Abstract
We and others have shown that the minor, nonconserved allele Gln381 of the Arg381Gln single-nucleotide polymorphism (rs11209026G>A) of the IL-23 receptor gene (IL23R) protects against psoriasis. Moreover, we have recently shown impaired IL-23-induced IL-17A production and STAT-3 phosphorylation in Th17 cells generated in vitro from healthy individuals heterozygous for the protective A allele (GA). However, the biological effect of this variant has not been determined in homozygous carriers of the protective A allele (AA), nor in psoriatic patients. Here we expand our functional investigation of the IL23R Arg381Gln gene variant to include AA homozygous individuals. By using isolated memory CD4+ T cells, we found attenuated IL-23-induced Th17 response in heterozygous individuals. Moreover, we found that AA homozygous individuals were strikingly unresponsive to IL-23, with minimal or no IL-17A and IL-17F production and failure of human memory Th17 cell survival/expansion. Finally, IL-23-induced Th17 response was also attenuated in age- and sex-matched GA versus GG psoriatic patients undergoing systemic treatment. Taken together, our data provide evidence for an allele-dosage effect for IL-23R Gln381 and indicate that common gene alleles associated with complex diseases might have biological effects of considerable magnitude in homozygous carriers.
Introduction
Psoriasis is a chronic immune-mediated inflammatory skin disease, characterized by inflamed scaly plaques, epidermal hyperplasia, and inflammatory infiltrate (Nestle et al., 2009). A pathogenic cross talk between innate and adaptive cells, including keratinocytes, dendritic cells, and T cells, underpins a dysregulated immune response leading to aberrant epidermal proliferation (Guttman-Yassky et al., 2011).
Advances have been made in understanding the genetic basis of the disease, which is inherited as a complex trait resulting from gene–gene and gene–environment interactions (Elder et al., 2010). Human leukocyte antigen-C and more than 30 other genes, mainly belonging to the IL-23/T helper (Th)-17 axis, the NF-κB pathway, and the epidermal differentiation complex, have been identified by means of linkage analysis and/or by genome-wide association studies as psoriasis susceptibility genes (Perera et al., 2012; Tsoi et al., 2012).
Insights gleaned from clinical and experimental studies confirm genetic data supporting a critical role for the IL-23/Th17 axis in disease pathogenesis (Fitch et al., 2007; Di Cesare et al., 2009). IL-23 is a heterodimeric IL-23p19/IL-12p40 cytokine, signals through its heterodimeric IL-23R complex, and is essential for Th17 cell effector function and pathogenicity. Th17 cells, producing IL-17A, IL-17F, IL-22, and IFN-γ (Volpe et al., 2009; Boniface et al., 2010), are the third arm of the adaptive immune system and protect against bacterial and fungal infections, e.g., infections due to Staphylococcus aureus and Candida albicans (Miller and Cho, 2011; Hernandez-Santos and Gaffen, 2012). However, Th17 cells have been increasingly implicated in autoimmunity (Miossec et al., 2009).
Increased IL-23 levels, as well as Th17 cells and cytokines, are present in psoriatic skin (Wilson et al., 2007; Lowes et al., 2008; Tonel et al., 2010). Experimental models of psoriasis-like skin inflammation (Chan et al., 2006; van der Fits et al., 2009; Rizzo et al., 2011) and selective targeting of IL-23 in a clinically relevant xenotransplantation model (Tonel et al., 2010) support the importance of the IL-23/Th17 axis. IL-23 and Th17 cytokines are downregulated after successful therapies (Gottlieb et al., 2005; Zaba et al., 2007), and ustekinumab, secukinumab, ixekizumab, and brodalimumab monoclonal antibodies directed against IL-12/23p40, IL-17A, and IL-17RA, respectively, are effective in psoriasis (Griffiths et al., 2010; Hueber et al., 2010; Leonardi et al., 2012; Papp et al., 2012).
We and others have shown that genes belonging to the IL-23 pathway, including IL23R, IL12B, and IL23A, are associated with psoriasis (Capon et al., 2007; Cargill et al., 2007; Nair et al., 2009). One of the most robust and independently replicated genome-wide association study findings is the nonsynonymous Arg381Gln single-nucleotide polymorphism (SNP) (rs11209026G>A) in the IL23R gene (Capon et al., 2007; Cargill et al., 2007; Nair et al., 2009), with the minor nonconserved Gln381 allele conferring approximately twofold greater protection against psoriasis. The IL23R GA/Arg381Gln SNP also confers an approximately threefold greater protection against Crohn's disease (Duerr et al., 2006) and twofold against ankylosing spondylitis (Rueda et al., 2008), thus suggesting protection against multiple immune-mediated diseases by impaired IL-23-induced Th17 responses. We have recently addressed this hypothesis in a comprehensive functional characterization of the IL23R GA/Arg381Gln gene variant in healthy volunteers (Di Meglio et al., 2011). We showed that although the frequency and overall activity of Th17 cells were not affected, IL-23-induced Th17 effector function is indeed impaired in the presence of the protective 381A/Gln allele. In vitro–polarized Th17 cells derived from IL23R GA/Arg381Gln heterozygous individuals (GA) displayed significantly reduced IL-23-induced STAT-3 activation and IL-17A production, as compared with homozygous carriers of the nonprotective IL23R 381Arg allele (GG). Our findings have been replicated by two independent groups, also showing reduced responsiveness to IL-23 in healthy heterozygous individuals (Pidasheva et al., 2011; Sarin et al., 2011).
Such functional studies have not been undertaken so far in either homozygous carriers of the protective 381A/Gln allele (AA) or in psoriatic patients.
Here we took advantage of the large Cambridge BioResource of volunteers to expand our functional investigation of the IL23R GA/Arg381Gln SNP in a largely independent cohort of healthy individuals. Importantly, this cohort included nine individuals carrying the AA genotype, which, to our knowledge, has not been previously functionally investigated. In keeping with our previous study, we found attenuated IL-23-induced Th17 response in memory Th cells of GA heterozygous individuals. Moreover, we found that AA homozygous individuals were strikingly unresponsive to IL-23, with minimal or no IL-17A and IL-17F production and failure of memory Th17 cell survival/expansion. Finally, to address its clinical relevance, we investigated the effect of the IL23R GA/Arg381Gln SNP in psoriatic patients undergoing systemic treatment and found impaired IL-23-induced Th17 response in GA heterozygous psoriatic patients.
Results
IL23R A/Gln381 heterozygous and homozygous carriers have normal Th17 cell development
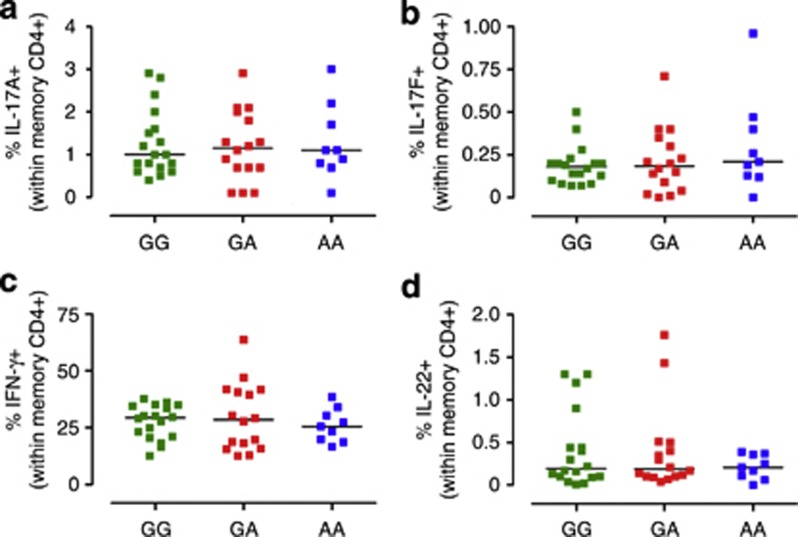
We have previously shown that the frequency of circulatory Th17 cells is similar in IL23R GG/Arg381 common homozygous and IL23R GA/Arg381Gln protected heterozygous in normal healthy individuals (Di Meglio et al., 2011). Here we sought to confirm and expand these findings in a largely independent cohort of normal healthy individuals, which included nine subjects homozygous for the minor A/Gln381 allele (demographics in Supplementary Table S1 online). Figure 1 a and b shows that in keeping with our earlier study, the frequency of memory CD4+ T cells producing Th17 signature cytokines IL-17A and IL-17F in response to phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation did not differ in the three groups. Frequency of cells producing IFNγ or IL-22, proinflammatory cytokines produced not only by Th17 cells but also by Th1 and Th22 cells, was also not affected by the IL23R genotype (Figure 1c and d). Therefore, we conclude that both heterozygous and homozygous carriers of the minor IL23R A/Gln381 allele have normal Th17 cell development, as frequency of circulatory Th17 cell was not affected. Furthermore, in keeping with our earlier study, we found no difference either in the frequency of memory CD4+IL-23R+T cells or in IL-23R surface expression in the different genetic groups (Supplementary Figure S1 online).
Figure 1.
IL-23R A/Gln381 heterozygous and homozygous carriers have normal Th17 cell development. Peripheral blood mononuclear cells from healthy individuals homozygous for the common G allele (GG, green squares), heterozygous GA (red squares), or homozygous for the protective A (AA, blue squares) were assayed for intracellular cytokines, following phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation for 5 hours. The frequency of IL-17A+ (a), IL-17F+ (b), IFN-γ+ (c), and IL-22+ (d) cells within memory CD4+ gate is shown. Each symbol represents one individual donor. Horizontal bars represent means (a, c) or medians (b, d). One-way analysis of variance, followed by Bonferroni post test (a, c) or Kruskal–Wallis test and Dunn's multiple-comparison test (b, d), was performed, yielding P>0.05 for all comparisons.
IL-23R A/Glu381 allele promotes IL-23 unresponsiveness in human memory Th 17 cells
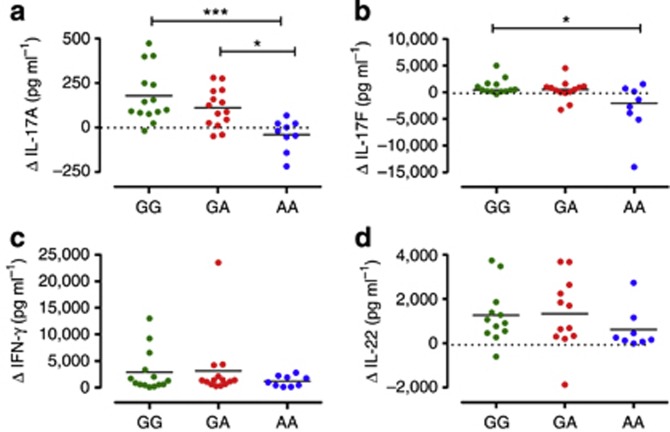
Next, we sought to test the effect of the IL23R GA/Arg381Gln SNP in IL-23-mediated Th17 responses. We have previously shown that in vitro–polarized Th17 cells from GA heterozygous individuals have impaired response to IL-23 with significantly reduced IL-17A production (Di Meglio et al., 2011). Here we used a simplified in vitro system in which IL-23-mediated Th17 responses are induced in memory CD4+T cells cultured with or without IL-23 in the presence of TCR stimulation for 72 hours (Supplementary Figure S1 online). GG homozygous memory Th cells responded to IL-23 stimulation by secreting Th17 cytokines, and this secretion was attenuated in GA heterozygous, although it did not reach statistical significance (Δ IL-17A mean: GG 179 pg ml−1, from −19 to 473 pg ml−1, and GA 110 pg ml−1, from −49 to 279 pg ml−1; Δ IL-17F median: GG 428 pg ml−1, from −351 to 5,002 pg ml−1, and GA 534 pg ml−1, from −3269 to 4514 pg ml−1; Figure 2a and b). However, IL-23-induced IL-17A and IL-17F production was nearly abolished in AA homozygous memory Th cells that were virtually unresponsive to IL-23 stimulation (Δ IL-17A mean: AA −41 pg ml−1, from −219 to 66.8 pg ml−1, P<0.001 vs. GG, P<0.05 vs. GA; Δ IL-17F median: AA −2,050 pg ml−1, from −14,047 to 1,534 pg ml−1, P< 0.05 vs. GG; Figure 2a and b). Neither IFN-γ nor IL-22 production in response to IL-23 was affected by the IL23R genotype, although a downward trend in IL-22 production was observed in AA homozygous (Figure 2c and d).
Figure 2.
IL-23R A/Glu381 allele promotes IL-23 unresponsiveness in human memory Th17 cells. Supernatants of memory CD4+ T cells from healthy individuals homozygous for the common G allele (GG, green dots), heterozygous GA (red dots), or homozygous for the protective A allele (AA, blue dots), cultured with or without IL-23 for 72 hours, were assayed for IL-17A (a), IL-17F (b), IFN-γ (c), and IL-22 (d) secretion. Data are shown as differential cytokine production (Δ) in cells cultured with versus without IL-23. Each symbol represents one individual donor. Dotted line denotes Δ=0. Horizontal bars represent means (a) or medians (b, c, d). One-way analysis of variance, followed by Bonferroni post test (a) or Kruskal–Wallis test and Dunn's multiple-comparison test (b, c, d), was performed. *P<0.05, ***P<0.001. P>0.05 for all other comparisons.
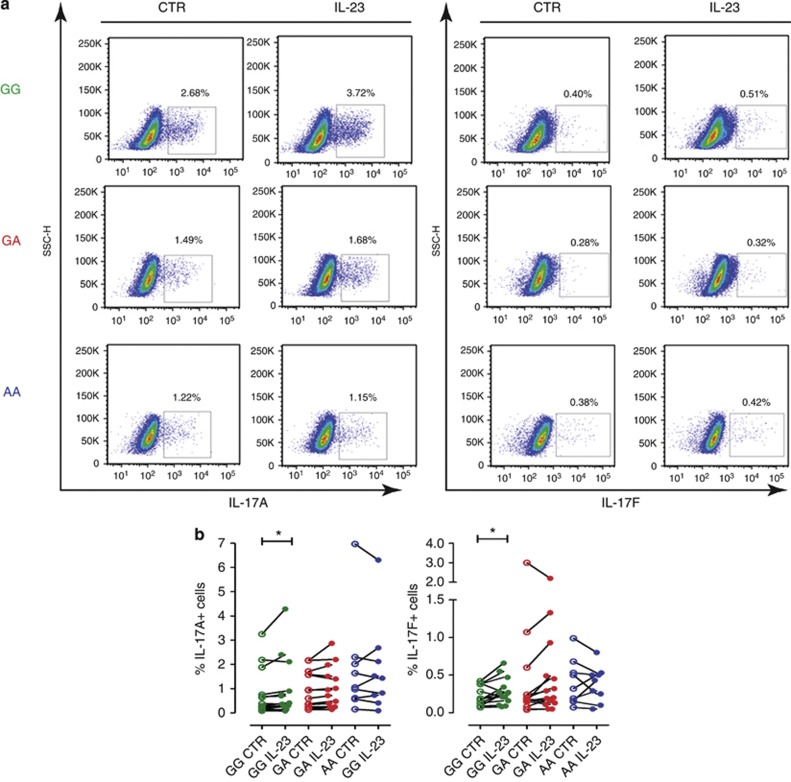
A critical role of IL-23/IL-23R signaling in favoring terminal differentiation, survival, and pathogenicity of effector Th17 cells has been demonstrated (McGeachy et al., 2009). Therefore, we asked whether the IL23R A/Gln 381 allele affects cell expansion/survival. To this end, we polyclonally restimulated memory Th cells previously cultured with or without IL-23, and determined the frequency of Th17 cells. As shown in Figure 3a and b, the frequency of IL-17A+ or IL-17F+ memory Th cells from GG homozygous carriers significantly increased (P<0.05) in the presence of IL-23. On the contrary, the frequency of IL-17A+ and IL-17F+ cells did not increase in either GA heterozygous or AA homozygous donors, suggesting impaired IL-23-driven survival/expansion of memory Th17 cells. Neither frequency of IFNγ+ nor IL-22+ cells was affected by the IL23R genotype (Figure 3).
Figure 3.
IL-23R A/Glu381 allele impairs IL-23-driven survival/expansion of human memory Th17 cells. Memory CD4+ T cells from healthy individuals homozygous for the common G allele (GG, green), heterozygous GA (red), or homozygous for the protective A allele (AA, blue), cultured with or without IL-23 for 72 hours, were assayed for intracellular cytokines IL-17A, IL-17F, IFN-γ, and IL-22, after phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation for 6 hours. (a) Representative dot plots of GG, GA, and AA individual donors. (b) Each connected symbol represents paired samples from one individual donor. Wilcoxon signed rank test (IL-17A: GG and AA, IL-17F: GA) or paired Student's t-test (IL-17A: GA, IL-17F:GG, AA) was performed. *P<0.05. P>0.05 for all other paired comparisons.
Taken together, we found that the minor A/Gln 381 allele modulates responsiveness to IL-23 in human memory Th17 cells, possibly affecting Th17 cell expansion/survival.
Functional investigation of the IL-23R GA/Arg381Gln SNP in psoriatic patients
Although the genetic association of the IL23R GA/Arg381Gln SNP with psoriasis has been extensively replicated, its functional investigation has not been undertaken in psoriasis patients so far.
Therefore, we performed such an investigation in psoriatic patients, either GG homozygous or GA for the IL23R GA/Arg381Gln SNP.
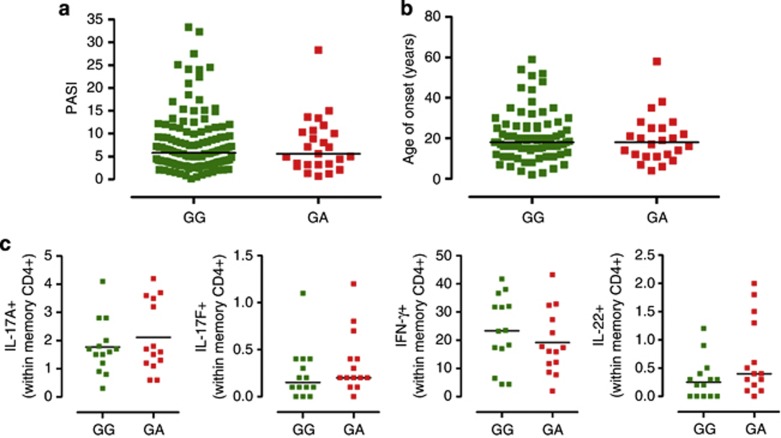
First, we sought to determine whether the IL23R GA/Arg381Gln SNP had any effect on disease severity and age of onset. We found that neither Psoriasis Area Severity Index, as measurement of disease severity (median Psoriasis Area Severity Index GG: 5.8, GA: 5.6; Figure 4a), nor age of onset (median onset GG: 18, GA: 18, Figure 4b) significantly differed in a large patients' cohort having the same median age. Moreover, there was no statistical difference in the frequency of cases of severe psoriasis (Psoriasis Area Severity Index ⩾15) in the two genetic groups (data not shown).
Figure 4.
Effect of IL23R A/Glu381 allele on disease phenotype and Th17 cell development in psoriasis patients. (a) Psoriasis Area Severity Index (PASI) of psoriatic patients homozygous for the common G allele (GG, green dots) and heterozygous (GA, red squares) for the protective A allele. (b) Age at disease onset of psoriatic patients homozygous GG (green squares) and heterozygous GA (red squares). (c) Peripheral blood mononuclear cells from psoriatic patients homozygous GG (green squares) and heterozygous GA (red squares) were assayed for intracellular cytokines, after phorbol 12-myristate 13-acetate (PMA)/ionomycin stimulation for 5 hours. Frequency of IL-17A+, IL-17F+, IFN-γ+, and IL-22+ cells within memory CD4+ gate is shown. Each symbol represents one individual donor; horizontal bars represent mean (c: IL-17A+) or medians (a, b, c: IL-17F+, IFNγ+, IL-22+). Unpaired Student's t-test (c: IL-17A+) or Mann–Whitney test (a, b, c: IL-17F+, IFNγ+, IL-22+) was performed, yielding P>0.05 for all comparisons.
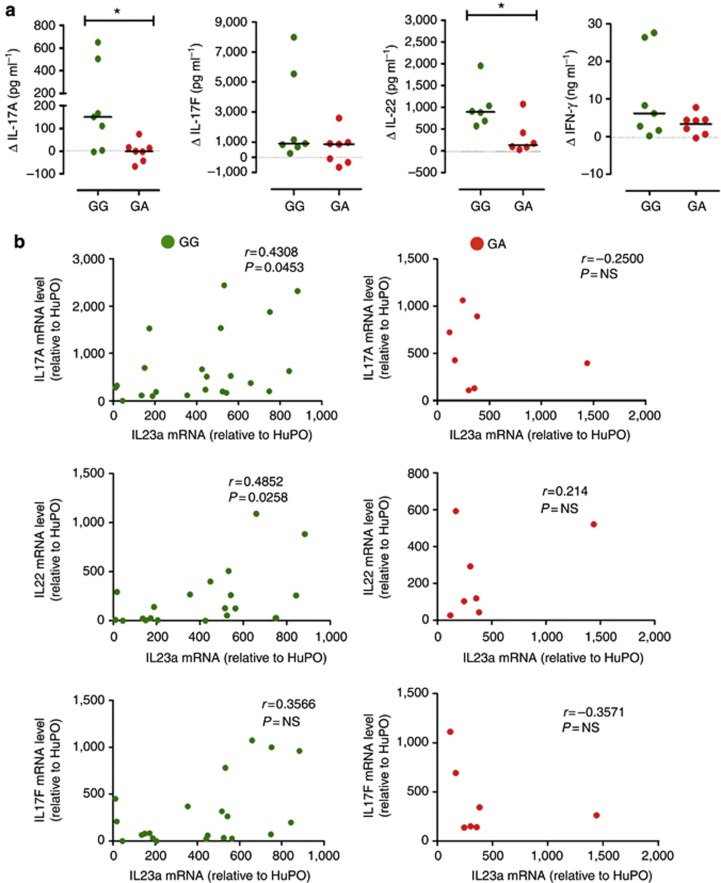
Next, we measured the frequency of circulatory Th17 cells in peripheral blood mononuclear cells (PBMCs) of age-, gender-, and treatment-matched psoriasis patients carrying the two IL23R genotypes (demographics in Supplementary Table S2 online) and found that the frequency of IL-17A+, IL-17F+, IFNγ+, and IL-22+ Th cells did not differ between the GG and GA groups (Figure 4c–f). Moreover, no difference was found in either the frequency of IL-23R+ memory T cells or in IL-23R surface expression (Supplementary Figure S3 online). However, by assessing IL-23-mediated Th17 responses in memory Th cells of age-, gender-, and treatment-matched patients (demographics in Supplementary Table S3 online), we found that GA patients had reduced production of IL-23-induced cytokines, with significantly reduced IL-17A and IL-22 (Δ IL-17A median: GG 151 pg ml−1, from −3.2 to 649 pg ml−1, and GA 0.2 pg ml−1, from −67 to 75 pg ml−1, P< 0.05; Δ IL-22 median: GG 892 pg ml−1, from 572 to 1,953 pg ml−1, and GA 134 pg ml−1, from 31 to 1,070 pg ml−1, P<0.05; Figure 5a–d). Moreover, increase of IL-17F+ cell frequency upon IL-23 culture was significantly reduced in GA heterozygous as compared with GG, and a downward trend was also observed in IL-17A+ and IL-22+ cell frequency (Supplementary Figure S2 online, P<0.05), further suggesting impaired IL-23-driven survival/expansion of memory Th17 cells.
Figure 5.
IL23R A/Glu381 allele reduces IL-23 responsiveness in human memory Th17 cells and interrupts the IL-23/th17 axis in the skin of heterozygous psoriatic patients. (a) Memory CD4+ T cell supernatants from psoriatic patients homozygous GG (green dots) and heterozygous GA (red dots), cultured with or without IL-23 for 72 hours, were assayed for IL-17A, IL-17F, IFN-γ, and IL-22 secretion. Data are shown as differential cytokine production (Δ) in cells cultured with versus without IL-23. Each symbol represents one individual donor. Dotted line denotes Δ=0. Horizontal bars represent medians. Mann–Whitney test was performed; *P<0.05. P>0.05 for all other comparisons. (b) IL-17A, IL-17F, IL-22, and IL-23p19 mRNA levels in psoriasis skin biopsies, relative to human acidic ribosomal protein (HuPO), were measured by quantitative reverse-transcriptase–PCR, and Spearman's correlation between IL-23p19 and IL-17A (top), IL-22 (middle), and IL-17F (bottom) skin mRNA level of GG (left, green dots) or GA (right, red dots) patients was performed.
Finally, we measured IL-17A, IL-17F, IL-22, and IFN-γ and IL-23p19 mRNA levels in psoriasis skin biopsies (demographics in Supplementary Table S4 online). We found no significant difference in skin mRNA of any of the cytokines examined in GA as compared with GG patients (data not shown), thus suggesting that skin cytokines were not affected by either the IL23R genotype or the different treatments. Next, we asked whether there was any correlation between IL-23p19 levels and those of Th17 cytokines in the skin of psoriasis patients. We found that although there was a positive correlation between IL-23p19 and IL-17A levels, as well as IL-22 mRNA levels, in GG patients (IL-23p19/IL-17A Spearman's r=0.4308, P<0.0453; IL-23p19/IL-22 Spearman's r=0.4852, P<0.0258), this correlation was absent in GA patients (IL-23p19/IL-17A Spearman's r=−0.25, P=not significant; IL-23p19/IL-22 Spearman's r=0.214, P=not significant Figure 5e and f), suggesting interruption of the IL-23/Th17 axis in the skin of GA carriers. No correlation was found between IL-23p19 and IL-17F or IFN-γ mRNA level in any genetic group (Figure 5g and data not shown).
Taken together, our data suggest that although not affecting disease severity, the IL23R GA/Arg381Gln SNP still results in attenuated IL-23 signaling and impaired Th17 response, both in blood and in the skin of psoriatic patients.
Discussion
The IL23R GA/Arg381Gln SNP, protecting against several immune-mediated inflammatory disease, such as psoriasis, Crohn's disease, and ankylosing spondylitis, is one of the most replicated and one of the very few that has been functionally validated (Di Meglio et al., 2011; Pidasheva et al., 2011; Sarin et al., 2011).
Here we expand our previous functional investigation using a largely independent cohort of healthy individuals, including carriers of the AA genotype, which, to our knowledge, has not been previously functionally investigated, as well as psoriatic patients. We found attenuated IL-23 signaling in healthy heterozygous individuals, with impaired Th17 cytokine production in memory Th cells. Remarkably, AA homozygous individuals were unresponsive to IL-23, with minimal or no IL-17A and IL-17F production and failure of memory Th17 cell survival/expansion in the presence of IL-23. Moreover, IL-23-induced Th17 responses were also attenuated in age-, gender-, and treatment-matched GA versus GG psoriatic patients.
Unlike Mendelian disorders in which rare disease alleles have major effect, the alleles underlying complex genetic disorders are relatively common and only modestly contribute to disease risk, thus hampering their functional investigation. We and others have recently performed such type of study in healthy GA heterozygous individuals (Di Meglio et al., 2011; Pidasheva et al., 2011; Sarin et al., 2011). However, similar studies have not been undertaken so far in either homozygous carriers of the protective A allele or in psoriatic patients, possibly owing to the rare frequency of the AA genotype (<1% AA homozygous in individuals of Western European descent, HapMap project, public release number 27) and to the likely presence of several confounding genetic and environmental factors in patients. Here we took advantage of the large Cambridge BioResource of volunteers and included in our study nine individuals homozygous for the protective A allele, even though they comprise only 0.36% of the Cambridge population (Nutland and Todd, unpublished).
In keeping with our previous study, we found no difference in either the frequency of IL-23R+ memory Th cells or in IL-23R surface expression in the different genetic groups also in this data set. Moreover, we also confirmed that IL-23 signaling has no role in Th17 lineage commitment, as the frequency of circulating Th17 cells did not differ. However, by using memory Th cells, we found that AA homozygous individuals were unresponsive to IL-23, whereas heterozygous individuals displayed an attenuated response to IL-23, although not statistically significant as in our earlier study in polarized Th17 cells (Di Meglio et al., 2011), possibly because of the different experimental system used. Nevertheless, the striking allele-dosage effect in IL-23 responsiveness, far exceeding the relatively small odds ratio conferred by IL23R GA/Arg381Gln, further supports its protective role in immune-mediated diseases.
Th17 cells and cytokines are increasingly implicated as major culprits in autoimmunity as opposed to their protective role in host immunity against fungal and bacterial infections (Miossec et al., 2009). Mendelian defects in several Th17-related genes, such as IL12b, IL12rb, STAT3, IL17ra, IL17F, cause severe immunodeficiency, marked susceptibility to, and recurrence of, bacterial and fungal infections (de Beaucoudrey et al., 2008; Puel et al., 2011). The lack of Th17 cytokines observed in these cases impairs neutrophil recruitment, macrophage activation, and AMP production by keratinocytes and neutrophils, thus interrupting the cross talk between adaptive and innate immunity required for full pathogen clearance. In contrast, the almost complete ablation of IL-23-induced Th17 response we observed in AA individuals does not seem to predispose to a significant increase in recurring fungal infections, as ascertained by a self-administered questionnaire to our healthy volunteers (data not shown), suggesting that alternative signals can deliver adequate Th17 responses. Although limited to a small number of participants, this finding is clinically relevant in the context of IL-23p19 or IL-23R targeting in immune-mediated diseases, and is in line with the long-term safety data for anti-IL-12/23p40 showing no increased susceptibility to infections (Papp et al., 2013). Interestingly, a study assessing the prognostic significance of genetic variants in the IL-23/Th17 axis for the outcome of T cell–depleted allogeneic stem cell transplantation found that the donor IL23R GA/Arg381Gln genotype was associated with decreased risk of fungal infections and improved patient overall survival, although increased risk of cytomegalovirus infections was detected (Carvalho et al., 2010).
To our knowledge, the functional effect of the IL23R Arg381Gln SNP in individuals affected by psoriasis was previously unreported. We performed our analysis in age-, gender-, and treatment-matched patients as the best possible means of stratification. We are aware that treatment matching may not entirely rule out the effect of therapy on the parameters analyzed and that the IL23R genotype itself might affect response to therapy. Nevertheless, we found attenuated IL-23-induced Th17 responses, with significant reduction in IL-17A and IL-22 production, as well as reduced expansion of IL-17F+ cells in GA patients. The effect of the IL23R SNP on IL-22 production had not been appreciated in healthy donors so far (Figures 2d and 3b and Di Meglio et al., 2011). A possible explanation of why this effect is found only in psoriasis patients could lie in their hypersensitivity to IL-23, as suggested by the increased IL-23R surface expression we have recently shown in the patients versus healthy control (Tonel et al. 2010). Moreover, we could detect a positive correlation between IL-23p19 skin mRNA level and IL-17A and IL-22 in GG, but not in GA patients, thus implying that the IL-23/Th17 axis was impaired in protective genotype carriers.
Th17 cytokines are pivotal in psoriasis immunopathogenesis. IL-17A and IL-17F stimulate keratinocytes to produce neutrophil-recruiting chemokines and AMPs; IL-22 induces epidermal hyperplasia by impairing keratinocyte differentiation and synergizes with IL-17A to induce AMPs (Di Cesare et al., 2009). Circulating Th17 cells are increased in psoriasis and are believed to contribute to the systemic inflammatory disease occurring in patients (Kagami et al., 2010). However, the attenuation of the IL-23/Th17 axis in the GA patients does not seem to have an impact on disease severity or time of onset, most likely because of other genetic and/or environmental factors involved. Interestingly, Bergboer et al. (2012) recently reported that IL23R GA/Arg381Gln SNP is associated with pediatric-onset, but not with adult-onset, psoriasis in a small-scale study. Moreover, our study has shown a putative protective effect of IL23R GA/Arg381Gln SNP in psoriatic patients, raising the question of whether this could be exploited therapeutically. Despite our increased understanding of psoriasis pathogenesis, at least 20% of patients do not respond to anti-tumor necrosis factor and anti-IL-12/23 therapy, possibly because of underdosing in overweight patients (Clark and Lebwohl, 2008), but also suggesting that key pathogenic mechanisms are still ill understood and the known mechanisms might not apply to every individual. As genome-wide association studies identify more psoriasis susceptible genes, the next challenge is not only their functional validation but most and foremost their ultimate application to deliver better patient care. With an increasing number of promising therapeutics aimed at targeting the IL-23/Th17 axis being developed and tested in the clinic, the IL23R GA/Arg381Gln SNP is an ideal candidate to probe as a biomarker for response to treatment. Such studies would be of relevance also to other immune-mediated inflammatory disorders, such as Crohn's disease and ankylosing spondylitis, owing to shared genetic variants, immunological pathways, and therapeutic targets.
Taken together, we have confirmed that the IL23R GA/Arg381Gln SNP confers protection against immune-mediated inflammatory disease by impairing or ablating IL-23-induced Th17 response in both healthy volunteers and psoriatic patients. Therefore, our study paves the way for larger-scale studies in psoriatic patients, aimed to translate insights gleaned from “gene-to-function” studies in the healthy population to stratified medicine approaches delivering the promise of personalized medicine.
Materials and Methods
Subjects
Healthy individuals of European descent were recruited and genotyped for the IL23R GA/Arg381Gln variant in house as previously described (Di Meglio et al., 2011) or through the Cambridge Bioresource. Functional studies were conducted with 45 donors (22 men and 23 women; mean age 43 years, range 24–67 years). Collectively, 174 psoriatic patients of European descent were recruited at St. John's Institute of Dermatology and Guy's and St. Thomas' Hospital, after an examination by expert clinicians. In-house genotyping for IL23R GA/Arg381Gln resulted in the identification of 149GG and 25GA patients. Psoriasis Area Severity Index was obtained for 156 patients; age of disease onset was recorded for 107 patients. Functional studies were conducted with 58 patients (44 men and 14 women; mean age 44 years, range 25–76 years). Full demographics of healthy donors and psoriatic patients are in Supplementary Tables S1–S4 online. Our study was conducted in accordance with the Declaration of Helsinki Principles, with written informed consent obtained from each volunteer and approved by the institutional review board of Guy's and St. Thomas' Hospital (Guy's Research Ethics Committee, Ethics Committee Code: 06/Q0704/18).
Isolation and activation of PBMCs
PBMCs were purified by centrifugation on a density gradient (Lymphoprep, PAA, Pasching, Austria), frozen down in RPMI 1640 (Life Technologies, Carlsbad, CA)/11.25% HSA solution (Gemini Bio-Products, West Sacramento, CA)/10% DMSO (Sigma, St. Louis, MO) and stored in liquid nitrogen. Cells were thawed and rested overnight in complete RPMI 1640 medium containing 10% heat-inactivated human AB serum (Biowhittaker, Walkersville, MD), 50 IU ml−1 penicillin, 50 μg ml−1 streptomycin, and 2 mM L-glutamine (all from Life Technologies) (10% hAB-cRPMI) at 37 °C in humidified 5% CO2/95% air and then activated with PMA (100 ng ml−1, Sigma)/ionomycin (1 μg ml−1, Calbiochem, Darmstadt, Germany) or left unstimulated, in the presence of Golgi Stop (3μM, BD Biosciences, San Jose, CA) and Brefeldin A (10 ng ml−1, BD Biosciences), for 5 hours.
Multiparameter flow cytometry
Dead cells were excluded from the analysis by staining with Live Dead Yellow (Life Technologies). Cells were stained for surface markers, fixed and permeabilized in BD Human FoxP3 Buffer set (BD Biosciences), stained for intracellular cytokines, and acquired on a BD SORP Fortessa (BD Biosciences). Antibodies used are listed in Supplementary Table S5 online. FACS data analysis was performed using the Diva (BD Biosciences) and FlowJo (TreeStar, Ashland,OR) software.
Isolation and activation of CD4+CD45RO+RA− memory T cells
Memory (CD4+CD45RO+RA−) Th cells were isolated from peripheral blood with Rosette Sep Human CD4+T cells enrichment cocktail (StemCells Technologies, Grenoble, France), followed by centrifugation through Lymphoprep and further purification by negative selection using magnetic beads (Dynabeads Pan Mouse IgG, Life Technologies) and anti-CD45RA (Diaclone Gen-Probe Incorporated, San Diego, CA). Purity of CD4+CD45RO+CD45RA− T cells was over 95%.
Memory Th cells were cultured in duplicate at a density of 5 × 105 cellsperml in U-bottomed 5-ml tubes in 10% hAB-cRPMI and activated with anti-CD3/anti-CD28-coated beads (1 bead per 10 cells, Dynabeads CD3/CD28 T cell Expander, Life Technologies) with or without recombinant human IL-23 (100 ng ml−1, R&D Systems, Minneapolis, MN) for 72 hours. On day 4, the supernatant was collected and stored at −80 °C. Cells from duplicated cultures were pulled together and restimulated in fresh 10% hAB-RPMI medium with PMA (10 ng ml−1)/ionomycin (500 ng ml−1), in the presence of Golgi Stop and Brefeldin A, for 6 hours. Multiparameter Flow Cytometry staining was performed as described for PBMCs.
Determination of cytokine levels
IL-17A and IFN-γ cytokine levels in cell supernatants were assayed using the Milliplex MAP Human Cytokine/Chemokine Kit (Merck Millipore, Billerica, MA) and acquired on a FlexiMap 3D flow-based sorting and detection analyzer (Luminex Corporation, Austin, TX). IL-22 and IL-17F cytokine levels were assayed using commercially available ELISA kits (R&D Systems).
RNA extraction and quantitative reverse-transcriptase–PCR
Total RNA from psoriasis skin biopsies was obtained using TRIzol (Life Technologies) and reverse-transcribed into complementary DNA. IL-23, IL-17A, IL-17F, IL-22, and IFNγ expression was assessed by real-time quantitative reverse-transcriptase–PCR using Taqman assays (Life Technologies). For each sample, mRNA abundance was normalized to the amount of human acidic ribosomal protein (HuPO). Data analysis was performed using the ΔΔCt method; results are expressed as relative mRNA levels.
Statistical analysis
For PBMC intracellular cytokine production experiments, frequencies of cytokine-producing cells for each donor belonging to different genetic groups were assessed for normal Gaussian distribution with D'Agostino–Pearson omnibus normality test and analyzed by unpaired two-tailed Student's t-test or Mann–Whitney's test, or by one-way analysis of variance, followed by Bonferroni post test or Kruskal–Wallis test followed by Dunn's multiple-comparison test, as appropriate, by using Prism version 5.0 (GraphPad Software, La Jolla, CA). For cytokine secretion experiments, cell supernatants were assayed in duplicate, and mean±SEM was calculated. Results, expressed as differential cytokine production (Δ) in cells cultured with versus without IL-23, were assessed for normal Gaussian distribution and then analyzed by unpaired two-tailed Student's t-test, Mann–Whitney's test, one-way analysis of variance, followed by Bonferroni post test or Kruskal–Wallis test followed by Dunn's multiple-comparison test, as appropriate. For survival/expansion experiments of cultured memory Th cells, frequencies of cytokine-producing cell for each donor belonging to the different genetic groups were assessed for normal Gaussian distribution and analyzed by Wilcoxon signed rank test. Spearman's correlation was used to correlate skin IL-23 mRNA levels with those of IL-17A, IL-22, IL-17F, and IFNγ. Values of P<0.05 were considered significant.
Acknowledgments
We gratefully acknowledge the participation of all our in-house healthy volunteers, Cambridge BioResource subjects, and psoriatic patients attending St. John's Institute of Dermatology clinic; their support, trust, and cooperation were essential for the collection of the data used in this study. We thank A. Di Cesare, D. Smyth, and the Diabetes and Inflammation Laboratory's sample processing team for DNA sample preparation and for genotype double scoring. We thank H. Sreeneebus, S. Jones, G. Perera, P. Tagart, J.Rice, J. Casey, and the Skin Therapy Research Unit at St. John's Institute of Dermatology for blood and/or skin sample collection. We thank U. Laggner and C.-C. Chu for their contribution to blood sample processing, L. Dunajova for her contribution to RNA preparation, M. Woodburn for his contribution to sample management, and A. Lindsay for administrative support. We thank S. Heck and P.J. Chana from the Biomedical Research Centre Flow Cytometry Core Laboratory for their assistance. We acknowledge support by the following grant funding bodies: Wyeth Advances in Psoriasis Research Grant Programme Award (F.O.N. and P.D.M.), Wellcome Trust Programme GR078173MA (F.O.N.), Medical Research Council UK Programme G0601387 (F.O.N.), NIHR Cambridge Biomedical Research Centre (Cambridge BioResource), and Medical Research Council Clinical Training Research Fellowship (R.K.M.). The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St. Thomas' NHS Foundation Trust, and King's College London.
Glossary
- PBMC
peripheral blood mononuclear cell
- PMA
phorbol 12-myristate 13-acetate
- SNP
single-nucleotide polymorphism
- Th
T helper
F.O.N. has provided consultant advice to Celgene, Abbott Laboratories, Pfizer, Novartis, and Janssen Cilag. F.O.N and P.D.M. have been awarded an unrestricted Advances in Psoriasis Research Grant Programme Award from Wyeth, now Pfizer. All other authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Bergboer JG, Oostveen AM, de Jager ME, et al. Paediatric-onset psoriasis is associated with ERAP1 and IL23R loci, LCE3C_LCE3B deletion and HLA-C*06. Br J Dermatol. 2012;167:922–925. doi: 10.1111/j.1365-2133.2012.10992.x. [DOI] [PubMed] [Google Scholar]
- Boniface K, Blumenschein WM, Brovont-Porth K, et al. Human Th17 cells comprise heterogeneous subsets including IFN-gamma-producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679–87. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- Capon F, Di Meglio P, Szaub J, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201–6. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–90. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Cunha C, Di Ianni M, et al. Prognostic significance of genetic variants in the IL-23/Th17 pathway for the outcome of T cell-depleted allogeneic stem cell transplantation. Bone Marrow Transplant. 2010;45:1645–52. doi: 10.1038/bmt.2010.28. [DOI] [PubMed] [Google Scholar]
- Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2577–87. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443–6. doi: 10.1016/j.jaad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–50. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–50. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- Di Meglio P, Di Cesare A, Laggner U, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS ONE. 2011;6:e17160. doi: 10.1371/journal.pone.0017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JT, Bruce AT, Gudjonsson JE, et al. Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol. 2010;130:1213–26. doi: 10.1038/jid.2009.319. [DOI] [PubMed] [Google Scholar]
- Fitch E, Harper E, Skorcheva I, et al. Pathophysiology of psoriasis: recent advances on IL-23 and Th17 cytokines. Curr Rheumatol Rep. 2007;9:461–7. doi: 10.1007/s11926-007-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb AB, Chamian F, Masud S, et al. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J Immunol. 2005;175:2721–9. doi: 10.4049/jimmunol.175.4.2721. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–28. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–32. doi: 10.1016/j.jaci.2011.01.054. [DOI] [PubMed] [Google Scholar]
- Hernandez-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–35. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–83. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–9. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–11. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–24. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11:505–518. doi: 10.1038/nri3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from five years of follow-up. Br J Dermatol. 2013;168:844–54. doi: 10.1111/bjd.12214. [DOI] [PubMed] [Google Scholar]
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–9. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol. 2012;7:385–422. doi: 10.1146/annurev-pathol-011811-132448. [DOI] [PubMed] [Google Scholar]
- Pidasheva S, Trifari S, Phillips A, et al. Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381Q. PLoS ONE. 2011;6:e25038. doi: 10.1371/journal.pone.0025038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–8. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo HL, Kagami S, Phillips KG, et al. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186:1495–1502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- Rueda B, Orozco G, Raya E, et al. The IL23R Arg381Gln non-synonymous polymorphism confers susceptibility to ankylosing spondylitis. Ann Rheum Dis. 2008;67:1451–4. doi: 10.1136/ard.2007.080283. [DOI] [PubMed] [Google Scholar]
- Sarin R, Wu X, Abraham C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc Natl Acad Sci USA. 2011;108:9560–5. doi: 10.1073/pnas.1017854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonel G, Conrad C, Laggner U, et al. Cutting edge: A critical functional role for IL-23 in psoriasis. J Immunol. 2010;185:5688–91. doi: 10.4049/jimmunol.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi LC, Spain SL, Knight J, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet. 2012;44:1341–8. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–45. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Volpe E, Touzot M, Servant N, et al. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood. 2009;114:3610–4. doi: 10.1182/blood-2009-05-223768. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183–94. doi: 10.1084/jem.20071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.