Abstract
Background: Prior research suggests that vitamin D protects against lung cancer only among certain subgroups.
Objectives: We investigated whether vitamin D intake was associated with lung cancer and explored whether vitamin A intake modified the association.
Design: Prospective cohort data from 128,779 postmenopausal women, including 1771 incident lung cancers in the Women's Health Initiative (Clinical Trials and Observational Study) 1993–2010, were analyzed. Twelve percent of women received active intervention (1 g Ca + 400 IU vitamin D3/d) in the Calcium/Vitamin D Trial. Baseline total intake included both dietary intake (from food-frequency questionnaires) and supplement intake (from bottle labels). HRs were estimated by Cox proportional hazard models.
Results: No significant association was observed overall. Among never smokers, a total vitamin D intake ≥400 IU/d was significantly associated with lower risks of lung cancer (HR: 0.37; 95% CI: 0.18, 0.77 for ≥800 compared with <100 IU/d; P-trend = 0.01). No significant effect modification of total vitamin A intake on the association between total vitamin D intake and lung cancer was found. However, the Calcium/Vitamin D Trial active intervention was significantly associated with a lower lung cancer risk only among women with a vitamin A intake <1000 μg/d retinol activity equivalents (HR: 0.69; 95% CI: 0.50, 0.96; P-interaction = 0.09).
Conclusions: Vitamin D intake was associated with a lower lung cancer risk in never-smoking, postmenopausal women. Lower vitamin A intake may be important for a beneficial association of 1 g Ca + 400 IU vitamin D3 supplementation with lung cancer. This trial was registered at clinicaltrials.gov as NCT00000611.
INTRODUCTION
Lung cancer is the leading cause of cancer death in women in the United States (1). In addition to smoking cessation, novel prevention strategies are needed because half of lung cancer cases in women are not attributed to smoking (2).
Whereas associations of vitamin D intake or status with several cancer sites have been proposed (3), current evidence is controversial (4), and few studies have examined the association between lung cancer and vitamin D. Two Finnish studies showed nonsignificant, inverse associations of serum concentrations of 25-hydroxyvitamin D—the standard biomarker for assessing vitamin D status—with lung cancer risk overall, although the associations were significant in subgroups, including those whose blood was drawn during darker months (5) and in women (6). In US populations, high serum 25-hydroxyvitamin D concentrations were significantly associated with higher lung cancer mortality in men (7), but with lower lung cancer mortality in nonsmoking men and women (8). Another US study showed a nonsignificant, inverse association between lung cancer incidence and plasma 25-hydroxyvitamin D concentrations predicted from vitamin D intake and demographic and lifestyle factors in men (9). Finally, in the Women's Health Initiative (WHI)4, randomized, placebo-controlled trial supplementation with daily calcium carbonate (1 g) and vitamin D3 (400 IU) in otherwise healthy postmenopausal women resulted in fewer lung cancers in the supplement group [109 (0.09% annualized rate) compared with 126 (0.10%); HR: 0.86; 95% CI: 0.67, 1.12], but the difference was not statistically significant (P = 0.26) (10).
In addition to the 36,282 postmenopausal women participating in the WHI Calcium/Vitamin D supplementation trial, 125,526 other postmenopausal women participated in the WHI Observational Study or the 2 other WHI Clinical Trials. In this study, we used the entire WHI population to determine whether total vitamin D intake (diet plus supplements) was associated with lung cancer risk. In addition, recent data suggest that excess circulating vitamin A may attenuate a beneficial association of 25-hydroxyvitamin D concentrations with lung cancer mortality (8). The biological mechanism involves excess cellular 9-cis-retinoic acid, an active metabolite of vitamin A, competing for retinoid X receptor with vitamin D receptor (11, 12). We investigated this hypothesis by examining effect modification of vitamin A intake on the association between vitamin D intake and lung cancer.
SUBJECTS AND METHODS
WHI overview
Eligible, interested, and consenting women aged 50–79 y at baseline joined the WHI between 1993 and 1998, either in one of the clinical trials (n = 68,132) or the observational study (n = 93,676). The 3 clinical trial components included trials of hormone therapy for women with or without a uterus (without a uterus, estrogen only compared with placebo, n = 10,739; with a uterus, estrogen plus progesterone compared with placebo, n = 16,608) and dietary modification behavioral intervention, ie, a low-fat dietary pattern compared with a comparison group (n = 48,835). The third trial was offered to women participating in one of the hormone therapy trials or the dietary modification trial: Calcium/Vitamin D supplementation compared with placebo (n = 36,282) (13). A partial factorial design was used for the clinical trial program, whereby participants could be randomly assigned to 1, 2, or all 3 of the components, thus providing a cost-efficient model.
Study participants
The current study included all WHI participants in the Clinical Trials and Observational Study. We excluded participants who had 1) a history of conditions that affect vitamin D and/or calcium metabolism, including ulcerative colitis, Crohn disease, part of the intestines removed, high blood calcium, liver diseases, dialysis for kidney failure, and a malignancy other than nonmelanoma skin carcinoma (14, 15) (n = 22,955); 2) an implausible BMI (in kg/m2; <15.0 or >50.0; n = 854) and/or an estimated energy intake from a baseline food-frequency questionnaire [<600 or >4000 kcal/d (n = 4598)]; and 3) missing data on baseline intake from diet (n = 299), supplement use (n = 2), follow-up time (n = 697), or covariates for multivariate analyses (n = 4698). As a result, 128,799 participants entered statistical analyses.
Outcome ascertainment
Participants reported lung cancer diagnoses at each follow-up semiannually in the Clinical Trials and annually in the Observational Study. Trained study physicians, blinded to WHI study components and randomization allocations, at local clinics confirmed and adjudicated cases by reviewing medical records (16). Tumor histologic subtype was coded by using the Surveillance, Epidemiology, and End Results guidelines (17). As of 30 September 2010, the current study included 1701 incident cases of lung cancer; 99.5% (1693) cases had tumor histologic data. Median follow-up was 12.7 y, and 6.7% of women were lost to follow-up.
Assessment of dietary and supplemental intake
Dietary intake at baseline was assessed by a self-administered food-frequency questionnaire (FFQ) developed specifically for the WHI (18). Among the subgroup of women who also completed an additional dietary intake assessment, correlation coefficients between the FFQ and 8 d of dietary intake (four 24-h recalls and a 4-d food record) were 0.70 for vitamin D, 0.30 for retinol, and 0.52 for β-carotene. Nutrient values were calculated based on the Nutrition Data Systems for Research version 2006, University of Minnesota Nutrition Coordinating Center food and nutrient database augmented with manufacturers’ data. Information on usual use of vitamin and mineral supplements was collected by a simplified inventory system (19). Participants were asked to bring their supplement bottles to the baseline clinic visit, and trained staff entered doses of vitamins and minerals based on the bottle labels. Only supplements used once per week or more were transcribed. The frequency (pills per week) and duration (months taken last year and total years taken) of use were also queried. The median duration of vitamin D supplement use was 5 y (IQR: 2–11 y) among the users.
For both Clinical Trials and Observational Study participants, the average daily intake of total vitamin D, vitamin A, and calcium were calculated by summing food and supplement sources together. Vitamin A was expressed as μg retinol activity equivalent (RAE) because it consists of a wide range of compounds, including retinol and carotenoids. The calculations of RAE for dietary and supplemental intake were as follows (20):
 |
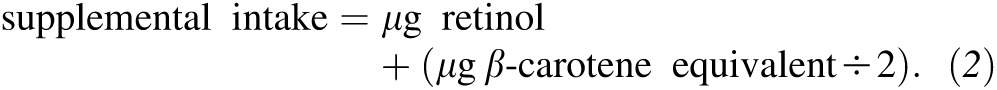 |
For participants in the active intervention arm of the Calcium/Vitamin D Trial, we did not combine their vitamin D intake from the intervention supplementation (400 IU/d) with the estimated average daily intake from food and supplements because the intervention began 12–24 mo after baseline and continued for 8 y with close monitoring. We therefore treated the Calcium/Vitamin D Trial as a separate indicator variable in regression models. Calcium/Vitamin D Trial participants were allowed to continue their own personal use of calcium and vitamin supplements as long as the dose of vitamin D did not exceed 600 IU (1000 IU from 1999 onward). Adherence to intervention was assessed by weighing returned pill bottles; ≥50% of the participants adhered to ≥80% or more of the study medication throughout the trial (21).
Covariate assessment
Covariates—including age, race-ethnicity, education level, hormone use, smoking habits, and physical activity at baseline—were collected by standardized, self-administered questionnaires. Participants were asked whether they had smoked ≥100 cigarettes in their lifetime and if they smoked currently to identify current and former smokers; an individual was a former smoker if she did not smoke currently but had smoked in the past. The number of cigarettes smoked per day and the number of years as a regular smoker were also queried. Never smokers were defined as those who had not smoked >100 cigarettes in their lifetime (22). Weight and height at baseline were measured by trained staff. Baseline data on environmental tobacco smoke (ETS) exposure at home and at work since age 18 y and number of months and average duration of doing yard work (such as mowing, raking, gardening, or shoveling snow) were collected only in the Observational Study. Information on sun exposure history, including time spent outdoors and current usual sunscreen use was collected from the follow-up year 4 questionnaire in the Observational Study (23). Time spent outdoors in summer and other seasons was queried both for the current year (ie, the time of WHI assessment) and between 30 and 39 y of age.
Statistical analysis
Lung cancer risks were estimated for categorical (<100, 100 to <200, 200 to <400, 400 to <600, 600 to <800, and ≥800 IU/d) and linear (per 100-IU/d increment) total vitamin D intakes in separate models. We chose these cutoffs because they reflect population intake levels and are relevant to maintaining desirable vitamin D status for bone health: 200 IU/d, approximately the median level of US women in all ages; 400 IU/d, the Estimated Average Requirement for all ages; 600 and 800 IU/d, the Recommended Dietary Allowance for ≤70 y and ≥71 y, respectively (24). The categories were restratified into fewer categories for analyses by histology (<100, 100 to <400, and ≥400 IU/d) and for effect modification analyses (<400 and ≥400 IU/d) to maintain sufficient numbers of lung cancer cases in each stratum. HRs and 95% CIs for lung cancer were estimated by Cox's proportional hazards models. Participants contributed follow-up time from the enrollment to the date of lung cancer diagnosis, date of death from causes other than lung cancer, the last documented follow-up contact, or 30 September 2010, whichever came first. The proportionality assumption was examined by testing whether scaled Schoenfeld residuals for total vitamin D intake were associated with survival time (25); the assumption was fulfilled (P = 0.81). Multivariate models for assessing the association of total vitamin D intake included baseline covariates that were chosen a priori: age (continuous), region (northeast, south, midwest, west), race-ethnicity (non-Hispanic White, black/African American, Hispanic, other), education level (high school or less, school after high school, college degree or higher, unknown), treatment assignments of the Hormone Therapy trials (26), Calcium/Vitamin D Trial active intervention, BMI (<25.0, 25.0 to <30.0, or ≥30.0), frequency of walking outside ≥10 min (rarely/never, 1–3 times/mo, 1 time/wk, 2–3 times/wk, 4–6 times/wk, or ≥7 times/wk), smoking status (current, former, or never), number of cigarettes smoked per day (<1, 1–4, 5–14, 15–24, 25–34, 35–44, or ≥45), total years of smoking (<5, 5–9, 10–19, 20–29, 30–39, 40–49, or ≥50), total vitamin A intake (<700, 700 to <2000, or ≥2000 μg/d RAE), total calcium intake (<800, 800 to <1500, 1500 mg/d), and energy intake (continuous). Study center region and time of walking outdoors were proxies for sunlight exposure (27, 28). Although the use of hormone therapy may increase serum 25-hydroxyvitamin D concentrations (27), there was no interaction of the Hormone Therapy Trial interventions with total vitamin D intake (P = 0.59; likelihood ratio test) or the Calcium/Vitamin D Trial intervention (P = 0.63; likelihood ratio test) for lung cancer risk. Additional baseline variables, including WHI study components, treatment assignments of the Dietary Modification Trial, use of oral contraceptives, use of hormone replacement therapy, history of nonmelanoma skin cancer, and alcohol use, made no meaningful contribution to models or changes to risk estimates in all women or never smokers. Thus, only the a priori set of covariates was included in the final models. Linear trends of risk estimates were examined by Wald tests (1 df) of an ordinal variable of total vitamin D intake categories. Cox models were performed for all women and by a priori smoking status subgroups (current, former, and never smokers) whenever possible. Calcium/Vitamin D Trial active intervention was modeled as a time-dependent variable, allowing the hazard of lung cancer to vary before, during, and after (2005 onward) the trial (25). Risks for a histologic subtype of lung cancer were estimated by competing risk models, censoring the other subtypes in addition to deaths and the end of follow-up (25). We evaluated whether preclinical lung cancer affected vitamin D intake by excluding women with a diagnosis of lung cancer made within 2 y of study entry. To evaluate the potential healthy user effect of multivitamin supplement use (29), lung cancer risks were estimated among participants who did not use multivitamin supplements.
To evaluate effect modification, we stratified the associations of total vitamin D intake and Calcium/Vitamin D Trial active intervention by total vitamin A intake category (≥3000, 2999–1000, <1000 μg/d RAE). We considered total vitamin A intake ≥3000 μg/d RAE as excess intake because it is the Tolerable Upper Intake Level for preventing liver toxicity in adults (20). Also, a vitamin A intake ≥3000 μg/d RAE can lead to excess circulating vitamin A (ie, serum retinyl esters concentrations ≥7.0 μg/dL) in elderly (30–32). The lower cutoff of 1000 μg/d RAE was chosen because the level is relatively close to the Dietary Reference Intake (900 and 700 μg/d RAE for males and females, respectively) (20). Interaction was examined by likelihood ratio tests (1 df) on separate models before and after entering a cross-product term of total vitamin D intake or Calcium/Vitamin D Trial active intervention and total vitamin A intake (all ordinal variables).
Sensitivity analyses were conducted by further considering ETS and sun exposure as confounders in Observational Study participants. First, we additionally included ever ETS exposure at home (yes or no) and at work (yes or no) (33), months of yard work per year (<1, 1–6, or ≥7 mo), and weekly duration of yard work (<0.5, 0.5–2, or >2 h) in main-effect models. Second, the main effect was stratified by time spent outdoors (<0.5, 0.5–2, or >2 h/d), additionally adjusting usual sunscreen use (no use, sun protective factor 2–24, or sun protective factor ≥25). Participants in this analysis contributed person-years from the date when the year 4 questionnaire was returned. In addition, we performed lag analyses for the Calcium/Vitamin D Trial active intervention by assessing the effect 1 and 2 y after the randomization and by extending the follow-up to years 1, 2, and 3 in the posttrial period. Also, because high calcium intake is associated with a lower risk of lung cancer (34, 35), we stratified data by total calcium intake (≥1 or <1 g/d) to investigate whether it modified the association of the Calcium/Vitamin D Trial active intervention with lung cancer risk. All statistical tests were 2-sided; statistical significance was defined as P < 0.05. Statistical analyses were conducted by using STATA (version 12.0; StataCorp).
RESULTS
Select baseline characteristics of the participants by their total vitamin D intake are shown in Table 1. Higher vitamin D intake was more likely to be observed among those of older age, with a lower BMI, living in northeast or midwest regions, who were non-Hispanic whites, with high education attainment, not participating in the Hormone Therapy Trials or Calcium/Vitamin D Trial, walking outside more frequently, and who never smoked (all P < 0.001).
TABLE 1.
Baseline characteristics of participants in the WHI Clinical Trials and Observational Study, 1993–20101
| Total vitamin D intake (IU/d) |
|||||||
| Total | <100 | 100 to <200 | 200 to <400 | 400 to <600 | 600 to <800 | ≥800 | |
| No. of participants | 128,779 | 20,003 | 28,484 | 24,042 | 31,567 | 16,651 | 8032 |
| Total vitamin D intake (IU/d) | 370.9 ± 277.02 | 66.8 ± 21.9 | 146.3 ± 28.4 | 281.6 ± 56.9 | 508.5 ± 51.4 | 682.5 ± 56.3 | 1004.9 ± 298.1 |
| Dietary vitamin D intake (IU/d) | 175.0 ± 120.8 | 66.6 ± 21.9 | 142.2 ± (32.1) | 228.6 ± 85.1 | 157.1 ± 115.1 | 243.7 ± 117.6 | 329.2 ± 224.3 |
| Supplemental vitamin D intake (IU/d) | 195.9 ± 246.7 | 0.2 ± 3.0 | 4.2 ± 20.5 | 53.0 ± 85.4 | 351.4 ± 122.4 | 438.8 ± 115.3 | 675.7 ± 362.4 |
| Vitamin D supplement use (%) | 48.1 | 0.5 | 4.6 | 31.4 | 91.7 | 97.4 | 97.4 |
| Total vitamin A intake (μg/d RAE) | 1711.7 ± 1705.6 | 692.3 ± 953.4 | 907.7 ± 1054.5 | 1241.0 ± 1432.9 | 2434.6 ± 1682.8 | 2770.7 ± 1570.0 | 3473.3 ± 2242.2 |
| Total retinol intake (μg/d) | 962.5 ± 1056.4 | 345.6 ± 638.5 | 519.5 ± 771.8 | 736.0 ± 775.3 | 1334.2 ± 1064.2 | 1556.2 ± 972.3 | 2057.0 ± 1512.5 |
| Vitamin A supplement use (%) | 47.9 | 9.1 | 10.9 | 23.6 | 87.5 | 95.2 | 94.4 |
| Multivitamin supplement use (%) | 39.2 | 0.2 | 1.4 | 11.2 | 79.9 | 90.1 | 88.0 |
| Age (y) | 63.0 ± 7.2 | 62.1 ± 7.3 | 62.7 ± 7.2 | 63.0 ± 7.1 | 63.3 ± 7.2 | 63.9 ± 7.0 | 63.9 ± 7.1 |
| BMI (kg/m2) | 27.8 ± 5.6 | 28.0 ± 5.6 | 28.3 ± 5.7 | 28.1 ± 5.7 | 27.4 ± 5.4 | 27.5 ± 5.4 | 27.2 ± 5.4 |
| Region (%) | |||||||
| Northeast | 23.5 | 20.2 | 23.9 | 24.6 | 23.5 | 25.6 | 22.9 |
| South | 25.0 | 29.6 | 27.6 | 24.2 | 22.9 | 21.5 | 22.0 |
| Midwest | 22.2 | 20.0 | 20.9 | 24.3 | 21.7 | 24.1 | 23.6 |
| West | 29.3 | 30.2 | 27.6 | 26.9 | 31.9 | 28.8 | 31.5 |
| Race-ethnicity (%) | |||||||
| Black/African American | 8.4 | 16.1 | 10.2 | 7.3 | 6.6 | 3.7 | 3.8 |
| Hispanic/Latino | 3.8 | 6.4 | 4.4 | 3.6 | 3.1 | 2.3 | 2.1 |
| Non-Hispanic white | 83.3 | 71.6 | 80.8 | 84.9 | 86.0 | 90.6 | 90.2 |
| Other3 | 4.5 | 5.8 | 4.6 | 4.3 | 4.3 | 3.4 | 3.9 |
| Education (%) | |||||||
| High school or less | 21.9 | 26.6 | 24.1 | 21.0 | 20.6 | 18.4 | 17.3 |
| School after high school | 37.4 | 39.2 | 37.4 | 36.7 | 37.5 | 36.7 | 36.7 |
| College degree or higher | 40.0 | 33.4 | 37.8 | 41.6 | 41.3 | 44.4 | 45.4 |
| Unknown | 0.7 | 0.8 | 0.7 | 0.7 | 0.7 | 0.6 | 0.6 |
| Participated in the Hormone Therapy Trial (%) | 18.0 | 30.7 | 19.9 | 18.2 | 16.6 | 15.5 | 14.8 |
| Participated in the Calcium/Vitamin D Trial (%) | 24.4 | 25.1 | 26.5 | 26.3 | 23.3 | 22.0 | 19.4 |
| Frequency of walking outside >10 min (%) | |||||||
| Rarely/never | 17.0 | 20.6 | 18.2 | 16.5 | 16.0 | 14.8 | 14.5 |
| 1–3 times/mo | 15.1 | 16.2 | 15.7 | 15.1 | 14.9 | 13.8 | 13.8 |
| 1 time/wk | 10.9 | 10.4 | 11.2 | 11.5 | 10.4 | 11.0 | 10.3 |
| 2–3 times/wk | 27.3 | 25.3 | 26.9 | 27.6 | 27.9 | 28.1 | 28.5 |
| 4–6 times/wk | 21.6 | 19.8 | 20.3 | 21.3 | 22.5 | 23.5 | 23.8 |
| ≥7 times/wk | 8.1 | 7.7 | 7.7 | 8.0 | 8.2 | 8.7 | 9.2 |
| Smoking status (%) | |||||||
| Current smoker | 7.3 | 10.1 | 8.5 | 6.7 | 6.7 | 5.3 | 5.4 |
| Former smoker | 40.3 | 39.1 | 40.0 | 38.9 | 41.5 | 41.8 | 40.6 |
| Never smoker | 52.4 | 50.8 | 51.6 | 54.4 | 51.9 | 52.9 | 54.0 |
All characteristics were significantly different by vitamin D intake category (all P < 0.001, chi-square tests for categorical variables and F tests for continuous variables). RAE, retinol activity equivalent; WHI, Women's Health Initiative.
Mean ± SD (all such values).
Includes American Indian, Alaska Native, Asian, Pacific Islander, other races, and unknown.
Multivariate-adjusted associations of total vitamin D intake with lung cancer risk are shown in Table 2. No significant associations were observed among all women, current smokers, or former smokers. Among never smokers, lower risks of lung cancer were observed among those with total vitamin D intake ≥100 IU/d. Compared with <100 IU/d, total vitamin D intake ≥400 IU/d was significantly associated with lower lung cancer risks (HR: 0.55; 95% CI: 0.31, 0.83), 0.55 (0.31, 0.96), and 0.37 (0.18, 0.77) for 400 to <600, 600 to <800, and ≥800 IU/d, respectively; P-trend = 0.01). The observed associations did not materially change after further adjustment for ETS exposure and time doing yard work (see Supplemental Table 1 under “Supplemental data” in the online issue) among multivitamin nonusers (see Supplemental Table 2 under “Supplemental data” in the online issue) or after exclusion of lung cancer cases diagnosed within the first 2 y of follow-up (data not shown). In the analysis of histologic subtypes (Table 3), total vitamin D intake ≥400 compared with <100 IU/d was significantly associated with lower risks of non–small cell lung cancer; HR: 0.37; 95% CI: 0.22, 0.64; P-trend < 0.001) among never smokers (P-interaction by smoking status = 0.029). No significant associations were observed for squamous cell carcinoma and small cell lung cancer with total vitamin D intake ≥400 compared with <100 IU/d (Table 3) or ≥400 compared with <400 IU/d (see Supplemental Table 3 under “Supplemental data” in the online issue).
TABLE 2.
Multivariate-adjusted lung cancer risk by total vitamin D intake category: WHI Clinical Trials and Observational Study, 1993–2010 (n = 128,779)1
| Total vitamin D intake (IU/d)2 |
||||||||
| Per 100 IU | <100 | 100 to <200 | 200 to <400 | 400 to <600 | 600 to <800 | ≥800 | P-trend | |
| All women (n) | 1701 | 291 | 385 | 283 | 418 | 218 | 106 | |
| HR (95% CI) | 1.00 (0.97, 1.02) | 1.00 (Ref) | 0.94 (0.80, 1.10) | 0.90 (0.74, 1.09) | 0.88 (0.73, 1.07) | 0.90 (0.71, 1.14) | 0.92 (0.69, 1.21) | 0.39 |
| Current smokers (n) | 527 | 99 | 130 | 87 | 119 | 65 | 27 | |
| HR (95% CI) | 0.99 (0.94, 1.04) | 1.00 (Ref) | 1.15 (0.88, 1.52) | 1.29 (0.91, 1.81) | 1.04 (0.73, 1.49) | 1.40 (0.91, 2.17) | 1.03 (0.60, 1.76) | 0.53 |
| Former smokers (n) | 896 | 147 | 201 | 135 | 236 | 112 | 62 | |
| HR (95% CI) | 1.01 (0.98, 1.04) | 1.00 (Ref) | 0.91 (0.73, 1.14) | 0.77 (0.59, 1.01) | 0.91 (0.70, 1.19) | 0.81 (0.59, 1.11) | 1.07 (0.74, 1.55) | 0.76 |
| Never smokers (n) | 278 | 45 | 54 | 61 | 63 | 41 | 14 | |
| HR (95% CI) | 0.94 (0.88, 1.01) | 1.00 (Ref) | 0.68 (0.45, 1.03) | 0.71 (0.45, 1.12) | 0.55 (0.31, 0.83) | 0.55 (0.31, 0.96) | 0.37 (0.18, 0.77) | 0.01 |
Adjusted for age, region, race-ethnicity, education, Hormone Therapy Trial treatment assignment, Calcium/Vitamin D Trial active intervention (time-dependent), BMI, smoking status (for all women only), number of cigarettes per day (for all women and current and former smokers), duration of regular smoking in years (for all women and current and former smokers), frequency of walking outside for >10 min, total vitamin A intake, total calcium intake, and energy intake. Ref, referent; WHI, Women's Health Initiative.
Baseline total vitamin D intake was assessed for all WHI participants. The intake does not include the active intervention (1 g Ca + 400 IU vitamin D3/d) of the Calcium/Vitamin D Trial. P = 0.07 for the interaction of total vitamin D intake and smoking for lung cancer risk (likelihood ratio test).
TABLE 3.
Multivariate-adjusted lung cancer risk within histologic subtypes by total vitamin D intake category: WHI Clinical Trials and Observational Study, 1993–20101
| Total vitamin D intake (IU/d)3 |
|||||
| Histologic subtype2 | Per 100 IU | <100 | 100 to <400 | ≥400 | P-trend |
| Non–small cell lung cancer | |||||
| All women (n) | 1104 | 198 | 419 | 487 | |
| HR (95% CI) | 0.98 (0.95, 1.01) | 1.00 (Ref) | 0.85 (0.71, 1.03) | 0.80 (0.63, 1.01) | 0.06 |
| Current smokers (n) | 306 | 56 | 123 | 127 | |
| HR (95% CI) | 0.99 (0.93, 1.05) | 1.00 (Ref) | 1.25 (0.88, 1.77) | 1.19 (0.76, 1.86) | 0.43 |
| Former smokers (n) | 604 | 108 | 216 | 280 | |
| HR (95% CI) | 0.99 (0.95, 1.03) | 1.00 (Ref) | 0.76 (0.59, 0.98) | 0.80 (0.59, 1.09) | 0.21 |
| Never smokers (n) | 194 | 34 | 80 | 80 | |
| HR (95% CI) | 0.92 (0.85, 1.00) | 1.00 (Ref) | 0.63 (0.40, 0.99) | 0.37 (0.22, 0.64) | <0.001 |
| Adenocarcinoma | |||||
| All women (n) | 785 | 139 | 306 | 340 | |
| HR (95% CI) | 0.97 (0.93, 1.00) | 1.00 (Ref) | 0.88 (0.70, 1.10) | 0.77 (0.59, 1.02) | 0.07 |
| Current smokers, n | 176 | 33 | 75 | 68 | |
| HR (95% CI) | 0.98 (0.90, 1.07) | 1.00 (Ref) | 1.48 (0.95, 2.30) | 1.34 (0.74, 2.45) | 0.27 |
| Former smokers (n) | 437 | 75 | 159 | 203 | |
| HR (95% CI) | 0.98 (0.93, 1.03) | 1.00 (Ref) | 0.79 (0.58, 1.07) | 0.83 (0.57, 1.19) | 0.36 |
| Never smokers (n) | 172 | 31 | 72 | 69 | |
| HR (95% CI) | 0.91 (0.83, 0.99) | 1.00 (Ref) | 0.62 (0.39, 1.00) | 0.34 (0.19, 0.60) | <0.001 |
| Squamous cell carcinoma4 | |||||
| All women (n) | 236 | 45 | 77 | 114 | |
| HR (95% CI) | 1.03 (0.97, 1.09) | 1.00 (Ref) | 0.69 (0.45, 1.04) | 0.86 (0.52, 1.44) | 0.64 |
| Current smokers (n) | 105 | 17 | 37 | 51 | |
| HR (95% CI) | 1.03 (0.94, 1.13) | 1.00 (Ref) | 1.00 (0.52, 1.90) | 1.27 (0.57, 2.79) | 0.54 |
| Former smokers (n) | 120 | 26 | 37 | 57 | |
| HR (95% CI) | 1.02 (0.94, 1.11) | 1.00 (Ref) | 0.51 (0.29, 0.90) | 0.57 (0.29, 1.15) | 0.13 |
| Small cell lung cancer4 | |||||
| All women (n) | 176 | 35 | 69 | 72 | |
| HR (95% CI) | 1.00 (0.93, 1.09) | 1.00 (Ref) | 0.87 (0.55, 1.37) | 0.79 (0.44, 1.40) | 0.42 |
| Current smokers (n) | 93 | 21 | 40 | 32 | |
| HR (95% CI) | 0.91 (0.81, 1.03) | 1.00 (Ref) | 0.80 (0.44, 1.46) | 0.49 (0.23, 1.06) | 0.07 |
| Former smokers (n) | 77 | 14 | 28 | 35 | |
| HR (95% CI) | 1.07 (0.98, 1.16) | 1.00 (Ref) | 0.97 (0.48, 1.96) | 1.30 (0.53, 3.22) | 0.57 |
Adjusted for age, region, race-ethnicity, education, Hormone Therapy Trials treatment assignment, Calcium/Vitamin D Trial active intervention (time-dependent), BMI, smoking status (for all women only), number of cigarettes per day (for all women and current and former smokers), duration of regular smoking in years (for all women and current and former smokers), frequency of walking outside for >10 min, total vitamin A intake, total calcium intake, and energy intake. Ref, referent; WHI, Women's Health Initiative.
Histologic subtypes were based on the WHO Classification of Tumors for tumors of the lung. Non–small cell lung cancer includes squamous cell carcinoma, adenocarcinoma, large cell carcinoma, sarcomatoid carcinoma, and pleomorphic carcinoma.
The intake does not include the active intervention (1 g Ca + 400 IU vitamin D3/d) of the Calcium/Vitamin D Trial. P = 0.029 for the interactions of total vitamin D intake and smoking for non–small cell lung cancer, 0.12 for adenocarcinoma, 0.07 for squamous cell carcinoma, and 0.33 for small cell lung cancer (likelihood ratio tests; df = 1).
Data for never smokers are not shown because of the small number of cases in the histologic subtype (n < 30).
Among all women, no significant effect modification of total vitamin A intake was observed for total vitamin D intake and lung cancer risk associations (Table 4). Women randomly assigned to the Calcium/Vitamin D Trial active intervention had a nonsignificant, 13% lower lung cancer risk compared with those randomly assigned to placebo plus those not in the trial (HR: 0.87; 95% CI: 0.70, 1.07). The association remained nonsignificant when the effect was lagged for 2 y after the randomization (HR: 0.85; 95% CI: 0.66, 1.08) or when the follow-up was extended for 1 y in the posttrial period (HR: 0.84; 95% CI: 0.69, 1.03) (data not shown). The association was not materially different by baseline total calcium intake (see Supplemental Table 4 under “Supplemental data” in the online issue). In the analyses of effect modification of total vitamin A intake, the risk of lung cancer associated with the active intervention was significantly lower among women with total vitamin A intake <1000 μg/d RAE (HR: 0.69; 95% CI: 0.50, 0.96; P-interaction = 0.09; Table 4). Significant effect modification by total vitamin A intake was observed among current smokers. Among current smokers with total vitamin A intake ≥3000 μg/d RAE, the Tolerable Upper Intake Level specified by the Institutes of Medicine, the active intervention was significantly associated with a higher lung cancer risk (HR: 2.26; 95% CI: 1.02, 5.01), but not among those with total vitamin A intake <3000 μg/d RAE (P-interaction = 0.01).
TABLE 4.
Effect modification of total vitamin A intake on the associations of total vitamin D intake and Calcium/Vitamin D Trial active intervention with lung cancer risk in the WHI Clinical Trials and Observational Study: 1993–2010 (n = 128,779)1
| Total vitamin D intake (IU/d)2 |
Calcium/Vitamin D Trial active intervention3 |
|||||||
| <400 |
≥400 |
No |
Yes |
|||||
| n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | |
| All women | ||||||||
| Main effect | 959 | 1.00 (Ref) | 742 | 0.95 (0.83, 1.10) | 1599 | 1.00 (Ref) | 102 | 0.87 (0.70, 1.07) |
| Vitamin A intake (μg/d RAE) | ||||||||
| ≥3000 | 50 | 1.00 (Ref) | 165 | 0.73 (0.53, 1.02) | 201 | 1.00 (Ref) | 14 | 1.22 (0.69, 2.15) |
| 2999–1000 | 196 | 1.00 (Ref) | 544 | 1.05 (0.86, 1.27) | 693 | 1.00 (Ref) | 47 | 1.02 (0.75, 1.39) |
| <1000 | 713 | 1.00 (Ref) | 33 | 0.96 (0.66, 1.38) | 705 | 1.00 (Ref) | 41 | 0.69 (0.50, 0.96) |
| P-interaction4 | 0.25 | 0.09 | ||||||
| Current smokers | ||||||||
| Main effect | 316 | 1.00 (Ref) | 211 | 0.94 (0.72, 1.23) | 485 | 1.00 (Ref) | 42 | 1.00 (0.72, 1.40) |
| Vitamin A intake (μg/d RAE) | ||||||||
| ≥3000 | 14 | 1.00 (Ref) | 41 | 0.50 (0.26, 0.98) | 46 | 1.00 (Ref) | 9 | 2.26 (1.02, 5.01) |
| 2999–1000 | 55 | 1.00 (Ref) | 159 | 1.05 (0.73, 1.51) | 197 | 1.00 (Ref) | 17 | 1.05 (0.62, 1.78) |
| <1000 | 247 | 1.00 (Ref) | 11 | 1.04 (0.64, 1.70) | 242 | 1.00 (Ref) | 16 | 0.72 (0.41, 1.22) |
| P-interaction | 0.26 | 0.01 | ||||||
| Former smokers | ||||||||
| Main effect | 483 | 1.00 (Ref) | 413 | 1.05 (0.86, 1.27) | 852 | 1.00 (Ref) | 44 | 0.76 (0.55, 1.04) |
| Vitamin A intake (μg/d RAE) | ||||||||
| ≥3000 | 27 | 1.00 (Ref) | 100 | 0.86 (0.56, 1.33) | 123 | 1.00 (Ref) | 4 | 0.65 (0.23, 1.78) |
| 2999–1000 | 107 | 1.00 (Ref) | 294 | 1.07 (0.82, 1.39) | 380 | 1.00 (Ref) | 21 | 0.92 (0.58, 1.46) |
| <1000 | 349 | 1.00 (Ref) | 19 | 1.04 (0.64, 1.70) | 349 | 1.00 (Ref) | 19 | 0.67 (0.41, 1.07) |
| P-interaction | 0.48 | 0.98 | ||||||
| Never smokers | ||||||||
| Main effect | 160 | 1.00 (Ref) | 118 | 0.69 (0.49, 0.97) | 262 | 1.00 (Ref) | 16 | 0.91 (0.54, 1.54) |
| Vitamin A intake (μg/d RAE) | ||||||||
| ≥3000 | 9 | 1.00 (Ref) | 24 | 0.53 (0.24, 1.16) | 32 | 1.00 (Ref) | 1 | 0.75 (0.10, 5.80) |
| 2999–1000 | 34 | 1.00 (Ref) | 91 | 0.86 (0.53, 1.39) | 116 | 1.00 (Ref) | 9 | 1.20 (0.59, 2.45) |
| <1000 | 117 | 1.00 (Ref) | 3 | 0.46 (0.14, 1.49) | 114 | 1.00 (Ref) | 6 | 0.68 (0.29, 1.59) |
| P-interaction | 0.94 | 0.70 | ||||||
Total vitamin D intake and Calcium/Vitamin D Trial active intervention (time dependent) were mutually adjusted. Regression models additionally included age, region, race-ethnicity, education, Hormone Therapy Trials treatment assignment, BMI, smoking status (for all women only), number of cigarettes per day (for all women and current and former smokers), duration of regular smoking in years (for all women and current and former smokers), frequency of walking outside for >10 min, total vitamin A intake (for main effects only), total calcium intake, and energy intake. RAE, retinol activity equivalent; Ref, referent; WHI, Women's Health Initiative.
The intake does not include the active intervention (1 g Ca + 400 IU vitamin D3/d) of the Calcium/Vitamin D Trial. P = 0.71 for the 3-factor interaction of total vitamin D intake, total vitamin A intake, and smoking (Wald test).
Women with exposure to the Calcium/Vitamin D active intervention were those randomly assigned to the calcium and vitamin D arm; women without exposure to that trial were the remainder of the Observational Study and Clinical Trial individuals, including those randomly assigned to the Calcium/Vitamin D placebo arm. Women in the active intervention arm received 1 g Ca + 400 IU vitamin D3/d during the Calcium/Vitamin D Trial, 1994–2005. P = 0.13 for the 3-factor interaction of Calcium/Vitamin D active intervention, total vitamin A intake, and smoking (Wald test).
Likelihood ratio tests for the cross-product term of total vitamin D intake or Calcium/Vitamin D Trial active intervention and total vitamin A intake (all ordinal variables; df = 1).
Among Observational Study participants with less time spent outdoors (<0.5 h/d) in summer this year, we observed an inverse association of borderline significance between total vitamin D intake and lung cancer risk (P-trend = 0.063; Table 5). This association significantly differed from that among participants with more time spent outdoors (0.5–2 compared with >2 h/d; P-interaction = 0.02).
TABLE 5.
Association of total vitamin D intake with lung cancer risk stratified by time spent outdoors in summer and other seasons this year and an age of 30 to 39 years in participants in the WHI Observational Study who completed the year 4 follow-up questionnaire (n = 56,003)1
| Total vitamin D intake (IU/d) |
|||||||||
| <100 |
100 to <400 |
≥400 |
|||||||
| N | n | HR (95% CI) | n | HR (95% CI) | n | HR (95% CI) | P-trend | P-interaction2 | |
| Time outdoors/summer/this year | |||||||||
| <0.5 h/d | 17,166 | 32 | 1.00 (Ref) | 84 | 0.87 (0.56, 1.36) | 92 | 0.63 (0.37, 1.06) | 0.063 | 0.02 |
| 0.5–2 h/d | 28,121 | 47 | 1.00 (Ref) | 117 | 0.93 (0.65, 1.34) | 145 | 0.71 (0.46, 1.09) | 0.094 | |
| >2 h/d | 10,716 | 16 | 1.00 (Ref) | 39 | 0.99 (0.53, 1.87) | 49 | 1.22 (0.59, 2.54) | 0.553 | |
| Time outdoors/other seasons/this year | |||||||||
| <0.5 h/d | 20,964 | 39 | 1.00 (Ref) | 96 | 0.91 (0.61, 1.36) | 110 | 0.67 (0.41, 1.09) | 0.085 | 0.046 |
| 0.5–2 h/d | 28,623 | 47 | 1.00 (Ref) | 123 | 0.92 (0.64, 1.32) | 150 | 0.73 (0.48, 1.11) | 0.111 | |
| >2 h/d | 6416 | 9 | 1.00 (Ref) | 21 | 0.94 (0.41, 2.17) | 26 | 1.19 (0.45, 3.11) | 0.695 | |
| Time outdoors/summer/aged 30–39 y | |||||||||
| <0.5 h/d | 7388 | 16 | 1.00 (Ref) | 16 | 0.26 (0.12, 0.56) | 33 | 0.32 (0.14, 0.71) | 0.012 | 0.54 |
| 0.5–2 h/d | 31,254 | 47 | 1.00 (Ref) | 138 | 1.14 (0.80, 1.63) | 160 | 0.90 (0.59, 1.36) | 0.470 | |
| >2 h/d | 17,361 | 32 | 1.00 (Ref) | 86 | 0.98 (0.64, 1.51) | 93 | 0.75 (0.45, 1.27) | 0.255 | |
| Time outdoors/other seasons/aged 30–39 y | |||||||||
| <0.5 h/d | 13,268 | 21 | 1.00 (Ref) | 47 | 0.69 (0.40, 1.21) | 68 | 0.68 (0.36, 1.29) | 0.299 | 0.49 |
| 0.5–2 h/d | 33,145 | 54 | 1.00 (Ref) | 148 | 1.06 (0.76, 1.48) | 164 | 0.76 (0.51, 1.14) | 0.125 | |
| >2 h/d | 9590 | 20 | 1.00 (Ref) | 45 | 0.83 (0.47, 1.47) | 54 | 0.74 (0.37, 1.45) | 0.378 | |
Adjusted for age, region, race-ethnicity, education, BMI, smoking status, number of cigarettes per day, duration of regular smoking in years, frequency of walking outside for >10 min, total vitamin A intake, total calcium intake, energy intake, and usual use of sun screening. Ref, referent; WHI, Women's Health Initiative.
Likelihood ratio tests for the cross-product term of total vitamin D intake and time spent outdoors, both as ordinal variables (df = 1).
DISCUSSION
In the WHI, we found that among never-smoking, postmenopausal women, total vitamin D intake ≥400 IU/d was associated with a significantly lower risk of lung cancer. To our knowledge, this was the first study to report dietary vitamin D intake and lung cancer risk in postmenopausal women. The finding among never smokers has been suggested in a serological investigation of serum 25-hydroxyvitamin D concentrations and lung cancer mortality (8). Studying the association among never smokers is important because smoking is a strong risk factor for lung cancer and may influence vitamin D metabolism (36)—a possible explanation for the null results in previous research (5–7).
The anticarcinogenic functions of vitamin D in regulating cell proliferation and angiogenesis are relevant to lung tumorigenesis. Vitamin D inhibits lung cancer signaling pathways, including mutations in epidermal growth factor receptor, Wnt-β-catenin dysregulation, and vascular endothelial growth factor (37, 38). Also, vitamin D promotes G1 cell-cycle arrest through signaling cyclin-dependent kinase inhibitors p21 and p27 (39). Both p21 and p27 proteins cooperate closely with the ras oncogene family (40, 41), and K-ras often mutates in adenocarcinoma (42). Our observation that vitamin D intake was inversely associated with adenocarcinoma among never smokers but not with other histologic types of lung cancer or among smokers provides important biological implications. Adenocarcinoma occurs in never smokers more often than in smokers, compared with other histologic subtypes (2). Thus, one could hypothesize that vitamin D may be more effective at preventing or reversing the mutations that are not tobacco-related in adenocarcinoma compared with tobacco-induced mutations.
The importance of vitamin D intake should be emphasized. At the baseline of the WHI Calcium/Vitamin D Trial, total vitamin D intake was shown to be an important determinant of serum 25-hydroxyvitamin D concentrations (β-coefficient = 5.05 nmol/L per 40 IU/d of total vitamin D intake; P < 0.0001) (27). Our analyses showed that vitamin D intake–associated lung cancer risk was more protective among women with shorter times than among those with longer times spent outdoors in summer. This is reasonable because the contribution of diet to vitamin D status increases when sun exposure decreases (43).
We observed suggestive evidence that total vitamin A intake may modify the association of Calcium/Vitamin D Trial active intervention, but not that of total vitamin D intake, with lung cancer risks. This suggests 2 challenging issues regarding the nature and assessment of dietary intake when we assess nutrient-nutrient interactions. First, vitamin D and vitamin A intakes were correlated in both food and supplements; thus, a relatively small number of participants had high vitamin D but low vitamin A intakes, which might have affected statistical power. Second, measurement errors from FFQs are also likely correlated between these 2 micronutrients, and the correlated errors may attenuate their estimated associations with lung cancer (44). Adjustment for energy intake may only partially account for the correlated errors. The Calcium/Vitamin D Trial provided direct supplementation of calcium plus vitamin D, which was unlikely to correlate with total vitamin A intake. Thus, although the vitamin D dose (400 IU/d) given in the trial was modest (45), we were able to observe the effect modification. Therefore, future studies should make efforts to reduce both correlations and measurement errors, such as incorporating biomarker data, in assessing dietary intake (44).
Our analyses show suggestive evidence that lower total vitamin A intake may be beneficial for a lower lung cancer risk associated with the Calcium/Vitamin D Trial active intervention among all participants and among current smokers. However, the subgroup observations, ie, data by participants’ smoking status, must be interpreted with caution because of the small numbers of lung cancer cases in the intervention arm of the Calcium/Vitamin D Trial. In addition, we were unable to isolate the effect of vitamin D from that of calcium in the Calcium/Vitamin D Trial supplementation. This limited our inferential ability because a high dietary calcium intake has been linked to lower risks of total lung cancer and adenocarcinoma in smokers and nonsmokers (34, 35). Furthermore, we observed a positive association of the Calcium/Vitamin D Trial active intervention with lung cancer risk among current smokers with excess total vitamin A intake. The biological mechanism is unclear. Because β-carotene supplementation increases lung cancer risk in current smokers and the effect is likely to be independent of effects of vitamin A intake (46, 47), we additionally included intake of β-carotene from supplements (μg/d, continuous scale) in the regression model, but the risk estimate was not materially altered (HR: 2.23; 95% CI: 1.00, 4.95; data not shown). Nevertheless, our stratified analysis suggests that a lower total calcium intake (<1 g/d) may be important to the increased lung cancer risk (for Calcium/Vitamin D Trial active intervention: HR, 5.91; 95% CI, 1.22, 28.71; see Supplemental Table 4 under “Supplemental data” in the online issue), but the observation is based on small numbers of lung cancer cases and warrants replication.
The major strengths of our study included the prospective design, detailed exposure measurement on vitamin D intake from both food and supplements, use of clinically relevant cutoffs of vitamin D intakes, and a large number of incident lung cancer cases for stratified analyses by smoking status and histologic subtype. Nevertheless, several limitations should be noted. First, the dietary data collected are subject to measurement error (48). Because the measurement error for dietary retinol intake was larger than that for β-carotene, it might have attenuated the accuracy of total vitamin A intake calculated as RAE. In addition, self-report data are vulnerable to recall bias and social desirability bias. Also, the accuracy of the potencies on supplement labels is of concern (49). Furthermore, nondifferential misclassification of the outcome is possible. Lung cancer was not a predefined study outcome in the WHI. Chest radiology imaging was not specified by the protocol at study entry or serially. Moreover, we were unable to eliminate possible residual confounding. Last, the generalizability of the WHI might be limited because the study enrolled postmenopausal women who volunteered rather than women selected from a random sample of the population. The WHI did not collect data from nonvolunteers, but the lower prevalence of current smoking among the participants suggested a healthy volunteer effect (50). In addition, we may have limited our ability to generalize our findings to younger women or men. For example, estrogen may influence both lung cancer risk and vitamin D metabolism (51, 52). Also, women and men have different clinical characteristics of lung cancer, because never-smoking women are more likely to develop adenocarcinoma than are never-smoking men (51).
In conclusion, a vitamin D intake of ≥400 IU/d from food and supplements was associated with a lower risk of lung cancer risk among never-smoking, postmenopausal women. A lower vitamin A intake (<1000 μg/d RAE for all women and <3000 μg/d RAE for current smokers) may be important to achieve a lower lung cancer risk associated with 1 g Ca + 400 IU vitamin D3 supplementation.
Supplementary Material
Acknowledgments
We thank the participants in the WHI for their contributions and the Program Office: National Heart, Lung, and Blood Institute, Bethesda, MD (Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, Nancy Geller); Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, WA (Garnet Anderson, Ross Prentice, Andrea LaCroix, Charles Kooperberg); the Investigators and Academic Centers: Brigham and Women's Hospital, Harvard Medical School, Boston, MA (JoAnn E Manson); MedStar Health Research Institute/Howard University, Washington, DC (Barbara V Howard); Stanford Prevention Research Center, Stanford, CA (Marcia L Stefanick); Ohio State University, Columbus, OH (Rebecca Jackson); University of Arizona, Tucson/Phoenix, AZ (Cynthia A Thomson); University at Buffalo, Buffalo, NY (Jean Wactawski-Wende); University of Florida, Gainesville/Jacksonville, FL (Marian Limacher); University of Iowa, Iowa City/Davenport, IA (Robert Wallace); University of Pittsburgh, Pittsburgh, PA (Lewis Kuller); and Wake Forest University School of Medicine, Winston-Salem, NC (Sally Shumaker).
The authors’ responsibilities were as follows—T-YDC: designed the study, analyzed the data, and had primary responsibility for the final content; AZL and SAAB: provided essential materials; T-YDC, AZL, SAAB, RTC, GYFH, and MLN: wrote the manuscript; and GEG, MDT, and YZ: provided critical comments on the study design, statistical methods, and manuscript preparation. All authors read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: ETS, environmental tobacco smoke; FFQ, food-frequency questionnaire; RAE, retinol activity equivalent; WHI, Women's Health Initiative.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29 [DOI] [PubMed] [Google Scholar]
- 2.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers—a different disease. Nat Rev Cancer 2007;7:778–90 [DOI] [PubMed] [Google Scholar]
- 3.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control 2005;16:83–95. [DOI] [PubMed]
- 4.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:827–38 [DOI] [PubMed] [Google Scholar]
- 5.Weinstein SJ, Yu K, Horst RL, Parisi D, Virtamo J, Albanes D. Serum 25-hydroxyvitamin D and risk of lung cancer in male smokers: a nested case-control study. PLoS ONE 2011;6:e20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilkkinen A, Knekt P, Heliövaara M, Rissanen H, Marniemi J, Hakulinen T, Aromaa A, et al. Vitamin D status and the risk of lung cancer: a cohort study in Finland. Cancer Epidemiol Biomarkers Prev 2008;17:3274–8 [DOI] [PubMed] [Google Scholar]
- 7.Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum Vitamin D and cancer mortality in the NHANES III study (1988-2006). Cancer Res 2010;70:8587–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng TY, Neuhouser ML. Serum 25-hydroxyvitamin D, interaction with vitamin A and lung cancer mortality in the U.S. population. Cancer Causes Control 2012;23:1557–65 [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451–9 [DOI] [PubMed] [Google Scholar]
- 10.Brunner RL, Wactawski-Wende J, Caan BJ, et al. The effect of calcium plus vitamin D on risk for invasive cancer: results of the Women's Health Initiative (WHI) Calcium Plus Vitamin D Randomized Clinical Trial. Nutr Cancer 2011;63:827–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlberg C, Bendik I, Wyss A, et al. Two nuclear signaling pathways for vitamin D. Nature 1993;361:657–60 [DOI] [PubMed] [Google Scholar]
- 12.Thompson PD, Jurutka PW, Haussler CA, Whitfield GK, Haussler MR. Heterodimeric DNA binding by the vitamin D receptor and retinoid X receptors is enhanced by 1,25-dihydroxyvitamin D3 and inhibited by 9-cis-retinoic acid. Evidence for allosteric receptor interactions. J Biol Chem 1998;273:8483–91 [DOI] [PubMed] [Google Scholar]
- 13.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol 2003;13(suppl):S18–77 [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81 [DOI] [PubMed] [Google Scholar]
- 15.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol 2010;5(suppl 1):S23–30 [DOI] [PubMed] [Google Scholar]
- 16. Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol 2003;13(9 suppl):S122–8. [DOI] [PubMed]
- 17.Adamo MBJC, Ruhl JL, Dickie LA. SEER Program coding and staging manual. Bethesda, MD: National Cancer Institute, 2010 (NIH publication no. 10-5581)
- 18. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87. [DOI] [PubMed]
- 19.Patterson RE, Levy L, Tinker LF, Kristal AR. Evaluation of a simplified vitamin supplement inventory developed for the Women's Health Initiative. Public Health Nutr 1999;2:273–6 [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press, 2001 [PubMed] [Google Scholar]
- 21.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354(7):684–96. [DOI] [PubMed]
- 22.Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tob Control 2009;18:317–23 [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg CA, Khandekar J, Greenland P, Rodabough RJ, McTiernan A. Cutaneous melanoma in postmenopausal women after nonmelanoma skin carcinoma: the Women's Health Initiative Observational Study. Cancer 2006;106:654–63 [DOI] [PubMed] [Google Scholar]
- 24.Institute of Medicine Dietary Reference Intake for calcium and vitamin D. Washington, DC: The National Academics Press, 2011 [Google Scholar]
- 25.Kleinbaum D, Klein M. Survival analysis. A self-learning text. New York, NY: Springer, 2011 [Google Scholar]
- 26.Chlebowski RT, Anderson GL, Manson JE, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial. J Natl Cancer Inst 2010;102:1413–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millen AE, Wactawski-Wende J, Pettinger M, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr 2010;91:1324–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kluczynski MA, Lamonte MJ, Mares JA, et al. Duration of physical activity and serum 25-hydroxyvitamin D status of postmenopausal women. Ann Epidemiol 2011;21:440–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011;26:546–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krasinski SD, Russell RM, Otradovec CL, et al. Relationship of vitamin A and vitamin E intake to fasting plasma retinol, retinol-binding protein, retinyl esters, carotene, alpha-tocopherol, and cholesterol among elderly people and young adults: increased plasma retinyl esters among vitamin A-supplement users. Am J Clin Nutr 1989;49:112–20 [DOI] [PubMed] [Google Scholar]
- 31.Stauber PM, Sherry B, VanderJagt DJ, Bhagavan HN, Garry PJ. A longitudinal study of the relationship between vitamin A supplementation and plasma retinol, retinyl esters, and liver enzyme activities in a healthy elderly population. Am J Clin Nutr 1991;54:878–83 [DOI] [PubMed] [Google Scholar]
- 32.Ballew C, Galuska D, Gillespie C. High serum retinyl esters are not associated with reduced bone mineral density in the Third National Health And Nutrition Examination Survey, 1988-1994. J Bone Miner Res 2001;16:2306–12 [DOI] [PubMed] [Google Scholar]
- 33.US Department of Health and Human Services The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Chapter 7. Cancer among adults from exposure to secondhand smoke. Washington, DC: US DHHS, 2006 [Google Scholar]
- 34.Mahabir S, Forman MR, Dong YQ, Park Y, Hollenbeck A, Schatzkin A. Mineral intake and lung cancer risk in the NIH-American Association of Retired Persons Diet and Health study. Cancer Epidemiol Biomarkers Prev 2010;19:1976–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takata Y, Shu XO, Yang G, et al. Calcium intake and lung cancer risk among female non-smokers: a report from the Shanghai Women's Health Study. Cancer Epidemiol Biomarkers Prev 2013;22:50–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsunawa M, Amano Y, Endo K, et al. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol Sci 2009;109:50–8 [DOI] [PubMed] [Google Scholar]
- 37.Königshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol 2010;42:21–31 [DOI] [PubMed] [Google Scholar]
- 38.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007;7:684–700 [DOI] [PubMed] [Google Scholar]
- 39.Hershberger PA, Modzelewski RA, Shurin ZR, Rueger RM, Trump DL, Johnson CS. 1,25-Dihydroxycholecalciferol (1,25-D3) inhibits the growth of squamous cell carcinoma and down-modulates p21(Waf1/Cip1) in vitro and in vivo. Cancer Res 1999;59:2644–9 [PubMed] [Google Scholar]
- 40.Tanaka T, Slamon DJ, Battifora H, Cline MJ. Expression of p21 ras oncoproteins in human cancers. Cancer Res 1986;46:1465–70 [PubMed] [Google Scholar]
- 41.Serres MP, Zlotek-Zlotkiewicz E, Concha C, et al. Cytoplasmic p27 is oncogenic and cooperates with Ras both in vivo and in vitro. Oncogene 2011;30:2846–58. [DOI] [PubMed] [Google Scholar]
- 42.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sorensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr 2001;86(suppl 1):S97–103 [DOI] [PubMed] [Google Scholar]
- 44.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011;103:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev 2012;33:456–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150–5 [DOI] [PubMed] [Google Scholar]
- 47.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35 [DOI] [PubMed] [Google Scholar]
- 48.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am J Epidemiol 2008;167:1247–59 [DOI] [PubMed] [Google Scholar]
- 49.Denham BE. Dietary supplements–regulatory issues and implications for public health. JAMA 2011;306:428–9 [DOI] [PubMed] [Google Scholar]
- 50.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol 2003;13(Suppl):S107–21 [DOI] [PubMed] [Google Scholar]
- 51.Alberg AJ, Wallace K, Silvestri GA, Brock MV. Invited commentary: the etiology of lung cancer in men compared with women. Am J Epidemiol 2013;177:613–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hoof HJ, van der Mooren MJ, Swinkels LM, Sweep CG, Merkus JM, Benraad TJ. Female sex hormone replacement therapy increases serum free 1,25-dihydroxyvitamin D3: a 1-year prospective study. Clin Endocrinol (Oxf) 1999;50:511–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


