Abstract
Background: The association of excess body weight across a lifetime with pancreatic cancer has not been examined extensively.
Objective: We determined the association for body mass index (BMI) at different ages and adiposity duration and gain with incident pancreatic adenocarcinoma in the NIH-AARP Diet and Health Study cohort.
Design: Participants aged 50–71 y completed questionnaires at baseline (1995–1996) and 6 months later that queried height and weight history. We calculated HRs and 95% CIs by using Cox proportional hazards models adjusted for age, smoking, sex, and intakes of energy and total fat.
Results: Over an average follow-up of 10.5 y, 1206 and 2122 pancreatic cancer cases were identified in the subcohort who completed the second questionnaire (n = 273,975) and the baseline cohort (n = 501,698), respectively. Compared with normal weight, overweight or obesity at ages 18, 35, 50, or >50 y (baseline BMI) was significantly associated with pancreatic cancer, with HRs ranging from 1.15 to 1.53. A longer duration of BMI (in kg/m2) >25.0 was significantly associated with pancreatic cancer (overall HR per 10-y increment of duration: 1.06; 95% CI: 1.02, 1.09), with individuals who reported diabetes having the greatest risk (HR per 10-y increment of duration: 1.18; 95% CI: 1.05, 1.32; P-interaction = 0.01) and rates. A substantial gain in adiposity (>10 kg/m2) after age 50 y was significantly associated with increased pancreatic cancer risk. The etiologic fraction of pancreatic cancer explained by adiposity at any age was 14% overall and 21% in never smokers.
Conclusion: Overweight and obesity at any age are associated with increased pancreatic cancer.
INTRODUCTION
Cigarette smoking, diabetes mellitus, and obesity are among the few consistent and modifiable risk factors for pancreatic cancer (1–4). Although overweight and obesity at an older age are positively associated with pancreatic cancer in many studies, the association between adiposity at younger ages and across a lifetime and risk of pancreatic cancer has not been examined extensively, particularly in prospective studies (5). We conducted an analysis in a large cohort, the NIH-AARP Diet and Health Study, to examine BMI at different ages (18, 35, 50, and >50 y), adiposity duration, and weight change during a lifetime in relation to incident pancreas adenocarcinoma. To the best of our knowledge, this is the first prospective study to examine adiposity across multiple times in life (namely, early adulthood, midlife, and older age), BMI change, as well as adiposity duration as risk factors for pancreatic cancer.
SUBJECTS AND METHODS
Study population
The NIH-AARP Diet and Health Study is a large prospective study in AARP members established in 1995–1996 (6). Self-administered questionnaires eliciting information on demographic characteristics, dietary intake over the previous 12 mo, and numerous health-related factors, including current weight and height, were mailed to AARP members aged 50–71 y, who resided in 6 US states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and 2 metropolitan areas (Atlanta, GA, and Detroit, MI). The questionnaire was returned and satisfactorily completed by 566,402 members (6). Six months after the baseline questionnaire was sent, baseline respondents were sent a second risk factor questionnaire that queried information on height at age 18 y and weight at ages 18, 35, and 50 y (6). In total, 334,906 participants completed the second questionnaire. The study was approved by the National Cancer Institute Special Studies Institutional Review Board, and informed consent was obtained from all participants.
We excluded subjects who had questionnaires completed by proxy respondents (n = 15,760), who had prevalent cancers as determined by the cancer registry data (n = 8587), who had missing height or weight (n = 13,240), and who were missing smoking data (n = 19,338). Among those who completed the second questionnaire, we further excluded participants with missing BMI at different ages (n = 37,076). Our final subcohort for analyses with complete BMI data at ages 18, 35, and 50 y consisted of 273,975 individuals (165,135 men, 108,840 women).
For analyses of baseline BMI at ages >50 y in Tables 2 and 3 only, we used the larger baseline cohort but excluded participants censored during the first year of follow-up (n = 6650 total) to avoid the influence of subclinical disease on current weight and in those <51 y old (n = 1126). Our final baseline analytic cohort consisted of 501,698 individuals (296,448 men, 205,250 women).
TABLE 2.
HRs and 95% CIs of BMI history for men and women at ages 18, 35, 50, and >50 y1
| Multivariable HR (95% CI) |
|||||
| Cases | Age-adjusted HR (95% CI) | Multivariable HR (95% CI) | Additionally adjusted for baseline BMI | BMI (kg/m2) ≥25 | |
| n/person-years | |||||
| BMI (in kg/m2) at 18 y2 | |||||
| <18.5 | 188/380,405 | 1.08 (0.92, 1.27) | 1.08 (0.92, 1.27) | 1.10 (0.93, 1.29) | 1.08 (0.92, 1.27) |
| ≥18.5 and <22.5 | 652/1,429,772 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| ≥22.5 and <25.0 | 216/442,799 | 1.10 (0.95, 1.29) | 1.07 (0.92, 1.25) | 1.05 (0.90, 1.23) | 1.07 (0.92, 1.25) |
| ≥25.0 and <27.5 | 91/185,345 | 1.16 (0.93, 1.45) | 1.11 (0.89, 1.39) | 1.08 (0.86, 1.35) | 1.25 (1.04, 1.49) |
| ≥27.5 | 59/90,518 | 1.62 (1.24, 2.12) | 1.56 (1.19, 2.03) | 1.52 (1.16, 2.00) | |
| P-trend | 0.0009 | 0.005 | 0.01 | 0.02 | |
| BMI at 35 y2 | |||||
| <18.5 | 34/75,215 | 1.06 (0.75, 1.50) | 1.04 (0.73, 1.48) | 1.04 (0.73, 1.48) | |
| ≥18.5 and <22.5 | 405/926,741 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |
| ≥22.5 and <25.0 | 350/730,737 | 1.10 (0.95, 1.27) | 1.08 (0.94, 1.25) | 1.08 (0.94, 1.25) | |
| ≥25.0 and <30.0 | 346/659,859 | 1.26 (1.09, 1.46) | 1.22 (1.05, 1.41) | 1.24 (1.08, 1.43) | |
| ≥30.0 | 71/136,267 | 1.40 (1.09, 1.81) | 1.37 (1.06, 1.79) | ||
| P-trend | 0.0002 | 0.001 | 0.002 | ||
| BMI at 50 y2 | |||||
| <18.5 | 27/47,626 | 1.27 (0.86, 1.87) | 1.26 (0.85, 1.85) | 1.26 (0.85, 1.85) | |
| ≥18.5 and <25.0 | 532/1,171,086 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |
| ≥25.0 and <30.0 | 499/988,218 | 1.17 (1.03, 1.32) | 1.13 (1.00, 1.29) | 1.15 (1.02, 1.30) | |
| ≥30.0 | 148/321,888 | 1.24 (1.03, 1.49) | 1.22 (1.02, 1.47) | ||
| P-trend | 0.004 | 0.01 | 0.02 | ||
| BMI at baseline: >50 y3 | |||||
| <18.5 | 25/44,834 | 1.25 (0.84, 1.87) | 1.18 (0.79, 1.75) | 1.16 (0.78, 1.73) | |
| ≥18.5 and <25.0 | 689/1,528,071 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |
| ≥25.0 and <30.0 | 934/1,876,406 | 1.07 (0.97, 1.18) | 1.09 (0.98, 1.20) | 1.12 (1.02, 1.23) | |
| ≥30.0 and <35.0 | 340/691,319 | 1.12 (0.98, 1.27) | 1.14 (1.00, 1.30) | ||
| ≥35.0 | 134/273,965 | 1.24 (1.03, 1.50) | 1.29 (1.07, 1.55) | ||
| P-trend | 0.04 | 0.01 | 0.01 | ||
Cox proportional hazard models were used to calculate HRs with age as the time metric. P-trend values were based on a median value of BMI within each category and exclude participants with a BMI <18.5 for each respective age group. Multivariable HRs were additionally adjusted for smoking (never, quit ≥10 y ago, quit 5–9 y ago, quit 1–4 y ago, quit <1 y ago, or current and smokes ≤20 or >20 cigarettes/d), energy (continuous), energy-adjusted total fat (continuous), and sex.
Subcohort of 273,977 participants and 1206 pancreatic cancer cases for models at ages 18, 35, and 50 y.
BMI at >50 y based on a baseline cohort of 501,702 participants and 2122 pancreatic cancer cases and excludes participants who were censored during the first year of follow-up.
TABLE 3.
HRs and 95% CIs for pancreatic cancer per 5-unit increase in BMI (kg/m2) at ages 18, 35, and 50 y overall and by sex, diabetes, and smoking history1
| BMI (kg/m2) at different ages |
||||
| Characteristics | 18 y | 35 y | 50 y | >50 y2 |
| All participants | ||||
| No. of cases | 1018 | 1172 | 1179 | 2097 |
| HR | 1.11 (1.01, 1.21) | 1.09 (1.01, 1.18) | 1.10 (1.03, 1.17) | 1.05 (1.01, 1.09) |
| Sex | ||||
| Male | ||||
| No. of cases | 673 | 768 | 776 | 1355 |
| HR | 1.10 (0.97, 1.24) | 1.12 (1.01, 1.23) | 1.15 (1.06, 1.27) | 1.10 (1.04, 1.17) |
| Female | ||||
| No. of cases | 345 | 404 | 403 | 742 |
| HR | 1.12 (0.98, 1.27) | 1.05 (0.93, 1.20) | 1.03 (0.93, 1.15) | 1.02 (0.96, 1.08) |
| History of diabetes | ||||
| No diabetes | ||||
| No. of cases | 890 | 1030 | 1036 | 1821 |
| HR | 1.05 (0.94, 1.18) | 1.05 (0.96, 1.15) | 1.08 (1.00, 1.17) | 1.03 (0.99, 1.08) |
| Diabetes | ||||
| No. of cases | 128 | 142 | 143 | 276 |
| HR | 1.20 (1.02, 1.42) | 1.10 (0.93, 1.31) | 1.00 (0.84, 1.19) | 1.04 (0.93, 1.16) |
| Smoking status | ||||
| Never | ||||
| No. of cases | 293 | 347 | 349 | 627 |
| HR | 1.09 (0.92, 1.30) | 1.06 (0.92, 1.23) | 1.08 (0.96, 1.22) | 1.11 (1.05, 1.17)3 |
| Former | ||||
| No. of cases | 399 | 466 | 469 | 796 |
| HR | 1.14 (1.00, 1.30) | 1.11 (0.98, 1.25) | 1.17 (1.06, 1.30) | 1.06 (0.99, 1.14) |
| Current and recent quitter | ||||
| No. of cases | 326 | 359 | 361 | 674 |
| HR | 1.07 (0.91, 1.27) | 1.10 (0.97, 1.26) | 1.01 (0.88, 1.15) | 0.97 (0.89, 1.05) |
All analyses were based on a subcohort of 273,975 participants and pancreatic cancer cases except for the association between baseline BMI at ages >50 y. Cox proportional hazards models used age as the time metric and adjusted for smoking (never, quit ≥10 y ago, quit 5–9 y ago, quit 1–4 y ago, quit <1 y ago, or current and smokes ≤20 or >20 cigarettes/d), energy (continuous), energy-adjusted total fat (continuous), and sex (in sex-combined models). HRs based on BMI per 5-kg/m2 increase exclude subjects with a BMI <18.5 for each respective age (18, 35, ≤50, >50 y).
Significant interaction for BMI at age >50 y and smoking (P-interaction = 0.01). No other significant interaction by sex, diabetes history, or smoking for all BMI age categories (P-interaction > 0.05).
BMI at ages >50 y based on a baseline cohort of 501,698 participants and excludes participants who were censored during the first year of follow-up.
Cohort follow-up and case ascertainment
Cancer cases were identified by linking cohort members to cancer registries covering the 8 states, as well as Arizona, Texas, and Nevada, and to the US National Death Index from 1995 through 2006 (7). The vital status of cohort participants was also ascertained by linkage to the Social Security Administration Death Master File. For these analyses, our outcome of interest was incident adenocarcinoma of the exocrine pancreas [International Classification of Diseases for Oncology, Third Edition (code C250–C259)]. Our case definition excluded pancreatic endocrine tumors, sarcomas, and lymphomas (histology types, 8150, 8151, 8153, 8155, 8240).
BMI exposure variables
We calculated BMI by using weight (kg) divided by height (m) squared. BMI at age 18 y was computed by using height at age 18 y, whereas BMI at the older ages was computed by using baseline height at ages 50–71 y. BMI (in kg/m2) categories at ages 18, 35, 50, and >50 y were consistent with the WHO obesity classifications of <18.5 (underweight), 18.5 to <25 (normal), 25 to <30 (overweight), 30 to <35 (moderate obesity), and ≥35 (severe obesity) (8). We used normal BMI (18.5 to <25.0) as the referent category for our most of our analyses. Because the majority of participants reported lower weights at ages 18 and 35 y, we separated BMI in the normal weight and overweight ranges by using finer categories within the WHO categories. Within each age category, continuous BMI was per 5-kg/m2 unit increase excluding individuals with a BMI <18.5. The HRs for BMI gain and BMI loss were determined in separate analyses. BMI change was calculated as the difference between reported BMI at different ages. We calculated duration in years of being overweight (BMI >25) by using linear interpolation methods to assign years between consecutive BMI measures, with BMI <25 being assigned zero. Weighted cumulative overweight years (OWY; similar to pack-years) considers the degree of overweight over time and was calculated by multiplying the duration (y) of high BMI by the difference (in BMI units) above normal BMI (≥25.0) for each age-specific BMI. A continuous duration of BMI ≥25 was per 10-y increment and cumulative OWY was per 100 OWY units. Quintiles for years overweight and cumulative OWY were based on the distribution of the cohort who ever had overweight, with a separate category for never overweight.
Statistical analysis
For the baseline cohort, cases and person-years were accrued from 1 y after the date of receipt of the baseline questionnaire until the earliest of the following dates: December 2006, diagnosis of pancreatic cancer, death, or moving out of the registry area. For the subcohort, cases and person-years were accrued from the date of receipt of the second questionnaire until the same exit dates specified above. Cox proportional hazard models, with age as the time metric, were used to generate HRs and 95% CIs. Trends were calculated by using the median value of each category. Potential confounding variables were included in the model if their inclusion changed the risk estimate for the BMI variable ≥10% or if they were putative risk factors for pancreatic cancer. The dietary variables were energy, adjusted by using the density method. Variables included in the final multivariable models were smoking (never, quit ≥10 y ago, quit 5–9 y ago, quit 1–4 y ago, quit <1 y ago, or current and smokes ≤20 or >20 cigarettes/d), total fat (g · 1000 kcal−1 · d−1) and energy intake (kcal/d), and sex. Self-reported diabetes (yes or no), a putative pancreatic cancer risk factor, is potentially on the causal pathway between BMI and pancreatic cancer and either changed slightly (<10%) or did not change risk estimates; therefore, it was not included in our final models. We also evaluated our primary associations by using proportional subdistribution hazard models for survival data (10, 11).
We used 3-knot splines to model nonlinear relations between BMI change in the different ages and pancreatic cancer risk. We assessed a priori interactions in stratified analyses by sex, smoking status (never, quit >10 y ago, and recent/current smoker), diabetes, and follow-up time; and tested for significance by using cross-product terms composed of median trend variables or continuous BMI and the categorical modifying variable. Because there was no significant interaction by sex (P-interaction > 0.05), we present most of our results with men and women combined.
Absolute incidence rates for pancreatic cancer standardized within 5-y age categories to the age distribution of the NIH-AARP population adjusted by sex were calculated for adiposity duration. We calculated the adjusted population attributable risk and 95% CI to estimate the percentage of pancreatic cancer cases that would have been eliminated if participants never achieved overweight and/or obesity at any age, assuming a causal relation between overweight/obesity and pancreatic cancer by using the methods described by Spiegelman et al (9).
All statistical analyses were performed by using Statistical Analysis Systems software (version 8.2; SAS Institute), and P values for statistical tests were 2-tailed. Results were considered significant if the associated P value was ≤0.05.
RESULTS
During 11.2 y of follow-up (median: 10.5 y), 1359 men and 763 women with incident pancreatic cancer were identified in the baseline cohort; 787 of these men and 419 of these women had also completed the second questionnaire that ascertained information on BMI at different ages. The pancreatic cancer incidence rates for men and women in the baseline cohort were 51.6 (95% CI: 48.8, 54.3) and 41.7 (95% CI: 38.7, 44.7) cases per 100,000 person-years, respectively; corresponding rates in the subcohort were 50.4 (95% CI: 46.9, 54.0) and 41.2 (95% CI: 37.2, 45.1) cases per 100,000 person-years.
The selected characteristics of cohort participants by duration of adiposity in the subcohort who completed the second questionnaire are shown in Table 1. Those who were ever overweight or obese were less likely to be college graduates, never or current smokers, physically active, and nondiabetic; they also had higher intakes of total and saturated fat and red meat. Those with the longest duration of overweight or obesity were more often men who had a higher proportion of diabetes, a higher baseline BMI, and who represented a lower proportion of current smokers and heavy drinkers.
TABLE 1.
Selected characteristics of the AARP cohort according to lifetime BMI and onset of overweight or obesity1
| Quintile of no. of years of overweight or obesity [BMI (in kg/m2) >25] |
||||||
| Characteristics | Never overweight2 (n = 83,268) | >0 and <10.1 y (n = 38,141) | ≥10.1 and <18.9 y (n = 38,142) | ≥18.9 and <27.8 y (n = 38,141) | ≥27.8 and <37.3 y (n = 38,140) | >37.3 (n = 38,143) |
| No. of years overweight | 0 ± 0.02 | 5.0 ± 0.03 | 14.5 ± 0.03 | 23.3 ± 0.03 | 32.6 ± 0.03 | 43.6 ± 0.02 |
| Age (y) | 62.3 ± 0.02 | 61.2 ± 0.03 | 61.4 ± 0.03 | 62.0 ± 0.03 | 61.8 ± 0.03 | 64.2 ± 0.03 |
| Sex (% male) | 47.0 | 55.5 | 57.3 | 66.0 | 73.6 | 77.9 |
| White (%) | 94.3 | 93.9 | 92.5 | 92.7 | 93.5 | 94.3 |
| College graduate (%) | 46.5 | 42.9 | 39.2 | 39.3 | 41.2 | 42.0 |
| Smoking history (%) | ||||||
| Never smoker | 40.3 | 34.0 | 34.3 | 34.6 | 35.4 | 35.0 |
| Former smoker | 45.1 | 54.3 | 55.4 | 56.1 | 55.3 | 56.6 |
| Current smoker | 14.6 | 11.7 | 10.3 | 9.3 | 9.3 | 8.4 |
| Self-reported diabetes (%) | 2.9 | 4.2 | 6.5 | 10.7 | 14.5 | 18.7 |
| BMI (kg/m2) | ||||||
| Age 18 y | 19.8 ± 0.007 | 20.2 ± 0.01 | 20.6 ± 0.01 | 21.1 ± 0.01 | 22.5 ± 0.02 | 25.5 ± 0.02 |
| Age 35 y | 21.1 ± 0.008 | 21.9 ± 0.01 | 22.8 ± 0.01 | 24.3 ± 0.01 | 26.9 ± 0.02 | 29.1 ± 0.02 |
| Age 50 y | 21.9 ± 0.008 | 23.3 ± 0.02 | 25.7 ± 0.01 | 27.5 ± 0.02 | 29.2 ± 0.02 | 30.5 ± 0.02 |
| Baseline | 22.5 ± 0.007 | 25.9 ± 0.01 | 28.0 ± 0.02 | 29.1 ± 0.02 | 30.4 ± 0.03 | 31.5 ± 0.03 |
| Change: 18 y to baseline | 2.7 ± 0.009 | 5.7 ± 0.02 | 7.4 ± 0.03 | 8.0 ± 0.03 | 7.9 ± 0.03 | 6.0 ± 0.03 |
| Cumulative OWY | 0 ± 0 | 20.3 ± 0.24 | 167 ± 0.76 | 446 ± 0.75 | 745 ± 1.1 | 1152 ± 1.5 |
| Physical activity3 (MET-h/wk) | 44.2 ± 0.08 | 40.9 ± 0.12 | 38.4 ± 0.12 | 37.8 ± 0.12 | 37.6 ± 0.12 | 37.9 ± 0.12 |
| Daily dietary intake4 | ||||||
| Fat (g/1000 kcal) | 32.1 ± 0.03 | 33.3 ± 0.04 | 33.7 ± 0.04 | 34.0 ± 0.04 | 34.3 ± 0.04 | 34.2 ± 0.04 |
| Saturated fat (g/1000 kcal) | 9.9 ± 0.01 | 10.4 ± 0.02 | 10.5 ± 0.02 | 10.6 ± 0.02 | 10.7 ± 0.02 | 10.6 ± 0.02 |
| Energy (kcal) | ||||||
| Men | 2005 ± 4.7 | 2024 ± 5.7 | 2053 ± 6.0 | 2057 ± 6.0 | 2071 ± 6.3 | 1998 ± 5.9 |
| Women | 1538 ± 3.1 | 1554 ± 5.8 | 1591 ± 6.3 | 1617 ± 6.6 | 1636 ± 6.9 | 1622 ± 7.2 |
| Red meat (g/1000 kcal) | ||||||
| Men | 32.6 ± 0.10 | 37.0 ± 0.13 | 38.6 ± 0.14 | 39.6 ± 0.14 | 40.1 ± 0.14 | 39.4 ± 0.14 |
| Women | 25.9 ± 0.08 | 25.7 ± 0.16 | 30.9 ± 0.17 | 31.4 ± 0.17 | 32.2 ± 0.18 | 32.3 ± 0.18 |
| Alcohol use: >3 drinks/d (%) | ||||||
| Men | 11.3 | 11.9 | 12.3 | 11.5 | 11.0 | 9.7 |
| Women | 3.5 | 3.4 | 2.9 | 2.7 | 2.2 | 1.4 |
Values are means ± SDs or proportions; n = 273,977. Generalized linear models were used to estimate means and SEs for continuous variables and frequencies for proportions within each BMI category. MET-h, metabolic equivalent task hours; OWY, overweight years.
Never overweight and ever underweight category includes participants who ever reported BMI <18.5 and never reported BMI >25.0.
Metabolic equivalent during the past 10 y: n = 162,508 men and n = 106,912 women with complete physical activity data.
Dietary variables adjusted for energy except for alcohol.
Risk associated with BMI at ages 18, 35, 50, and >50 y
Increasing BMI at age 18 y was associated with a significantly greater pancreatic cancer risk compared with those with a BMI of 18.5–22.4 (Table 2). This association was not attenuated after additional adjustment for baseline BMI. Compared with those with a BMI of 18.5–22.4, those who were overweight or obese at age 35 had a significant and elevated pancreatic cancer risk. Compared with participants with a normal BMI within each age-specific stratum, those who were obese at age 50 y and those who were severely obese at ages >50 y also showed significantly increased pancreatic cancer risk with significant trends. Our results were similar when we used proportional subdistribution hazard models for survival data (10, 11).
The overall HRs per 5-unit increase in BMI were significantly associated with pancreatic cancer for each age stratum (Table 3). The association for BMI at age 18 y was strongest among those who reported diabetes at baseline compared with those without diabetes. The association for BMI at ages >50 y was stronger among never smokers compared with former and current smokers. There were no other significant interactions by sex, smoking status, or diabetes.
Risk associated with gain or loss in BMI over time
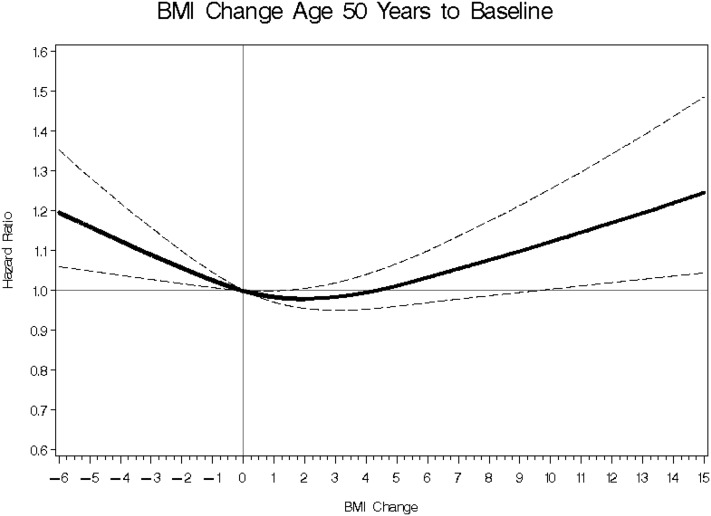
A 3-knot spline was used to model the nonlinear relation between BMI change in the different ages and risk of pancreatic cancer. The test for nonlinearity reached significance for BMI change between age 50 y and baseline (P = 0.0001), such that compared with no BMI change (zero), we observed significant positive associations for BMI gain >10 or any weight loss (Figure 1). None of the other differences in BMI between ages were significantly associated with pancreatic cancer.
FIGURE 1.
BMI (in kg/m2) change between age 50 y and baseline and pancreatic cancer risk. The figure shows a 3-knot spline of the nonlinear relation for BMI change and risk of pancreatic cancer adjusted for smoking (never, quit ≥10 y ago, quit 5–9 y ago, quit 1–4 y ago, quit <1 y ago, or current and smokes ≤20 or >20 cigarettes/d), energy (continuous), energy-adjusted total fat (continuous), and sex. The solid line represents the OR, and the dashed lines represent 95% CIs. The test for nonlinearity reached significance (P = 0.0001), such that compared with no change in BMI (referent zero), any BMI loss or BMI gain >10 was significantly associated with greater pancreatic cancer risk.
Risk associated with duration of being overweight and cumulative OWY
A longer duration of overweight was associated with greater pancreatic cancer risk (Table 4), particularly among those who reported diabetes at baseline (Table 5). For every 10 y of having a BMI ≥25, pancreatic cancer risk increased 6% overall and 18% among those diagnosed with diabetes but not as strongly among participants without diabetes (P-interaction = 0.01). Participants with diabetes and the longest duration of adiposity also had the highest incidence rate for pancreatic cancer (incidence rate per 100,000 person-years: 85.8; 95% CI: 61.9, 109.7) compared with those with less duration of adiposity or without diabetes. A similar pattern was observed for cumulative OWY. Among those with ≥4 y of follow-up (n = 825 cases), a significant 2-fold increased risk remained among participants with diabetes and the longest duration of adiposity, whereas no significant associations were observed among those with adiposity without diabetes (see Supplemental Table S1 under “Supplemental data” in the online issue). In contrast, for the cases that occurred during the first 4 y of follow-up (n = 381 cases), significantly increased (∼50%) risks were observed only among nondiabetic participants with shorter duration of overweight or obesity and cumulative adiposity (Supplemental Table S1). Our results were similar when we used proportional subdistribution hazard models for survival data (see Supplemental Tables S2–S4 under “Supplemental data” in the online issue) (10, 11).
TABLE 4.
Incidence rates, HRs, and 95% CIs of duration of overweight or obesity across a lifetime1
| Cases | Incidence rate (95% CI)2 | HR (95% CI)3 | |
| n/person-years | |||
| Years overweight4 | |||
| Never overweight | 317/774,273 | 37.5 (33.3, 41.7) | 1.00 (referent) |
| >0 and <10.1 y | 156/354,539 | 44.0 (37.1, 50.9) | 1.16 (0.96, 1.41) |
| ≥10.1 and <18.9 y | 170/354,362 | 47.5 (40.3, 54.6) | 1.25 (1.04, 1.51) |
| ≥18.9 and <27.8 y | 162/352,253 | 42.1 (35.4, 48.8) | 1.14 (0.94, 1.38) |
| ≥27.8 and <37.3 y | 186/350,058 | 50.0 (42.2, 57.8) | 1.32 (1.10, 1.59) |
| ≥37.3 y | 215/343,333 | 49.0 (40.6, 57.4) | 1.31 (1.09, 1.56) |
| P-trend5 | 0.002 | ||
| Continuous, per 10 y | 1206/2,528,844 | 1.06 (1.02, 1.09) | |
| Cumulative OWY4 | |||
| Never overweight | 317/774,273 | 37.5 (33.3, 41.7) | 1.00 (referent) |
| >0 and <24 OWY | 186/353,197 | 52.7 (45.1, 60.2) | 1.23 (1.03, 1.48) |
| ≥24 and <415 OWY | 130/355,857 | 46.7 (37.6, 55.9) | 1.14 (0.93, 1.40) |
| ≥415 and <663 OWY | 195/350,578 | 52.1 (44.5, 59.9) | 1.23 (1.03, 1.48) |
| ≥663 and <949 OWY | 163/351,861 | 50.6 (42.0, 59.1) | 1.20 (0.99, 1.46) |
| ≥949 OWY | 215/343,072 | 57.5 (49.1, 65.8) | 1.35 (1.13, 1.62) |
| P-trend5 | 0.006 | ||
| Continuous, per 100 units OWY | 1206/2,528,844 | 48.4 (45.4, 51.3) | 1.02 (1.01, 1.03) |
All analyses were limited to the subcohort of 273,977 participants and 1206 pancreatic cancer cases. OWY, overweight years.
Sex-adjusted, age-standardized incidence rates per 100,000 person-years.
Cox proportional hazard models were used to calculate HRs with age as the time metric. Adjusted for smoking (never, quit ≥10 y ago, quit 5–9 y ago, quit 1–4 y ago, quit <1 y ago, or current and smokes ≤20 or >20 cigarettes/d), energy (continuous), energy-adjusted total fat (continuous), and sex.
Quintiles for years overweight and cumulative OWY were based on the distribution of the cohort who ever had overweight with a separate category for never overweight. Weighted cumulative OWY (similar to pack-years) are based on the degree of overweight over time.
Based on the median of each category.
TABLE 5.
Incidence rates, HRs, and 95% CIs of duration of overweight or obesity across a lifetime by history of diabetes1
| Cases |
Incidence rate (95% CI)2 |
HR (95% CI)3 |
||||
| No diabetes | Diabetes | No diabetes | Diabetes | No diabetes | Diabetes | |
| n/person-years | ||||||
| Years overweight4 | ||||||
| Never overweight | 308/752,914 | 9/21,358 | 38.6 (34.3, 42.9) | 38.8 (9.9, 67.6) | 1.00 (referent) | 0.88 (0.45, 1.71) |
| >0 and <10.1 y | 148/340,307 | 8/14,231 | 45.0 (37.5, 52.5) | 43.0 (10.5, 75.4) | 1.15 (0.95, 1.40) | 1.31 (0.65, 2.64) |
| ≥10.1 and <18.9 y | 157/332,470 | 13/21,892 | 46.9 (39.4, 54.4) | 63.6 (27.5, 99.7) | 1.24 (1.02, 1.50) | 1.43 (0.82, 2.49) |
| ≥18.9 and <27.8 y | 142/316,371 | 20/35,882 | 42.1 (34.8, 49.5) | 47.6 (25.5, 69.8) | 1.12 (0.92, 1.37) | 1.32 (0.84, 2.07) |
| ≥27.8 and <37.3 y | 157/301,682 | 29/48,377 | 49.1 (40.2, 58.1) | 52.5 (31.1, 73.9) | 1.29 (1.06, 1.57) | 1.48 (101, 2.17) |
| ≥37.3 y | 150/283,341 | 65/59,993 | 39.2 (30.7, 47.7) | 85.8 (61.9, 109.7) | 1.10 (0.90, 1.34) | 2.27 (1.73, 2.98) |
| P-trend5 | 0.14 | 0.003 | ||||
| Continuous, per 10 y | 1062/2,327,086 | 144/201,733 | 48.4 (45.4, 51.3) | 71.4 (59.7, 83.1) | 1.03 (0.99, 1.07) | 1.18 (1.05, 1.32) |
| Cumulative OWY4 | ||||||
| Never overweight | 308/752,914 | 9/21,358 | 38.6 (34.3, 42.9) | 38.8 (9.9, 67.6) | 1.00 (referent) | 0.88 (0.46, 1.72) |
| >0 and <24 OWY | 175/337,176 | 11/16,004 | 46.5 (39.4, 53.6) | 53.5 (17.3, 89.6) | 1.22 (1.01, 1.47) | 1.44 (0.79, 2.63) |
| ≥24 and <415 OWY | 120/334,241 | 10/21,616 | 42.2 (34.0, 50.4) | 46.8 (16.9, 76.7) | 1.13 (0.91, 1.40) | 1.44 (0.79, 2.63) |
| ≥415 and <663 OWY | 172/315,450 | 23/35,128 | 45.3 (37.4, 53.3) | 57.6 (31.3, 83.9) | 1.21 (1.00, 1.46) | 1.39 (0.91, 2.12) |
| ≥663 and <949 OWY | 135/306,953 | 28/44,908 | 44.4 (35.2, 53.5) | 56.2 (32.2, 80.2) | 1.14 (0.93, 1.40) | 1.60 (1.08, 2.36) |
| ≥949 OWY | 152/280,352 | 63/62,720 | 42.1 (33.6, 50.6) | 82.3 (59.5, 105.2) | 1.17 (0.96, 1.42) | 2.18 (1.65, 2.87) |
| P-trend5 | 0.31 | 0.003 | ||||
| Continuous, per 100 units OWY | 1062/2,327,086 | 144/201,733 | 48.4 (45.4, 51.3) | 71.4 (59.7, 83.1) | 1.01 (0.99, 1.02) | 1.03 (1.01, 1.07) |
All analyses were limited to the subcohort of 273,977 participants and 1206 pancreatic cancer cases. OWY, overweight years.
Sex-adjusted, age-standardized incidence rates per 100,000 person-years.
Cox proportional hazard models were used to calculate HRs with age as the time metric. Adjusted for smoking (never, quit ≥10 y ago, quit 5–9 y ago, quit 1–4 y ago, quit <1 y ago, or current and smokes ≤20 or >20 cigarettes/d), energy (continuous), energy-adjusted total fat (continuous), and sex (in sex-combined models). Duration of overweight by diabetes, P-interaction = 0.01. Cumulative overweight by diabetes, P-interaction = 0.02.
Quintiles for years overweight and cumulative OWY were based on the distribution of the cohort who ever had overweight with a separate category for never overweight. Weighted cumulative OWY (similar to pack-years) are based on the degree of overweight over time.
Based on the median of each category.
The proportion of our population ever having a BMI >25.0 was 70%, and the HR for ever having a BMI >25.0 was 1.25 (95% CI: 1.08, 1.41). The population attributable risk for being ever overweight or obese at any age was 14% (95% CI: 0.05, 0.22) overall, 21% (95% CI: 0.06, 0.34) among never smokers, 18% (95% CI: 0.03, 0.32) among former smokers who quit >10 y before baseline, 1% (95% CI: 0.14, 0.16) among recent smokers, 45% (95% CI: 0.05, 0.73) among those who reported baseline diabetes, and 10% (95% CI: 0.01, 0.19) among nonpatients with diabetes.
DISCUSSION
In this large cohort of middle-aged and older adults, excess body weight (BMI >25) at any age was positively associated with pancreatic cancer. Longer adiposity duration and cumulative duration that considered the degree of excess body fat were significantly associated with pancreatic cancer, with individuals having diabetes and longer adiposity experiencing the highest risk and rates. The etiologic fraction of pancreatic cancer explained by overweight or obesity at any age in our population was 14% overall, 18% in former smokers, and 21% in never smokers. BMI gain of >10 or any loss between the ages of 50 y and baseline was significantly associated with increased pancreatic cancer risk. Therefore, excess body mass represents an important potentially modifiable risk factor that, if prevented, could substantially decrease the burden of pancreatic cancer.
To our knowledge, this is the first prospective study to examine adiposity across multiple times in life, namely early adulthood, midlife, and older age, thus allowing us to calculate cumulative duration of overweight/obesity. We are also unaware of previous prospective studies that reported etiologic fractions for lifetime overweight/obesity and pancreatic cancer. Our results confirm the positive associations between excess weight in early adulthood and pancreatic cancer observed in some previous studies. Nine studies, 5 case-control (5, 12–15) and 4 prospective (16–19), have examined BMI during adolescence or early adulthood and risk of pancreatic cancer, with 5 showing significant positive associations (5, 12, 15, 18, 19). One of these was a pooled analysis of 14 cohorts that examined BMI in early adulthood (age 18 or 21 y) and showed a significant 20% increased risk per 5-unit increase in BMI (19). Another was a multicenter case-control study which reported that high compared with low BMI at ages 20 and 40 y and both ages was significantly associated with a 36%, 57%, and 86% elevated pancreatic cancer risk, respectively (15). Within a cohort of 720,000 Israeli men with BMIs measured at ages 16 and 19 y, adolescent overweight as defined by a BMI >85th percentile of the US-CDC adolescence reference distribution showed a significant 2-fold pancreatic cancer risk (18). Neither of these later 2 studies determined associations between BMI and pancreatic cancer at older ages.
Our study and most other prospective studies showed positive associations between middle-aged and older adult BMI and pancreatic cancer, with the risk being stronger among nonsmokers (19). One case-control study found that significant increases in risk associated with increasing BMI during early adulthood and middle age diminished and became nonsignificant for BMI at ages >40 y, particularly among women and never smokers (5). It is possible that these differing results may be explained by biases related to recalling older adult weight after being diagnosed with pancreatic cancer or reverse causation in the case-control study because pancreatic cancer is most often diagnosed at advanced stages and is preceded by significant weight loss.
We observed a nonlinear association with BMI change between age 50 y and baseline, such that any weight loss and substantial gain in adiposity during older ages was significantly associated with greater pancreatic cancer risk; however, we observed no associations for BMI changes occurring at younger ages. Our finding with weight loss after age 50 y is consistent with the aforementioned weight loss that occurs during latent pancreatic cancer. Previous studies that examined change in weight or BMI across a lifetime have mostly shown no association (20). One pooled analysis of BMI change between early adulthood and older age reported a significant 40% increased risk among participants whose BMI decreased by >2 or increased by >10 compared with those with BMI changes of ±2 (19). In contrast, BMI change between ages 18 y and baseline was not associated with pancreatic cancer in our study. Our results suggest that weight gain later in life, closer to the time of pancreatic cancer diagnosis, may promote or stimulate pancreatic tumor growth more strongly than weight gain occurring earlier in life. Given the characteristics of the NIH-AARP study population, however, we are only able to evaluate associations for pancreatic cancer occurring at ages >50 y. Future studies should examine whether weight gain earlier in life is associated with pancreatic cancer occurring at younger ages (<50 y).
The pattern of pancreatic cancer associations that we observed for absolute and cumulative duration of adiposity, as well as recent weight gain, and pancreatic cancer might be explained by the growth-promoting effects of insulin. Overweight and obesity lead to insulin resistance and eventually diabetes (21). Biomarkers of insulin resistance have been associated with increased pancreatic cancer risk in prospective epidemiologic studies (2, 3, 22–25). Experimental studies have shown that insulin has mitogenic effects on pancreatic cancer cell lines (26) and peripheral insulin resistance promotes ductal pancreatic carcinogenesis in animals (27–31). Experimental and epidemiologic studies also suggest that administration of certain antidiabetic drugs such as metformin, an insulin sensitizer, decreases pancreatic cancer risk and progression, whereas insulin and insulin secretagogues increase risk (5, 21, 30, 32, 33). Alternatively, rodent studies have shown that circulating stromal and vascular progenitor cells derived from white adipose tissue are recruited by tumors and enhance tumor vascularization, growth, and progression (34–36). This might be relevant for pancreatic cancer given the proximity of the pancreas to visceral intraabdominal adipose tissue (34) and that intraabdominal body fat has been associated with pancreatic cancer (19, 37–39).
In the present study, baseline BMI appeared to be more clearly associated with pancreatic cancer among participants who were never smokers. Many studies have observed stronger positive associations with adult BMI among never smokers compared with smokers or the overall population (19, 41). Cigarette smoke contains a multitude of potential carcinogens that may contribute to pancreatic carcinogenesis. Therefore, it is possible that adiposity does not play a substantial role in the etiology of pancreatic cancer among older current smokers.
An important strength of our study is its large prospective design with detailed information about body weight at different ages collected on the same individuals, which was assessed before cancer diagnosis, thereby reducing the influence of reverse causation and differential biases. Seventy percent of our study population was ever overweight or obese, a proportion similar to that of the American population (41); this suggests that our cohort is representative of the US population with respect to the prevalence of overweight and obesity.
Our study also has limitations. Our case definition included nonmicroscopically confirmed cancer, which could contribute to misclassification of case status and some attenuation of risk estimates (42). Measurement error related to self-reported height and weight could contribute to inaccurate associations, particularly because participants had to recall their weight from the distant past. However, others have shown that self-reported body weight can be recalled with fairly good accuracy and reproducibility (43–49). The misclassification of reported body weight, particularly among those who are overweight or obese (because this group tends to underreport true weight), would attenuate the associations. Because we tested many associations, it is possible that some of the significant associations that we observed may be due to chance. However, we had one primary hypothesis, namely overweight and obesity is associated with pancreatic cancer. In addition, our results for overweight and obesity and pancreatic cancer risk are consistent with those of other studies, particularly cohort studies (20).
In conclusion, we observed significant positive associations between overweight and obesity in early, middle, and older age and subsequent pancreatic cancer. Individuals with the longest duration of overweight and diabetes had the greatest risk and rates. Our results are relevant given the increasing rates of obesity, diabetes, and pancreatic cancer in the United States (42, 50), particularly among individuals who were overweight at younger ages. Maintaining a healthy weight and avoidance of adiposity might prevent pancreatic cancer.
Supplementary Material
Acknowledgments
Cancer incidence data from Arizona were collected by the Arizona Cancer Registry; from Georgia by the Georgia Center for Cancer Statistics; from California by the California Department of Health Services, Cancer Surveillance Section; from Michigan by the Michigan Cancer Surveillance Program; from Florida by the Florida Cancer Data System under contract to the Department of Health; from Louisiana by the Louisiana Tumor Registry; from New Jersey by the New Jersey State Cancer Registry; from North Carolina by the North Carolina Central Cancer Registry; from Pennsylvania by the Division of Health Statistics and Research, Pennsylvania Department of Health; and from Texas by the Texas Cancer Registry. The views expressed herein are solely those of the authors and do not necessarily reflect those of the cancer registries or contractors. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. We are indebted to the participants of the NIH-AARP Diet and Health Study for their outstanding cooperation. We acknowledge Arthur Schatzkin, the visionary who founded the NIH-AARP Diet and Health Study, and the contributions of Barry Graubard for his assistance and discussions regarding the statistical analysis and splines.
The authors’ responsibilities were as follows—RZS-S: conceived and designed the study, performed statistical analyses, drafted and revised the manuscript, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis; AH and RZS-S: acquired the data; RZS-S and CS: analyzed the data; RZS-S, CS, SM, and DTS: interpreted the data; and all authors: contributed substantive interpretation of and editorial comments on the manuscript drafts. None of the authors declared a conflict of interest.
REFERENCES
- 1.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, Albanes D. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005;294:2872–8 [DOI] [PubMed] [Google Scholar]
- 3.Michaud DS, Wolpin B, Giovannucci E, Liu S, Cochrane B, Manson JE, Pollak MN, Ma J, Fuchs CS. Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 2007;16:2101–9 [DOI] [PubMed] [Google Scholar]
- 4.Anderson KE, Mack TM, Silverman DT. Cancer of the Pancreas. : Schottenfeld D, Fraumeni JF, eds. Cancer epidemiology and prevention. 3rd ed. New York, NY: Oxford University Press, 2006:721–63 [Google Scholar]
- 5.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009;301:2553–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health–American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–25 [DOI] [PubMed] [Google Scholar]
- 7.Michaud DS, Midthune D, Hermansen S, Leitzmann M, Harlan LC, Kipnis V, Schatzkin A. Comparison of cancer registry case ascertainment with SEER estimates and self-reporting in a subset of the NIH-AARP Diet and Health Study. J Registr Manag 2005;32:70–5 [Google Scholar]
- 8. WHO Expert Committee. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1–452. [PubMed]
- 9.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control 2007;18:571–9 [DOI] [PubMed] [Google Scholar]
- 10.Lin G, So Y, Johnston G. Analyzing survival data with competing risks using SAS software. SAS Global Forum 2012. Cary, NC: SAS Institute, 2012.
- 11.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberle CA, Bracci PM, Holly EA. Anthropometric factors and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes Control 2005;16:1235–44 [DOI] [PubMed] [Google Scholar]
- 13.Fryzek JP, Schenk M, Kinnard M, Greenson JK, Garabrant DH. The association of body mass index and pancreatic cancer in residents of southeastern Michigan, 1996-1999. Am J Epidemiol 2005;162:222–8 [DOI] [PubMed] [Google Scholar]
- 14.Ji BT, Hatch MC, Chow WH, McLaughlin JK, Dai Q, Howe GR, Gao YT, Fraumeni JF., Jr Anthropometric and reproductive factors and the risk of pancreatic cancer: a case-control study in Shanghai, China. Int J Cancer 1996;66:432–7 [DOI] [PubMed] [Google Scholar]
- 15.Urayama KY, Holcatova I, Janout V, Foretova L, Fabianova E, Adamcakova Z, Ryska M, Martinek A, Shonova O, Brennan P, et al. Body mass index and body size in early adulthood and risk of pancreatic cancer in a central European multicenter case-control study. Int J Cancer 2011;129:2875–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev 2005;14:459–66 [DOI] [PubMed] [Google Scholar]
- 17.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921–9 [DOI] [PubMed] [Google Scholar]
- 18.Levi Z, Kark JD, Afek A, Derazne E, Tzur D, Furman M, Gordon B, Barchana M, Liphshitz I, Niv Y, et al. Measured body mass index in adolescence and the incidence of pancreatic cancer in a cohort of 720,000 Jewish men. Cancer Causes Control 2012;23:371–8 [DOI] [PubMed] [Google Scholar]
- 19.Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, English DR, Folsom AR, Freudenheim JL, Fuchs CS, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer 2011;129:1708–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WCRF/AICR. Continuous update project for pancreatic cancer. World Cancer Research Fund 2012. Available from: http://www.dietandcancerreport.org/cup/current_progress/pancreatic_cancer.php (cited December 2012)
- 21.Bao B, Wang Z, Li Y, Kong D, Ali S, Banerjee S, Ahmad A, Sarkar FH. The complexities of obesity and diabetes with the development and progression of pancreatic cancer. Biochim Biophys Acta 2011;. 1815:135–46. [DOI] [PMC free article] [PubMed]
- 22.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA 2000;283:2552–8 [DOI] [PubMed] [Google Scholar]
- 23.Stattin P, Bjor O, Ferrari P, Lukanova A, Lenner P, Lindahl B, Hallmans G, Kaaks R. Prospective study of hyperglycemia and cancer risk. Diabetes Care 2007;30:561–7 [DOI] [PubMed] [Google Scholar]
- 24.Grote VA, Rohrmann S, Nieters A, Dossus L, Tjonneland A, Halkjaer J, Overvad K, Fagherazzi G, Boutron-Ruault MC, Morois S, et al. Diabetes mellitus, glycated haemoglobin and C-peptide levels in relation to pancreatic cancer risk: a study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Diabetologia 2011;54:3037–46 [DOI] [PubMed] [Google Scholar]
- 25.Grote VA, Rohrmann S, Dossus L, Nieters A, Halkjaer J, Tjonneland A, Overvad K, Stegger J, Chabbert-Buffet N, Boutron-Ruault MC, et al. The association of circulating adiponectin levels with pancreatic cancer risk: a study within the prospective EPIC cohort. Int J Cancer 2012;130:2428–37 [DOI] [PubMed] [Google Scholar]
- 26.Hennig R, Ding XZ, Adrian TE. On the role of the islets of Langerhans in pancreatic cancer. Histol Histopathol 2004;19:999–1011 [DOI] [PubMed] [Google Scholar]
- 27.Bell RH, Jr, McCullough PJ, Pour PM. Influence of diabetes on susceptibility to experimental pancreatic cancer. Am J Surg 1988;155:159–64 [DOI] [PubMed] [Google Scholar]
- 28.Pour PM, Kazakoff K, Carlson K. Inhibition of streptozotocin-induced islet cell tumors and N-nitrosobis(2-oxopropyl)amine-induced pancreatic exocrine tumors in Syrian hamsters by exogenous insulin. Cancer Res 1990;50:1634–9 [PubMed] [Google Scholar]
- 29.Pour PM, Stepan K. Modification of pancreatic carcinogenesis in the hamster model. VIII. Inhibitory effect of exogenous insulin. J Natl Cancer Inst 1984;72:1205–8 [PubMed] [Google Scholar]
- 30.Schneider MB, Matsuzaki H, Haorah J, Ulrich A, Standop J, Ding XZ, Adrian TE, Pour PM. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 2001;120:1263–70 [DOI] [PubMed] [Google Scholar]
- 31.Zyromski NJ, Mathur A, Gowda GA, Murphy C, Swartz-Basile DA, Wade TE, Pitt HA, Raftery D. Nuclear magnetic resonance spectroscopy-based metabolomics of the fatty pancreas: implicating fat in pancreatic pathology. Pancreatology 2009;9:410–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–61 [DOI] [PubMed] [Google Scholar]
- 33.Bodmer M, Becker C, Meier C, Jick SS, Meier CR. Use of antidiabetic agents and the risk of pancreatic cancer: a case-control analysis. Am J Gastroenterol 2012;107:620–6 [DOI] [PubMed] [Google Scholar]
- 34.Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, Debeb B, Woodward W, Schmandt R, Broaddus R, et al. Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res 2012;18:771–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellows CF, Zhang Y, Chen J, Frazier ML, Kolonin MG. Circulation of progenitor cells in obese and lean colorectal cancer patients. Cancer Epidemiol Biomarkers Prev 2011;20:2461–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res 2009;69:5259–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berrington de Gonzalez A, Spencer EA, Bueno-de-Mesquita HB, Roddam A, Stolzenberg-Solomon R, Halkjaer J, Tjonneland A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, et al. Anthropometry, physical activity, and the risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 2006;15:879–85. [DOI] [PubMed]
- 38.Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, Fuchs CS, Gross MD, Jacobs EJ, Lacroix AZ, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, Schairer C, Michaud DS, Hollenbeck A, Schatzkin A, Silverman DT. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health–AARP Diet and Health Cohort. Am J Epidemiol 2008;167:586–97 [DOI] [PubMed] [Google Scholar]
- 40.Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol 2012;23:843–52 [DOI] [PubMed] [Google Scholar]
- 41.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7 [DOI] [PubMed] [Google Scholar]
- 42.Verhage BA, Schouten LJ, Goldbohm RA, van den Brandt PA. Anthropometry and pancreatic cancer risk: an illustration of the importance of microscopic verification. Cancer Epidemiol Biomarkers Prev 2007;16:1449–54 [DOI] [PubMed] [Google Scholar]
- 43.Klipstein-Grobusch K, Kroke A, Boeing H. Reproducibility of self-reported past body weight. Eur J Clin Nutr 1998;52:525–8 [DOI] [PubMed] [Google Scholar]
- 44.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology 1995;6:61–6 [DOI] [PubMed] [Google Scholar]
- 45.Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol 1990;132:1156–63 [DOI] [PubMed] [Google Scholar]
- 46.Kovalchik S. Validity of adult lifetime self-reported body weight. Public Health Nutr 2009;12:1072–7 [DOI] [PubMed] [Google Scholar]
- 47.Kyulo NL, Knutsen SF, Tonstad S, Fraser GE, Singh PN. Validation of recall of body weight over a 26-year period in cohort members of the Adventist Health Study 2. Ann Epidemiol 2012;22:744–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norgan NG, Cameron N. The accuracy of body weight and height recall in middle-aged men. Int J Obes Relat Metab Disord 2000;24:1695–8 [DOI] [PubMed] [Google Scholar]
- 49.Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol 1991;18:155–66 [DOI] [PubMed] [Google Scholar]
- 50.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA 2012;307:483–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.