or pulmonary hypertension (32–34). VWFpp was not different among groups. The VWFpp/Ag ratio was significantly lower in all subjects with asthma and subjects with severe asthma compared with normal subjects, and in subjects with severe asthma compared with subjects with nonsevere asthma. P-selectin was not significant between these groups, but there were trends to higher values in subjects with asthma compared with normal subjects and in subjects with severe compared with nonsevere asthma. Although others have found increases in P-selectin after certain provocations in asthma (37–39), most have not demonstrated significant steady-state differences between subjects with asthma and normal subjects or between groups of subjects with asthma. PF4 was higher in severe asthma than in nonsevere asthma, as described previously (48). Percentage defect volume on HPHe-MRI was significantly higher in subjects with nonsevere asthma than in normal subjects. Percentage area with low density on MDCT less than −856 HU at FRC was higher in severe than in nonsevere asthma. Percentage area with low density less than −950 HU at TLC was higher in all asthma and in subjects with severe asthma compared with normal subjects. In a somewhat larger study reported in abstract form (58) (n = 101, of which 74 = nonsevere, 16 = severe, and 11 = control subjects, some subjects overlapping with the current manuscript) of regional imaging metrics, it was similarly found that area of low density on MDCT less than −856 HU at FRC was significantly greater in severe than in nonsevere asthma, and also that defect volume on HPHe-MRI was significantly greater in all asthma than in control subjects.
TABLE 4.
ENDOTHELIAL CELL AND PLATELET ACTIVATION MARKERS, AND IMAGING METRICS IN SEVERE ASTHMA RESEARCH PROGRAM SUBJECTS WITH SEVERE ASTHMA, NONSEVERE ASTHMA, OR NO ASTHMA
| Plasma Protein or Imaging Metric | Severe Asthma | Nonsevere Asthma | All Asthma | No Asthma |
|---|---|---|---|---|
| VWF:Ag, U/dl (mean ± SD) |
115 ± 51 |
96 ± 29 |
102 ± 38 |
87 ± 21 |
| VWFpp, U/dl (mean ± SD) |
107 ± 47 |
115 ± 36 |
112 ± 40 |
118 ± 37 |
| VWFpp/Ag (mean ± SD) |
0.97 ± 0.20*,† |
1.24 ± 0.34 |
1.15 ± 0.33‡ |
1.36 ± 0.37 |
| P-selectin, ng/ml (mean ± SD) |
29 ± 15 |
25 ± 12 |
26 ± 13 |
21 ± 11 |
| PF4, ng/ml (median [25th, 75th percentiles]) |
14 (5.2, 38)§ |
6.3 (3.6, 13)‡ |
7.1 (4.0, 17) |
12 (8.0, 21) |
| Percentage defect volume on HPHe-MRI (median [25th, 75th percentiles]) |
1.6 (0.0, 5.6) |
2.1 (0.6, 4.6)‡ |
1.8 (0.5, 4.6) |
0.8 (0.2, 1.5) |
| Percentage area of low density on MDCT less than −856 HU at FRC (median [25th, 75th percentiles]) |
13 (4.7, 31)§ |
6.2 (1.9, 11) |
7.0 (2.9, 14) |
6.9 (2.9, 8.2) |
| Percentage area of low density on MDCT less than −950 HU at TLC (median [25th, 75th percentiles]) | 1.8 (0.6, 3.8)‡ | 1.1 (0.4, 4.0) | 1.2 (0.5, 3.9)‡ | 0.8 (0.4, 1.2) |
Definition of abbreviations: HPHe-MRI = hyperpolarized helium-3 magnetic resonance imaging; HU = Hounsfield unit; MDCT = multidetector computed tomography; PF4 = platelet factor 4; VWF:Ag = von Willebrand factor mature protein; VWFpp = von Willebrand factor propeptide.
n (number of subjects) = 21, 42, and 15 in the three groups for the plasma markers; 10, 40, and 12 for MRI; 15, 28, and 9 for MDCT at FRC; and 21, 38, and 13 for MDCT at TLC.
P less than or equal to 0.001 versus nonsevere asthma.
P less than or equal to 0.01 versus no asthma.
P less than or equal to 0.05 versus no asthma.
P less than or equal to 0.05 versus nonsevere asthma.
Most subjects with asthma in our study were also included in a larger population from the multicenter SARP study that were classified into five asthma phenotype groups using unsupervised hierarchical cluster analysis (cluster 1 = mild allergic asthma; 2 = mild-moderate allergic asthma; 3 = more severe older-onset asthma; 4 = severe variable allergic asthma; 5 = severe fixed-airflow asthma) (9, 59). Figure E1 in the online supplement shows plasma marker concentrations in the different clusters. We pooled clusters 3–5 here because of low numbers of subjects in each of these clusters. VWFpp (see Figure E1A), VWFpp/Ag (see Figure E1B), VWF:Ag (not shown), or PF4 (not shown) were not significantly different among clusters. Plasma P-selectin was significantly different among clusters with the highest concentrations in clusters 3–5 (see Figure E1C), indicating that P-selectin is associated with more severe disease.
Because CS use may reduce vascularity in asthma (19, 23–25), the possibility existed that systemic CS might also impact imaging signals and plasma marker concentrations. To address this issue, we compared subjects who were receiving oral CS with those who were not (see Table E1). P-selectin, PF4, and area of low density less than −856 HU at FRC were higher in subjects on oral CS versus those not on oral CS, possibly because the subjects on oral CS have more severe underlying disease. No marker or imaging parameter was significantly lower in those on oral CS, suggesting that CS does not suppress the plasma markers or imaging signals.
Correlations
Because of the variations in plasma protein concentrations and imaging data among subjects (Table 4), we compared these measurements directly to explore potential correlations, as described below. Examples of HPHe-MRI images in Figures 1A and 1B reflect the observed trend toward increased ventilation defect volume (arrows in Figure 1A) in asthma and with increased VWFpp or VWFpp/Ag. Examples of images of MDCT at TLC in Figures 1C and 1D depict low-density pixels color-coded in yellow and reflect the overall trend of decreased lung density observed in asthma, particularly severe asthma, and with increased plasma P-selectin.
VWFpp and VWFpp/Ag Correlate with Defect Volume on HPHe-MRI
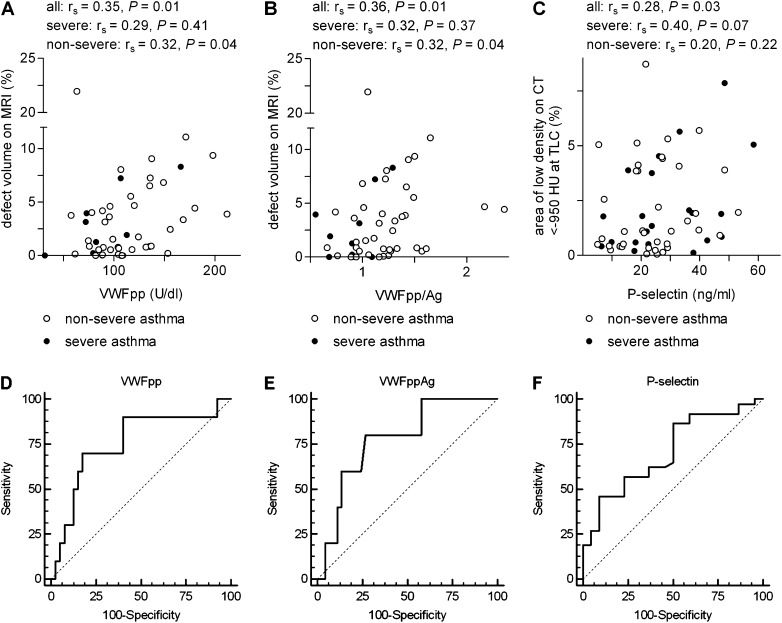
VWFpp and VWFpp/Ag correlated significantly with percentage defect volume on HPHe-MRI in asthma (Table 5; Figures 3A and 3B). The rs (Spearman rank correlation coefficient) values were similar in severe and nonsevere asthma and reached significance in nonsevere asthma (Figures 3A and 3B). When the subjects with asthma were divided in two groups by median percentage defect volume, subjects with greater defect volume had significantly higher VWFpp and a trend to higher VWFpp/Ag than those with lesser or no defect volume (Table 6; see Figures E2A and E2B). VWF:Ag did not correlate with percentage defect volume (Table 5) or differ significantly between the two groups defined by defect volume (Table 6). Similarly, P-selectin and PF4 neither correlated with defect volume nor differed between the groups (Tables 5 and 6). VWFpp/Ag correlated only with airway wall area in third-generation right upper lobe segment (rs = 0.30; P = 0.02), whereas VWFpp did not (rs = 0.05; P = 0.72); VWFpp/Ag or VWFpp did not correlate with airway wall area of six other segments. VWFpp did not correlate with FVC, whereas VWFpp/Ag correlated directly with FVC (see Table E2). VWFpp or VWFpp/Ag did not correlate with FEV1 or FEV1/FVC (see Table E2), even though defect volume correlated inversely with FEV1 and FEV1/FVC (see Table E2) in a manner similar to correlations described before between ventilation defects or defect volume and FEV1 or FEV1/FVC (2, 7). VWFpp did not correlate significantly with TLC, residual volume/TLC, FRC/TLC, or FVC/slow vital capacity (not shown). VWFpp/Ag correlated with TLC (% pred., rs = 0.32; P = 0.02), but not with the other lung volumes (not shown). To summarize, VWFpp or VWFpp/Ag correlated with defect volume on HPHe-MRI, indicating that VWFpp is associated with ventilation defects.
TABLE 5.
CORRELATIONS BETWEEN ENDOTHELIAL CELL AND PLATELET ACTIVATION MARKERS AND IMAGING METRICS IN SEVERE ASTHMA RESEARCH PROGRAM SUBJECTS WITH ASTHMA
| Plasma Protein | Plasma Protein versus Defect Volume on HPHe-MRI |
Plasma Protein versus Area of Low Density on MDCT Less than −856 HU at FRC |
Plasma Protein Versus Area of Low Density on MDCT Less than −950 HU at TLC |
|||
|---|---|---|---|---|---|---|
| rs | P | rs | P | rs | P | |
| VWF:Ag |
0.10 |
0.49 |
0.02 |
0.91 |
0.05 |
0.73 |
| VWFpp |
0.35 |
0.01 |
−0.10 |
0.53 |
0.03 |
0.84 |
| VWFpp/Ag |
0.36 |
0.01 |
−0.16 |
0.29 |
−0.10 |
0.46 |
| P-selectin |
−0.04 |
0.80 |
0.10 |
0.52 |
0.28 |
0.03 |
| PF4 | −0.08 | 0.58 | 0.23 | 0.14 | 0.04 | 0.75 |
Definition of abbreviations: HPHe-MRI = hyperpolarized helium-3 magnetic resonance imaging; HU = Hounsfield unit; MDCT = multidetector computed tomography; PF4 = platelet factor 4; rs = Spearman rank correlation coefficient; VWF:Ag = von Willebrand factor mature protein; VWFpp = von Willebrand factor propeptide.
n = 50 for MRI, 43 for MDCT at FRC, and 59 for MDCT at TLC.
Figure 3.
Correlations between imaging metrics and von Willebrand factor (VWF) or P-selectin in Severe Asthma Research Program subjects with asthma and receiver operating characteristic (ROC) curves for the ability of VWF and P-selectin to predict defect volume on hyperpolarized helium-3 magnetic resonance imaging (HPHe-MRI) and low-attenuation area on multidetector computed tomography (MDCT), respectively. (A and B) Percentage defect volume on HPHe-MRI versus plasma concentration of VWF propeptide (VWFpp) (A) or VWFpp/VWF mature protein (VWF:Ag) (B). (C) Percentage area of signal density on MDCT less than −950 HU at TLC versus plasma concentration of soluble P-selectin. (D) ROC curve for the ability of VWFpp to predict percentage defect volume on HPHe-MRI greater than 6%; area under curve (AUC) = 0.76, P = 0.007; for criterion greater than 131.6 U/dl, sensitivity = 70% and specificity = 82%. (E) ROC curve for the ability of VWFpp/Ag to predict percentage defect volume on HPHe-MRI greater than 8.25%, AUC = 0.78, P = 0.008; for criterion greater than 1.27, sensitivity = 80% and specificity = 73%. (F) ROC curve for the ability of P-selectin to predict percentage area of signal density of MDCT less than −950 HU greater than 0.8%; AUC = 0.71, P = 0.002; for criterion greater than 29 ng/ml, sensitivity = 46% and specificity = 91%. P = probability, rs = Spearman rank correlation coefficient. U = unit in which pooled normal plasma has 100 U/dl. Ratio was calculated from U/dl VWFpp divided by U/dl VWF:Ag.
TABLE 6.
ENDOTHELIAL CELL AND PLATELET ACTIVATION MARKERS IN SEVERE ASTHMA RESEARCH PROGRAM SUBJECTS WITH ASTHMA WITH GREATER OR LESSER DEFECT VOLUME ON HPHE-MRI
| Plasma Protein | Defect Volume on HPHe-MRI Greater than Median of 1.8% | Defect Volume on HPHe-MRI Less than Median of 1.8% | P |
|---|---|---|---|
| VWF:Ag, U/dl (mean ± SD) |
102 ± 32 |
95 ± 31 |
0.40 |
| VWFpp, U/dl (mean ± SD) |
124 ± 43 |
99 ± 26 |
0.05 |
| VWFpp/Ag (mean ± SD) |
1.26 ± 0.40 |
1.09 ± 0.25 |
0.07 |
| P-selectin, ng/ml (mean ± SD) |
24 ± 13 |
25 ± 13 |
0.96 |
| PF4, ng/ml (median [25th, 75th percentiles]) | 6.9 (3.3, 24) | 5.2 (4.0, 15) | 0.99 |
Definition of abbreviations: HPHe-MRI = hyperpolarized helium-3 magnetic resonance imaging; PF4 = platelet factor 4; U = unit; VWF:Ag = von Willebrand factor mature protein; VWFpp = von Willebrand factor propeptide.
n = 25 in each group.
To investigate the use of VWFpp or VWFpp/Ag as continuous variables in predicting defect volume on HPHe-MRI, we performed ROC curve analysis. As shown in Figures 3D and 3E, VWFpp and VWFpp/Ag significantly (P = 0.007 and 0.008) predicted defect volume on HPHe-MRI of greater than 6% and greater than 8.25%, respectively, with areas under curve (AUC) of 0.76 and 0.78. AUC values greater than 0.70 were obtained for VWFpp or VWFpp/Ag using different cutoffs for percentage defect volume ranging from 4–8% (see Table E3).
P-Selectin Correlates with Area of Low Density on MDCT at TLC
Plasma P-selectin correlated significantly with area of low density on MDCT less than −950 HU at TLC in all subjects with asthma (Table 5, Figure 3C). The rs value was greater in severe than in nonsevere asthma (Figure 3C). P-selectin did not correlate with area of low density less than −856 HU at FRC (Table 5). Subjects with asthma with area of low density less than −950 HU at TLC above the median had higher P-selectin than subjects with percentage area below the median (Table 7; see Figure E2C). VWF forms and PF4 did not correlate with area of low density on MDCT at TLC (Table 5) and were not higher in subjects with greater area of low density (Table 7). P-selectin did not correlate with airway wall area measurements (not shown). P-selectin did not correlate with measurements of lung volumes (not shown) or lung function (see Table E2); even though area of low density on MDCT less than −950 HU at TLC correlated with FEV1/FVC (see Table E2) as found previously (12, 14). The MDCT less than −850 HU at FRC was also associated with FEV1/FVC (see Table E2) as found before (3), and with other obstructive measures (see Table E2), but did not correlate with any vascular endothelial marker (Table 5). ROC analysis demonstrated that P-selectin significantly (P = 0.002) predicted low-attenuation area on MDCT at TLC of greater than 0.8% with an AUC of 0.71 (Figure 3F). AUC values greater than or equal to 0.65 were obtained for cutoffs of 0.6–1.5% (see Table E4).
TABLE 7.
ENDOTHELIAL CELL AND PLATELET ACTIVATION MARKERS IN SEVERE ASTHMA RESEARCH PROGRAM SUBJECTS WITH ASTHMA WITH GREATER OR LESSER AREA OF LOW DENSITY ON MDCT LESS THAN −950 HU AT TLC
| Plasma Protein | Area of Low Density on MDCT Less than −950 HU at TLC Greater than Median of 1.16% | Area of Low Density on MDCT Less than −950 HU at TLC Less than median of 1.16% | P |
|---|---|---|---|
| VWF:Ag, U/dl (mean ± SD) |
100 ± 28 |
102 ± 48 |
0.49 |
| VWFpp, U/dl (mean ± SD) |
106 ± 27 |
117 ± 49 |
0.48 |
| VWFpp/Ag (mean ± SD) |
1.10 ± 0.32 |
1.21 ± 0.33 |
0.12 |
| P-selectin, ng/ml (mean ± SD) |
29 ± 14 |
22 ± 12 |
0.05 |
| PF4, ng/ml (median [25th, 75th percentiles]) | 6.9 (4.3, 22) | 11 (3.6, 17) | 0.99 |
Definition of abbreviations: HU = Hounsfield unit; MDCT = multidetector computed tomography; PF4 = platelet factor 4; U = unit; VWF:Ag = von Willebrand factor mature protein; VWFpp = von Willebrand factor propeptide.
n = 29 in Group 1 (area > median) and 30 in Group 2 (area < median).
VWF and P-Selectin after Whole-Lung Antigen Challenge
Plasma preparation and imaging were performed on different visits (Table 1). To examine within-individual variability and stability of markers during a maneuver that might be expected to perturb endothelial cells and cause regulated release of contents of Weibel-Palade granules, we studied 12 subjects with mild allergic asthma undergoing whole-lung inhaled antigen challenge, a model of asthma exacerbation (47).
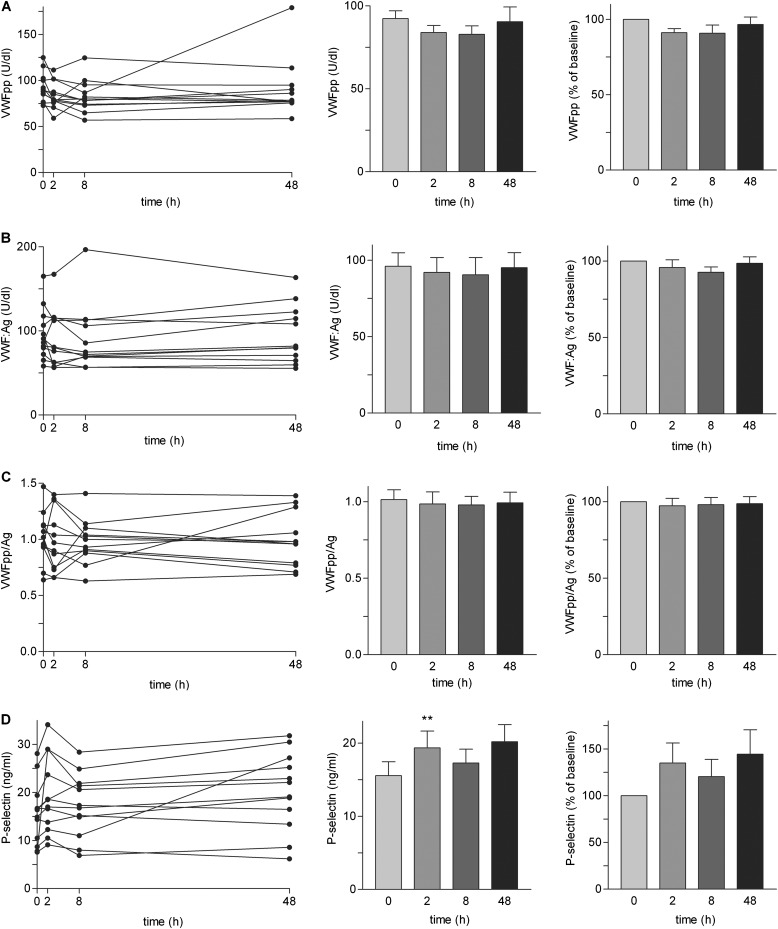
Mean coefficients of variation (SD/mean) for VWFpp, VWF:Ag, VWFpp/Ag, and P-selectin among the different time-points in our subjects were 11%, 9%, 11%, and 18%, respectively; mean coefficients of variation among the different subjects at the same time-points were 23%, 37%, 23%, and 40%. Thus, differences in VWF and P-selectin among subjects were greater than differences among time-points. There was no increase in VWFpp (Figure 4A), VWF:Ag (Figure 4B), or VWFpp/Ag (Figure 4C) after challenge. There was a small but significant increase (mean 1.2-fold in absolute and 1.3-fold in relative values) in P-selectin at 2 hours compared with before challenge (Figure 4D). The small increase in P-selectin is likely caused by platelet activation, inasmuch as such activation has previously been reported after whole-lung antigen challenge as assessed by increases in plasma β-thromboglobulin and PF4 and approximately 1.5-fold increase in P-selectin at 0.5 or 6 hours (37). Thus, although whole-lung challenge leads to platelet activation, it does not cause Weibel-Palade granule secretion.
Figure 4.
Concentrations of von Willebrand factor propeptide (VWFpp), VWF mature protein (VWF:Ag), and P-selectin after whole-lung inhaled antigen challenge in subjects with mild allergic asthma. (A) VWFpp, (B) VWF:Ag, (C) VWFpp/Ag, and (D) P-selectin. Panels show individual values (left), means of absolute values (center), and means of values expressed as percentage of baseline (right). U = unit. **P less than or equal to 0.01 for increase versus baseline at 0 hours. n = 12.
Discussion
In this study, we measured regional lung function using HPHe-MRI and structure using MDCT in patients with asthma. Specifically, we investigated the relationship between regional defect volume on HPHe-MRI and low-attenuation area on MDCT with circulating blood biomarkers reflecting alterations of vascular endothelial cells that are typically activated during inflammation and repair. We found that circulating VWF propeptide (VWFpp) and the propeptide/mature protein ratio (VWFpp/Ag), indicators of vascular endothelial cell perturbation, correlated with and predicted lung ventilation defects detected by HPHe-MRI. Surprisingly, P-selectin, an indicator of vascular endothelial cell and platelet activation, correlated with and predicted a distinctly different abnormality manifested as a diffuse pattern of reduced parenchymal density on MDCT acquired at TLC. Therefore, VWFpp, VWFpp/Ag, and P-selectin warrant further investigation as possible surrogate biomarkers for specific airway structural alterations in asthma.
Because plasma concentrations of VWFpp and P-selectin are both associated with endothelial cell activation through release of Weibel-Palade granules, it is surprising that these two blood-based measures correlate with different imaging metrics. This raises challenging questions about how the features of the asthmatic lung reported by the imaging techniques relate to determinants of circulating VWFpp and P-selectin. The lack of correlation between plasma PF4 and MDCT data indicates that endothelial cells are largely responsible for the increase in plasma P-selectin, although there may also be a small contribution from P-selectin derived from platelets. The fact that whole-lung antigen challenge did not result in increases of VWFpp or VWF:Ag or increases in P-selectin other than those that can be attributed to platelet activation indicates that the observed correlations result from stable and not acute change in lung structure and function.
In asthma, MDCT at end expiration (FRC) detects changes in the more distal small airways that collapse at lower lung volume and prevent air from leaving (1, 6, 9). Lung volume affected by air trapping is generally estimated by calculating the percentage less than −856 HU at FRC. A regional mosaic of lower local density occurs at FRC because of increased volume of trapped air, which has a calibrated density of approximately −1,000 HU. Regional air trapping distal to obstructed small airways at the level of secondary lobules is often made more visually apparent when adjacent to normal lung regions in which the mean density of residual air, blood vessels, and supporting tissue is approximately −800 HU. At TLC, the HU numbers are expected to be lower because the lung is fully inflated. Regionally, lung density is less affected by air trapping at TLC because all normal airways and secondary lobules are typically recruited. In emphysema, density decreases in the lung parenchyma at TLC are used to estimate change caused by hyperinflation and loss of normal lung architecture, using an empirical threshold of the percentage of lung tissue less than −950 HU (10, 11). In nonsmokers with asthma, a mosaic of similarly attenuated areas on MDCT at TLC has also been observed and has been proposed to be associated with hyperinflation, airflow limitation, and altered terminal airspace geometry (6, 12–17). In addition, hyperinflation has been implicated as a major determinant of altered physiology in subjects with asthma (60).
HPHe-MRI can be thought of as being similar to nuclear scintigraphy lung ventilation scans but with higher contrast and improved spatial resolution caused by the use of HPHe as a contrast agent (5) and ability to obtain three-dimensional image resolution. In normal ventilated lung, the HPHe contrast agent should distribute throughout the available alveolar and bronchial areas. In obstructed lung, regions fed by partially or completely obstructed airways do not fill, leading to a pattern with low or no signal regions adjacent to locally high-signal regions fed by unobstructed airways. The impact of structural defects on ventilation caused by partial or near complete obstruction of the airways is not as well seen on MDCT. Thus, whereas MDCT lung density evaluates regional air trapping at FRC and possibly hyperinflation at TLC (6, 12–17), HPHe-MRI measures the change in signal intensity related to gas ventilation in the air-trapped regions of the distal small airways and the larger central airways (2, 4–7). A large defect percentage in the lung volume measured on HPHe-MRI may be an indicator of more central airway involvement and more pronounced functional narrowing that falls beyond the limit of structural abnormalities detectable with MDCT. Although a single mucous plug could be present at one time-point and resolve shortly thereafter, 40–60% of ventilation defects detected by HPHe-MRI persist in the same locations on repeated study (61). In the cross-sectional imaging studies described here, it is not possible to separate reversible from more persistent defects.
Why do VWFpp markers correlate exclusively with HPHe-MRI measurements while P-selectin correlates exclusively with MDCT measurements at TLC? Despite their common origins in endothelial cells, control and pathways of synthesis and secretion of VWF:Ag, VWFpp, and P-selectin are different. VWF:Ag and VWFpp are constitutively secreted in tandem and packaged together in tubular structures within the Weibel-Palade granule, whereas P-selectin is a type I membrane protein with its extracellular domain jutting into the granule (Figure 2A) (30, 31). Regulated release of VWFpp, VWF:Ag, and P-selectin from Weibel-Palade granules into plasma may be controlled differentially (62). VWFpp is secreted efficiently from endothelial cells, whereas VWF:Ag may bind to subendothelial extracellular matrix of endothelial cells and enter plasma (30), and P-selectin must be proteolytically released after externalization (Figure 2A) (35, 36). VWF expression is variable among vascular beds in ways that are incompletely understood (63). In addition, Weibel-Palade granules are enriched in arterioles and venules of the pulmonary circulation but lacking in capillary cells even though the capillary cells express VWF and P-selectin (64). Animal model studies indicate that repeated lung antigen challenges increase vascularity and VWF expression (27, 65) and that this occurs in a vascular endothelial growth factor–dependent manner (65), whereas distention or stretching of endothelial cells (66) or inflammation (67) increases P-selectin expression.
In Figures 2B and 2C, we propose an explanation for the differential associations between VWFpp or P-selectin with imaging metrics. We depict increased plasma VWFpp as originating from endothelial cells of the more numerous and hypertrophied vessels in inflamed bronchi (Figure 2B) and increased plasma P-selectin as originating in endothelial cells of blood vessels of abnormal terminal airway structure detected by MDCT at TLC (Figure 2C). The changes shown in Figure 2B seem to occur in nonsevere and severe asthma inasmuch as VWFpp concentration and the HPHe-MRI abnormalities were not higher in severe than in nonsevere asthma and the rs values for the correlation between VWFpp and the HPHe-MRI signal were similar for both groups. The increased vascularity is proposed to contribute to the obstruction and ventilation defects affecting air flow in the larger and more central airways that are detected by HPHe-MRI. There is precedence for chronically increased VWFpp compared with VWF:Ag because of decreased survival and increased clearance of VWF:Ag in type 1C von Willebrand disease (53, 68).
Figure 2C is proposed to explain the association of plasma P-selectin concentration with MDCT findings. In this model, the pulmonary vasculature within the abnormal terminal airway structure detected by MDCT at TLC is populated by endothelial cells that are more susceptible to secretion of P-selectin. The changes depicted in Figure 2C seem to occur in more severe disease inasmuch as P-selectin was higher in more severe asthma clusters, the MDCT signal at TLC was highest in severe asthma, and P-selectin and the abnormal MDCT signal at TLC correlated best in severe asthma. However, we are not aware of pathologic studies of lung tissue biopsies linking abnormal terminal airway structure with the diffuse pattern of attenuation area at TLC observed in this study. Clearly, further work is necessary to test and refine the proposed model.
Limitations of this study include the fact that the current study was cross-sectional in time. The whole-lung antigen challenge data support the stability of endothelial cells in subjects with mild asthma over 48 hours after a major provocation. It is reasonable to surmise that the plasma biomarkers report chronic pulmonary changes that may evolve over years. It is likely that longitudinal changes of VWFpp from baseline may correlate better with other manifestations of asthma on an individual basis. Indeed, it is remarkable that we could discern correlations between VWFpp and HPHe-MRI signals given the wide range of normal concentrations of VWF and without control of medications. Concentration of normal plasma VWF:Ag is sensitive to polymorphisms in VWF itself, proteins determining ABO blood type, and secretion and turnover of plasma VWF (69). Studies are ongoing to follow VWFpp and P-selectin longitudinally and relate changes to medications. Other limitations are that 83% of the subjects with asthma studied were ethnically white and non-Hispanic, and that the severe asthma, nonsevere asthma, and normal groups differed in age. However, we did not find any significant interactions between age and the associations of plasma markers and imaging metrics (i.e., the associations between plasma markers and imaging metrics were similar in age-adjusted and non–age-adjusted models [not shown]). Future work should test the observed relationships in broader populations with different genetic backgrounds.
Potential impact and importance of our findings include the prospects that serial measurements of VWFpp or VWFpp/Ag may complement imaging in longitudinal assessment of asthma and strategies to treat asthma. HPHe-MRI is limited by cost and availability of He and MDCT is limited by cost and the need to minimize lifelong radiation exposure. VWFpp or VWFpp/Ag may provide a gauge of evolving airway injury, possibly leading to a more progressed disease state, whereas P-selectin may be sensitive to chronically altered structure of the peripheral airway. These possibilities need to be investigated in future studies. Also importantly, we have identified a biologic correlate, increased plasma P-selectin, of the low-attenuation areas detected by MDCT at TLC, which is a poorly understood aspect of asthma that needs more attention. Finally, our findings should focus more attention on mediators and consequences of vascular perturbation and endothelial activation in asthma and regional differences in vessels of the asthmatic lung as proposed in our model.
Acknowledgments
Acknowledgment
The authors thank Daniel Kolk, Erin Billmeyer, and Holly Eversoll for patient recruitment, screening, and assessments; Gina Crisafi, Katie Gaworski, and Helen Holden for preparing plasma samples; Kristin Gunderson for help with P-selectin and platelet factor 4 ELISAs; Crystal Perry for performing von Willebrand factor ELISAs and analyses; GE Healthcare for loan of the 3He polarizer; Kelli Hellenbrand and Sara John for magnetic resonance technical support; Jionghan Dai and Eric Peterson for contributions in imaging data acquisition; Amruta Dattawadkar for providing images; Gina Crisafi, Helen Holden, and Holly Eversoll for providing assessment data and data on inhaled corticosteroid use; Maura Robinson and Ruthie Knowles at the SARP Data Coordinating Center for help with the central database; Janice Yakey for safety and protocol coordination; Gina Crisafi and Cheri Swenson for administrative help; and Becky Kelly for discussions.
Footnotes
Supported by grant R01 HL69116 (W.W.B.), grant 1 U10 HL109168 (N.N.J.), Program Project grant P01 HL088594 (N.N.J., D.F.M.), General Clinical Research Center grant M01 RR03186, and Clinical and Translational Science Award grant UL1 RR25011 from the National Institutes of Health; and a grant from The Hartwell Foundation (S.B.F.).
Author Contributions: M.W.J. oversaw P-selectin and platelet factor 4 ELISAs, performed P-selectin and platelet factor 4 analyses, performed statistical analysis, and wrote the manuscript. S.J.K. performed imaging analysis. M.L.S. contributed to the writing of the manuscript. M.D.E. performed statistical analysis and provided advice on statistics. R.L.S. provided pulmonary function predicted values, assessment data, and data on inhaled corticosteroid use; performed statistical analysis; and helped revise the manuscript before submission. L.C.D. performed statistical analysis, supervised whole-lung antigen challenge, and helped revise the manuscript before submission. W.W.B. and N.N.J. conceived, designed, and supervised the Severe Asthma Research Program in Wisconsin and helped revise the manuscript before submission. R.R.M. and his laboratory developed and performed von Willebrand factor ELISAs and analyses, and R.R.M. helped revise the manuscript before submission. D.F.M. provided ongoing oversight of ELISA data acquisition and analyses and participated in the preparation and writing of the manuscript. S.B.F. conceived, designed, and supervised imaging analysis, and participated in the writing of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201301-0185OC on June 19, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, Zheng J, Schechtman KB, Ramkumar TP, Cochran R, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008;134:1183–1191. doi: 10.1378/chest.07-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fain SB, Gonzalez-Fernandez G, Peterson ET, Evans MD, Sorkness RL, Jarjour NN, Busse WW, Kuhlman JE. Evaluation of structure-function relationships in asthma using multidetector CT and hyperpolarized He-3 MRI. Acad Radiol. 2008;15:753–762. doi: 10.1016/j.acra.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busacker A, Newell JD, Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, Fain S, Wenzel S. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135:48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes JH, O’Halloran RL, Brodsky EK, Bley TA, Francois CJ, Velikina JV, Sorkness RL, Busse WW, Fain SB. Three-dimensional imaging of ventilation dynamics in asthmatics using multiecho projection acquisition with constrained reconstruction. Magn Reson Med. 2009;62:1543–1556. doi: 10.1002/mrm.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fain S, Schiebler ML, McCormack DG, Parraga G. Imaging of lung function using hyperpolarized helium-3 magnetic resonance imaging: review of current and emerging translational methods and applications. J Magn Reson Imaging. 2010;32:1398–1408. doi: 10.1002/jmri.22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro M, Fain SB, Hoffman EA, Gierada DS, Erzurum SC, Wenzel S National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Lung imaging in asthmatic patients: the picture is clearer. J Allergy Clin Immunol. 2011;128:467–478. doi: 10.1016/j.jaci.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lange EE, Altes TA, Patrie JT, Gaare JD, Knake JJ, Mugler JP, III, Platts-Mills TA. Evaluation of asthma with hyperpolarized helium-3 MRI: correlation with clinical severity and spirometry. Chest. 2006;130:1055–1062. doi: 10.1378/chest.130.4.1055. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman EA, Simon BA, McLennan G. State of the art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, et al. NHLBI Severe Asthma Research Program (SARP) Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gevenois PA, De Vuyst P, de Maertelaer V, Zanen J, Jacobovitz D, Cosio MG, Yernault JC. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 11.Bankier AA, Madani A, Gevenois PA. CT quantification of pulmonary emphysema: assessment of lung structure and function. Crit Rev Computed Tomogr. 2002;43:399–417. [PubMed] [Google Scholar]
- 12.Mitsunobu F, Mifune T, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, Harada S, Takata S, Tanizaki Y. Influence of age and disease severity on high resolution CT lung densitometry in asthma. Thorax. 2001;56:851–856. doi: 10.1136/thorax.56.11.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsunobu F, Mifune T, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, Harada S, Tanizaki Y. Low-attenuation areas of the lungs on high-resolution computed tomography in asthma. J Asthma. 2001;38:413–422. doi: 10.1081/jas-100001496. [DOI] [PubMed] [Google Scholar]
- 14.Mitsunobu F, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, Nishida K, Takata S, Yokoi T, Mishima M, Tanizaki Y. Complexity of terminal airspace geometry assessed by computed tomography in asthma. Am J Respir Crit Care Med. 2003;167:411–417. doi: 10.1164/rccm.2112070. [DOI] [PubMed] [Google Scholar]
- 15.Mitsunobu F, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, Nishida N, Nagata T, Takata S, Tanizaki Y. Decreased computed tomographic lung density during exacerbation of asthma. Eur Respir J. 2003;22:106–112. doi: 10.1183/09031936.03.00081702. [DOI] [PubMed] [Google Scholar]
- 16.Mitsunobu F, Tanizaki Y. The use of computed tomography to assess asthma severity. Curr Opin Allergy Clin Immunol. 2005;5:85–90. doi: 10.1097/00130832-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Ueda T, Niimi A, Matsumoto H, Takemura M, Hirai T, Yamaguchi M, Matsuoka H, Jinnai M, Muro S, Chin K, et al. Role of small airways in asthma: investigation using high-resolution computed tomography. J Allergy Clin Immunol. 2006;118:1019–1025. doi: 10.1016/j.jaci.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 19.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Yamada G, Saikai T, Hashimoto M, Tanaka S, Suzuki K, Fujii M, Takahashi H, Abe S. Increased airway vascularity in newly diagnosed asthma using a high-magnification bronchovideoscope. Am J Respir Crit Care Med. 2003;168:1495–1499. doi: 10.1164/rccm.200306-727OC. [DOI] [PubMed] [Google Scholar]
- 21.Bailey SR, Boustany S, Burgess JK, Hirst SJ, Sharma HS, Simcock DE, Suravaram PR, Weckmann M. Airway vascular reactivity and vascularisation in human chronic airway disease. Pulm Pharmacol Ther. 2009;22:417–425. doi: 10.1016/j.pupt.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Bischof RJ, Bourke JE, Hirst SJ, Meeusen EN, Snibson KJ, Van Der Velden J. Measurement and impact of remodeling in the lung: airway neovascularization in asthma. Proc Am Thorac Soc. 2009;6:673–677. doi: 10.1513/pats.200907-064DP. [DOI] [PubMed] [Google Scholar]
- 23.Paredi P, Barnes PJ. The airway vasculature: recent advances and clinical implications. Thorax. 2009;64:444–450. doi: 10.1136/thx.2008.100032. [DOI] [PubMed] [Google Scholar]
- 24.Ribatti D, Puxeddu I, Crivellato E, Nico B, Vacca A, Levi-Schaffer F. Angiogenesis in asthma. Clin Exp Allergy. 2009;39:1815–1821. doi: 10.1111/j.1365-2222.2009.03385.x. [DOI] [PubMed] [Google Scholar]
- 25.Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, Genovese A. Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy. 2010;65:946–958. doi: 10.1111/j.1398-9995.2010.02372.x. [DOI] [PubMed] [Google Scholar]
- 26.Park HS, Kim SY, Kim SR, Lee YC. Targeting abnormal airway vascularity as a therapeutical strategy in asthma. Respirology. 2010;15:459–471. doi: 10.1111/j.1440-1843.2010.01724.x. [DOI] [PubMed] [Google Scholar]
- 27.Asosingh K, Cheng G, Xu W, Savasky BM, Aronica MA, Li X, Erzurum SC. Nascent endothelium initiates Th2 polarization of asthma. J Immunol. 2013;190:3458–3465. doi: 10.4049/jimmunol.1202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bochner BS, Schleimer RP.The role of adhesion molecules in human eosinophil and basophil recruitment J Allergy Clin Immunol 199494427–438.quiz 439 [DOI] [PubMed] [Google Scholar]
- 29.Granger DN, Kubes P. The microcirculation and inflammation: modulation of leukocyte-endothelial cell adhesion. J Leukoc Biol. 1994;55:662–675. [PubMed] [Google Scholar]
- 30.van Mourik JA, Romani de Wit T. Von Willebrand factor propeptide in vascular disorders. Thromb Haemost. 2001;86:164–171. [PubMed] [Google Scholar]
- 31.Haberichter SL, Shi Q, Montgomery RR. Regulated release of VWF and FVIII and the biologic implications. Pediatr Blood Cancer. 2006;46:547–553. doi: 10.1002/pbc.20658. [DOI] [PubMed] [Google Scholar]
- 32.Boldy DA, Short PE, Cowen P, Hill FG, Chambers DC, Ayres JG. Plasma levels of von Willebrand factor antigen in acute bronchitis and in a normal population. Respir Med. 1998;92:395–400. doi: 10.1016/s0954-6111(98)90281-5. [DOI] [PubMed] [Google Scholar]
- 33.Rubin DB, Wiener-Kronish JP, Murray JF, Green DR, Turner J, Luce JM, Montgomery AB, Marks JD, Matthay MA. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990;86:474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes AA, Maeda NY. Circulating von Willebrand factor antigen as a predictor of short-term prognosis in pulmonary hypertension. Chest. 1998;114:1276–1282. doi: 10.1378/chest.114.5.1276. [DOI] [PubMed] [Google Scholar]
- 35.André P. P-selectin in haemostasis. Br J Haematol. 2004;126:298–306. doi: 10.1111/j.1365-2141.2004.05032.x. [DOI] [PubMed] [Google Scholar]
- 36.Kappelmayer J, Nagy B, Jr, Miszti-Blasius K, Hevessy Z, Setiadi H. The emerging value of P-selectin as a disease marker. Clin Chem Lab Med. 2004;42:475–486. doi: 10.1515/CCLM.2004.082. [DOI] [PubMed] [Google Scholar]
- 37.Kowal K, Pampuch A, Kowal-Bielecka O, DuBuske LM, Bodzenta-Łukaszyk A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin Exp Allergy. 2006;36:426–432. doi: 10.1111/j.1365-2222.2006.02446.x. [DOI] [PubMed] [Google Scholar]
- 38.Zietkowski Z, Skiepko R, Tomasiak MM, Bodzenta-Lukaszyk A. Soluble CD40 ligand and soluble P-selectin in allergic asthma patients during exercise-induced bronchoconstriction. J Investig Allergol Clin Immunol. 2008;18:272–278. [PubMed] [Google Scholar]
- 39.Yu H, Ren J, Wang F, Liu L, Wu S, Liu Y. [P-selectin and tachykinins in bronchial hyperresponsiveness of asthma] Zhonghua Nei Ke Za Zhi. 1999;38:228–230. [PubMed] [Google Scholar]
- 40.Gearing AJ, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993;14:506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- 41.McEver RP, Beckstead JH, Moore KL, Marshall-Carlson L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hippenstiel S, Krüll M, Ikemann A, Risau W, Clauss M, Suttorp N. VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol. 1998;274:L678–L684. doi: 10.1152/ajplung.1998.274.5.L678. [DOI] [PubMed] [Google Scholar]
- 43.Kneuer C, Ehrhardt C, Radomski MW, Bakowsky U. Selectins—potential pharmacological targets? Drug Discov Today. 2006;11:1034–1040. doi: 10.1016/j.drudis.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Kanaji S, Fahs SA, Shi Q, Haberichter SL, Montgomery RR. Contribution of platelet vs. endothelial VWF to platelet adhesion and hemostasis. J Thromb Haemost. 2012;10:1646–1652. doi: 10.1111/j.1538-7836.2012.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Erzurum SC, Gaston BM, Israel E, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol. 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 47.Gauvreau GM, Evans MY. Allergen inhalation challenge: a human model of asthma exacerbation. Contrib Microbiol. 2007;14:21–32. doi: 10.1159/000107052. [DOI] [PubMed] [Google Scholar]
- 48.Johansson MW, Han ST, Gunderson KA, Busse WW, Jarjour NN, Mosher DF. Platelet activation, P-selectin, and eosinophil β1-integrin activation in asthma. Am J Respir Crit Care Med. 2012;185:498–507. doi: 10.1164/rccm.201109-1712OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Page C, Pitchford S. Platelets coming of age: implications for our understanding of allergic inflammation. Am J Respir Crit Care Med. 2013;187:459–460. doi: 10.1164/rccm.201301-0085ED. [DOI] [PubMed] [Google Scholar]
- 50.Johansson MW, Kruger SJ, Montgomery RR, Evans MD, Mosher DF, Busse WW, Fain SB, Jarjour NN. Markers of vascular perturbation correlate with airway structural change in asthma [abstract] Am J Respir Crit Care Med. 2011;183:A4371. doi: 10.1164/rccm.201301-0185OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansson MW, Han S-T, Gunderson KA, Montgomery RR, Busse WW, Jarjour NN, Mosher DF. Platelet activation, P-selectin mobilization, and eosinophil beta1 integrin activation occur in asthma and are associated with clinical phenotypes [abstract] Am J Respir Crit Care Med. 2011;183:A4335. [Google Scholar]
- 52.American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 53.Haberichter SL, Balistreri M, Christopherson P, Morateck P, Gavazova S, Bellissimo DB, Manco-Johnson MJ, Gill JC, Montgomery RR. Assay of the von Willebrand factor (VWF) propeptide to identify patients with type 1 von Willebrand disease with decreased VWF survival. Blood. 2006;108:3344–3351. doi: 10.1182/blood-2006-04-015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siegel S.Nonparametric statistics for the behavioral sciences. New York, NY: McGraw-Hill; 1956 [Google Scholar]
- 55.Nusinow SR, Federici AB, Zimmerman TS, Curd JG. Increased von Willebrand factor antigen in the plasma of patients with vasculitis. Arthritis Rheum. 1984;27:1405–1410. doi: 10.1002/art.1780271211. [DOI] [PubMed] [Google Scholar]
- 56.Sadler JE. Von Willebrand disease type 1: a diagnosis in search of a disease. Blood. 2003;101:2089–2093. doi: 10.1182/blood-2002-09-2892. [DOI] [PubMed] [Google Scholar]
- 57.Sadler JE. Low von Willebrand factor: sometimes a risk factor and sometimes a disease. Hematology (Am Soc Hematol Educ Program) 2009:106–112. doi: 10.1182/asheducation-2009.1.106. [DOI] [PubMed] [Google Scholar]
- 58.Kruger SJ, Niles D, Evans MD, Jarjour NN, Busse WW, Dattawadkar A, Sorkness RL, Fain SB. MRI and CT imaging of lung structure and function show regional differences in mild/moderate and severe asthma [abstract] Am J Respir Crit Care Med. 2011;183:A5170. [Google Scholar]
- 59.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown RH, Pearse DB, Pyrgos G, Liu MC, Togias A, Permutt S. The structural basis of airways hyperresponsiveness in asthma. J Appl Physiol. 2006;101:30–39. doi: 10.1152/japplphysiol.01190.2005. [DOI] [PubMed] [Google Scholar]
- 61.de Lange EE, Altes TA, Patrie JT, Battiston JJ, Juersivich AP, Mugler JP, III, Platts-Mills TA. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology. 2009;250:567–575. doi: 10.1148/radiol.2502080188. [DOI] [PubMed] [Google Scholar]
- 62.Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2006;107:2736–2744. doi: 10.1182/blood-2004-07-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu J, Yuan L, Molema G, Regan E, Janes L, Beeler D, Spokes KC, Okada Y, Minami T, Oettgen P, et al. Vascular bed-specific regulation of the von Willebrand factor promoter in the heart and skeletal muscle. Blood. 2011;117:342–351. doi: 10.1182/blood-2010-06-287987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ochoa CD, Wu S, Stevens T. New developments in lung endothelial heterogeneity: von Willebrand factor, P-selectin, and the Weibel-Palade body. Semin Thromb Hemost. 2010;36:301–308. doi: 10.1055/s-0030-1253452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Y, Wang J, Li H, Han X. Found in inflammatory zone 1 induces angiogenesis in murine models of asthma. Lung. 2008;186:375–380. doi: 10.1007/s00408-008-9099-1. [DOI] [PubMed] [Google Scholar]
- 66.Moldobaeva A, Jenkins J, Wagner E. Effects of distension on airway inflammation and venular P-selectin expression. Am J Physiol Lung Cell Mol Physiol. 2008;295:L941–L948. doi: 10.1152/ajplung.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stevens T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc Am Thorac Soc. 2011;8:453–457. doi: 10.1513/pats.201101-004MW. [DOI] [PubMed] [Google Scholar]
- 68.Eikenboom J, Federici AB, Dirven RJ, Castaman G, Rodeghiero F, Budde U, Schneppenheim R, Batlle J, Canciani MT, Goudemand J, et al. MCMDM-1VWD Study Group. VWF propeptide and ratios between VWF, VWF propeptide, and FVIII in the characterization of type 1 von Willebrand disease. Blood. 2013;121:2336–2339. doi: 10.1182/blood-2012-09-455089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith NL, Chen MH, Dehghan A, Strachan DP, Basu S, Soranzo N, Hayward C, Rudan I, Sabater-Lleal M, Bis JC, et al. Wellcome Trust Case Control Consortium. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: the CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation. 2010;121:1382–1392. doi: 10.1161/CIRCULATIONAHA.109.869156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corrigan CJ, Wang W, Meng Q, Fang C, Wu H, Reay V, Lv Z, Fan Y, An Y, Wang YH, et al. T-helper cell type 2 (Th2) memory T cell-potentiating cytokine IL-25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci USA. 2011;108:1579–1584. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]