Abstract
Background: The relation between sugar-sweetened beverages (SSBs) and body weight remains controversial.
Objective: We conducted a systematic review and meta-analysis to summarize the evidence in children and adults.
Design: We searched PubMed, EMBASE, and Cochrane databases through March 2013 for prospective cohort studies and randomized controlled trials (RCTs) that evaluated the SSB-weight relation. Separate meta-analyses were conducted in children and adults and for cohorts and RCTs by using random- and fixed-effects models.
Results: Thirty-two original articles were included in our meta-analyses: 20 in children (15 cohort studies, n = 25,745; 5 trials, n = 2772) and 12 in adults (7 cohort studies, n = 174,252; 5 trials, n = 292). In cohort studies, one daily serving increment of SSBs was associated with a 0.06 (95% CI: 0.02, 0.10) and 0.05 (95% CI: 0.03, 0.07)-unit increase in BMI in children and 0.22 kg (95% CI: 0.09, 0.34 kg) and 0.12 kg (95% CI: 0.10, 0.14 kg) weight gain in adults over 1 y in random- and fixed-effects models, respectively. RCTs in children showed reductions in BMI gain when SSBs were reduced [random and fixed effects: −0.17 (95% CI: −0.39, 0.05) and −0.12 (95% CI: −0.22, −0.2)], whereas RCTs in adults showed increases in body weight when SSBs were added (random and fixed effects: 0.85 kg; 95% CI: 0.50, 1.20 kg). Sensitivity analyses of RCTs in children showed more pronounced benefits in preventing weight gain in SSB substitution trials (compared with school-based educational programs) and among overweight children (compared with normal-weight children).
Conclusion: Our systematic review and meta-analysis of prospective cohort studies and RCTs provides evidence that SSB consumption promotes weight gain in children and adults.
INTRODUCTION
As the search for solutions to the worldwide epidemic of obesity continues, the relation between consumption of sugar-sweetened beverages (SSBs)4 and body weight has become a matter of much public and scientific interest. SSBs are composed of energy-containing sweeteners such as sucrose (50% glucose, 50% fructose), high-fructose corn syrup (HFCS; most often 45% glucose and 55% fructose), or fruit juice concentrates that are added to the beverage by manufacturers, establishments, or individuals and usually contain >25 kcal per 8 fluid ounces. Although temporal patterns from the United States have shown a decrease in added sugar consumption between 2000 and 2008, primarily from reductions in SSBs, average intakes still exceed recommended limits and SSBs continue to be the largest contributor to added sugar and top sources of calories in the US diet (1). Globally, intake of SSBs has been increasing steadily, because of rapid urbanization and heavy marketing in low- and middle-income countries (2).
Within the past 2 decades, a number of epidemiologic studies both in children and adults have evaluated the association between SSB intake and weight gain and obesity. In general, findings from large observational studies support a link between SSB consumption and development of obesity (3, 4). However, controversy remains whether the association is causal and whether public action should be taken on the basis of the observational evidence. Recently, several randomized controlled trials (RCTs) have been performed to evaluate whether adding SSBs into the habitual diet can increase body weight or if substituting SSBs by other low- or noncaloric beverages can reduce weight gain or facilitate weight loss. The results have been mixed as a result of heterogeneity in study design, sample size, and study duration.
For clinicians and policymakers to make informed evidence-based recommendations about SSBs, the totality of the available evidence needs to be examined in a thorough and systematic manner. Thus, we conducted a systematic review and meta-analyses of prospective cohort studies and RCTs in children and adults to provide a comprehensive summary of the literature evaluating SSBs and body weight gain.
METHODS
Literature search
Standard methods were used for conducting and reporting meta-analyses (5). Relevant articles were identified by searching PubMed (http://www.ncbi.nlm.nih.gov/pubmed; since 1966), EMBASE (http://www.embase.com; since 1947), and the Cochrane library (http://www.thecochranelibrary.com/; since 1951) databases from the index date through March 2013 for studies evaluating the association between SSBs and body weight in children and adults. Our search strategy combined various terms for SSBs (eg, carbonated beverages, sweetened beverages, soda, sports drink, fruit drink) and body weight (ie, body weight, BMI, overweight, obesity), related cardiometabolic outcomes (ie, diabetes mellitus, insulin resistance, cardiovascular diseases, hypertension), and study design/epidemiologic methods (ie, epidemiologic studies, cohort, case-control, clinical trials) by using exploded versions of nedical subject headings terms and corresponding key words in titles and abstracts. Additional articles were identified from reference lists of included studies and relevant reviews. Full details on our search terms and strategy are shown in Supplemental Table 1 under “Supplemental data” in the online issue. The current meta-analysis focused on outcomes related to body weight. Our search strategy included terms for cardiometabolic outcomes because some of these studies also report outcomes for body weight.
Study selection
Studies were considered for inclusion in our meta-analysis if they met the following criteria: 1) were original research, ie, not a review, abstract, editorial, letter, or commentary; 2) were prospective cohort studies or clinical trials conducted in children or adults; 3) reported multivariable-adjusted coefficients for the association between SSBs and body weight (any available metric) from prospective cohort studies or the difference in changes in body weight (any available metric) between intervention and control groups from clinical trials; 4) did not combine SSBs with other beverages, foods, or lifestyle factors as a composite exposure; and 5) had a control group and intervened for at least 2 wk in clinical trials. We restricted publications to the English language, and we did not consider cross-sectional or ecologic studies because they are highly prone to confounding and reverse causation. Titles and abstracts of identified studies were screened, and potentially relevant articles were selected for full-text review, which was performed independently by 2 investigators (VSM and AP). Discrepancies were resolved by consensus or consultation with a third author (FBH).
Data extraction
For each article identified, we extracted information on study characteristics (authors, publication year, geographic location, sample size, and duration), participant characteristics (sex, age, and baseline body weight), SSB assessment method [food-frequency questionnaire (FFQ), 24-h recall, or diet record], type of SSB and serving size, body weight assessment method (measured, self-report), intervention design (crossover trial, parallel trial, or cluster RCT), intervention and control modality, and analysis strategy (statistical models, adjustment for total energy and covariates). For prospective cohort studies, we extracted multivariable-adjusted β coefficients and corresponding SEs for the association between SSBs and any available measure of body weight. Because total energy intake partly mediates the association between SSBs and weight, where possible we extracted estimates that were not adjusted for total energy. For RCTs, we extracted means and SDs of changes in body weight (any available metric) from baseline to the end of follow-up for intervention and control regimens. If a trial did not report the SD for the measurement of change, we imputed this value by using the correlation coefficient method referenced in the Cochrane Handbook for Systematic Reviews of Interventions (6). We used a correlation coefficient of 0.95 because the correlation between body weights at the 2 time points was assumed to be very high.
Data synthesis and analysis
For a number of studies it was necessary to obtain data from authors or apply scaling factors and transformations with various assumptions to generate consistent units for the meta-analyses (see Supplemental Table 2 under “Supplemental data” in the online issue). For prospective cohort studies in children, our primary estimate of interest was the predicted change in BMI per one 12-oz–serving/d increment of SSBs during the time period specified in each study. Studies that reported serving sizes in units other than 12 oz were scaled accordingly. Studies by Blum et al (7), Newby et al (8), and Mundt et al (9) were scaled from 1-oz servings to 12-oz servings. Studies by Striegel-Moore et al (10), Johnson et al (11), Libuda et al (12), and Olsen et al (13) were scaled from 100 g/d, 180 g/d, 1 MJ/d, and 10 g/d to one 12-oz serving/d, respectively. For studies that did not specify a serving size (14–19), we assumed the standard serving size of 12 oz. Two studies were converted from servings per week to servings per day (15, 19). Studies reporting estimates using BMI z score (7, 12, 13, 18, 20, 21) were converted to BMI by using the LMS method developed by Cole (22), and studies reporting estimates of fat mass (kg) were converted to BMI by dividing the coefficients by average height in meters squared (9, 11). Finally, studies reporting estimates categorically (18, 19) were converted into continuous variables by assigning medians to each intake category, which were plotted against weight change by using least squares linear regression to obtain the slope (β) and SE. This transformation makes the assumption of linearity. Because studies evaluating change in SSB intake in relation to change in weight have some features of a quasi-experimental design, we conducted a separate meta-analysis for the 1-y change in BMI per 1-serving/d increment of SSBs by using studies that reported change versus change estimates (12, 14–17, 19, 21). Units were converted to 1-y change by dividing β coefficients by the time period specified in each study (see Supplemental Table 2 under “Supplemental data” in the online issue).
For trials in children, our primary estimate of interest was the mean difference in BMI between intervention and control regimens (see Supplemental Table 2 under “Supplemental data” in the online issue).
For prospective cohort studies in adults, our primary estimate of interest was the 1-y change in weight (kg) per 1-serving/d increment of SSBs by using studies that reported change in weight in relation to change in SSBs (23–29). Units were converted to 1-y change by dividing coefficients by the time period specified in each study, and the serving size was assumed to be 12 oz, consistent with most cans and glasses. Two studies (23, 27) were converted from servings per week to servings per day, and one study (28) was converted from change in 1 percentage unit of SSBs to servings per day. The study by Palmer et al (25) was converted into continuous data by assigning medians to each intake category, which were plotted against weight change by using least squares linear regression to obtain the slope (β) and SE. Data were converted from pounds to kilograms by multiplying coefficients by 0.45. For trials in adults, the unit of interest was the mean difference in weight in kilograms from baseline to end of follow-up between intervention and controls (see Supplemental Table 2 under “Supplemental data” in the online issue).
Summary estimates were calculated by combining inverse-variance–weighted study-specific estimates using random-effects models, which accounts for between-study heterogeneity and is generally considered the more conservative method (6). Fixed-effects models were also evaluated for comparison. Forest plots were used to visualize individual and summary estimates, and the Cochrane Q test and I2 statistic were used to evaluate between-study heterogeneity (30, 31). An I2 value >50% was generally considered to be high (32). Potential sources of heterogeneity, including adjustment for total energy, duration, age, dietary assessment method, sample size, and baseline weight status, were explored by using univariate meta-regressions and stratified analyses (36). We also tested the influence of individual studies on the results in sensitivity analysis (36). The potential for publication bias was evaluated by using Begg's test and visual inspection of funnel plots (33, 34). All analyses were performed by using Stata 10.0 (StataCorp).
Risk of bias assessment
Study-level risk of bias was assessed by 2 authors (VSM and AP), and disagreements in ratings were discussed until consensus. For cohort studies, the Newcastle Ottawa scale was used (35), which assesses 3 broad areas: the selection of exposed and unexposed participants, the comparability of the groups, and the assessment of the outcome. A star was awarded for high quality in each area, with a maximum of 4 stars for the “Selection” category, 2 stars for “Comparability,” and 3 stars for “Outcome.” For comparability, we awarded a star for studies that provided coefficients that did not adjust for total energy intake (36) and for those that adjusted for age and other important factors. Studies with a score ≥7 were considered as good quality and those with a score <5 were considered as poor quality. RCTs were reviewed by using the Cochrane Collaboration's risk of bias tool (6), which rates 7 domains (sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessment, completeness of outcome data, selective reporting of outcomes, and other threats to validity, eg, contamination of intervention, baseline imbalance, and carry-over effect in crossover trials) as having a low risk of bias, a high risk of bias, or an unclear risk of bias.
RESULTS
Literature search
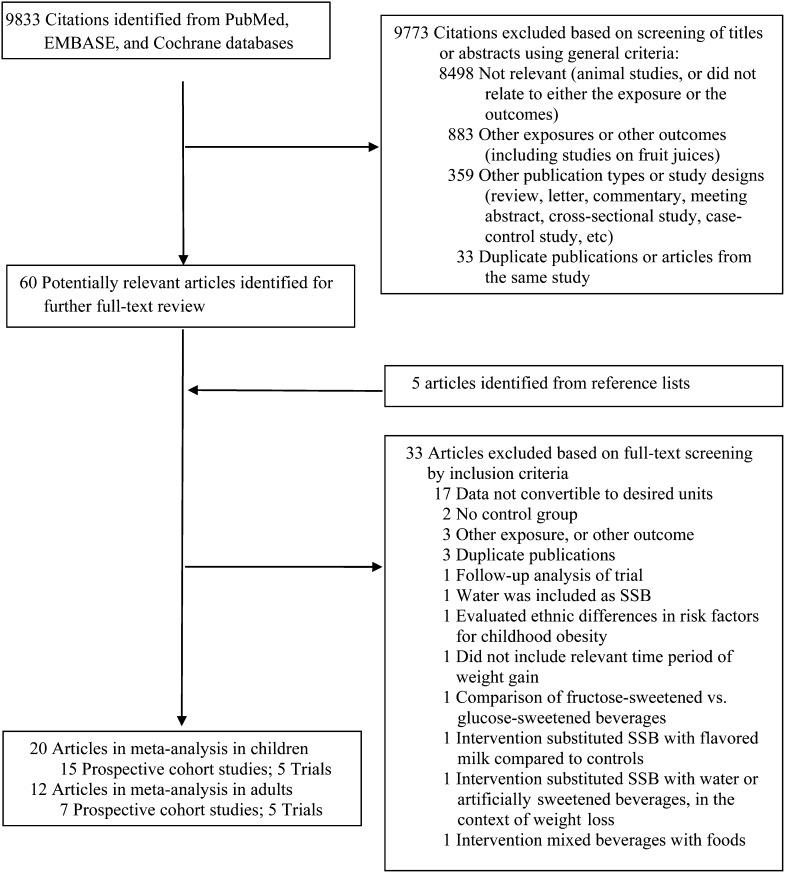
Our search strategy identified 9833 unique citations, of which 60 were selected for full-text review after screening titles and abstracts, plus an additional 5 articles identified from reference lists (Figure 1). After reviewing full texts, 33 articles were additionally excluded. Among cohort studies in children, 11 studies were excluded because we were not able to obtain data in the necessary units from transformations or author correspondence: 4 presented ORs (37–40), 2 did not report longitudinal data for SSBs (41, 42), one presented data as frequencies by weight change group (43), one presented SSBs in grams of carbohydrate per day by BMI gainers/losers (44), one did not present weight change data for all categories of beverage intake (45), one modeled SSBs dichotomously (46), and one presented standardized β coefficients (47). Among RCTs in children, 2 studies were excluded because one was a follow-up of an included trial (48) and the other was conducted in a duplicate study population (49). Another study was excluded because it substituted SSBs with flavored milk (50).
FIGURE 1.
Flowchart of study search and selection. PubMed, http://www.ncbi.nlm.nih.gov/pubmed; EMBASE, http://www.embase.com; Cochrane, http://www.thecochranelibrary.com/. SSB, sugar-sweetened beverage.
Among cohort studies in adults, 6 studies were excluded because of unavailability of data or heterogeneity of outcome measures: 2 reported ORs (51, 52), one reported results stratified by weight gain before baseline (53), 2 reported BMI rather than body weight (54, 55), and one presented data in a figure that could not be extracted (56). Two studies were additionally excluded because they were conducted in duplicate study populations (57, 58). Among intervention studies in adults, the study by Raben et al (59) was excluded because the intervention combined beverages and foods. We did not include the trial by Tate et al (60) because unlike other trials, which evaluated the effects of adding SSBs on body weight, this study substituted SSBs with water or artificially sweetened beverages in a context of active weight-loss intervention (60). Therefore, the data could not be combined because of the different study questions. After final exclusions, 32 original articles were included in our meta-analyses: 20 in children (15 prospective cohort studies and 5 trials) and 12 in adults (7 prospective cohort studies and 5 trials). The excluded studies were evaluated qualitatively.
Study characteristics
Characteristics of the prospective cohort studies included in our meta-analyses are shown in Tables 1 and 2. Among the 15 cohort studies in children, the majority were from the United States (n = 10), Europe (n = 4), and Canada (n = 1), with ages at baseline ranging from 2 to 16 y (Table 1). The number of participants in each study ranged from 141 to 11,703, with durations of follow-up ranging from 6 mo to 14 y. Studies used a variety of methods to assess diet, including FFQs (n = 5), 24-h recalls (n = 4), diet and lifestyle questionnaires (n = 3), and diet records (n = 3); and all studies adjusted for additional diet or lifestyle risk factors, although 2 studies did not adjust for age (13, 20) and one study adjusted only for age and time (12). Only 3 studies adjusted for total energy intake (7, 8, 10). Among the 7 cohort studies in adults, the majority were conducted in black or white populations from the United States (n = 6) and one study was from the Netherlands (Table 2). Cohorts ranged in size from 173 to 120,877 participants, with durations of follow-up ranging from 1 to 20 y. Two studies were conducted exclusively in overweight or obese women (27, 28), and one study was conducted in participants with prehypertension or stage 1 hypertension (26). All but one study (28) used an FFQ to assess diet, and all studies adjusted for additional diet and lifestyle factors, although one study did not adjust for age (27). None of the studies adjusted for total energy intake.
TABLE 1.
Characteristics of studies included in meta-analysis of prospective cohort studies in children1
| Reference | Study population and location | Sample size | Mean ± SD baseline age or age range | Duration | Dietary assessment method | Outcome assessment method | Study question | Covariates | Adjusted for energy |
| Ludwig, 2001 (16) | Intervention and Evaluation Project, Planet Health, Massachusetts | 548 | 11.7 ± 0.8 y | 19 mo | FFQ | BMI based on measured height and weight | Change in SSBs from baseline to end of follow-up and BMI at end of follow-up | Baseline BMI, age, sex, ethnicity, school, percentage of energy from fat and energy-adjusted fruit juice intake at baseline and change from baseline to follow-up, physical activity, television, and change in television from baseline to follow-up | No |
| Berkey, 2004 (17) | Growing Up Today Study (GUTs), 50 states, USA | 11,703 | 9–14 y | 2 y | 132-item FFQ | Self-reported BMI | 1-y change in SSB intake and change in BMI | Age, Tanner stage, race, menarche (girls), prior BMI z score, height growth, milk type, physical activity, inactivity, diet soda juice, milk | No |
| Newby, 2004 (8) | North Dakota, Women, Infants, and Children (WIC), USA | 1345 | 2–5 y | 6–12 mo | 84-item FFQ | BMI based on measured height and weight | SSB intake at baseline and change in BMI between baseline and end of follow-up | Age, sex, total energy, ethnicity, residence, level of poverty, maternal education, and birth weight | Yes |
| Blum, 2005 (7) | Nebraska schoolchildren, USA | 166 | 9.3 ± 1.0 y | 2 y | 24-h diet recall | BMI z score based on measured height and weight | SSB intake and year 2 BMI z score | Baseline BMI z score, age, sex, age × sex, baseline and year 2 milk, juice, diet soda, SSBs, total calories | Yes |
| Mundt, 2006 (9) | University of Saskatchewan's Pediatric Bone Mineral Accrual Study, Canada | 208 | 8–15 y | 7 y | 24-h diet recall | Fat mass (kg) using dual-energy X-ray absorptiometry | SSB intake at each measurement occasion and change in fat mass (kg) over time | Age, fat-free mass, physical activity, adjusted total energy adjusted for SSBs | Yes, but SSBs removed from total energy |
| Striegal-Moore,2006 (10) | National Heart, Lung, and Blood Institute Growth and Health Study, California, Ohio, Maryland, USA | 2379 girls | 9–10 y | 10 y | 3-d diet records | BMI based on measured height and weight | SSB intake at each measurement occasion and change in BMI over time | Milk, diet soda, fruit juice, fruit drinks, coffee/tea, site, visit (proxy for age), race, and total energy | Yes |
| Viner, 2006 (18) | 1970 British cohort, UK | 4461 | 16 y | 14 y | Questionnaire, intake the day before | BMI z score based on measured height and weight at baseline and self-reported at end of follow-up | SSB at baseline and BMI z score at the end of follow-up | Baseline BMI z score, sex, SES, and height at baseline and end of follow-up | No |
| Johnson, 2007 (11) | Avon Longitudinal Study of Parents and Children, UK | 682 | 5 y | 4 y | Parent-administered 3-d unweighed diet records | Fat mass (kg) using dual-energy X-ray absorptiometry | SSB intake at age 5 y and fat mass (kg) at age 9 y | Sex, height at 9 y, BMI at baseline, television, maternal education, paternal class, maternal BMI, paternal BMI, misreporting of energy intake, dietary energy density, percentage of energy from fat, and fiber density | No |
| Laurson, 2008 (15) | Idaho, Montana, Wyoming, rural USA | 268 | 10 y | 18 mo | Diet and lifestyle questionnaire | BMI based on measured height and weight | Change in SSB intake and change in BMI from baseline to end of follow-up | Age, baseline BMI, change in height, ethnicity, and state of residence | No |
| Libuda, 2008 (12) | DONALD study, Germany | 244 | Girls: 11.8 ± 1.5 y; boys: 11.9 ± 1.6 y | 5 y | Self- and parent-administered 3-d weighed diet records | BMI-SDS based on measured height and weight | 1-y change in SSB intake and change in BMI-SDS | Time and age | No |
| Vanselow, 2009 (19) | Project EAT (Eating Among Teens) II Minnesota, USA | 2294 | 14.9 y | 5 y | 149-item FFQ | Self-reported BMI | SSBs at end of follow-up and change in BMI from baseline to end of follow-up | Age, sex, race, SES, baseline BMI, baseline SSBs | No |
| Carlson, 2012 (21) | Control group of a community-based obesity-prevention program California, USA | 254 | 6.7 ± 0.7 y | 2 y | Parent-administered diet and lifestyle questionnaire | BMI z score based on measured height and weight | Change in SSB intake and change in BMI z score from baseline to end of follow-up | Age, sex, ethnicity, parent education, height | No |
| Laska, 2012 (14) | Identifying Determinants of Eating and Activity (IDEA) and the Etiology of Childhood Obesity (ECHO) studies, Minnesota, USA | 693 | 14.6 y | 2 y | 24-h diet recall | BMI based on measured height and weight | Change in SSB intake and change in BMI from baseline to end of follow-up | Physical activity, puberty, race, parental education, eligibility for free/reduced-price lunch, age, and study | No |
| Olsen, 2012 (13) | European Youth Heart Study, Denmark | 359 | 9.6 y | 6 y | 24-h diet recall, supplemented with FFQ, diet record | BMI z score based on measured height and weight | SSB intake at baseline and change in BMI z score between baseline and follow-up | Baseline anthropometric measures, total intake of complex carbohydrates, total intake of fat, maternal SES, maternal SES × sex, started puberty, physical activity, sex, intake of solid sucrose | No |
DONALD, DOrtmund Nutritional and Anthropometric Longitudinally Designed; FFQ, food-frequency questionnaire; SDS, SD score; SES, socioeconomic status; SSB, sugar-sweetened beverage.
TABLE 2.
Characteristics of studies included in meta-analysis of prospective cohort studies in adults1
| Reference | Study population and location | Sample size | Mean ± SD baseline age and/or age range | Duration | Dietary assessment method | Outcome assessment method | Study question | Covariates | Adjusted for energy |
| French, 1994 (23) | Healthy Worker Project, USA | 3552 | Women: 37.3 ± 10.7 y; men: 39.1 ± 9.8 y | 2 y | 18-item FFQ | Measured by investigators | Change in SSB consumption and body weight changes (lb) from baseline to end of follow-up | Age, education, marital status, job, treatment group, dieting history, baseline weight, physical activity, smoking change, certain food items (dairy, grains, sweets, alcohol, meat, eggs, fats, French fries) | No |
| Nooyens, 2005 (24) | The Doetinchem Cohort Study, Netherlands | 288 men | 50–65 y | 5 y | 178-item FFQ | Measured by investigators | Change in SSB consumption and body weight changes (kg) from baseline to end of follow-up | Retirement, type of job, interaction between retirement and type of job, age, smoking, base level of the behavior, physical activity, potatoes, fruit, breakfast, fiber density | No |
| Palmer, 2008 (25) | Black Women's Health Study (BWHS), USA | 43,960 women | 21–69; 38.4 ± ∼10.0 y | 6 y | 68-item FFQ at baseline and 6 y later | Self-reported | Change in SSB consumption and body weight changes (kg) from baseline to end of follow-up | Age; smoking; years of education; physical activity; family history of diabetes; baseline BMI; intake of red meat, processed meat, cereal fiber, and coffee; glycemic index; changes in physical activity; cigarette smoking; dietary factors from 1995 to 2001; and the other types of beverages | No |
| Stookey, 2008 (28) | The Stanford A TO Z weight-loss intervention, USA | 173 premenopausal overweight women | 25–50 y | 1 y | Three unannounced 24-h diet recalls at baseline and follow-up | Measured by investigators | Change in SSB consumption and body weight changes (kg) from baseline to end of follow-up | Age, race-ethnicity, baseline status, diet treatment group, energy expenditure, energy intake from food, and food macronutrient and water composition | No |
| Chen, 2009 (26) | PREMIER: Lifestyle Interventions for Blood Pressure Control trial, USA | 810 adults with prehypertension or stage 1 hypertension | 50 ± 8.9 y; 25–79 | 1.5 y | Two 24-h recalls at baseline, 6 mo, and 18 mo | Measured by investigators | Change in SSB consumption and body weight changes (kg) from baseline to end of follow-up | Baseline sex, race, age, income, education, marital and employment status, BMI status, intervention group, concurrent change in fitness, physical activity, and changes in other beverage intakes (diet drinks, milk, coffee and tea, alcoholic beverages) | No |
| Mozaffarian, 2011 (29) | NHS, NHS II, and HPFS, USA | NHS: 50,422; NHS II: 47,898; HPFS: 22,557 | NHS: 52.2 ± 7.2 y; NHS II: 37.5 ± 4.1 y; HPFS: 50.8 ± 7.5 y | NHS: 20 y; NHS II: 12 y; HPFS: 20 y | 133–165-item FFQ | Self-reported | Change in SSB consumption and body weight changes (lb) from baseline to the end of follow-up over 4-y periods | Age, baseline BMI at the beginning of each 4-y period, sleep duration, changes in physical activity, alcohol use, television use, smoking, and all of the dietary factors | No |
| Barone Gibbs, 2012 (27) | Women on the Move through Activity and Nutrition, (WOMAN) Study, USA | 481 overweight and obese postmenopausal women | 57 ± 2.9 | 4 y | 32-item FFQ | Measured by investigators | Change in SSB consumption and body weight changes (kg) from baseline to end of follow-up | Group, baseline weight, baseline eating behavior values, baseline physical activity (author correspondence) | No |
FFQ, food-frequency questionnaire; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; SSB, sugar-sweetened beverage.
Characteristics of the RCTs included in our meta-analyses are shown in Tables 3 and 4. Among the 5 trials conducted in children and adolescents, 2 were from the United States, 2 were from Europe, and one was from Brazil (Table 3). All of these studies evaluated the effect of reducing intake of SSBs on body weight. Two studies were school-based interventions using focused nutrition education (61, 62) for 1 school year among 644–1140 children aged 8–10 y, and 3 studies were randomized trials replacing SSBs with noncaloric beverages (63–65) for 25 wk to 18 mo among 103–641 children ranging in age from 8 to 16 y. One trial used a double-blind design (65), and one was conducted exclusively in overweight adolescents (64).
TABLE 3.
Characteristics of studies included in meta-analysis of randomized controlled trials in children
| Reference | Study population and location | Sample size | Mean age | Baseline BMI | Duration | Design | Study question | Intervention | Control |
| James, 2004 (61) | The Christchurch Obesity Prevention Project in Schools (CHOPPS), UK | 644; 29 clusters | Intervention: 8.7 yControl: 8.7 y girls, 8.6 y boys | Intervention: BMI (in kg/m2) of 17.4Control: BMI of 17.6 | One school year | Parallel-cluster randomized intervention | School-based education program aimed at reducing SSBs1 and weight | Focused educational program on nutrition to discourage consumption of SSBs | Not specified |
| Ebbeling 2006 (63) | Beverages and Student Health, Massachusetts, USA | 103 | Intervention: 16.0 yControl: 15.8 y | Intervention: BMI of 25.7Control: BMI of 24.9 | 25 wk | Parallel, randomized intervention | Replacement of SSBs with noncaloric beverage on weight | Four 12-oz servings of noncaloric beverages/d, provided by weekly home deliveries, motivational phone calls, mailed fridge magnets with intervention messages | Asked to continue their usual beverage consumption habits |
| Sichieri, 2008 (62) | Schoolchildren, Brazil | 1140; 47 clusters | Intervention: 10.9 yControl: 10.9 y | Intervention: BMI of 18.3Control: BMI of 18.2 | One school year | Parallel-cluster randomized controlled intervention | School-based education program aimed at reducing SSBs and weight | Focused nutrition education with emphasis on decreasing SSBs and increasing water intake | Control clusters received 2 general information sessions about health and given material about healthy diets |
| de Ruyter, 2012 (65) | Double-blind, Randomized Intervention Study in Kids (DRINK), Netherlands | 641 | Intervention: 8.2 yControl: 8.2 y | Intervention: BMI of 16.9Control: BMI of 16.8 | 18 mo | Parallel, double- blind, randomized intervention | Replacement of SSBs with noncaloric beverage on weight | One 8-oz can of artificially sweetened beverage/d (0 calories, 35 mg sucralose, 12 mg acesulfame potassium) provided at school | One 8-oz can of SSB/d (104 kcal, 26 g sucrose) provided at school |
| Ebbeling, 2012 (64) | Overweight adolescents, Massachusetts, USA | 244 | Intervention: 15.3 yControl: 15.2 y | Intervention: BMI of 30.4Control: BMI of 30.1 | 1 y | Parallel, randomized intervention | Replacement of SSBs with noncaloric beverage on weight | Home deliveries of noncaloric beverages, motivational phone calls, check-in visits, mailed written intervention messages | Supermarket gift cards as a retention strategy |
SSB, sugar-sweetened beverage.
TABLE 4.
Characteristics of studies included in meta-analysis of randomized controlled trials in adults
| Reference | Study population | Sample size | Mean ± SD baseline age and/or age range | Mean ± SD baseline BMI and/or BMI range | Duration | Design | Study question | Intervention | Control |
| Tordoff, 1990 (69) | USA | 9 women and 21 men | Women: 28.2 ± 2.7 y; men: 22.9 ± 0.8 y | Women: 25.4 ± 1.4; men: 25.1 ± 0.5 | 3 wk | Crossover | Adding HFCS1 soda to the normal diet and changes in body weight compared with diet soda with aspartame | 1135 mL soda including 133 g HFCS/d (530 kcal) | 1135 mL diet soda including 590 mg aspartame/d, no calories |
| Reid, 2007 (66) | UK | 133 women | 31.8 ± 9.1 y; 20–55 y | 22.5 ± 2.8; range: <25 | 4 wk | Parallel | Adding sucrose beverages to the normal diet and changes in body weight compared with artificially sweetened beverages | 1 L sucrose-sweetened drinks (1800 kJ/d) | 1 L artificially sweetened drinks (67 kJ/d) |
| Reid, 2010 (67) | UK | 53 overweight women | 34.5 ± 11.0 y in the intervention group and 32.9 ± 8.8 y in the control group; 20–55 y | 27.2 ± 2.06 in the intervention group and 27.8 ± 1.8 in the control group; 25–30 | 4 wk | Parallel | Adding sucrose beverages to the normal diet and changes in body weight compared with artificially sweetened beverages | 1 L sucrose-sweetened drinks (1800 kJ/d) | 1 L artificially sweetened drinks (67 kJ/d) |
| Aeberli, 2011 (70) | Switzerland | 29 healthy-weight men | 26.3 ± 6.6 y | 22.4 ± 1.9 | 3 wk | Crossover | Adding sucrose beverages to the normal diet and changes in body weight compared with dietary advice | 600 mL drinks including 80 g sucrose/d | Dietary advice aimed at reducing free fructose intake |
| Maersk, 2012 (68) | Denmark | 30 women and 17 men who were overweight | Mean: ∼39 y; 20–50 y | Mean: ∼32; 26–40 | 6 mo | Parallel | Adding sucrose beverages to the normal diet and changes in body weight compared with 3 other beverages (milk, diet soda, and water) | 1 L sucrose-sweetened regular cola/d (1800 kJ) | 1 L semiskim milk/d (1900 kJ) or still mineral water (0 kJ) or aspartame-sweetened diet cola (15 kJ) |
HFCS, high-fructose corn syrup.
The majority of trials conducted in adults were from Europe (2 United Kingdom, 2 Denmark, 1 Switzerland) and one was from the United States, with sample sizes ranging from 29 to 133 (Table 4). All studies evaluated the effect of adding SSBs to the diet on body weight. Most studies compared SSBs (sucrose- or HFCS-sweetened beverages) with artificially sweetened beverages in either a parallel (66–68) or crossover (69, 70) design, for 3 wk to 6 mo, with intervention doses ranging from 600 mL to >1 L SSBs/d. One study included semiskim milk and mineral water in addition to artificially sweetened beverages as control regimens (68), and one study compared SSBs with dietary advice (70). Three (59, 67, 68) of the 5 studies were conducted exclusively in overweight individuals.
Risk of bias
Risk of bias is summarized for cohort studies in Supplemental Table 3 under “Supplemental data” in the online issue. Among studies in children, scores ranged from 4 (8) to 8 (14, 16, 21) out of a possible score of 9. Sixty percent of studies received a score ≥7, denoting good quality (9, 11, 12, 14, 16, 17, 19–21), whereas 40% were considered to be of poorer quality (7, 8, 10, 13, 15, 18). Among the 7 studies in adults, 3 studies received a score of 6, and 4 studies received a score ≥7 (25, 26, 28, 29).
Risk of bias summaries for RCTs are shown in Supplemental Table 4 under “Supplemental data” in the online issue. For studies in children and adults, risk of bias tended to be low or unclear for most domains assessed. A quantitative summary for each domain can be found in the footnote to the table.
SSBs and body weight in children
Prospective cohort studies
On the basis of data from 20 comparisons of the 15 studies (25,745 children and adolescents), we found a positive association between SSB consumption and BMI. The pooled estimate for the change in BMI (in kg/m2) during the time period specified in each study associated with each one 12-oz serving/d increase in SSBs was 0.07 (95% CI: 0.01, 0.12; random-effects model; Figure 2). Results from the fixed-effects model (0.16; 95% CI: 0.15, 0.16) differed from the random-effects model and was most likely a result of the high degree of between-study heterogeneity (I2 = 91.6%, P-heterogeneity < 0.001). Meta-regressions for duration (P = 0.51), age (P = 0.70), adjustment for total energy (P = 0.37), use of an FFQ (P = 0.43), and sample size (P = 0.95) were not significant, suggesting that these factors may not be substantial sources of heterogeneity. However, when we stratified the analysis by whether a study had adjusted for total energy, the estimate was greater in studies that did not adjust for total energy (0.08; 95% CI: 0.02, 0.14; I2 = 91.1%; n = 17) compared with those that did (0.04; 95% CI: 0.00, 0.07; I2 = 0%; n = 3). In general, studies with greater statistical weight (>5%) tended to have positive associations, except for the study by Mundt et al (9). This study (9) along with the study by Johnson et al (11), evaluated fat mass (kg) as the outcome, which may not be comparable to BMI despite our scaling. We made the assumption that differences in fat mass are equal to differences in body weight, which may not be the case. Excluding these studies that estimated BMI from fat mass (9, 11) slightly increased the strength of the estimate (0.09; 95% CI: 0.04, 0.15) but had no impact on heterogeneity (I2 = 90.3%). However, excluding the study by Viner and Cole (18), which had the greatest statistical weight, reduced heterogeneity by ∼23% (I2 = 68.3%), yielding more comparable estimates between the random-effects (0.05; 95% CI: 0.01, 0.10) and fixed-effects (0.04; 95% CI: 0.02, 0.06) models.
FIGURE 2.
Changes in BMI (95% CI) per 1-serving/d increase in sugar-sweetened beverages during the time period specified in each study from prospective cohort studies in children. Horizontal lines denote 95% CIs; solid diamonds represent the point estimate of each study. Open diamonds represent pooled estimates, and the dashed line denotes the point estimate of the pooled results from the random-effects model (D+L). Study weights are from the random-effects analysis (D+L). Pooled estimates from the random-effects analysis (D + L) and the fixed-effects analysis (I-V) are shown based on 15 cohort studies (n = 25,745). The I2 and P values for heterogeneity are shown. D+L, DerSimonian and Laird; I-V, inverse variance.
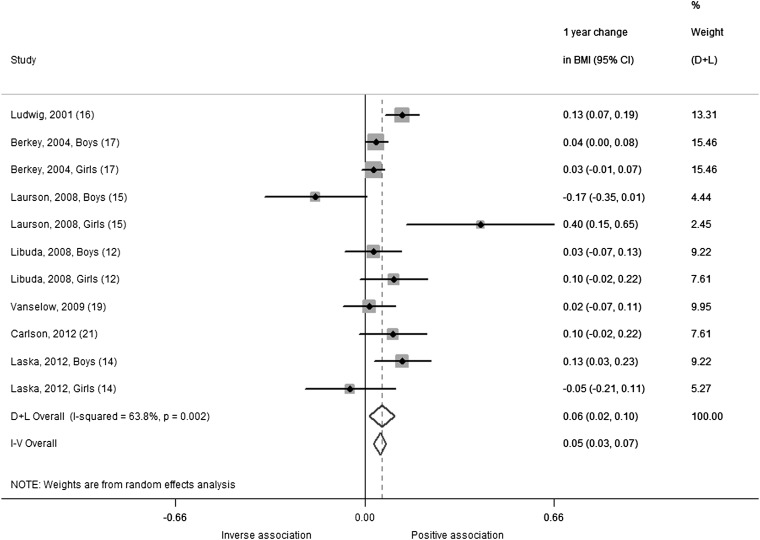
Our analysis of 1-y change in BMI included 7 studies with 11 comparisons in 15,736 children and adolescents. The summary estimate indicated that BMI increased by 0.06 (95% CI: 0.02, 0.10; random-effects model) for each additional daily 12-oz serving of SSBs over a 1-y period (Figure 3). Results from the fixed-effects model were similar (0.05; 95% CI: 0.03, 0.07), and significant heterogeneity was observed (I2 = 63.8%; P-heterogeneity = 0.002). Removing the study by Laurson et al (15) as an outlier reduced heterogeneity (I2= 44.4%; P-heterogeneity = 0.07) but did not change the summary estimate (0.06; 95% CI: 0.03, 0.09).
FIGURE 3.
One-year changes in BMI (95% CI) per 1-serving/d increase in sugar-sweetened beverages from prospective cohort studies in children using a change versus change analysis strategy. Horizontal lines denote 95% CIs; solid diamonds represent the point estimate of each study. Open diamonds represent pooled estimates, and the dashed line denotes the point estimate of the pooled result from the random-effects model (D+L). Weights are from the random-effects analysis (D+L). Pooled estimates from the random-effects analysis (D+L) and the fixed-effects analysis (I-V) are shown based on 7 cohort studies (n = 16,004). The I2 and P values for heterogeneity are shown. D+L, DerSimonian and Laird; I-V, inverse variance.
Trials
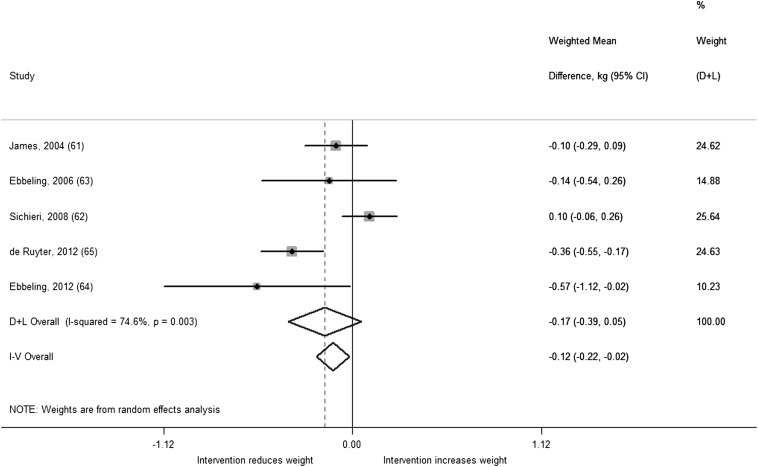
A total of 5 studies including 2772 children and adolescents were included in our analysis of SSB trials and body weight. On the basis of these data, we found a nonsignificant difference in change in BMI from reducing SSB consumption [weighted mean difference (WMD): −0.17; 95% CI: −0.39, 0.05; I2 = 74.6%; P-heterogeneity = 0.003] in the random-effects model (Figure 4). Results from the fixed-effects model were significant (−0.12; 95% CI: −0.22, −0.02). This difference is likely a result of the random-effects model giving greater statistical weight to smaller studies and having wider CIs in the presence of heterogeneity compared with the fixed-effects model. Meta-regressions for intervention modality (education or beverage substitution; P = 0.08), duration (P = 0.18), and age (P = 0.84) were not significant, although power to detect a difference was low with only 5 studies. When we stratified our analysis by intervention modality, we observed a significant weight reduction among the 3 studies that provided noncaloric beverages as substitutes for SSBs (63–65): the summary estimate was −0.34 (95% CI: −0.50, −0.18; I2 = 0%). In contrast, we did not find a significant intervention effect in the 2 studies that used focused school-based education (61, 62) to discourage SSB consumption (0.01; 95% CI: −0.19, 0.20; I2 = 59.6%). For the study by James et al (61), although the difference in BMI change did not reach significance, there was a significant difference in the prevalence of childhood overweight and obesity between intervention (0.2% reduction) and control clusters (7.5% increase). This suggests that the intervention may be more effective in preventing weight gain in higher risk children. Heterogeneity was reduced when we removed the study by Sichieri et al (62), which had the largest sample size in the meta-analysis, from the analysis (−0.25; 95% CI: −0.43, −0.06; I2 = 43.8%; P-heterogeneity = 0.15).
FIGURE 4.
Weighted mean differences in BMI change (95% CI) between the intervention and control regimens from randomized controlled trials in children. Interventions evaluated the effect of reducing sugar-sweetened beverages. Horizontal lines denote 95% CIs; solid diamonds represent the point estimate of each study. Open diamonds represent pooled estimates of the intervention effect, and the dashed line denotes the point estimate of the pooled result from the random-effects model (D+L). Weights are from the random-effects analysis (D+L). Pooled estimates from the random-effects analysis (D+L) and the fixed-effects analysis (I-V) are shown based on 5 randomized controlled trials (n = 2772). The I2 and P values for heterogeneity are shown. D+L, DerSimonian and Laird; I-V, inverse variance.
All of the studies except for the one by Sichieri et al (62) showed a beneficial effect or trend of interventions to reduce SSB intake on weight. The study by Sichieri et al (62) was a school-based intervention that used focused education to discourage consumption of carbonated SSBs, but according to the authors, students compensated by increasing their consumption of sugar-added juices and fruit drinks, which may explain the lack of findings. However, in subgroup analysis, children who were overweight at baseline showed greater BMI reduction in the intervention group, which was significant among girls (62). Similarly, Ebbeling et al (63) found more pronounced benefits of the intervention among adolescents who were overweight at baseline, and another study by Ebbeling et al (64), which was conducted exclusively in overweight adolescents, showed the strongest intervention effect among studies included in our analysis. Combining Ebbeling et al (64) with the subgroup findings from Ebbeling et al (63), we observed an increased benefit of substituting noncaloric beverages for SSBs on weight gain (−0.64; 95% CI: −1.07, −0.21), suggesting that this type of intervention may have greater impact on those who are overweight. We were not able to include the subgroup findings from Sichieri et al (62) in this secondary analysis because the data were not available in the necessary units.
SSBs and body weight in adults
Prospective cohort studies
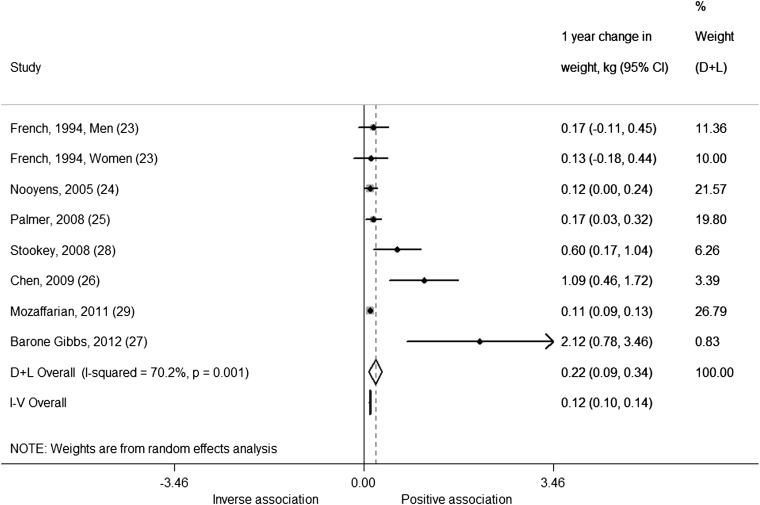
Our analysis of 1-y change in weight (kg) in adults was based on 7 studies, including 8 comparisons and 170,141 men and women. We found that each serving per day increase in SSBs was associated with an additional weight gain of 0.22 kg over 1 y (0.22 kg; 95% CI: 0.09, 0.34 kg; I2 = 70.2%; P-heterogeneity < 0.001) from the random-effects model (Figure 5). The estimate from the fixed-effects model was significant but not as strong (0.12 kg; 95% CI: 0.10, 0.14 kg). This is probably because the random-effects model gives greater weight to smaller studies compared with the fixed-effects model and there are a couple of small studies that are outliers (estimates that fall outside of the 95% CI of other estimates included in the analysis), such as Barone Gibbs et al (27) and Chen et al (26). Meta-regressions for age at baseline (P = 0.32), duration (P = 0.37), use of an FFQ to assess diet (P = 0.26), sample size (P = 0.48), and baseline weight status (P = 0.10) were not significant. However, when we stratified the analysis by baseline weight status, we observed greater although nonsignificant weight gain in the 2 studies (27, 28) conducted in overweight populations (1.22 kg; 95% CI: −0.23, 2.68 kg; I2 = 77.5%) compared with nonoverweight populations (0.15 kg; 95% CI: 0.06, 0.24 kg; I2 = 50.3%). Excluding the study by Barone Gibbs et al (27) from the overall analysis as an outlier reduced heterogeneity somewhat (I2 = 59.8%). Excluding the study by Mozaffarian et al (29) from the overall analysis, which had the largest sample size in the meta-analysis, increased summary estimates for both the random-effects model (0.31 kg; 95% CI: 0.11, 0.50 kg) and the fixed-effects model (0.18 kg; 95% CI: 0.10, 0.26 kg) but did not reduce heterogeneity (I2 = 71.3%).
FIGURE 5.
One-year changes (95% CI) in weight (kg) per 1-serving/d increase in sugar-sweetened beverages from prospective cohort studies in adults using a change versus change analysis strategy. Horizontal lines denote 95% CIs; solid diamonds represent the point estimate of each study. Open diamonds represent pooled estimates, and the dashed line denotes the point estimate of the pooled result from the random-effects model (D+L). Weights are from the random-effects analysis (D+L). Pooled estimates from the random-effects analysis (D+L) and the fixed-effects analysis (I-V) are shown based on 7 cohort studies (n = 174,252). The I2 and P values for heterogeneity are shown. D+L, DerSimonian and Laird; I-V, inverse variance.
Trials
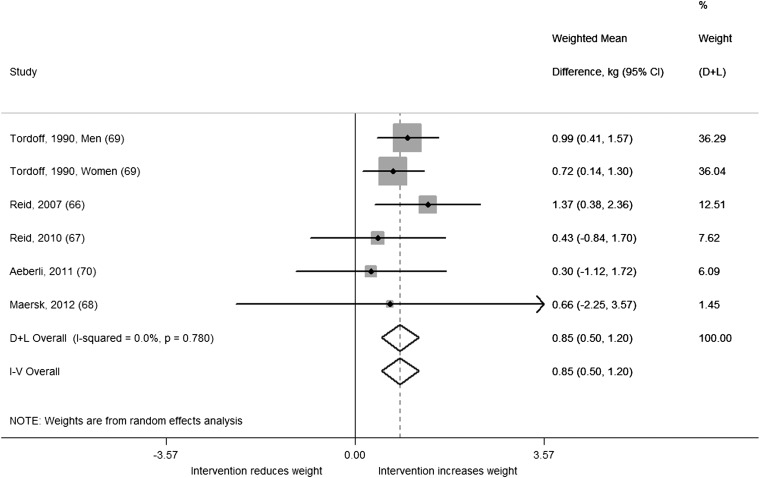
A total of 5 studies including 6 comparisons with 292 men and women were included in our analysis of trials in adults. We found a significant difference in change in body weight (kg) between intervention and control regimens (WMD: 0.85; 95% CI: 0.50, 1.20; I2 = 0.0%; P-heterogeneity = 0.78) from the random-effects model (Figure 6). The estimate from the fixed-effects model was identical. All studies observed significantly greater weight gain or trends toward greater weight gain in intervention compared with control regimens, and there was no evidence of heterogeneity. When we stratified our analysis by baseline weight status, we observed greater weight gain in intervention compared with control regimens among the 3 studies conducted in nonoverweight populations (WMD: 0.89; 95% CI: 0.52, 1.26; I2 = 0.0%;) compared with the 2 studies conducted in overweight populations (WMD: 0.47; 95% CI: −0.70, 1.63; I2 = 0.0%;). Adding the study by Raben et al (59) to the analysis, which was excluded because the intervention contained some foods in addition to beverages (∼70% beverages and 30% food), increased the overall estimate but introduced some heterogeneity (WMD: 1.06; 95% CI: 0.54, 1.58; I2 = 46.3%; P-heterogeneity = 0.08).
FIGURE 6.
Weighted mean differences (95% CI) in weight change (kg) between the intervention and control regimens from randomized controlled trials in adults. Interventions evaluated the effect of adding sugar-sweetened beverages. Horizontal lines denote 95% CIs; solid diamonds represent the point estimate of each study. Open diamonds represent pooled estimates of the intervention effect, and the dashed line denotes the point estimate of the pooled result from the random-effects model (D+L). Weights are from the random-effects analysis (D+L). Pooled estimates from the random-effects analysis (D+L) and the fixed-effects analysis (I-V) are shown based on 5 randomized controlled trials (n = 292). The I2 and P values for heterogeneity are shown. D+L, DerSimonian and Laird; I-V, inverse variance.
Publication bias
Visual inspection of funnel plots (see Supplemental Figures 1–5 under “Supplemental data” in the online issue) along with Begg's test suggested that publication bias was unlikely in our analyses in children (all prospective cohort studies, P = 0.12; prospective cohort studies evaluating change versus change, P = 0.88; trials, P = 0.47) and in trials in adults (P = 0.59). However, for cohorts in adults there was suggestion of publication bias (P = 0.02). This may be due to the lack of estimates in the bottom right quadrant of the funnel plot, indicating a lack of publication of small, null studies. However, this is complicated by the narrow spread of studies about the plot, which is likely a result of the preponderance of large studies.
Qualitative review of studies not included in meta-analyses
A number of prospective cohort studies evaluating SSB consumption and body weight in both children and adults were excluded from our meta-analyses because we were not able to obtain data in the necessary units from either transformations or author correspondence. Among these studies in children, 9 of 11 supported the findings from our meta-analysis of a positive association between SSBs and body weight (37–39, 41, 42, 44–47), whereas 2 did not find an association (40, 43). Four studies found significant positive associations between SSB consumption and weight gain (44–47), with one study reporting associations for only boys (46). Four studies found positive associations between SSB consumption and risk of developing overweight or obesity (37–39, 42), with one study reporting significant associations only among children who were at risk of becoming overweight at baseline (37). One small study (n = 49) found a positive association between SSB consumption and change in waist circumference but not BMI z score among children followed from age 3 to 6 y (41). Among studies that did not find an association between SSBs and childhood body weight, Wijga et al (40) suggested that their lack of findings among 1871 Dutch children followed from age 5 to 8 y might have been a result of reverse causation and selective underreporting by parents of children who became overweight. In the study by Sugimori et al (43), which was conducted in a cohort of 8170 Japanese children followed from age 3 to 6 y, consumption amounts may have been too low to observe significant between-group differences.
Similar to studies in children, the majority (4 of 6) of cohort studies in adults that were excluded as a result of difficulty in obtaining optimal units found positive associations between SSBs and body weight in either primary analysis or subgroup findings (52, 53, 55, 56), whereas 2 studies did not find significant associations (51, 54). Among studies that evaluated baseline SSB consumption and weight change, Bes-Rastrollo et al (53) found that higher SSB consumption was associated with significant weight gain among subjects with previous weight gain (≥3 kg in 5 y before baseline) in a cohort of 7194 adults from Spain followed for over 2 y. Odegaard et al (56) found that individuals with higher SSB consumption had a subtle but significant increase in weight (0.53 kg) compared with those who did not consume soft drinks (P < 0.001) in a large cohort (n = 43,580) of Chinese Singaporeans with a mean weight change of 0.10 kg over 5.7 y. In contrast, Fowler et al (54) did not find an association between SSBs and change in BMI in a small (n = 3371) US cohort. The authors did, however, find a positive association between artificially sweetened beverages and BMI change, which they largely ascribed to reverse causation. Two studies (52, 55) evaluating baseline SSB intake and risk of obesity found significant positive associations, although the association was significant only in women in the study by Inoue et al (55): a Japanese cohort that included >75% women. The study by Kvavvik et al (51), which evaluated change in SSBs and risk of obesity in a small cohort from Norway (n = 422), found that risk was increased for long-term high-SSB consumers (≥3 servings/wk) compared with long-term low consumers, although this finding was not significant. Two large cohort studies (57, 58), which were excluded because they were conducted in duplicate populations of Mozaffarian et al (29), found significant positive associations between SSB consumption and weight change.
Among trials in children not included in our meta-analysis, one found an adverse effect of SSBs and body weight (49), whereas 2 did not find significant effects (48, 50), although the study by James et al (48) was a follow-up analysis of a previous school-based intervention (61). This study (48), along with the recent RCT by Ebbeling et al (64), examined the sustained effects of their interventions on body weight at 2 and 1 y postintervention, respectively. Both of these studies found that the beneficial effects of the interventions dissipated after the interventions had ended. We combined these studies and observed a summary WMD in BMI between the intervention and control of −0.26 (95% CI: −0.53, 0.03), suggesting that, despite a beneficial trend, the interventions did not have a sustained effect on weight gain, highlighting the importance of active intervention. The study by Albala et al (50), which evaluated replacing SSBs with flavored milk beverages providing 80 kcal and 11 g carbohydrate/serving, did not find a beneficial intervention effect on body weight. In contrast, the study by Sichieri et al (49), which was excluded from our meta-analysis because it was a duplicate study population, confirmed that consumption of SSBs is a significant risk factor for BMI gain.
Among 2 studies that were excluded from our analysis of trials in adults, one found an adverse effect of SSBs on body weight (59), whereas the other evaluated a different study question related to weight loss (60). The study by Raben et al (59) was excluded because the intervention combined beverages and foods but found that body weight and fat mass increased in overweight participants who consumed sucrose (mostly from beverages) and decreased in those who consumed artificial sweeteners after 10 wk. The study by Tate et al (60) found that participants who were assigned to caloric beverage replacement with water and diet beverages compared with controls were twice as likely to have achieved a 5% weight loss during 6 mo, although no significant between-group differences in weight reduction were found.
DISCUSSION
Findings from our systematic review and meta-analyses of prospective cohort studies and trials showed an overall positive association between consumption of SSBs and body weight gain in both children and adults with the exception of trials in children from the random-effects model. On the basis of the totality of the available evidence from prospective cohort studies, a 1-serving/d increase in SSBs was associated with a 0.06-unit increase in BMI over a 1-y period among children and adolescents and an additional weight gain of 0.12 to 0.22 kg (∼0.25–0.50 lb) over 1 y among adults. In children, it is difficult to gauge the impact of our findings, because weight gain in childhood varies as a function of age, maturation, and growth velocity. Adult weight gain in the general population is a gradual process, occurring over decades and averaging ∼1 lb/y (29). Thus, eliminating SSBs from the diet could be an effective way to prevent age-related weight gain.
Our findings from trials generally support those from prospective cohort studies. Trials in children were of 2 modalities, either reducing SSBs by substitution with noncaloric beverages or school-based education programs aimed at discouraging intake of SSBs. In sensitivity analysis, we showed that the substitution trials, which included 2 recent trials that were the most rigorous to date (64, 65), resulted in significantly less BMI gain compared with the education interventions. Some of the trials in our analysis were “effectiveness trials” of behavioral modification (eg, school-based education programs), which are useful in evaluating real-world scenarios for policy decisions. However, these studies evaluate intervention modalities more so than causal relations because their findings are greatly affected by intervention intensity and adherence. Thus, a lack of benefit does not mean that the relation between SSBs and weight gain is not causal but rather that the given modality might not be effective at changing behaviors.
The current set of analyses support findings from our previous systematic review in children and adults (3) and meta-analysis in children (4), both of which reported a significant link between SSB consumption and weight gain. Our previous meta-analysis (4) was a reanalysis of an article that did not find an association between SSBs and BMI in children resulting from methodologic errors and inclusion of coefficients that adjusted for total energy intake (71). In contrast to these previous meta-analyses (4, 71), here we conducted separate analyses for prospective cohort studies and trials, qualitatively reviewed studies that were not included in our analyses, and independently evaluated prospective cohort studies that used a change versus change analysis. This type of analysis has some of the features of a quasi-experimental design, although it lacks the element of randomization in a clinical trial. An advantage of this design is the generalizability to a noncontrolled setting, relative to a controlled setting, because participants are able to change their diet and lifestyle without investigator-driven intervention. We also included a number of more recent cohort studies (11–15, 18, 19, 21) and trials (62, 64, 65) in children that were not included in these previous analyses. To our knowledge, this is the first meta-analysis to evaluate prospective cohort studies of SSBs and body weight in adults. A previous meta-analysis of 6 trials found a significant dose-dependent increase in weight among studies that added SSBs to the diet but found no effect on BMI among another 6 trials that attempted to reduce SSBs (72). However, a significant benefit on body weight was observed among individuals who were overweight at baseline (72), a finding that we also observed in children. These analyses combined studies in children and adults and included various trials excluded from our analyses, such as a study that substituted flavored milk for SSBs (50), a doctoral dissertation, and a study of postintervention follow-up after completion of the trial (48). Our analyses also included more recent trials in children (64, 65) and adults (67, 68, 70).
The studies included in our meta-analyses varied substantially with respect to study design, exposure assessment, adjustment for covariates, and specific outcomes evaluated. Although we did not identify these factors as significant sources of heterogeneity, we cannot rule them out. Estimates from cohort studies are also likely to be underestimated because of random measurement error in SSB assessment. The relatively high degree of unexplained heterogeneity observed in our analyses may limit the validity of our summary estimates. In addition, the data transformations that we performed to obtain consistent units across studies may further limit the validity of our estimates by imposing various assumptions. Our assumption of a 12-oz serving size for some studies, which is consistent with most cans and glasses, may have introduced some random misclassification and further attenuated our estimates. Publication bias is always a potential concern in meta-analysis, but standard tests and visual inspection of funnel plots suggested that there was limited evidence for publication bias in most of our analyses. In addition, we were not able to include a number of studies in our analysis because of difficulty in obtaining consistent units; however, these studies were reviewed qualitatively. Ascertainment of unpublished results via author correspondence may have reduced the likelihood of publication bias, but it should be noted that our search was limited to English-language publications and non-English reports may exist.
Because observed associations between SSBs and weight may be confounded by other diet and lifestyle factors, some scholars have put into question the validity of findings from observational studies. However, all of the cohort studies in our meta-analyses adjusted for potential confounding by various diet and lifestyle factors, and for most, a positive association persisted, suggesting an independent effect of SSBs, although residual confounding by unmeasured or poorly measured factors cannot be dismissed. Results from rigorously conducted RCTs also support conclusions from our observational analyses, further lending to their validity. Risk of bias assessment suggested that most cohort studies were of good quality and the majority of trials had a low or unclear risk of bias for the domains that were evaluated. Longitudinal studies evaluating diet and weight may also be prone to reverse causation. Although it is not possible to completely eliminate this issue, studies with longer durations and repeated measures as in our change versus change analyses are less prone to this process (73).
SSBs can lead to weight gain through their high added-sugar content, low satiety, and an incomplete compensatory reduction in energy intake at subsequent meals after intake of liquid calories (3). On average, SSBs contain 140–150 calories and 35.0–37.5 g sugar per 12-oz serving. In addition, fructose from any sugar or HFCS has been shown to promote development of visceral adiposity and ectopic fat deposition (74–77). Odegaard et al (78) recently found in a cross-sectional analysis that increased SSB consumption was associated with an adverse abdominal adipose tissue deposition pattern. Numerous societies and organizations including the American Heart Association, the American Academy of Pediatrics, and the US 2010 Dietary Guidelines technical review committee have called for reductions in intake of SSBs to help prevent obesity and improve overall health. Our meta-analyses offer additional support for these recommendations. Our results also suggest the need for targeted strategies to reduce SSB consumption among high-risk populations, particularly children who are already overweight to prevent further weight gain, and highlight the importance of sustained strategies. The studies included in our analyses evaluated risk or prevention of weight gain rather than weight loss. From a public health point of view, identifying dietary determinants of weight gain is critical for reducing obesity prevalence because once an individual becomes obese, it is increasingly difficult to achieve and maintain weight loss (79).
In conclusion, our systematic review and meta-analyses provide additional evidence that SSB consumption is associated with weight gain in both children and adults. Our findings have broad implications for developing public health strategies and policies targeting SSBs for weight control and obesity prevention.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—VSM, AP, WCW, and FBH: designed the research; VSM and AP: conducted the analyses; VSM: wrote the manuscript; AP, WCW, and FBH: critically reviewed the manuscript; VSM and FBH: had primary responsibility for final content; and all authors: read and approved the final manuscript. The authors reported no conflicts of interest.
Footnotes
Abbreviations used: FFQ, food-frequency questionnaire; HFCS, high-fructose corn syrup; RCT, randomized controlled trial; SSB, sugar-sweetened beverage; WMD, weighted mean difference.
REFERENCES
- 1.Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States. Am J Clin Nutr 2011;94:726–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yngve A, Haapala I, Hodge A, McNeill G, Tseng M. Making soft drinks the dietary version of the cigarette. Public Health Nutr 2012;15:1329–30 [DOI] [PubMed] [Google Scholar]
- 3.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr 2009;89:438–9; author reply 9–40 [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Oxford, United Kingdom: The Cochrane Collaboration, 2011. Available from: http://handbook.cochrane.org/ (cited 27 May 2013)
- 7.Blum JW, Jacobsen DJ, Donnelly JE. Beverage consumption patterns in elementary school aged children across a two-year period. J Am Coll Nutr 2005;24:93–8 [DOI] [PubMed] [Google Scholar]
- 8.Newby PK, Peterson KE, Berkey CS, Leppert J, Willett WC, Colditz GA. Beverage consumption is not associated with changes in weight and body mass index among low-income preschool children in North Dakota. J Am Diet Assoc 2004;104:1086–94 [DOI] [PubMed] [Google Scholar]
- 9.Mundt CA, Baxter-Jones AD, Whiting SJ, Bailey DA, Faulkner RA, Mirwald RL. Relationships of activity and sugar drink intake on fat mass development in youths. Med Sci Sports Exerc 2006;38:1245–54 [DOI] [PubMed] [Google Scholar]
- 10.Striegel-Moore RH, Thompson D, Affenito SG, Franko DL, Obarzanek E, Barton BA, Schreiber GB, Daniels SR, Schmidt M, Crawford PB. Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr 2006;148:183–7 [DOI] [PubMed] [Google Scholar]
- 11.Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. Is sugar-sweetened beverage consumption associated with increased fatness in children? Nutrition 2007;23:557–63 [DOI] [PubMed] [Google Scholar]
- 12.Libuda L, Alexy U, Sichert-Hellert W, Stehle P, Karaolis-Danckert N, Buyken AE, Kersting M. Pattern of beverage consumption and long-term association with body-weight status in German adolescents—results from the DONALD study. Br J Nutr 2008;99:1370–9 [DOI] [PubMed] [Google Scholar]
- 13.Olsen NJ, Andersen LB, Wedderkopp N, Kristensen PL, Heitmann BL. Intake of liquid and solid sucrose in relation to changes in body fatness over 6 years among 8- to 10-year-old children: the European Youth Heart Study. Obes Facts 2012;5:506–12 [DOI] [PubMed] [Google Scholar]
- 14.Laska MN, Murray DM, Lytle LA, Harnack LJ. Longitudinal associations between key dietary behaviors and weight gain over time: transitions through the adolescent years. Obesity (Silver Spring) 2012;20:118–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurson K, Eisenmann JC, Moore S. Lack of association between television viewing, soft drinks, physical activity and body mass index in children. Acta Paediatr 2008;97:795–800 [DOI] [PubMed] [Google Scholar]
- 16.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 2001;357:505–8 [DOI] [PubMed] [Google Scholar]
- 17.Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res 2004;12:778–88 [DOI] [PubMed] [Google Scholar]
- 18.Viner RM, Cole TJ. Who changes body mass between adolescence and adulthood? Factors predicting change in BMI between 16 year and 30 years in the 1970 British Birth Cohort. Int J Obes (Lond) 2006;30:1368–74 [DOI] [PubMed] [Google Scholar]
- 19.Vanselow MS, Pereira MA, Neumark-Sztainer D, Raatz SK. Adolescent beverage habits and changes in weight over time: findings from Project EAT. Am J Clin Nutr 2009;90:1489–95 [DOI] [PubMed] [Google Scholar]
- 20.Phillips SM, Bandini LG, Naumova EN, Cyr H, Colclough S, Dietz WH, Must A. Energy-dense snack food intake in adolescence: longitudinal relationship to weight and fatness. Obes Res 2004;12:461–72 [DOI] [PubMed] [Google Scholar]
- 21.Carlson JA, Crespo NC, Sallis JF, Patterson RE, Elder JP. Dietary-related and physical activity-related predictors of obesity in children: a 2-year prospective study. Child Obes 2012;8:110–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr 1990;44:45–60 [PubMed] [Google Scholar]
- 23.French SA, Jeffery RW, Forster JL, McGovern PG, Kelder SH, Baxter JE. Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes Relat Metab Disord 1994;18:145–54 [PubMed] [Google Scholar]
- 24.Nooyens AC, Visscher TL, Schuit AJ, van Rossum CT, Verschuren WM, van Mechelen W, Seidell JC. Effects of retirement on lifestyle in relation to changes in weight and waist circumference in Dutch men: a prospective study. Public Health Nutr 2005;8:1266–74 [DOI] [PubMed] [Google Scholar]
- 25.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med 2008;168:1487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Appel LJ, Loria C, Lin PH, Champagne CM, Elmer PJ, Ard JD, Mitchell D, Batch BC, Svetkey LP, et al. Reduction in consumption of sugar-sweetened beverages is associated with weight loss: the PREMIER trial. Am J Clin Nutr 2009;89:1299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barone Gibbs B, Kinzel L, Gabriel K, Chang Y, Kuller L. Short- and long-term eating habit modification predicts weight change in overweight, postmenopausal women: results from the WOMAN Study. J Acad Nutr Diet. 2012;112:1347–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stookey JD, Constant F, Popkin BM, Gardner CD. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 2008;16:2481–8 [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011;364:2392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11:193–206 [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101 [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2011. Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.asp (cited 27 May 2013)
- 36.Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welsh JA, Cogswell ME, Rogers S, Rockett H, Mei Z, Grummer-Strawn LM. Overweight among low-income preschool children associated with the consumption of sweet drinks: Missouri, 1999-2002. Pediatrics 2005;115:e223–9 [DOI] [PubMed] [Google Scholar]
- 38.Dubois L, Farmer A, Girard M, Peterson K. Regular sugar-sweetened beverage consumption between meals increases risk of overweight among preschool-aged children. J Am Diet Assoc 2007;107:924–34, discussion 34–5 [DOI] [PubMed] [Google Scholar]
- 39.Huus K, Brekke HK, Ludvigsson JF, Ludvigsson J. Relationship of food frequencies as reported by parents to overweight and obesity at 5 years. Acta Paediatr 2009;98:139–43 [DOI] [PubMed] [Google Scholar]
- 40.Wijga AH, Scholtens S, Bemelmans WJE, Kerkhof M, Koppelman BB, Smit HA. Diet, screen time, physical activity, and childhood overweight in the general population and in high risk subgroups: prospective analyses in the PIAMA Birth Cohort. J Obes (Epub ahead of print 17 June 2010) [DOI] [PMC free article] [PubMed]
- 41.Kral TV, Stunkard AJ, Berkowitz RI, Stallings VA, Moore RH, Faith MS. Beverage consumption patterns of children born at different risk of obesity. Obesity (Silver Spring) 2008;16:1802–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim S, Zoellner JM, Lee JM, Burt BA, Sandretto AM, Sohn W, Ismail AI, Lepkowski JM. Obesity and sugar-sweetened beverages in African-American preschool children: a longitudinal study. Obesity (Silver Spring) 2009;17:1262–8 [DOI] [PubMed] [Google Scholar]
- 43.Sugimori H, Yoshida K, Izuno T, Miyakawa M, Suka M, Sekine M, Yamagami T, Kagamimori S. Analysis of factors that influence body mass index from ages 3 to 6 years: a study based on the Toyama cohort study. Pediatr Int 2004;46:302–10 [DOI] [PubMed] [Google Scholar]
- 44.Tam CS, Garnett SP, Cowell CT, Campbell K, Cabrera G, Baur LA. Soft drink consumption and excess weight gain in Australian school students: results from the Nepean study. Int J Obes (Lond) 2006;30:1091–3 [DOI] [PubMed] [Google Scholar]
- 45.Mrdjenovic G, Levitsky DA. Nutritional and energetic consequences of sweetened drink consumption in 6- to 13-year-old children. J Pediatr 2003;142:604–10 [DOI] [PubMed] [Google Scholar]
- 46.Feeley AB, Musenge E, Pettifor JM, Norris SA. Investigation into longitudinal dietary behaviours and household socio-economic indicators and their association with BMI Z-score and fat mass in South African adolescents: the Birth to Twenty (Bt20) cohort. Public Health Nutr 2013;16:693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiorito LM, Marini M, Francis LA, Smiciklas-Wright H, Birch LL. Beverage intake of girls at age 5 y predicts adiposity and weight status in childhood and adolescence. Am J Clin Nutr 2009;90:935–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James J, Thomas P, Kerr D. Preventing childhood obesity: two year follow-up results from the Christchurch obesity prevention programme in schools (CHOPPS). BMJ 2007;335:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sichieri R, Yokoo EM, Pereira RA, Veiga GV. Water and sugar-sweetened beverage consumption and changes in BMI among Brazilian fourth graders after 1-year follow-up. Public Health Nutr 2013;16:73–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albala C, Ebbeling CB, Cifuentes M, Lera L, Bustos N, Ludwig DS. Effects of replacing the habitual consumption of sugar-sweetened beverages with milk in Chilean children. Am J Clin Nutr 2008;88:605–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kvaavik E, Meyer HE, Tverdal A. Food habits, physical activity and body mass index in relation to smoking status in 40-42 year old Norwegian women and men. Prev Med 2004;38:1–5 [DOI] [PubMed] [Google Scholar]
- 52.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8 [DOI] [PubMed] [Google Scholar]
- 53.Bes-Rastrollo M, Sanchez-Villegas A, Gomez-Gracia E, Martinez JA, Pajares RM, Martinez-Gonzalez MA. Predictors of weight gain in a Mediterranean cohort: the Seguimiento Universidad de Navarra Study 1. Am J Clin Nutr 2006;83:362–70; quiz 94–5 [DOI] [PubMed] [Google Scholar]
- 54.Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16:1894–900 [DOI] [PubMed] [Google Scholar]
- 55.Inoue M, Toyokawa S, Inoue K, Suyama Y, Miyano Y, Suzuki T, Miyoshi Y, Kobayashi Y. Lifestyle, weight perception and change in body mass index of Japanese workers: MY Health Up Study. Public Health 2010;124:530–7 [DOI] [PubMed] [Google Scholar]
- 56.Odegaard AO, Koh WP, Arakawa K, Yu MC, Pereira MA. Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes: the Singapore Chinese Health Study. Am J Epidemiol 2010;171:701–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004;292:927–34 [DOI] [PubMed] [Google Scholar]
- 58.Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Int J Obes (Lond) 2013;Jan 15 (Epub ahead of print; DOI:10.1038/ijo.2012.225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9 [DOI] [PubMed] [Google Scholar]
- 60.Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, Diamond M, Wang X, Popkin B. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95:555–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomised controlled trial. BMJ 2004;328:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sichieri R, Paula Trotte A, de Souza RA, Veiga GV. School randomised trial on prevention of excessive weight gain by discouraging students from drinking sodas. Public Health Nutr 2009;12:197–202 [DOI] [PubMed] [Google Scholar]
- 63.Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics 2006;117:673–80 [DOI] [PubMed] [Google Scholar]
- 64.Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med 2012;367:1407–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012;367:1397–406 [DOI] [PubMed] [Google Scholar]
- 66.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr 2007;97:193–203 [DOI] [PubMed] [Google Scholar]
- 67.Reid M, Hammersley R, Duffy M. Effects of sucrose drinks on macronutrient intake, body weight, and mood state in overweight women over 4 weeks. Appetite 2010;55:130–6 [DOI] [PubMed] [Google Scholar]
- 68.Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9 [DOI] [PubMed] [Google Scholar]
- 69.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr 1990;51:963–9 [DOI] [PubMed] [Google Scholar]
- 70.Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, Berthold HK, Spinas GA, Berneis K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94:479–85 [DOI] [PubMed] [Google Scholar]
- 71.Forshee RA, Anderson PA, Storey ML. Sugar-sweetened beverages and body mass index in children and adolescents: a meta-analysis. Am J Clin Nutr 2008;87:1662–71 [DOI] [PubMed] [Google Scholar]
- 72.Mattes RD, Shikany JM, Kaiser KA, Allison DB. Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes Rev 2011;12:346–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu FB. New York, NY: Oxford University Press, 2008 [Google Scholar]
- 74.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, Keim NL, Cummings BP, Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab 2009;94:1562–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr 2008;87:1194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr 2008;88(suppl):1733S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Odegaard AO, Choh AC, Czerwinski SA, Towne B, Demerath EW. Sugar-sweetened and diet beverages in relation to visceral adipose tissue. Obesity (Silver Spring) 2012;20:689–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev 2013;14:606–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.