Abstract
Anti-epileptic drugs can be associated with a wide spectrum of cutaneous adverse reactions ranging from simple maculopapular rashes to more severe and life threatening reactions like Stevens-Johnson syndrome and toxic epidermal necrolysis. These rashes are well documented with older antiepileptic drugs like phenytoin, phenobarbitone and carbamazapine. Lamotrigine is a newer, unrelated antiepileptic drug that causes skin rashes in 3-10% of new users. Higher starting dose or rapid escalation, concurrent treatment with valproic acid, and a previous history of a rash with other antiepileptic drugs are well recognized risk factors for lamotrigine related serious rashes. We report two patients with toxic epidermal necrolysis, resulting from concomitant use of lamotrigine and valproic acid. It is emphasized that clinicians adhere to the recommended dosage guidelines and adopt a slow dose titration when initiating treatment with lamotrigine.
Keywords: Lamotrigine, toxic epidermal necrolysis, valproic acid
Introduction
What was known?
Anti-epileptic drugs are a known cause of serious adverse cutaneous drug reactions.
Lamotrigine is a new, anti-epileptic drug used in the treatment of partial and generalized seizures and maintenance treatment of bipolar I disorder.[1,2] It is a phenyltriazine that is extensively metabolized in liver by N-glucuronidation.[3] Common adverse effects of lamotrigine include headache, nausea, vomiting, tremors, anxiety and insomnia.[3] The major adverse drug reaction leading to lamotrigine discontinuation is skin rash, observed in 3-10% of users.[4] Lamotrigine related rashes are mostly simple maculopapular exanthems, observed within first eight weeks of treatment and resolve promptly on withdrawal of the drug.[3,4] However, in a small percentage of patients, lamotrigine can cause serious adverse cutaneous reactions like Stevens-Johnson syndrome and toxic epidermal necrolysis.[3,5,5] We report two cases of toxic epidermal necrolysis resulting from lamotrigine therapy and discuss the risk factors associated with the development of serious reactions, with emphasis on the importance of adhering to the recommended dosage guidelines.
Case Reports
Case 1
A 47-year-old female presented with fever, oral erosions and blisters all over the body for ten days. Patient was a known epileptic, on valproic acid 300 mg/day since four months. Two weeks prior to onset of symptoms she had been initiated on lamotrigine 50 mg twice daily by her physician. About 6 months ago, she had had carbamazepine induced maculopapular rash. Examination revealed multiple, flat target-like lesions, diffuse erythema, flaccid blisters and erosions over face, trunk and extremities including palms and soles [Figure 1]. Body surface involvement was around 60%. Nikolsky's sign was positive. Severe oral and genital erosions were noted along with conjunctivitis and eyelid oedema. Patient was febrile with normal systemic examination. Hemogram, liver and renal function tests, blood sugar and urinalysis were normal. The diagnosis of lamotrigine induced toxic epidermal necrolysis was established using the Naranjo adverse drug reaction probability scale.[6] Patient was admitted to intensive care unit, and lamotrigine and valproic acid were discontinued. Clobazam 20 mg/day was begun for seizure prophylaxis. In addition to supportive care, she was given prednisolone 60 mg/day. After a tumultuous course in the hospital extending up to 45 days, patient made an eventual recovery but was left with pigmentary changes, nail dystrophy, bilateral ectropion and corneal scarring.
Figure 1.
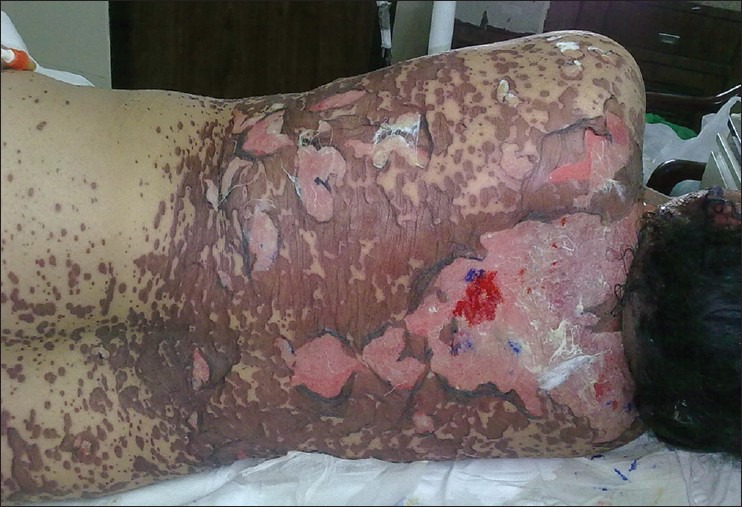
Erythematous papules, bullae, erosions and separation of necrotic epidermis
Case 2
A 26 year old female was under treatment with valproic acid 750 mg/day, propranolol 10 mg/day, clomipramine 10 mg/day, and risperidone 2 mg/day for bipolar mood disorder and obsessive compulsive disorder since two years. Four weeks prior to presentation, she had been initiated on lamotrigine at 25 mg/day, which was rapidly increased to 50 mg BD over 2 weeks and 100 mg BD over another 2 weeks, at which time she developed skin rash and oral erosions. Examination revealed a rash typical of toxic epidermal necrolysis with erythematous macules, papules, flaccid blisters, targetoid lesions, and erosions over face, trunk and palms involving around 30% of body surface area [Figure 2]. Nikolsky's sign was positive. Genital and oral erosions with hemorrhagic crusting over lips and severe conjunctival congestion were observed. Systemic examination was unremarkable. A causality assessment of this adverse event revealed a probable association between lamotrigine and toxic epidermal necrolysis based on the Naranjo probability scale.[6] Blood counts and basic biochemistry were normal. Lamotrigine and other drugs, except valproic acid, were stopped. She was given prednisolone 40 mg/day, in addition to supportive care. Prednisolone was tapered gradually, and the patient made an uneventful recovery and was discharged.
Figure 2.
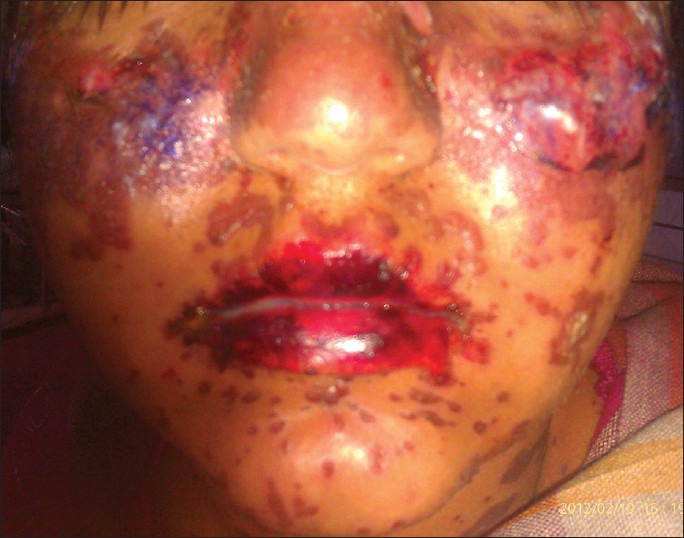
Multiple targetoid lesions, bullae, and erosions on the face and hemorrhagic crusting on the lips
Discussion
Antiepileptic drugs are frequently associated with idiosyncratic adverse skin reactions, which are a leading cause of withdrawal of the drug.[7] Among the traditional antiepileptic drugs, aromatic compounds like phenytoin, carbamazepine and phenobarbitone have been associated with a relatively high incidence of cutaneous reactions.[7] Of the nine newer antiepileptics introduced in the past decade, lamotrigine has been most associated with rash, in up to 3-10% of users in clinical trials.[4,7]
Most of the rashes with lamotrigine are benign and resolve promptly on discontinuation of treatment. The incidence of rash due to lamotrigine, associated with hospitalization in clinical trials was three of 1000 in adults (0.3%) and one of 100 in children (1%).[4] Several factors are likely to increase the development of serious rashes. Co-administration of valproic acid with lamotrigine significantly increases the risk for development of serious adverse cutaneous reactions.[8] It has been hypothesized that valproic acid interacts with lamotrigine by inhibiting its glucuronidation. The elimination half life of lamotrigine when used as monotherapy is 25-30 hours and it is more than doubled to 60 hours when combined with valproic acid.[3,4] It has also been suggested that a high starting dose and a rapid dose escalation may increase the occurrence of skin rashes with lamotrigine.[4,8] The risk of allergic reactions is decreased when treatment is started at a low dose and the doses are increased gradually following the recommended dosage guidelines, possibly because slow titration may allow desensitization to occur.[9] Both of our patients were on valproic acid when lamotrigine was initiated as an add-on therapy. High starting doses were employed and dose was also rapidly increased in both the cases.
History of a rash attributed to another antiepileptic drug results in a five times greater increase in the rate of an antiepileptic drug rash.[7] Hirsch et al. have concluded that a rash with another antiepileptic drug is the greatest risk factor for developing a rash with lamotrigine.[8] In the first patient reported here, there was a history of rash to carbamazepine. The phenomenon of cross reactivity may be a general feature of patients predisposed to drug rashes. Also a patient with a prior medication related rash would be more vigilant and likely to notice a drug related rash from a new medication.[8]
Females have a higher risk for antiepileptic skin reactions compared to males; probably due to hormonal factors.[10] It is notable that both of our patients were females. A causality assessment using Naranjo probability scale[6] was performed in both the patients that revealed a probable association between lamotrigine and toxic epidermal necrolysis in both of the cases. Our second patient was on several medications (valproic acid, propranolol, clomipramine, risperidone) since two years. These drugs have a very low potential for causing serious drug reactions like toxic epidermal necrolysis. The rates of skin rash with valproic acid are very low as compared to lamotrigine.[7,11] Also most of the rashes develop in first eight weeks of initiation of treatment. Thus it is unlikely that they were responsible for causing toxic epidermal necrolysis in the second patient.
The international prescribing guidelines regarding use lamotrigine as an adjunctive treatment with valproic acid, recommend that lamotrigine should be initiated at a dose of 12.5 mg once daily for first two weeks, followed by 25 mg once daily for next two weeks, and increased thereafter by 25 mg every other week until a maintenance dose of 100-200 mg daily.[3,4] Patients should be warned to follow the dosing instructions carefully. It must be pointed out, however, that serious reactions can occur with recommended dosing and titration schedules, and without concurrent valproic acid.[4] Increased vigilance is thus required when initiating lamotrigine in a patient and treatment should be discontinued if any rash appears.
What is new?
When lamotrigine is prescribed as an adjunctive treatment with valproic acid, dose titration should be slow and according to the recommended guidelines.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Dichter MA, Brodie MJ. New antiepileptic drugs. N Engl J Med. 1996;334:1583–90. doi: 10.1056/NEJM199606133342407. [DOI] [PubMed] [Google Scholar]
- 2.Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antiepileptics in the treatment of bipolar disease. J Clin Psychiatr. 1998;59(suppl 6):74–81. [PubMed] [Google Scholar]
- 3.Patsalos PN, Bourgeois BFD. 1st ed. Cambridge: Cambridge University Press; 2010. Lamotrigine. In, The Epilepsy Prescribers Guide to Antiepileptic Drugs; pp. 111–23. [Google Scholar]
- 4.Guberman AH, Besag FM, Brodie MJ, Dooley JM, Duchowny MS, Pellock JM, et al. Lamotrigine-associated rash: Risk/benefit considerations in adults and children. Epilepsia. 1999;40:985–91. doi: 10.1111/j.1528-1157.1999.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 5.Schlienger RG, Shapiro LE, Shear NH. Lamotrigine-induced severe cutaneous adverse reactions. Epilepsia. 1998;39(Suppl 7):S22–6. doi: 10.1111/j.1528-1157.1998.tb01681.x. [DOI] [PubMed] [Google Scholar]
- 6.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 7.Arif H, Buchsbaum R, Weintraub D, Koyfman S, Salas-Humara C, Bazil CW, et al. Comparison and predictors of rash associated with 15 antiepileptic drugs. Neurology. 2007;68:1701–9. doi: 10.1212/01.wnl.0000261917.83337.db. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch LJ, Weintraub DB, Buchsbaum R, Spencer HT, Straka T, Hager M, et al. Predictors of Lamotrigine-associated rash. Epilepsia. 2006;47:318–22. doi: 10.1111/j.1528-1167.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 9.Zaccara G, Franciotta D, Perucca E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007;48:1223–44. doi: 10.1111/j.1528-1167.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- 10.Alvestad S, Lydersen S, Brodtkorb E. Rash from antiepileptic drugs: Influence by gender, age, and learning disability. Epilepsia. 2007;48:1360–5. doi: 10.1111/j.1528-1167.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 11.Harden CL. Therapeutic safety monitoring: What to look for and when to look for it. Epilepsia. 2000;41(Suppl 8):S37–44. doi: 10.1111/j.1528-1157.2000.tb02945.x. [DOI] [PubMed] [Google Scholar]


