Abstract
Rebamipide is an amino acid analog of 2 (1H)-quinolinone. It is being introduced and used since 1980 for the treatment of peptic ulcer. Its therapeutic use in recurrent aphthous ulcer was not known. It acts by the decrease in oxygen radicals, increase in blood flow and production of protective prostaglandins in ulcer mucosa, which accelerates the process of healing. In this article, we focus on the pharmacodynamics, pharmacokinetics, side-effects and other therapeutic uses of Rebamipide. It will be a new and effective drug in the dermatologists’ drug armamentarium for the treatment of aphthous ulcers and related diseases.
Keywords: Aphthous ulcer, Behcet's syndrome, rebamipide
Introduction
What was known?
Rebamipide is an amino acide analogue used in the treatment of gastroduodenal ulcers.
Recurrent aphthous Ulcers (RAU) or stomatitis or mucositis are always problematic to the patient and the dermatologist. The exact cause of recurrent aphthae is unknown and many etiologies like herpes virus, autoimmunity, deficiency of vitamins like B1, B2, B12, zinc, and folic acid, drugs like captopril, gold salts, nicorandil, phenobarbitol, piroxicam, and stress have been indicated. The treatment modalities tried for recurrent aphthae are vitamins, antivirals, thalidomide, colchicine, topical and oral steroids, etc., Treatment of RAU has always been elusive and for years doctors have yearned for a ‘magic bullet’ that is suitable for all patients. Many treatment options have been tried which includes antibiotics (tetracycline, minocycline),[1] anti-inflammatory agents (topical and systemic corticosteroids),[2,3] immune modulators (thalidomide), pain relieving agents (lidocaine, benzocaine), zinc, vitamin B complex,[4] vitamin C, but none of them gives complete cure. Rebamipide 2-(4-chlorobenzoylamine)-3-[2-(1H)-quinolinon-4-yl] is a new mucoprotective agent which enhances preservation of existing epithelial cells and replacement of lost tissue through a multifactorial mode of action.[5]
Mechanism of action
The mechanism of action of rebamipide in RAU is by preservation of existing cells and replacement of lost tissue. Action of preservation of existing cells occurs through increase in the content of soluble mucus,[6,7] increase in the gastric concentrations of PGE2 and PGI2, down regulation of 15-hydroxyprostaglandin dehydrogenase,[8] increase in the mucosal blood flow through enhanced nitric oxide synthase activity, decrease in the expression of neutrophil adhesion molecules (CD11b/CD18), inhibition of the secretion of TNF-α by inhibiting the synthesis of inflammatory E-selectin and has a free radical scavenging effect on reactive oxygen species.[9]
Rebamipide helps in replacement of lost tissue by increasing the expression of epidermal growth factor (EGF) and EGF receptors.[10] These EGF causes angiogenesis, increased production of granulation tissue and epithelization of ulcer healing.
Dosage
The adult dosage of rebamipide is 100 mg orally three times daily.
RAU: 3 tablets/day for 7-14 days.
Behcet's disease: 3 tablets/day for 2 months.
Pharmacokinetics
The effective concentration of rebamipide is in the range of 1-1000 μm. Following table[11] briefly explains the pharmacokinetic parameter of ribamipide.
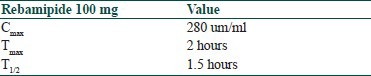
Up to 98.4% of ingested repbamipide is bound to plasma proteins. It is metabolized in the liver by human cytochrome P450 enzyme. The cytochrome P450 enzyme acts on rebamipide through hydroxylation and glucoronidation, resulting in the formation of 6-hydroxy and 8-hydroxyrebamipide.[12] The role of glucoronidation in the metabolism of rebamipide is very low and nonsignificant. Drug interactions of rebmipide with other drugs is very low and safely used concomitantly with other drugs.[9]
Indications of ribamipide in dermatology
Ribamipide has been used for the following indications,[13]
Stomatitis
Recurrent oral aphthae
Behcet's disease
Matsuda et al.,[14] in their multi-centric, double blind, placebo-controlled study, compared Ribamide 300 mg/day with placebo in 35 patients of Behcet's disease for 12-24 weeks. They found, rebamipide to be very effective in controlling the oral apthae and reducing the pain score when compared to placebo and ‘P’ value was significant (<0.01 between groups). They concluded that, rebamipide is an effective and safe drug in the treatment of recurrent oral ulcers.
Till now, there are no randomized, placebo-controlled studies of rebamipide in the treatment of recurrent oral aphthae.[15]
Contraindication
Rebamipide is contraindicated in patients with known history of drug hypersensitivity.
Pregnancy and lactation
It is a category C drug.[16] It should used with caution only when therapeutic benefits outweighs any potential risk. Lactation should be avoided when rebamipide is administered.
Use of rebamipide in children
Clinical evidence to support the use of rebamipide in children is insufficient.
Side-effects of rebamipide
Adverse drug reactions to rebamipide is not common, side effects seen are mild and can be corrected with dose adjustment. The common side-effects noticed after rebamipide use is gastrointestinal like constipation,[17] bloating, diarrhea, nausea and vomiting. Hypersensitivity and rash was seen in less than 1% of patients.
Mouth ulcers and quality of life
The pain of recurrent mouth ulcers is excruciating and increases after eating, drinking and talking. This affects the quality of life of an individual. The patients of severe RAU avoid eating, drinking, kissing and talking. Speech is painful and all these put-together cause depression in the individual.
Structure.
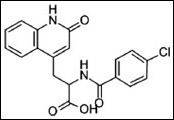
Rebamipide is chemically an amino acide analog of quinolinone 9[18]
Conclusion
The exact etiology of RAU is not-known and it can be multifactorial. The new drug, rebamipide addresses the problem in many angles and it has ulcer healing and ulcer protective functions. Rebamipide is safe, well tolerated and effective drug in the treatment of RAU and Behcet's disease. The administration of rebamipide is not cumbersome and does not have any specific adverse drug reaction. Therefore, rebamipide is an addition in the armamentarium of management of RAU and Behcets's disease.[18]
What is new?
Rebamipide is an effective and safe drug in the treatment of recurrent aphthous ulcers and Behcet's syndrome.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Barrons RW. Treatment strategies for recurrent oral aphthous ulcers. Am J Health Syst Pharm. 2001;58:41–50. [PubMed] [Google Scholar]
- 2.Femiano F, Gombos F, Scully C. Recurrent aphthous stomatitis unresponsive to topical steroids: A study of the comparative therapeutic effects of systemic prednisone and systemic sulodexide. Int J Dermatol. 2003;43:394–7. doi: 10.1046/j.1365-4362.2003.01853.x. [DOI] [PubMed] [Google Scholar]
- 3.Lo Muzio L, della Vella A, Mignogna MD, Pannone G, Bucci P, Bucci E, et al. The treatment of oral aphthous ulceration or erosive lichen planus with topical clobetasol propiopnated in three preparations: A clnical and pilot study on 54 patients. J Oral Pathol Med. 2001;30:611–7. doi: 10.1034/j.1600-0714.2001.301006.x. [DOI] [PubMed] [Google Scholar]
- 4.Piskin S, Sayan C, Durukan N, Senoi M. Serum iron, ferritin, folic acid and vitamin B12 levels in recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol. 2002;16:66–7. doi: 10.1046/j.1468-3083.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim HK, Kim JI, Kim JK, Han JY, Park SH, Choi KY, et al. Preventive effects of rebamipide on NSAID-induced gastric mucosal injury and reduction of gastric mucosal blood flow in healthy volunteers. Dig Dis Sci. 2007;52:1776–82. doi: 10.1007/s10620-006-9367-y. [DOI] [PubMed] [Google Scholar]
- 6.Matysiak-Budnik T, Heyman M, Mégraud F. Review article: Rebamipide and the digestive epithelial barrier. Aliment Pharmacol Ther. 2003;18:55–62. doi: 10.1046/j.1365-2036.18.s1.6.x. [DOI] [PubMed] [Google Scholar]
- 7.Haruma K, Ito M. Review article: Clinical significance of mucosal-protective agents: Acid, inflammation, carcinogenesis and rebamipide. Aliment Pharmacol Ther. 2003;18:153–9. doi: 10.1046/j.1365-2036.18.s1.17.x. [DOI] [PubMed] [Google Scholar]
- 8.Tanigawa T, Watanabe T, Ohkawa F, Nadatani Y, Otani K, Machida H, et al. Rebamipide, a mucoprotective drug, inhibits NSAIDs-induced gastric mucosal injury: Possible involvement of the downregulation of 15-hydroxyprostaglandin dehydrogenase. J Clin Biochem Nutr. 2011;48:149–53. doi: 10.3164/jcbn.10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai K, Sasabe H, Koga T, Konishi T. Mechanism of hydroxyl radical scavenging by rebamipide: Identification of mono-hydroxylated rebamipide as a major reaction product. Free Radic Res. 2004;38:487–94. doi: 10.1080/1071576042000209808. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara S, Morita Y, Toyonaga T, Kawakami F, Itoh T, Yoshida M, et al. A randomized controlled trial of rebamipide plus rabeprazole for the healing of artificial ulcers after endoscopic submucosal dissection. J Gastroenterol. 2011;46:595–602. doi: 10.1007/s00535-011-0372-3. [DOI] [PubMed] [Google Scholar]
- 11.Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: Overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43:5S–13. [PubMed] [Google Scholar]
- 12.Koyama H, Sasabe G, Miyamoto Involvement of cytochrome P450 in the metabolism of rebamipide by the human liver. Xenobiotica. 2002;32:573–86. doi: 10.1080/00498250210130591. [DOI] [PubMed] [Google Scholar]
- 13.Genta RM. Review article: The role of ribamipide in the management of inflammatory disease of the gasrointestinal tract. Aliment Pharmacol Ther. 2003;18(Suppl 1):8–13. doi: 10.1046/j.1365-2036.18.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda T, Ohno S, Hirohata S, Miyanaga Y, Ujihara H, Inaba G, et al. Efficacy of rebamipide as adjunctive therapy in the treatment of recurrent oral aphthous ulcers in patients with behcet's disease: A randomised, double-blind, placebo-controlled study. Drugs R D. 2003;4:19–28. doi: 10.2165/00126839-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Bruce A, Rogers RS., 3rd New and old therapeutics for oral ulcerations. Arch Dermatol. 2007;143:519–23. doi: 10.1001/archderm.143.4.519. [DOI] [PubMed] [Google Scholar]
- 16.Kim YJ, Cheon JH, Lee SK, Kim JH, Lee YC. Rebamipide may be comparable to H 2 receptor antagonist in healing iatrogenic gastric ulcers created by endoscopic mucosal resection: A prospective randomized pilot study. J Korean Med Sci. 2010;25:583–8. doi: 10.3346/jkms.2010.25.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SH, Cho CS, Lee OY, Jun JB, Lin SR, Zhou LY, et al. Comparison of prevention of NSAID-induced gastrointestinal complications by rebamipide and misoprostol: A randomized, multicenter, controlled trial-storm study. J Clin Biochem Nutr. 2007;40:148–55. doi: 10.3164/jcbn.40.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shunji H, Toshiro S, Ken-Ichi A, Hiroshi I, Emiko I, Miki A, et al. Effect of Rebamipide, a novel antiulcer agent, on Helicobacter pylori adhesion to gastric epithelial cells. Antimicrob Agents Chemother. 1998;42:1895–9. doi: 10.1128/aac.42.8.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]


