Abstract
The mechanism of mesenchymal stem cell therapy in acute kidney injury remains uncertain. Previous studies indicated that mesenchymal stem cells could attenuate inflammation-related organ injury by induction of regulatory T cells. Whether regulatory T-cell induction is a potential mechanism of mesenchymal stem cell therapy in ischemic acute kidney injury and how these induced regulatory T cells orchestrate local inflammation are unknown. Here we found that mesenchymal stem cells decrease serum creatinine and urea nitrogen levels, improve tubular injury, and downregulate IFN-γ production of T cells in the ischemic kidney. In addition to the lung, mesenchymal stem cells persisted mostly in the spleen. Mesenchymal stem cells increased the percentage of regulatory T cells in the spleen and the ischemic kidney. Antibody-dependent depletion of regulatory T cells blunted the therapeutic effect of mesenchymal stem cells, while coculture of splenocytes with mesenchymal stem cells caused an increase in the percentage of regulatory T cells. Splenectomy abrogated attenuation of ischemic injury, and downregulated IFN-γ production and the induction of regulatory T cells by mesenchymal stem cells. Thus, mesenchymal stem cells ameliorate ischemic acute kidney injury by inducing regulatory T cells through interactions with splenocytes. Accumulated regulatory T cells in ischemic kidney might be involved in the downregulation of IFN-γ production.
Keywords: ischemic acute kidney injury, mesenchymal stem cells, regulatory T cells, spleen
Ischemia/reperfusion injury (IRI) is a major cause of clinical acute kidney injury (AKI). Although vigorous research has been conducted, the mortality rate remains high.1 Stem cell therapy for AKI is now becoming viable. Several studies have shown that administration of mesenchymal stem cells (MSCs) leads to the amelioration of ischemic AKI.2, 3, 4 However, the mechanism remains uncertain, thus limiting the therapeutic application in clinical practice.
Inflammation is a major factor in ischemic AKI.5 Indirect6, 7, 8 and direct9 evidence from several studies have implicated T cells,9, 10, 11 especially IFN-γ-positive CD4+ T cells,10 as mediators in renal IRI. These data suggest that modulating T cells may yield novel therapies for renal disease. Regulatory T cells (Tregs) are lymphocytes with immunosuppressive properties that inhibit effector T cells both in vitro12 and in vivo.13 Tregs are commonly identified by their expression of CD4 and CD25 on the cell surface and upregulation of the transcription factor FoxP3. More recently, Tregs were identified in normal mouse kidneys by flow cytometry.14 It was demonstrated that Tregs directly inhibit innate immune responses in ischemic kidneys but had no role in the number of CD4+ T cells that infiltrated into the kidney. However, proinflammatory cytokines, such as IFN-γ production of CD4+ T cells, were not detected.15 Another study indicated that Tregs infiltrated ischemic kidneys during the healing process and promoted tissue repair, probably through the modulation of proinflammatory cytokines from effector T cells.16 Thus, infusion of Tregs15, 17 or pharmacological recruitment of Tregs18 protected against ischemic AKI. Furthermore, MSCs could induce naïve T cells into Tregs in vitro,19, 20, 21, 22 and Treg induction was the key mechanism of MSC therapy in many disease contexts.23, 24, 25, 26, 27, 28, 29 However, whether Treg induction is a potential mechanism of MSC therapy in AKI and how these induced Tregs orchestrate local inflammation were unknown.
The aim of this study was to explore the potential mechanism of MSC therapy in ischemic AKI. We found that MSCs attenuated IRI in a differentiation-independent manner, as infused MSCs persisted mostly in the lung and spleen and no signals were detected in the kidney. We then tested the hypothesis that Treg induction was involved in MSC therapy. MSCs increased the frequency of Tregs in the spleen and the ischemic kidney, and partial depletion of Tregs by PC61 blunted the therapeutic effect of MSCs. Furthermore, we found that the spleen was the crucial organ in MSC therapy, as coculture of splenocytes with MSCs showed increased Tregs, and splenectomy could abrogate the attenuation of ischemic injury, downregulation of IFN-γ production, and induction of Tregs by MSCs.
RESULTS
Infused MSCs attenuated ischemic AKI
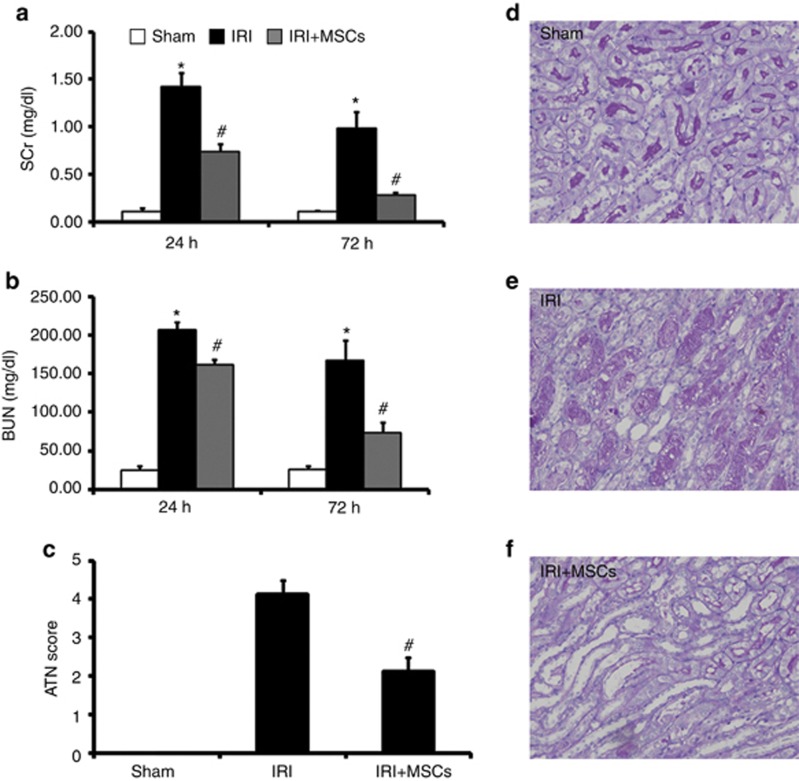
To determine whether MSCs could attenuate ischemic AKI, we subjected mice to bilateral IRI for 35 min. We administered MSCs (106 per mouse) 6 h after reperfusion. MSC infusion markedly reduced serum creatinine (SCr, Figure 1a) and blood urea nitrogen (BUN, Figure 1b) levels after 24 and 72 h of reperfusion, compared with mice given saline. As spontaneous restoration began at 72 h in our study, we chose 72 h as the end point in the following assays. Renal IRI caused tubular necrosis, tubular dilatation, and cast formation (Figure 1e). Infused MSCs markedly improved tubular injury (Figure 1f) and lowered the AKI score (Figure 1c) after 72 h of reperfusion.
Figure 1.
Mesenchymal stem cells (MSCs) ameliorate renal ischemia/reperfusion injury (IRI). In comparison with animals treated with saline, infused MSCs significantly reduced serum creatinine (SCr (a)) and blood urea nitrogen (BUN (b)) 24 h and 72 h after IRI. IRI could induce severe tubular injury (e). Moreover, MSC therapy markedly improved tubular injury (f) and reduced acute tubular necrosis (ATN) scores (c) 72 h after IRI. Sham-operated control (sham, d) had no effect on renal histopathological parameters (c and d). For SCr (a) and BUN (b), values are mean±s.e., n=8–10 in each group. *P<0.05 vs. sham, #P<0.05 vs. IRI treated with saline. For ATN scores (c), values are mean±s.e. Approximately 80 high-power fields (HPFs, × 400) per individual mouse (20 HPFs per slide, four slides per animal) were evaluated. n=6 in each group. #P<0.05 vs. IRI treated with saline.
MSCs suppress IRI-induced upregulation of IFN-γ of T cells infiltrating in ischemic kidney
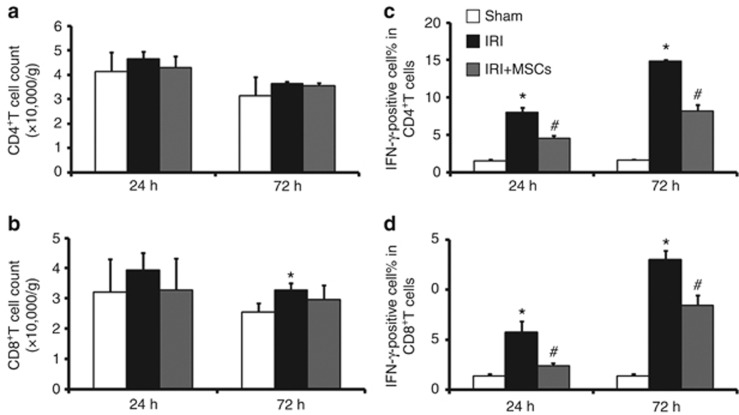
It is well known that CD4+ T-cell subsets, especially those secreting IFN-γ, have an important role in various IRI models.10 Recent studies indicated that CD8+ T cells might also be involved in ischemic AKI.9, 16, 30 To determine whether MSC-mediated renoprotection was associated with a reduction of T-cell subsets and IFN-γ production, the number and IFN-γ production of T-cell subsets in ischemic kidney was determined by flow cytometry. Although no increased presence of CD4+ T lymphocytes in the ischemic kidney was evident (Figure 2a) at both 24 h and 72 h of reperfusion, and the number of CD8+ T lymphocytes increased slightly at only 72 h (Figure 2b) after reperfusion, IFN-γ production by CD4+ (Figure 2c) and CD8+ (Figure 2d) T cells was significantly greater than that in the sham group at both time points. Despite the fact that MSC therapy did not influence the number of CD4+ (Figure 2a) and CD8+ (Figure 2b) T cells in the ischemic kidney, it reduced IFN-γ production, as the percentage of IFN-γ-positive CD4+(Figure 2c) and CD8+ (Figure 2d) T cells was less than that in saline-treated animals.
Figure 2.
Mesenchymal stem cells (MSCs) suppress ischemia/reperfusion injury (IRI)–induced upregulation of proinflammatory cytokine IFN-γ. IRI per se could only slightly increase the number of CD8+(B)T cells infiltrating in ischemic kidney after 72 h of reperfusion. MSCs had no roles in the number of CD4+ (a) and CD8+ (b) T cells infiltrating in ischemic kidney both after 24 and 72 h of reperfusion. In comparison with animals treated with saline, MSCs could reduce IFN-γ production of CD4+ (c) and CD8+ (d) T cells infiltating in ischemic kidney after both 24 and 72 h of reperfusion. The number and IFN-γ production of CD4+ and CD8+ T cells were measured by flow cytometry. For the percentage of IFN-γ-positive cells in CD4+ and CD8+ T cells, values are mean±s.e., n=5 in each group. *P<0.05 vs. sham, #P<0.05 vs. IRI treated with saline.
Distribution of intravenously infused MSCs
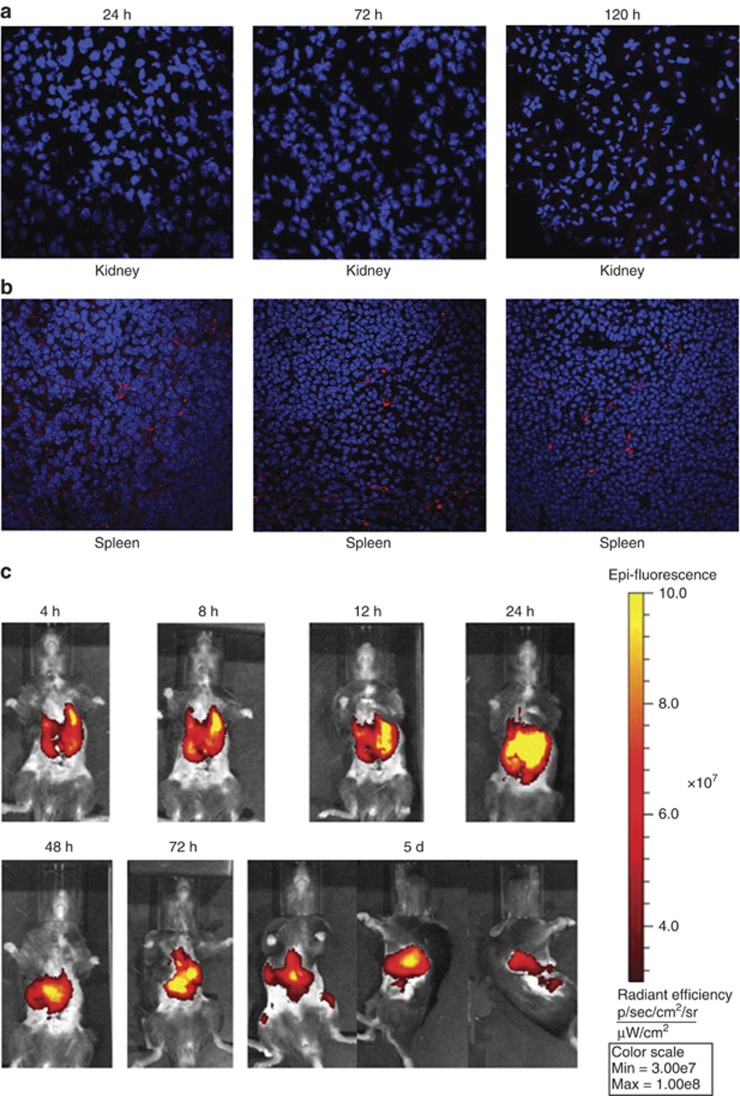
The location of MSCs was related to their functional mechanism. To evaluate the engraftment of RFP-labeled MSCs after injection into mice with AKI, MSCs were detected in frozen samples by a laser confocal microscope. Imaging was performed at 24, 72, and 120 h after cell injection. No signal was found in the kidney (Figure 3a) or heart (Supplementary Figure S1c online) throughout this period. However, signals were detected in the spleen (Figure 3b) and lung (Supplementary Figure S1a online) 24 h after injection and persisted for at least 120 h. Moreover, at 8 h after injection, signals were detected in the liver and persisted for 72 h (Supplementary Figure S1b online). The in vivo imaging results confirmed the presence of MSCs in the spleen and lung. MSCs were labeled with DiR, a near-infrared dye,31 and injected intravenously into mice. Imaging was performed at 4, 8, 12, 24, 48 72, and 120 h post cell injection by IVIS. Signals were detected in the spleen and lung after injection and persisted for at least 120 h (Figure 3c).
Figure 3.
Infused mesenchymal stem cells (MSCs) persist in spleen after ischemia/reperfusion injury (IRI). Intravenously delivered RFP-labeled MSCs were not detected in ischemic kidney at any time point (a), but persisted in spleen during the whole process, at least for 120 h after reperfusion (b). RFP-labeled MSCs (red) were detected by laser confocal microscopy. Nuclei were stained with DAPI (blue). Original magnification, × 600, n=3. (c) Intravenously delivered DiR-labeled MSCs (yellow and red) persisted in the spleen at least for 120 h after reperfusion. Signals were detected by IVIS in vivo imaging, n=3.
Infused MSCs increase the percentage of CD25+Foxp3+ T cells in ischemic kidney and spleen
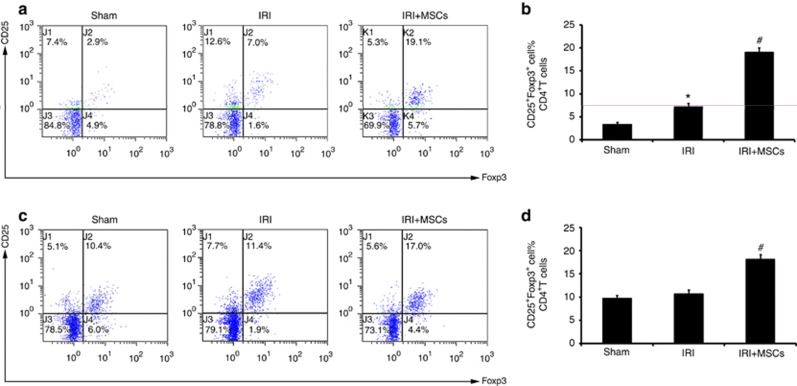
Tregs inhibited effector T cells both in vitro12 and in vivo.13 MSCs have been shown to induce Tregs both in vitro19, 20, 21, 22 and in vivo,23, 24, 25, 26, 27 and this evidence led us to count Tregs in ischemic AKI mice after 72 h of reperfusion. First, we confirmed the presence of Tregs in the ischemic kidney (Supplementary Figure S2b online) via immunofluorescence staining of Foxp3. Spleen samples were used as a positive control (Supplementary Figure S2a online). Flow cytometry assays showed that IRI alone increased the percentage of CD25+ Foxp3+ cells in CD4+ T cells in the kidney (Figure 4a), but not in the spleen (Figure 4c and d). When infused with 106 MSCs, the percentage of CD25+Foxp3+ Tregs in the total CD4+ T-cell compartment in the ischemic kidney (Figure 4a and b) and spleen (Figure 4c and d) increased significantly compared with saline-treated animals.
Figure 4.
Mesenchymal stem cells (MSCs) increase the percentage of CD25+Foxp3+ cells in CD4+ T cells in ischemic kidney and spleen. Ischemia/reperfusion injury (IRI) per se increased the percentage of CD25+Foxp3+ cells in CD4+ T cells (a and b) in ischemic kidney after 72 h of reperfusion. In comparison with animals treated with saline, MSCs increased the percentage of CD25+Foxp3+ cells in CD4+ T cells (a and b) after 72 h of reperfusion. IRI per se had no role in CD25+Foxp3+ cells in CD4+ T cells in the spleen (c and d) after 72 h of reperfusion. In comparison with animals treated with saline, MSCs increased the percentage of CD25+Foxp3+ cells in CD4+ T cells (c and d). The percentage of CD25+Foxp3+ cells in CD4+ T cells was measured by flow cytometry. For the percentage of CD25+Foxp3+ cells in CD4+ T cells, values are mean±s.e. n=5 in each group. *P<0.05 vs. sham, #P<0.05 vs. IRI treated with saline.
Depletion of CD25+ T cells partially inhibits the therapeutic effect of MSCs
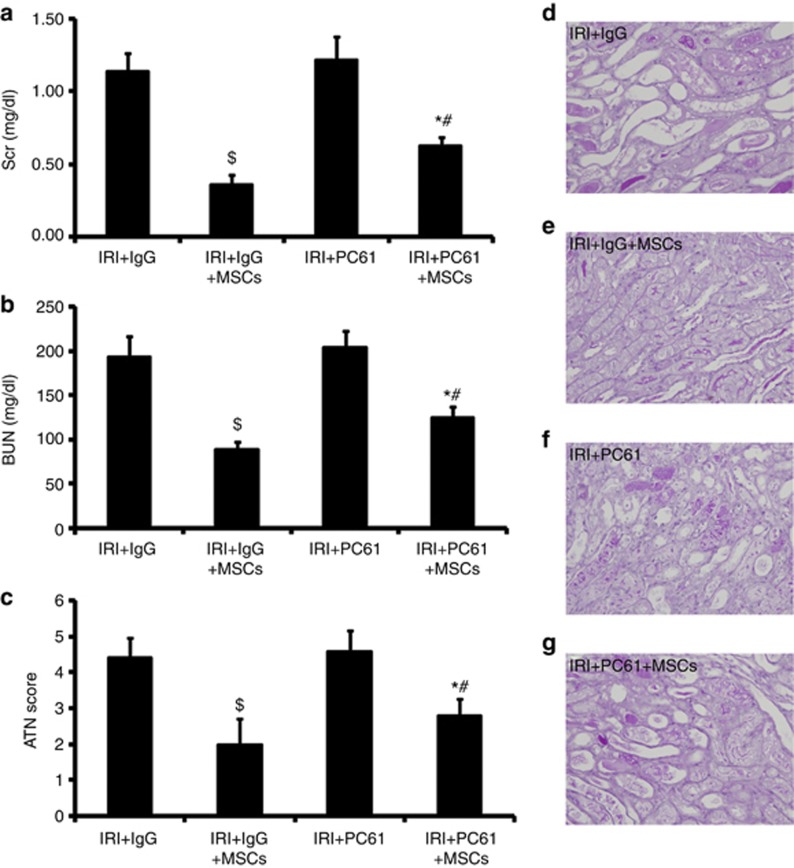
To further delineate their role in MSC-induced renoprotection, we determined whether depletion of Tregs would have an effect on this phenotype. CD25 (interleukin-2 receptor-a) is essential for the functional development and homeostasis of Tregs.32, 33 Recently, anti-CD25 mAb (i.e., PC61) was used to achieve partial depletion of Tregs34 without any significant effect on non-Tregs in the ischemic kidney, including B cells, activated effector T cells, and natural killer cells, indicating that PC61 treatment is specific for Tregs.17 Therefore, PC61 was used to partially deplete Tregs in our study. Rat IgG was used as an isotype control. We performed assays to detect the therapeutic effect of MSCs after PC61 or Rat IgG injection. In mice with IgG injection, MSCs significantly improved SCr (Figure 5a), BUN (Figure 5b), and tubular injury (Figure 5c–e) 72 h after IRI compared with saline-treated mice. In mice injected with PC61, MSCs slightly improved SCr (Figure 5a), BUN (Figure 5b), and tubular injury (Figure 5c) 72 h after IRI compared with saline-treated mice. However, in MSC-treated mice, PC61-injected animals exhibited higher levels of SCr (Figure 5a) and BUN (Figure 5b) and aggravation of tubular injury (Figure 5c) 72 h after IRI compared with IgG-injected mice. These results further indicated that the attenuation of MSC therapy was inhibited by Treg depletion.
Figure 5.
Depletion of Tregs blunts renoprotection of mesenchymal stem cells (MSCs). In mice with IgG injection, MSCs could significantly improve serum creatinine (SCr (a)), blood urea nitrogen (BUN (b)), and tubular injury (c–e) 72 h after IRI compared with saline-treated mice. In mice with PC61 injection, MSCs only slightly improved SCr (a), BUN (b), and tubular injury (c, f and g) 72 h after IRI in mice with saline-treated mice. However, in MSC-treated mice, animals with PC61 injection still showed higher levels of SCr (a) and BUN (b) and aggravation of tubular injury (c, e, and g) 72 h after IRI compared with mice with IgG injection. For SCr (a) and BUN (b), values are mean±s.e. n=5 in each group. For ATN scores (c), approximately 80 high-power fields (HPFs, × 400 ) per individual mouse (20 HPFs per slide, four slides per animal) were evaluated. Values are mean±s.e., n=5 in each group. $P<0.05 vs. IRI+IgG, *P<0.05 vs. saline-treated mice with PC61 injection; #P<0.05 vs. MSC-treated mice with IgG injection.
Coculture of splenocytes with MSCs increases the number of Tregs in vitro
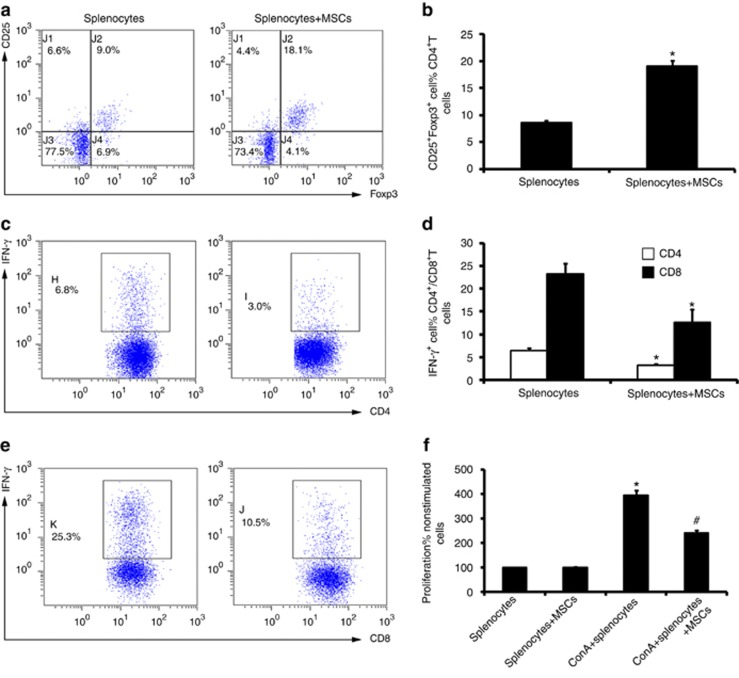
We hypothesized that MSCs could directly induce Treg expansion in the spleen, which would explain the increase of Tregs observed in vivo after MSC transplantation. As IL-2 is the best studied cytokine in terms of its impact on the development, homeostasis, and function of Tregs,35 we cocultured splenocytes with MSCs in low-dose IL-2-supplemented medium (10 U/ml) and analyzed the percentage of Tregs. After 72 h of MSC coculture, there was a marked increase in CD25+ Foxp3+ cells in the CD4+ T-cell population (Figure 6a and b) of unfractionated splenocytes compared with the control (i.e., splenocytes cultured alone in RPMI 1640 medium).
Figure 6.
Coculture with mesenchymal stem cells (MSCs) increases the percentage of Tregs in splenocytes. The percentage of CD25+Foxp3+ cells in CD4+ T cells was detected by flow cytometry. The percentage of Tregs significantly increased after coculture with MSCs for 72 h (a and b). The IFN-γ production by CD4+ and CD8+ T cells in splenocytes stimulating with PMA/Ionocymin was detected by intracelluar staining via flow cytometry. Splenocytes removed from the coculture system displayed a significantly lower capacity of IFN-γ secretion of both CD4+ (c and d) and CD8+ T cells (d and e). Proliferation of splenocytes stimulated by ConA (10 μg/ml) was detected by WST-8 kit. Splenocytes removed from the coculture system displayed a significantly lower proliferation rate (f) after stimulation. For the percentage of CD25+Foxp3+ cells in CD4+ T cells (a and b), values are mean±s.e. n=5 in each group. *P<0.05 vs. splenocytes culture alone. For the percentage of IFN-γ-positive cells in CD4+ and CD8+ T cells, values are mean±s.e. n=5 in each group. *P<0.05 vs. splenocyte culture alone. For the proliferation of splenocytes, values are mean±s.e., n=5 in each group. *P<0.05 vs. splenocyte culture alone; #P<0.05 vs. splenocytes stimulated by ConA.
To further define the function of those MSC-induced Tregs, we performed functional assays in terms of IFN-γ secretion of CD4+ and CD8+ T cells and splenocyte proliferation. Early in the IRI process, the activation of T cells is antigen independent.36 Therefore, we subjected splenocytes to PMA/Ionomycin and ConA stimulation. As mentioned above, as CD4+ and CD8+ T cells that secrete IFN-γ had vital roles in ischemic AKI, we performed an IFN-γ intracellular staining assay via PMA/Ionomycin stimulation. As PMA can downregulate CD4 expression, but has no effect on CD3 and CD8 expression,37 we labeled splenocytes with FITC-conjugated CD3 and PE-conjugated CD8 to define CD4+ and CD8+ T cells. Data suggested that splenocytes removed from the coculture system exhibited decreased IFN-γ secretion of either CD4+ (Figure 6c and d) or CD8+ T cells (Figure 6d and e) compared with the control (i.e., splenocytes cultured alone). The proliferation assay showed that splenocytes removed from coculture displayed a significantly lower proliferation rate (Figure 6f) after ConA (10 μg/ml) stimulation compared with that of the control (splenocytes cultured alone).
Surgical removal of the spleen abolishes MSC-induced renoprotection
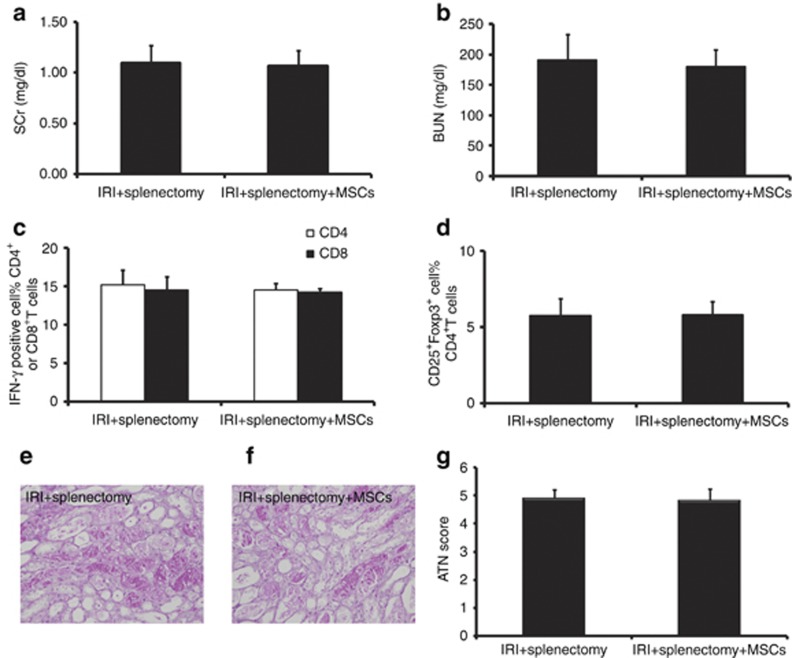
To further confirm whether Tregs were induced via an MSC–splenocyte interaction in the spleen, we performed splenectomy in mice before IRI. After splenectomy, we found no improvement in SCr (Figure 7a), BUN (Figure 7b), and tubular injury (Figure 7e–g). Meanwhile, we found no reduction in IFN-γ production by CD4+ and CD8+ T cells (Figure 7c), or increases in the percentage of Tregs (Figure 7d) in the ischemic kidney of MSC-treated mice. These results indicated that splenectomy completely abolished the effects of MSC therapy, which further confirmed that the induction of Tregs was dependent on MSC localization in the spleen, as well as interaction with splenocytes.
Figure 7.
Splenectomy abolishes mesenchymal stem cell (MSC)–induced renoprotection. In ischemia/reperfusion injury (IRI) mice with splenectomy, infused MSCs had no roles in serum creatinine (SCr (a)), blood urea nitrogen (BUN (b)), and tubular injury (e–g) 72 h after IRI. MSC therapy showed no reduction of IRI-induced upregulation of IFN-γ of CD4+ and CD8+ T cells (c) 72 h after IRI. Moreover, MSC treatment showed no effect on the percentage of Tregs in ischemic kidney (d) 72 h after IRI. The IFN-γ production of CD4+ and CD8+ T cells and the percentage of Tregs were measured by flow cytometry. For SCr (a) and BUN (b), values are mean±s.e., n=6 in each group. For the percentage of IFN-γ-positive cells in CD4+ and CD8+ T cells (c), values are mean±s.e., n=5 in each group. For the percentage of CD25+Foxp3+ cells in CD4+ T cells (d), values are mean±s.e. n=5 in each group. For ATN scores (g), ∼80 high-power fields (HPFs, × 400 ) per individual mouse (20 HPFs per slide, four slides per animal) were evaluated. Values are mean±s.e. n=5 in each group.
DISCUSSION
Our study highlighted several findings in the mechanism of MSC therapy in ischemic AKI: (1) intravenously infused MSCs persisted in the spleen for at least 120 h after reperfusion; (2) MSCs increased the percentage of Tregs in the spleen and the ischemic kidney; (3) MSC-mediated renoprotection was blunted by depletion of Tregs; (4) coculture splenocytes obtained from AKI mice with MSCs could not only increase the percentage of Tregs in splenocytes but could also inhibit the proliferation of splenocytes and IFN-γ production of T cells; (5) splenectomy could abrogate MSCs' therapeutic effect on AKI, downregualtion of IFN-γ, and induction of Tregs. Our data for the first time demonstrated that MSCs could attenuate ischemic AKI by inducing Tregs via interaction with splenocytes, which might be one of the potential mechanisms underlying MSC therapy in ischemic AKI.
It is well known that MSCs attenuate experimental ischemic AKI,38 and they lower ischemic AKI incidence in patients with cardiac surgery. However, the mechanisms remain unclear. The pathogenesis of IRI represents a complex interplay between biochemical, cellular, vascular endothelial, and tissue-specific factors, with inflammation being a common feature. T lymphocytes are important mediators of IRI in the kidney and other organs.36 We found that MSC therapy, despite showing no effects on the number of CD4+ and CD8+ T cells, significantly downregulated IFN-γ production both 24 and 72 h after reperfusion, compared with saline-treated mice. These data suggest that immunomodulation, especially inhibition of effector T cells, might have an important role in MSC therapy in AKI.
The distribution of MSCs in ischemic AKI mice is related to the mechanism of MSC therapy. Here, intravenously infused MSCs appeared in the lung and persisted for up to 120 h after injection, which was similar to another study.39 However, unlike former studies that showed only <5% of MSCs appearing in organs besides the lung,40 or evidence that suggested a transient fate of MSCs in spleen,26 our study showed that intravenously infused MSCs dispersed in the spleen and persisted at least up to 120 h. MSCs prefer to migrate into inflammatory sites.41 Severe ischemic AKI always leads to severe systemic inflammation and elevated IFN-γ production of splenic T cells during severe renal IRI,42 which may explain the quantity of MSCs persisting in the spleen in our study. Moreover, no signal was detected in the ischemic kidney throughout the study, which eliminated the possibility that the effect of MSCs was due to escape of a small number of MSCs from being trapped in the lung homed to the injured tissues and differentiated into kidney cell lineage.
How can these intravenously infused MSCs repair ischemic kidney injury and modulate systemic or local inflammation? MSCs might embed in the lung, and secrete soluble factors into the bloodstream that ameliorate renal injury and enhance repair of the ischemic kidney by suppressing inflammatory and immune reactions.3 However, it was suspected by Prockop et al., that there might be alternative possibilites in MSC therapy since soluble factors would have to be secreted in a transient burst, and probably at high concentrations and most soluble factors produce toxic effects if exogenously infused in high concentrations.39 In our study, MSCs persisted in the spleen, the most important peripheral lymphoid organ. Moreover, IFN-γ production in T cells was inhibited in the ischemic kidney. We hypothesize that an endogenous T-cell immunoregulatory system (e.g., Tregs) might be activated via an MSC–splenocyte interaction.
Is Treg induction important in MSC therapy? Treg infiltration into the ischemic kidney has been reported previously and was replicated in our study.16, 17 The involvement of Treg induction in MSC therapy was supported by the high frequencies of Tregs in the spleen and ischemic kidney. PC61 partially inhibited attenuation of MSCs in renal injury, further indicating a critical role for Treg induction in MSC therapy in ischemic AKI. Unlike natural Tregs originating in the thymus, CD25 expression on the surface of induced Tregs is variable,43 and depends on the disease setting and the site of regulatory activity.44 Therefore, induced Tregs were not completely depleted in the spleen or in the ischemic kidney in our study. In addition, MSCs might serve as guardians against excessive inflammatory responses in various modes such as converting macrophages to the phenotype that secretes IL-10,45, 46 which could explain why the MSC therapeutic effects were not completely abolished by PC61 injection. Collectively, these data suggested that Treg induction was one of the underlying mechanisms in the MSC-mediated protection against renal IRI.
Do MSCs induce Tregs in the spleen? Previous studies have demonstrated that MSCs could expand CD4+CD25highFoxp3+ regulatory T cells in peripheral blood lymphocytes via HLA-G5 secretion.21 We found the presence of an interaction between MSCs and splenocytes from AKI mice, i.e. splenocytes removed from coculture with MSCs showed a significant increase in the percentage of Tregs among the CD4+ T-cell population, less proliferation in response to ConA stimulation, and reduced IFN-γ production by CD4+ and CD8+ T cells after PMA/Ionomycin activation. These data would suggest that short-range secreted signals that locally increase the concentration of a paracrine mediator might also be involved in the reprogramming of non-Tregs. Furthermore, we discovered MSC therapy to be ineffective in splenectomized hosts. These results are similar to the phenomenon that splenectomy abolished MSC therapy in experimental enteritis.26 These data suggested that the spleen is a critical tissue in which MSCs interact with splenocytes in a therapeutic manner, which may have direct relevance to clinical settings as exclusionary criteria for human trials.26 In addition, splenectomy also abrogated MSC therapy in the downregulation of local IFN-γproduction and induction of Tregs. Considering that the downregulation of IFN-γproduction was accompanied with an increase of Tregs in ischemic kidney, and that this phenomenon no longer existed when increase of Tregs was abrogated after splenectomy, it was indicated that those induced regulatory T cells might be involved in the downregulation of IFN-γproduction of local T cells.
In conclusion, we explored mechanisms of MSC therapy in murine ischemic AKI. We found that MSC therapeutic effects, such as attenuation of IRI and downregulation of proinflammatory cytokines in T cells, appear to be mediated by the presence of MSCs in the spleen and consequent induction of Tregs via an MSC–splenocyte interaction. This mechanism may be common to MSC therapy for other inflammatory-related diseases.
MATERIALS AND METHODS
Animals and procedures
C57/BL6 mice (20–25 g) were purchased from the animal center at the Chinese PLA General Hospital. All animal procedures were approved by the Institutional Animal Care and Use Committee at the Chinese PLA General Hospital and Military Medical College. Renal ischemia (35 min) (ref. 10) and splenectomy26 were performed as described previously. At various time points after reperfusion, blood, kidney, spleen, and other organ samples were harvested for further processing. In vivo depletion of Tregs34 was achieved by intravenous injection of 100 μg of monoclonal anti-CD25 antibody (Clone PC61, Biolegend, San Diego, CA). Control mice were injected with Rat IgG (Biolegend).
Histopathological examination for acute tubular necrosis scores
Histological examinations for acute tubular necrosis scores were performed in a blinded manner, as described previously.47 Acute tubular necrosis severity was semiquantified using the following scoring system: 0=none, 1=<10% 2=11–25%, 3=26–45%, 4=46–75%, and 5=>76%.48 Approximately 80 high-power fields (HPFs, × 400) per individual mouse (20 HPFs per slide, four slides per animal) were evaluated (n=6 in each group).
Immunofluorescence staining
For immunofluorescence, sections were fixed in acetone at −20 °C for 10 min. This was followed by blocking with 1 × casein for 30 min at room temperature. Sections were incubated with anti-Foxp3 mAb (1/75, FJK-16s, eBiosciences, San Diego, CA) overnight at 4 °C in PBS–1% BSA.49 After washing the sections in PBS three times, they were incubated with FITC-conjugated AffiniPure donkey anti-rat IgG (H+L) (1/50; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. Rat IgG was used as an isotype-matched control. Sections were mounted with mounting medium containing DAPI (Zhongshan Goldenbridge Biotechnology, Beijing, China). Each tissue section was observed under a confocal laser scanning microscope (Olympus FluoView 1000, Tokyo, Japan) at magnifications of × 600 and × 2400, if necessary.
In vivo tracking of MSCs
In vivo fluorescence tracking of MSCs was performed as follows. RFP-labeled MSCs (106) were injected into mice 6 h after reperfusion via the tail vein (n=3). Heart, liver, spleen, lung, and kidney samples were harvested and snap-frozen with OCT (Sakura Finetek USA, Torrance, CA) compound (frozen sections), to follow the redistribution of MSCs over time up to 120 h. Slides were fixed in acetone at −20 °C for 10 min. After three washes with PBS, slides were stained with mounting medium containing DAPI (Zhongshan Goldenbridge Biotechnology). Slides were then observed under a confocal laser scanning microscope (Olympus FluoView 1000) at a magnification of × 600.
In vivo fluorescent dye tracking of MSCs was performed as follows. MSCs were labeled with near-infrared fluorescence lipophilic DiR (Molecular Probes, USA) before injection. IVIS Lumina II in vivo imaging system (Caliper Life Sciences, Hopkinton, MA) was used to follow the redistribution of MSCs over time in vivo. The excitation and emission filter set in the IVIS was 745 and 780 nm, respectively. DiR-labeled MSCs (106) were injected into mice 6 h after reperfusion via the tail vein (n=3) and assayed for up to 120 h thereafter (4, 8, 12, 24, 72, and 120 h).
Cell preparation
RFP-labeled mouse bone marrow–derived MSCs were obtained from Cyagen Biosciences (Cyagen Biosciences, Sunnyvale, CA). The culture process was performed according to the manufacturer's instructions.
Single-cell preparations from spleen and kidney were performed as described previously.11 Blood was flushed out with ice-cold PBS and then kidneys and spleens were harvested. Spleen tissue was removed, minced in PBS with 1% BSA, and lysed with RBC lysis buffer (TBD science, China) for 1–2 min, according to the manufacturer's protocols. Kidney was decapsulated, diced, and incubated (at 37 °C for 30 min) with collagense I (0.5 mg/ml; Sigma-Aldrich, Shanghai, China) and DNaseI (100 U/ml; Sigma-Aldrich) in HBSS. To remove debris, sample were filtered via a filter mesh (40 μm). Single cells were washed in PBS for supplementary application.
MSCs and splenocyte coculture
Splenocytes were isolated from mice by performing bilateral renal ischemia 6 h after reperfusion. Whole splenocytes (1 × 106 cells) were cultured alone or in coculture with MSCs at a 10:1 ratio of splenocyte to MSCs in RPMI 1640 medium with 10% fetal calf serum (Gibco BRL, Grand Island, NY), 100 kU/l penicillin (Gibco BRL), 100 mg/l streptomycin (Gibco BRL), 10 mmol/l N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES, Gibco BRL), 50 μmol/L 2-mercaptoethanol (-ME, Gibco BRL), and low-dose recombinant human IL-2 (10 U/ml; PeproTech China, Suzhou, Jiangsu Province, PR China). After 72 h of coculture, CD4, CD25, and Foxp3 expression by splenocytes was analyzed by flow cytometry.
Splencoyte proliferation assay
Briefly, after a 72-h coculture of splenocytes and MSCs, splenocytes were removed by aspiration. Splenocytes (5 × 105) in 200 μl of medium were placed on a 96-well microplate. After activation with ConA (10 μg/ml) for 72 h, cell proliferation was assessed using a WST-8 cell proliferation kit (Cell Counting Kit-8, Beyotime Institute of Biotechnology, Haimen, China), according to the manufacturer's protocols. Proliferation results are expressed as the absorbance of the culture medium at 450 nm.
Flow cytometric analysis
We performed surface staining with antibodies to CD45 (30-F11), CD3 (145-2C11), CD8 (53-6.7), CD4 (GK1.5), and CD25 (PC61) (eBiosciences), according to the manufacturer's instructions. We also performed intracellular staining for Foxp3 (FJK-16S; eBioscience), again according to the manufacturer's instructions. We performed intracellular staining for IFN-γ (XMG1.2; eBioscience) after stimulation with a cell stimulation cocktail (plus protein transport inhibitors) (eBiosciences) for 16 h, according to the manufacturer's protocol. Cells were detected using a flow cytometer (FC500 MPL, Beckman Coulter, Brea, CA) and analyzed with the corresponding CXP software.
Statistical analysis
Analysis was performed using the IBM SPSS Statistics 17.0.2 software (IBM Corporation, Armonk, NY). Results were presented as mean values±s.d. Multiple comparisons of parametric data were performed using one-way analysis of variance (ANOVA), followed by Student–Newman–Keuls post-hoc tests. Student's t-test was used to compare differences in means. Nonparametric data were compared with the Mann–Whitney U-test to identify differences between groups, and α was corrected by the number of comparisons (α/comparisons) to ensure α=0.05. P<0.05 was considered statistically significant for all analysis.
Acknowledgments
This research was supported by a grant from the National Basic Research Program of China (2011CB964904), a grant from the National Natural Science Foundation of China (81270819), the Fund of Chinese PLA 12th Five-Year Plan for Medical Sciences (BWS11J027), a grant from the National Basic Research Program of China (2011CB944000), a grant from National High Technology Research (2011AA020115), and Key Project in the National Science & Technology Pillar Program in the 12th Five-Year Plan period (2011BAI10B07)
All the authors declared no competing interests.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S1. Detection of intravenously infused mesenchymal stem cells (MSCs) in lung (A), liver (B), and heart (C) after ischemia/reperfusion injury (IRI).
Figure S2. Foxp3+ Treg detection in kidney.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Palevsky PM. Epidemiology of acute renal failure: the tip of the iceberg. Clin J Am Soc Nephrol. 2006;1:6–7. doi: 10.2215/CJN.01521005. [DOI] [PubMed] [Google Scholar]
- Togel F, Cohen A, Zhang P, et al. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–485. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semedo P, Palasio CG, Oliveira CD, et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009;9:677–682. doi: 10.1016/j.intimp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Zhuo W, Liao L, Xu T, et al. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int. 2011;86:191–196. doi: 10.1159/000319366. [DOI] [PubMed] [Google Scholar]
- Bajwa A, Kinsey GR, Okusa MD. Immune mechanisms and novel pharmacological therapies of acute kidney injury. Curr Drug Targets. 2009;10:1196–1204. doi: 10.2174/138945009789753174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Jain S, Pawluczyk IZ, et al. Inflammation and caspase activation in long-term renal ischemia/reperfusion injury and immunosuppression in rats. Kidney Int. 2005;68:2050–2067. doi: 10.1111/j.1523-1755.2005.00662.x. [DOI] [PubMed] [Google Scholar]
- Ysebaert DK, De Greef KE, Vercauteren SR, et al. Effect of immunosuppression on damage, leukocyte infiltration, and regeneration after severe warm ischemia/reperfusion renal injury. Kidney Int. 2003;64:864–873. doi: 10.1046/j.1523-1755.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- De Greef KE, Ysebaert DK, Dauwe S, et al. Anti-B7-1 blocks mononuclear cell adherence in vasa recta after ischemia. Kidney Int. 2001;60:1415–1427. doi: 10.1046/j.1523-1755.2001.00944.x. [DOI] [PubMed] [Google Scholar]
- Yokota N, Daniels F, Crosson J, et al. Protective effect of T cell depletion in murine renal ischemia-reperfusion injury. Transplantation. 2002;74:759–763. doi: 10.1097/00007890-200209270-00005. [DOI] [PubMed] [Google Scholar]
- Burne MJ, Daniels F, El Ghandour A, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108:1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascon DB, Lopez-Briones S, Liu M, et al. Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol. 2006;177:3380–3387. doi: 10.4049/jimmunol.177.5.3380. [DOI] [PubMed] [Google Scholar]
- Venken K, Thewissen M, Hellings N, et al. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Methods. 2007;322:1–11. doi: 10.1016/j.jim.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Nie CQ, Bernard NJ, Schofield L, et al. CD4+ CD25+ regulatory T cells suppress CD4+ T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infect Immun. 2007;75:2275–2282. doi: 10.1128/IAI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascon DB, Ascon M, Satpute S, et al. Normal mouse kidneys contain activated and CD3+CD4- CD8- double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol. 2008;84:1400–1409. doi: 10.1189/jlb.0907651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey GR, Huang L, Vergis AL, et al. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77:771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo MT, Jang HR, Bagnasco SM, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- Kinsey GR, Sharma R, Huang L, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai LW, Yong KC, Lien YH. Pharmacologic recruitment of regulatory T cells as a therapy for ischemic acute kidney injury. Kidney Int. 2012;81:983–992. doi: 10.1038/ki.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Gonzalez MA, Varela N, et al. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- Ye Z, Wang Y, Xie HY, et al. Immunosuppressive effects of rat mesenchymal stem cells: involvement of CD4+CD25+ regulatory T cells. Hepatobiliary Pancreat Dis Int. 2008;7:608–614. [PubMed] [Google Scholar]
- Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- Prevosto C, Zancolli M, Canevali P, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- Patel SA, Meyer JR, Greco SJ, et al. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- Madec AM, Mallone R, Afonso G, et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52:1391–1399. doi: 10.1007/s00125-009-1374-z. [DOI] [PubMed] [Google Scholar]
- Parekkadan B, Upadhyay R, Dunham J, et al. Bone marrow stromal cell transplants prevent experimental enterocolitis and require host CD11b+ splenocytes. Gastroenterology. 2011;140:966–975. doi: 10.1053/j.gastro.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Han ZB, Liao W, et al. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4CD25(+) Forkhead Boxp3 (FOXP3) (+) regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. 2011;27:587–596. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Annal Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Anderson P, Gonzalez MA, et al. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- Rabb H, Daniels F, O'Donnell M, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279:F525–F531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- Kalchenko V, Shivtiel S, Malina V, et al. Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing. J Biomed Opt. 2006;11:050507. doi: 10.1117/1.2364903. [DOI] [PubMed] [Google Scholar]
- Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, et al. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldjian E, McCarthy SA, Sharrow SO, et al. Nonequivalent effects of PKC activation by PMA on murine CD4 and CD8 cell-surface expression. FASEB J. 1988;2:2801–2806. doi: 10.1096/fasebj.2.12.3261700. [DOI] [PubMed] [Google Scholar]
- Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchuck SL, Zwezdaryk KJ, Coffelt SB, et al. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne-Taney MJ, Yokota N, Rabb H. Persistent renal and extrarenal immune changes after severe ischemic injury. Kidney Int. 2005;67:1002–1009. doi: 10.1111/j.1523-1755.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Andre-Schmutz I, Carnaud C, et al. Damage control, rather than unresponsiveness, effected by protective DX5+ T cells in autoimmune diabetes. Nat Immunol. 2001;2:1117–1125. doi: 10.1038/ni738. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YH, Yong KC, Cho C, et al. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney international. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.