Abstract
Background: Arginine is considered an essential amino acid during critical illness in children, and supplementation of arginine has been proposed to improve arginine availability to facilitate nitric oxide (NO) synthesis. Protein-energy–enriched enteral formulas (PE-formulas) can improve nutrient intake and promote anabolism in critically ill infants. However, the effect of increased protein and energy intake on arginine metabolism is not known.
Objective: We investigated the effect of a PE-formula compared with that of a standard infant formula (S-formula) on arginine kinetics in critically ill infants.
Design: A 2-h stable-isotope tracer protocol was conducted in 2 groups of critically ill infants with respiratory failure because of viral bronchiolitis, who received either a PE-formula (n = 8) or S-formula (n = 10) in a randomized, blinded, controlled setting. Data were reported as means ± SDs.
Results: The intake of a PE-formula in critically ill infants (aged 0.23 ± 0.14 y) resulted in an increased arginine appearance (PE-formula: 248 ± 114 μmol · kg−1 · h−1; S-formula: 130 ± 53 μmol · kg−1 · h−1; P = 0.012) and NO synthesis (PE-formula: 1.92 ± 0.99 μmol · kg−1 · h−1; S-formula: 0.84 ± 0.36 μmol · kg−1 · h−1; P = 0.003), whereas citrulline production and plasma arginine concentrations were unaffected.
Conclusion: In critically ill infants with respiratory failure because of viral bronchiolitis, the intake of a PE-formula increases arginine availability by increasing arginine appearance, which leads to increased NO synthesis, independent of plasma arginine concentrations. This trial was registered at www.trialregister.nl as NTR515.
INTRODUCTION
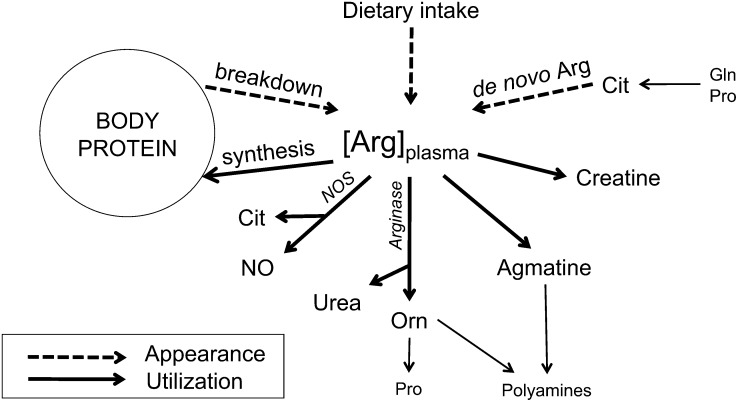
Arginine is an important amino acid during disease and healing. Arginine is needed for protein synthesis, ureagenesis, and the production of agmatine, creatine, polyamines, proline, and the signaling molecule nitric oxide (NO)6 (Figure 1) (1, 2). Hence, arginine has a function in wound healing, cell regeneration, immune function, and tissue perfusion as well as airway tone and inflammation. Plasma arginine can be derived from nutrition that is released from body protein or newly synthesized primarily in the kidneys from its sole precursor citrulline (de novo arginine synthesis) (Figure 1) (1, 2). The latter makes arginine a nonessential amino acid under healthy conditions. Citrulline is a nonprotein amino acid that is produced in the intestines, predominantly from glutamine and proline (1, 3). NO is produced during the conversion of arginine to citrulline by the enzyme nitric oxide synthase (NOS), of which 3 isoforms exist. The 2 isoforms nitric oxide synthase 1 (neuronal NOS) and nitric oxide synthase 3 (NOS3) (endothelial NOS) are constitutive enzymes; nitric oxide synthase 2 (inducible NOS) is induced to a great extent during inflammation (1).
FIGURE 1.
Schematic representation of plasma arginine appearance and use. Arginine appearance in the plasma pool results from dietary intake, de novo arginine synthesis from citrulline (which is produced in the gut from glutamine and proline) in the kidney, and arginine release from body protein by protein breakdown. Plasma arginine is used for body protein synthesis, NO synthesis via nitric oxide synthases, urea and ornithine production via arginases, and the production of agmatine and creatine. [Arg]plasma, plasma arginine concentration; Cit, citrulline; de novo Arg, de novo arginine synthesis; Gln, glutamine; NO, nitric oxide; NOS, nitric oxide synthases; Orn, ornithine; Pro, proline.
During conditions with increased metabolic needs, such as critical illness, arginine is considered a conditionally essential amino acid because arginine production does not meet the increased needs (2). In critically ill children, plasma arginine concentrations are decreased proportionally to the severity of inflammation as assessed with C-reactive protein (CRP) (4). Studies that have used stable-isotope techniques in critically ill adults and children have suggested that arginine becomes an essential amino acid in these conditions (5–8) because de novo arginine synthesis is reduced and arginine use increased. This process seems to be related to the severity of inflammation (7). Reduced arginine availability may lead to impaired NOS3 NO synthesis, which has been suggested to contribute to impaired microcirculation (9).
To improve NO synthesis in children with diseases with arginine deficiency or insufficient NO synthesis, arginine supplementation has been suggested (10–12). In clinical practice, nutritional support in the pediatric intensive care unit is usually provided by commercially available formulas. We have previously shown that a protein-energy–enriched enteral formula (PE-formula) compared with a standard infant formula (S-formula) resulted in a higher protein balance in critically ill infants with viral bronchiolitis (13, 14) and, hence, seems a more-potent tool than standard formulas to provide adequate nutritional support. However, the effect of such commercially available and generally used formulas on arginine metabolism is not known. We hypothesized that the PE-formula, which contains more arginine because of the higher protein content than that of the standard formula, in addition would stimulate arginine appearance and NO synthesis. Therefore, in the previously mentioned study (13, 14), we also investigated the effect of both formulas on arginine and citrulline kinetics by using a stable-isotope tracer methodology.
SUBJECTS AND METHODS
Subjects and setting
This study was part of a larger study on the safety and efficacy of a PE-formula compared with an S-formula, as previously reported (13, 14). Subjects were critically ill infants with respiratory failure because of respiratory syncytial virus (RSV) bronchiolitis who were admitted to the pediatric intensive care unit of either the Maastricht University Medical Center (MUMC), Maastricht, Netherlands, or the Erasmus MC – Sophia Children's Hospital, Rotterdam, Netherlands. Inclusion criteria were age from 4 wk to 12 mo, born term (>38 wk of gestation) or >40 wk postmenstrual age if born preterm, mechanical ventilation, arterial and venous catheter, hemodynamically stable (normal blood pressure with or without inotropics), ability to start feeding <24 h after admission, and an expected length of stay >96 h. Exclusion criteria were breastfeeding; parenteral nutrition other than intravenous glucose; (congenital) gastrointestinal, metabolic, or chromosomal disorder; and abnormal liver- or kidney-function tests.
Intervention
Within 24-h after admission, patients were randomly assigned to receive either a PE-formula (Infatrini; Nutricia Advanced Medical Nutrition) or S-formula (Nutrilon 1; Nutricia Advanced Medical Nutrition) via a nasogastric (MUMC) or nasoduodenal tube (Erasmus MC – Sophia Children's Hospital) as per standard in-house feeding protocols. Random assignment was completed by using permuted block random assignment with sealed envelopes. Patients, caregivers, nursing and medical staff, and investigators were blinded to the administered formula. The formula was introduced as soon as possible after admission and increased to the target intake (130 mL/kg) by steps of 25% of target volume per 12 h. Thereafter the formula was continuously provided for subsequent days. Arginine intake and intake of its precursors were from amino acids released after digestion and absorption of the whey and casein proteins in the formulas. Formulas did not contain citrulline, ornithine, or additional free amino acids. Descriptions of the contents of both formulas are shown in Table 1.
TABLE 1.
Composition of study formulas1
| Average content per 100 mL | PE-formula | S-formula |
| Energy (kcal) | 100 | 67 |
| Protein (g) (from cow milk) | 2.6 | 1.4 |
| Casein:whey (g) | 1.0:1.6 | 0.6:0.8 |
| Percentage of energy (protein:energy ratio) | 10 | 8 |
| Amino acids (mg) | ||
| l-Alanine | 117 | 55 |
| l-Arginine | 83 | 46 |
| l-Aspartic acid/l-asparagine | 225 | 120 |
| l-Cystine | 39 | 22 |
| l-Glutamic acid/l-glutamine | 556 | 260 |
| Glycine | 52 | 27 |
| l-Histidine | 60 | 35 |
| l-Isoleucine | 159 | 74 |
| l-Leucine | 278 | 130 |
| l-Lysine | 257 | 120 |
| l-Methionine | 68 | 34 |
| l-Phenylalanine | 112 | 55 |
| l-Proline | 198 | 110 |
| l-Serine | 156 | 69 |
| l-Threonine | 169 | 73 |
| l-Tryptophan | 39 | 21 |
| l-Tyrosine | 107 | 44 |
| l-Valine | 169 | 82 |
| Carbohydrates (g) | 10.3 | 7.5 |
| Percentage of energy | 41 | 45 |
| Fat (g) (from vegetable oil) | 5.4 | 3.5 |
| Percentage of energy | 49 | 47 |
Adapted from de Betue et al (14) with permission from the BMJ Publishing Group Ltd. The PE-formula (Infatrini) and S-formula (Nutrilon 1) were from Nutricia Advanced Medical Nutrition. PE-formula, protein-energy–enriched enteral formula; S-formula, standard infant formula.
Ethical approval was obtained from the local ethical committee and The Central Committee on Research Involving Human Subjects (The Hague, Netherlands). Written informed consent was obtained from parents or caregivers.
Study design
Body weight was assessed on admission, and pediatric risk of mortality (15) scores were calculated. To assess arginine, citrulline, and phenylalanine kinetics, a 2-h stable-isotope tracer protocol was conducted, and plasma amino acid concentrations were determined in the fed state at day 5 after admission to ensure the provision of enteral nutrition at the target intake and to allow for some time of adaptation.
Stable-isotope tracer protocol
The tracer protocol was based on 2-h protocols used for the determination of arginine and citrulline kinetics in healthy and critically ill adults in whom a steady state could be achieved at 60–120 min after infusion of arginine and citrulline tracers (7, 16).
Before the start of the tracer protocol, a baseline arterial blood sample was taken to determine background isotopic enrichments. Stable-isotope tracers were primed and subsequently continuously infused for 2 h with calibrated syringe pumps. Arterial blood was sampled at 60, 90, and 120 min for the measurement of isotopic enrichments to determine arginine, citrulline, and phenylalanine kinetics. Samples were transfer pipetted into cups containing heparin and EDTA put on ice, and centrifuged (3500 × g) for 10 min at 4°C. Plasma for stable-isotope analysis and plasma amino acid concentrations was deproteinized with 5% sulfosalicylic acid, snap frozen in liquid nitrogen, and stored at −80°C until analysis.
To assess arginine metabolism l-[guanidino-15N2- (5, 5)-2H2]arginine (infusion rate: 5.6 μmol · kg−1 · h−1) and l-[ureido-13C]citrulline ([13C]Cit) (infusion rate: 0.2 μmol · kg−1 · h−1), tracers were used. For the assessment of whole-body protein kinetics l-[ring-2H5]phenylalanine ([2H5]Phe) (infusion rate: 4.3 μmol · kg−1 · h−1) and l-[3,3-2H2]tyrosine ([2H2]Tyr) (infusion rate: 1.5 μmol · kg−1 · h−1) were used. Before continuous infusion, a bolus of l-[ring-2H4]tyrosine was infused to prime the pool of [2H4]Tyr coming from [2H5]Phe. To determine splanchnic extraction in the fed state, in protein-energy–enriched formula–fed infants with viral bronchiolitis (PE-group) and standard formula–fed infants with viral bronchiolitis (S-group) l-[13C]phenylalanine ([13C]Phe) (infusion rate: 8.1 μmol · kg−1 · h−1) was meanwhile infused enterally via a nasogastric tube. Tracers (all >98% mole percentage enrichment) were purchased as sterile pyrogen-free powders from Cambridge Isotope Laboratories and Buchem BV. Tracer solutions were prepared by a licensed pharmacist and dispensed by the clinical pharmacy of the MUMC after testing for sterility and pyrogenicity. Intravenous tracers were administered through a 2-μm-pore filter. A sample of the used tracer solution was stored for the determination of tracer concentrations.
Plasma amino acid concentrations and intestinal fatty acid binding protein
In the baseline sample, plasma amino acid concentrations were determined to get insight into the plasma concentrations of arginine and citrulline and related amino acids. Also, plasma CRP concentrations were measured in the baseline sample as a marker of severity of inflammation. Because we had previously shown that, in critically ill children, plasma citrulline concentrations are reduced (4), which may be related to intestinal damage (17), we measured plasma intestinal fatty acid binding protein (I-FABP) as a marker of enterocyte damage.
Sample analysis
Samples had been previously analyzed for phenylalanine and tyrosine kinetics as reported elsewhere (14). To determine arginine and citrulline kinetics, samples were reanalyzed with a different method, which will be described in the next paragraph, with concomitant reassessment of phenylalanine and tyrosine kinetics.
Exogenous amino acid stable-isotopomer enrichments [tracer-to-tracee ratios (TTRs)] were determined by using an liquid chromatography–electronspray ionization–mass spectrometry system (QTrap 5500 MS; AB Sciex) with liquid chromatography device ExpressHT Ultra LC (Eksigent Div; AB Sciex). Samples were standardized with 0.1 N HCl that contained a stable isotopomer of every amino acid as an internal standard. For internal standards, stable isotopomers with a high mass were chosen (at least m+5) to prevent the contribution of overlapping isotopomer distributions to isotopomers that were used as tracers in the experimental protocol. Samples were transfer pipetted onto strong-cation-exchange drip columns. Columns were washed with water and eluted with 2.5 mol/L ammonia. Eluates were desolvated in a centrifugal evaporator. Solid-residue tubes were capped for storage in the dark at room temperature. Within 3 d of liquid chromatography–electronspray ionization–mass spectrometry analysis, samples were derivatized with 9-fluorenylmethoxycarbonyl (Fmoc) and subsequently neutralized, after which 160 nL of the solution was injected onto a 50 × 0.5 mm HALO C18 column (Eksigent Div; AB Sciex) and kept at 35°C. Analytes were eluted with a segmentally linear gradient from 35% to 85% acetonitrile in water supplemented with ammonium acetate to 10 μmol/L and 5% isopropanol. Detection was done by using electrospray triple-quadrupole–tandem mass spectrometry in multiple-reaction monitoring mode. Fmoc amino acid derivatives were fragmented in the collision cell for detection of either free aminoacyl anions or a fragment larger by 26 atom mass units (coming from the Fmoc derivative), whichever gave the highest sensitivity. Thus, monitoring occurred for each amino acid, their tracers, and internal standards. TTRs were determined as tracer (labeled substance):tracee (unlabeled substance).
We used the SignalFinder algorithm in MultiQuant software (version 2.1; ABScieix) to quantify peaks. Because the SignalFinder integration algorithm calculates signal-to-noise more accurately (and, thus, more accurately predicts CVs), we used the 1-σ signal-to-noise approach and also have compared this approach to empirical data from our laboratory. We showed this approach to be valid in our hands. The lowest detectable TTR was 0.00003 for l-[15N2-(5,5)-2H2]citrulline and 0.00083 for l-[guanidino-13C]arginine.
Plasma amino acid concentrations and plasma CRP concentrations were determined as described previously (4). Plasma I-FABP concentrations were determined by using a highly specific in-house ELISA that selectively detected human I-FABP (standard: 12.5–800 pg/mL).
Calculations
Whole-body metabolism
The whole-body rate of appearance (Ra; flux or Q) of plasma arginine, citrulline, phenylalanine, and tyrosine were calculated from arterial isotopic enrichments of l-[guanidino-15N2-(5,5)-2H2]arginine, [13C]Cit, [2H5]Phe, [13C]Phe, and [2H2]Tyr, respectively, by using the standard steady state isotope-dilution equation (18):
where I is the rate of tracer infusion. Isotopic enrichment was calculated by correcting the measured TTRs at the plateau phase by subtracting the background TTR, which was determined in the sample obtained before the start of the tracer infusion. In the case of arginine and citrulline tracers, the contribution of overlapping isotopomer distributions of tracers with lower masses to the measured TTR was accounted for as previously described (19).
Whole-body arginine and citrulline metabolism
The rate of NO synthesis was calculated as the flux from arginine to citrulline (20) by using the following equation:
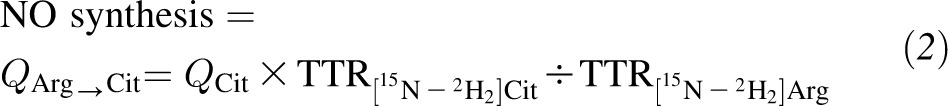 |
where QCit is the Ra of citrulline as calculated by using Equation 1 from [13C]Cit enrichment.
The rate of de novo arginine synthesis was calculated as the flux from citrulline to arginine (21) as follows:
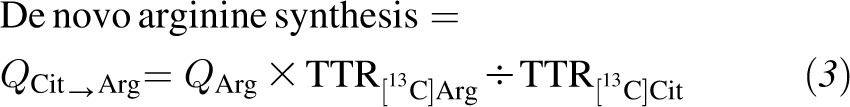 |
where QArg is the Ra of arginine as calculated by using Equation 1 from [15N2]Arg enrichment.
Arginine release from protein via protein breakdown (ArgRP) was calculated from:
Arginine clearance (in mL · kg−1 · min−1) was calculated as follows (6):
where Ra Arg is the total arginine appearance from arginine intake, protein breakdown, and de novo arginine synthesis, and plasma [Arg] is the plasma arginine concentration in micromoles per liter.
Arginine clearance represents the volume of plasma from which arginine is removed per time unit and, thus, indicates the capacity of arginine use. Arginine clearance involves all pathways of arginine disappearance (ie, arginine use by arginase and NOS and for protein synthesis and the production of agmatine and creatine).
Whole-body protein metabolism
Under steady state conditions, the Ra of amino acids in the plasma pool is equal to the rate of disappearance (Rd; flux or Q). In the fed state, the Ra equals the sum of the amino acid release from protein and the rate at which the amino acid enters the blood pool from the nutrition source. In the case of enteral nutrition, the rate at which the amino acid enters the blood pool from the nutrition source is the rate of enteral intake corrected for the proportion of the amino acid intake that is retained in the splanchnic area during the first pass (splanchnic extraction). Rd equals the sum of the rate of oxidation or hydroxylation (in the case of phenylalanine hydroxylation) and the rate at which the amino acid is used for protein synthesis.
Plasma phenylalanine-to-tyrosine flux, which indicates phenylalanine hydroxylation, was calculated by using following equation:
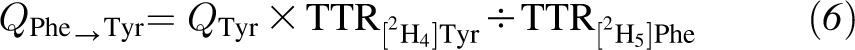 |
where QTyr is the Ra of tyrosine as calculated by using Equation 1 from [2H2]Tyr enrichment.
In the fed state, dietary Phe via the enteral route needs to be adjusted for the splanchnic extraction of Phe (SPEPhe) as previously described. The SPEPhe was calculated as follows:
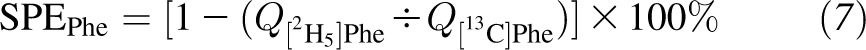 |
where Q[2H5]Phe is the Ra of phenylalanine derived from intravenously infused [2H5]Phe, and Q[13C]Phe is the Ra of phenylalanine derived from enterally administered [13C]Phe.
The Ra of phenylalanine that did not come from dietary intake [endogenous Ra Phe (Raend-Phe)], which represents whole-body protein breakdown (WbPB) was calculated as follows:
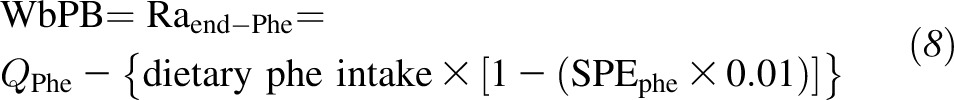 |
Whole-body protein synthesis (WbPS) was calculated as follows:
Net whole-body protein synthesis (netWbPS) can be calculated as follows:
Arginine release from protein was also calculated from Raend-Phe by assuming an average Phe content in human proteins of 280 μmol Phe/g protein (22) and an average Arg content of 362 μmol Arg/g protein (23).
Statistics
Data are presented as means ± SDs. A power analysis had been based on protein metabolism parameters in infants (24) because protein balance was the primary outcome measure of the larger study; with 0.80 sensitivity and a 2-tailed 0.05 significance, the number deemed necessary to detect a 20% difference in protein balance was 8.
Because of the small sample size, nonparametric tests were used. Comparisons between the PE-group and S-group were done by using Wilcoxon's rank-sum test with exact significance. Spearman's ρ correlation was used to assess associations between variables. Two-tailed P < 0.05 was considered significant. Analyses were done with IBM SPSS Statistics software (version 17; IBM).
RESULTS
Subjects
Twenty infants with viral bronchiolitis were enrolled, and 10 infants were assigned to each group. Two patients in the PE-group were excluded before day 5 because of a switch to a nonstudy formula after extubation before day 5. Thus, in 18 patients, the stable-isotope tracer protocol was conducted [8 patients in the PE-group; 10 patient in the S-group (see Supplemental Figure S1 under “Supplemental data” in the online issue for a flow diagram). An overview of baseline characteristics of both groups is shown in Table 2. Besides a lower gestational age in the PE-group, there were no significant differences in baseline characteristics. Both formulas were well tolerated as described previously (13). Volume intakes at day 5 were not different between both groups (Table 3). Energy and protein intakes at day 5 covered recommended allowances for healthy infants <6 mo of age (25, 26) in the S-group, whereas intake rates were significantly higher in the PE-group (Table 3). Arginine intake and intakes of its precursors glutamine and proline were significantly higher in the PE-group because of the significantly higher protein intake.
TABLE 2.
Baseline characteristics of critically ill infants receiving protein-energy–enriched formula or standard formula1
| PE-group (n = 8) | S-group (n = 10) | |
| Medical center (MUMC/Erasmus MC) (n) | 4/6 | 4/4 |
| Sex (M) [n (%)] | 2 (25) | 3 (30) |
| Age (mo) | 2.7 ± 1.42 | 3.0 ± 1.8 |
| Gestational age (wk) | 35.0 ± 3.3* | 37.3 ± 1.0 |
| Postmenstrual age (wk) | 46.8 ± 7.6 | 49.9 ± 8.2 |
| Weight (kg) | 4.0 ± 1.0 | 4.8 ± 1.2 |
| PRISM | 20.3 ± 4.6 | 18.6 ± 4.8 |
| C-reactive protein at admission (mg/L) | 77 ± 66 | 64 ± 64 |
| C-reactive protein at day 5 (mg/L) | 23 ± 20 | 28 ± 25 |
Comparisons were performed by using Wilcoxon's rank-sum test. *P < 0.05 compared with the S-group. Erasmus MC, Erasmus MC–Sophia Children's Hospital; MUMC, Maastricht University Medical Center; PE-group, protein-energy–enriched formula–fed infants with viral bronchiolitis; PRISM, Pediatric Risk of Mortality (15); S-group, standard formula–fed infants with viral bronchiolitis.
Mean ± SD (all such values).
TABLE 3.
Nutritional intake during the stable-isotope tracer protocol in critically ill infants receiving protein-energy–enriched formula or standard formula1
| PE-group (n = 8) | S-group (n = 10) | |
| Volume (mL · kg−1 · 24 h−1) | 121 ± 13 | 119 ± 13 |
| Energy (kcal · kg−1 · 24 h−1) | 119 ± 25‡ | 84 ± 15 |
| Protein (g· kg−1 · 24 h−1) | 3.1 ± 0.3‡ | 1.7 ± 0.2 |
| Arginine (μmol · kg−1 · h−1) | 24 ± 2.8‡ | 13 ± 1.7 |
| Glutamine (μmol · kg−1 · h−1) | 191 ± 23‡ | 88 ± 12 |
| Proline (μmol · kg−1 · h−1) | 86 ± 10‡ | 47 ± 6 |
All values are means ± SDs. Comparisons between groups were performed by using Wilcoxon's rank-sum test. ‡P < 0.001 compared with the S-group. PE-group, protein-energy–enriched formula–fed infants with viral bronchiolitis; S-group, standard formula–fed infants with viral bronchiolitis.
Measurements
Enrichments
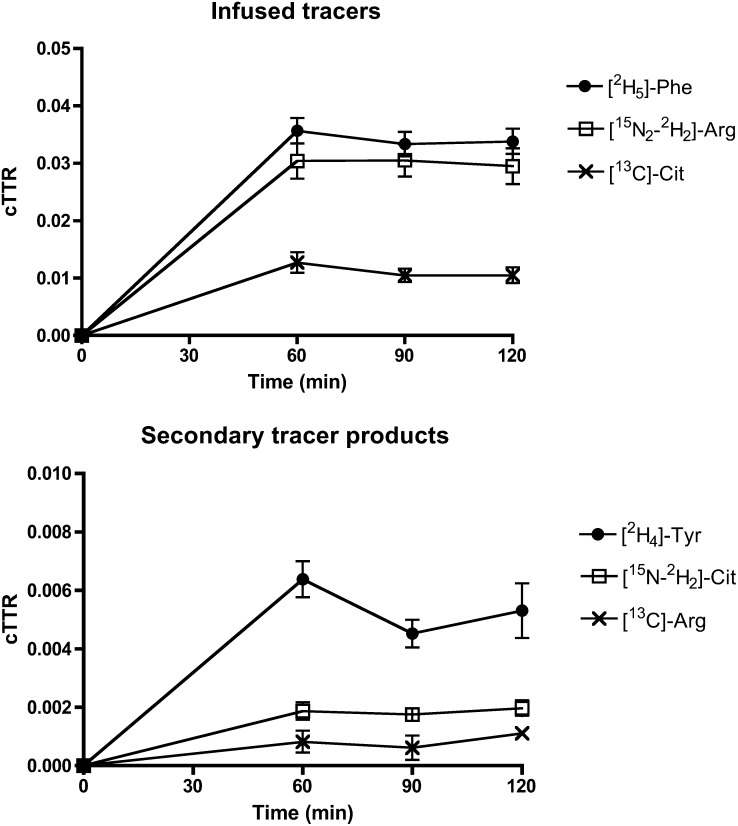
Enrichments of infused tracers are shown in Figure 2. A steady state of infused tracers and their secondary products was achieved. As regards the detection limits described in Subject and Methods, averages of enrichments that are reported in Figure 2 were well above the detection limit for l-[15N-2H2]citrulline, whereas for l-[13C]arginine, enrichments were near the detection limit. The latter was a result of a higher than anticipated citrulline production from glutamine and proline and from arginine as a byproduct of NO synthesis (Ra Cit). At the time of the design of the study, no data were available on Ra Cit and conversion rates to arginine (de novo arginine synthesis) in fed critically ill infants in a low inflammatory state. A high Ra Cit is characterized by relatively low [13C]-citrulline enrichments and consequently [13C]-arginine enrichments, which result from the conversion of [13C]-citrulline to [13C]-arginine. Thus, the enrichment of [13C]-arginine could only be picked up in 11 patients (n = 5 in the PE-group; n = 6 in the S-group), and de novo arginine synthesis could be calculated in those patients only.
FIGURE 2.
Mean (±SEM) enrichments of infused tracers and tracer products during the stable-isotope tracer protocol. For [13C]-Arg, n = 11; for all others, n = 18. Arg, arginine; Cit, citrulline; cTTR, tracer-to-tracee ratio corrected for background enrichment; Phe, phenylalanine; Tyr, tyrosine.
Arginine, citrulline, and phenylalanine kinetics
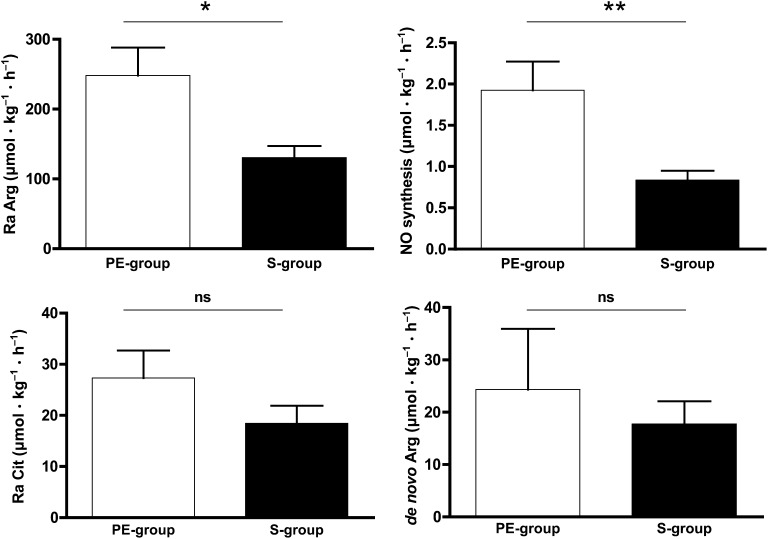
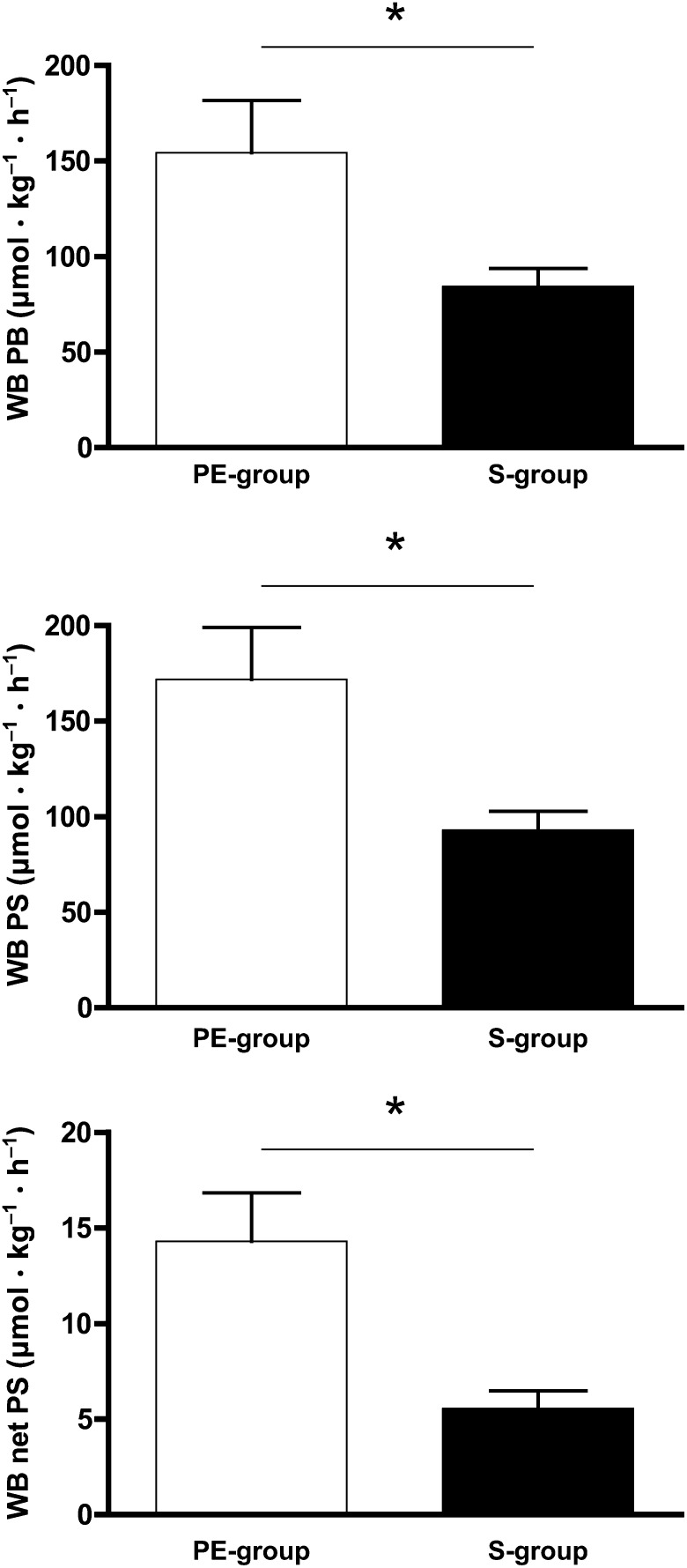
Arginine and citrulline kinetics in the PE-group and S-group are shown in Figure 3; whole-body protein kinetics as calculated from phenylalanine kinetics are represented in Figure 4.
FIGURE 3.
Mean (±SEM) arginine and citrulline kinetics in critically ill infants receiving protein-energy–enriched formula (n = 8) or standard formula (n = 10). For de novo arginine synthesis, n = 5 in the PE-group, and n = 6 in the S-group. *,**Comparisons between the PE-formula (n = 8) and S-formula (n = 10) were performed by using Wilcoxon's rank-sum test: * P < 0.05, **P < 0.001. de novo Arg, de novo arginine synthesis; NO, nitric oxide; PE-formula, protein-energy–enriched enteral formula; PE-group, protein-energy–enriched formula–fed infants with viral bronchiolitis; Ra Arg, rate of arginine appearance; Ra Cit, rate of citrulline appearance (citrulline production); S-formula, standard infant formula; S-group, standard formula–fed infants with viral bronchiolitis.
FIGURE 4.
Mean (±SEM) whole-body protein metabolism in critically ill infants receiving protein-energy–enriched formula (n = 8) or standard formula (n = 10). *Comparisons between the PE-formula and S-formula were performed by using Wilcoxon's rank-sum test, P < 0.05. PE-formula, protein-energy–enriched enteral formula; PE-group, protein-energy–enriched formula–fed infants with viral bronchiolitis (n = 8); S-formula, standard infant formula; S-group, standard formula–fed infants with viral bronchiolitis (n = 10); WB net PS, whole-body net protein synthesis; WB PB, whole-body protein breakdown; WB PS, whole-body protein synthesis.
Ra Arg was significantly higher with the PE-formula than with the S-formula (P = 0.021). Ra Cit was not different between both groups of infants (P = 0.146). In a subgroup of 11 patients (PE-formula: n = 5; S-formula: n = 6), de novo Arg synthesis was not significantly different either (P = 1.000). Ra Cit in this subgroup was 29 ± 17 μmol · kg−1 · h−1 in the PE-group and 22 ± 13 μmol · kg−1 · h−1 in the S-group (P = 0.537). Because of the low enrichment amounts of l-[13C]arginine close to the detection limit, these data on de novo arginine synthesis should merely be seen as indicative.
As concerns arginine use, NO synthesis was significantly increased with the PE-formula as compared with S-formula (P = 0.003) (Figure 3). Also, WbPS was significantly increased in the PE-group compared with the S-group (P = 0.043) (Figure 4). As previously reported (14), the increase in WbPS was higher than the increase in WbPB in the PE-group. Therefore, the PE-formula resulted in a higher net WbPS (P = 0.016).
Arginine clearance was significantly higher in the PE-group than S-group (48.3 ± 28.0 compared with 27.1 ± 8.9 mL · kg−1 · min−1, respectively; P = 0.012). Arginine release from protein as calculated from the Ra Arg, de novo Arg synthesis, and Arg intake was 201 ± 108 compared with 113 ± 58 μmol · kg−1 · h−1 (P = 0.177) for the PE-group and S-group, respectively, and when calculated from the Raend-Phe, arginine release was 231 ± 102 compared with 124 ± 43 μmol · kg−1 · h−1, respectively P = 0.012).
Plasma amino acid and I-FABP concentrations
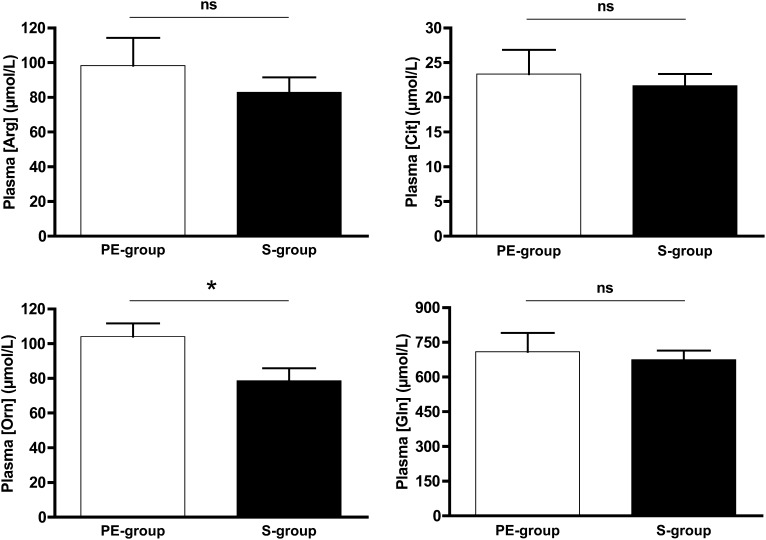
Plasma concentrations of arginine, citrulline, glutamine, and ornithine in the PE-group and S-group are shown in Figure 5. Plasma arginine (P = 0.573), citrulline (P = 0.829), and glutamine (P = 0.762) concentrations were not different between both groups, but plasma ornithine concentrations were significantly higher in the PE-group (P = 0.021). Arginine:ornithine ratios were not significantly different between groups (PE-group: 0.91 ± 0.28; S-group: 1.15 ± 0.41 μmol/L; P = 0.203). I-FABP concentrations were in the normal range and not significantly different between both groups [PE-group (n = 6): 283 ± 162; S-group (n = 5) 452 ± 222; P = 0.177).
FIGURE 5.
Mean (±SEM) plasma concentrations of amino acids involved in arginine metabolism in critically ill infants receiving protein-energy–enriched formula (n = 8) or standard formula (n = 10). *Comparisons between S-formula (n = 10) and PE-formula (n = 8) were performed by using Wilcoxon's rank-sum test, P < 0.05. PE-formula, protein-energy–enriched enteral formula; PE-group, protein-energy–enriched formula–fed infants with viral bronchiolitis; Plasma [Arg], plasma arginine concentration; Plasma [Cit], plasma citrulline concentration; Plasma [Gln], plasma glutamine concentration; Plasma [Orn], plasma ornithine concentration; S-formula, standard infant formula; S-group, standard formula–fed infants with viral bronchiolitis.
Correlations
Ra Arg was strongly associated with NO synthesis (r = 0.695, P = 0.001), WbPB (r = 0.946, P < 0.001), and de novo Arg synthesis (r = 0.655, P = 0.029) and tended to be associated with arginine intake (r = 0.467, P = 0.05). NO synthesis was also associated with protein intake and, therefore, arginine intake (r = 0.680, P = 0.002). Arginine clearance was associated with the NO synthesis (r = 0.492, P = 0.038) and WbPS (r = 0.777, P < 0.001) and inversely associated with the arginine:ornithine ratio (r = −0.575, P = 0.013).
Plasma arginine concentrations were inversely associated with CRP (r = −0.479, P = 0.044) and tended to be associated with NO synthesis (r = 0.439, P = 0.069). Plasma arginine, citrulline, glutamine or ornithine concentrations were not associated with protein intake. I-FABP concentrations were not associated with CRP, plasma citrulline concentrations, or Ra Cit.
DISCUSSION
In this study, we showed that the intake of an enteral protein-energy–enriched formula in critically ill infants with respiratory failure because of viral bronchiolitis resulted in increased arginine appearance compared with that with a standard formula. Moreover, the intake of the enteral protein-energy–enriched formula resulted in increased NO synthesis. This result was in line with our previous study in this study population that showed increased protein turnover, protein synthesis, and protein breakdown with the protein-energy–enriched formula, which led to an anabolic state (14). In addition, we previously showed that the formula was safe and well tolerated (13). Thus, our results imply that arginine availability can be increased with the protein-energy–enriched formula and facilitate important arginine functions. Arginine clearance was increased in the PE-group, which was most likely caused by increased NOS activity (increased NO production) and increased protein synthesis but possibly also by increased arginase activity, as suggested by increased plasma ornithine concentrations and the inverse association with arginine:ornithine ratios. Remarkably, plasma arginine concentrations did not significantly increase with the protein-energy–enriched formula. Therefore, an important implication of our results is that plasma arginine concentrations may not be a good predictor of arginine (dis)appearance in the fed state and, thus, of the availability of arginine for its functions.
Nutrition and arginine appearance and NO synthesis
To our knowledge, we are the first to describe these effects of an enteral formula in critically ill children. Yu et al (23) showed increased arginine and leucine appearance when providing total parenteral nutrition to burned children compared with a basal state without parenteral amino acids. Otherwise, in critically ill adults and children, arginine kinetics have been studied only in the fasted state to our knowledge. Studies in the fed state have been conducted in healthy adults. In line with our results, in healthy adults, arginine appearance increased from the fasted to the fed state with an arginine-rich diet (74.5 mg · kg−1 · d−1), was higher with the arginine-rich diet than with an arginine-free diet (20, 27, 28), and increased with an arginine-supplemented diet (561 mg · kg−1 · d−1) compared with a normal arginine diet (56 mg · kg−1 · d−1) (29). However, NO synthesis did not differ between the different diets (20, 29). Interventions in these studies were isonitrogenous and isocaloric, whereas in our study, the interventions differed in protein and energy intakes. Therefore, currently described changes may not have been attributable to a higher arginine intake alone but may have been influenced by intakes of other amino acids and more energy as well, in addition to differences in disease states. Consistent with our current findings, in a study in pigs, we observed that a reduced protein-energy intake (25% of the normal food intake) during 7 d resulted in reduced protein turnover, reduced arginine and citrulline appearance, and reduced NO synthesis in the fasted state (30). Indeed, in the current study, the stimulation of protein turnover, with a concomitant increased protein breakdown, seemed to be the largest drive behind the increased arginine appearance and NO synthesis, as shown by the strong associations between these variables.
Measurement of NO production
Different methods to measure NO production have been used, most often with plasma or urine concentrations of the NO metabolites nitrate/nitrite. However, these concentrations may be influenced by altered renal function and extracellular volume changes and do not represent acute changes in NO production (31). Other approaches include stable-isotope techniques (31), as we have used in the current study. Indeed, in critically ill adults and children, discrepancies between methods were shown, and it remains unclear whether NO production is unchanged, decreased, or increased during critical illness (5–8). A drawback of the stable-isotope methods is that arginine and NO metabolism are highly compartmentalized. Hence, the calculated NO production is the NO production from plasma-labeled arginine. In some tissues, the labeled arginine does not fully equilibrate with the pool in which NO is produced. Also, some cell types (eg, endothelial cells) have a complete arginine → NO + citrulline → arginine cycle within the same compartment. In this case, most of the arginine and citrulline will remain within the cell and will not fully equilibrate with plasma pools (32). Therefore, although plasma citrulline is not disposed of other than by de novo arginine synthesis, rates of de novo arginine synthesis in the current study were slightly lower than citrulline-appearance rates. Of note, because of the low enrichment amounts of arginine derived from citrulline, which were close to the detection limit, these data on de novo arginine synthesis should merely be seen as indicative.
Relevance of increased NO production in critically ill infants
From stable-isotope studies in critically ill adults and children, it has been consistently concluded that arginine becomes an essential amino acid during critical illness because of increased arginine use and insufficient arginine synthesis in part because of reduced de novo arginine synthesis that resulted from reduced citrulline production (5–8). This effect may possibly affect NO production. In addition, in septic patients, the ratio of arginine as a substrate and asymmetric dimethylarginine as an inhibitor of NOS is decreased and associated with increased mortality (33, 34). Therefore, the restoration of the arginine:asymmetric dimethylarginine ratio by arginine supplementation to normalize NO production has been proposed as a therapeutic target to improve the outcome (35). During critical illness, NO production by nitric oxide synthase 2 is increased, whereas the production by NOS3 is impaired because of alterations in arginine transporters by inflammatory cytokines (36). As a consequence, microcirculation and tissue oxygenation may be compromised (36). Thus, improving NO production, especially through NOS3, seems to be a target during critical illness. However, the benefit of arginine supplementation in critically ill patients has been questioned (37) because of a putative negative effect on hemodynamics. However, no adverse effects on hemodynamics were shown in critically ill adults who received continuous intravenous arginine supplementation (38).
The previously cited studies mostly concerned septic patients during severe systemic inflammation as shown by high plasma CRP concentrations when the largest derangements in arginine metabolism occur (4, 7). Subjects in the current study were in a low systemic inflammatory state as shown by low plasma CRP concentrations. In a previous study, we showed an inverse relation between plasma arginine and citrulline concentrations and plasma CRP concentrations (4). In critically ill children with viral disease, hence with low CRP concentrations, plasma arginine and citrulline concentrations were not severely reduced in the first 24-h after admission in the fasted state and increased during recovery in the fed state, although without statistical significance, to concentrations similar to these shown in the current study (4). Also, in a control group of critically ill adults with lower CRP concentrations than in the investigational group with sepsis, Luiking et al (9) showed that in the fasted state arginine metabolism was less affected than that in in the septic group. Especially the reduction in Ra Cit, which seemed to be an important factor in the development of arginine deficiency, was less apparent in patients with lower systemic inflammation (9). Therefore, it could be questioned whether arginine supplementation is relevant in critically ill infants with viral bronchiolitis. However, despite relatively low systemic inflammation, RSV bronchiolitis is characterized by severe local inflammation combined with airway reactivity leading to respiratory failure (39), which was indeed the case in our subjects who all required mechanical ventilation. In infants with acute RSV bronchiolitis referred to an emergency department, exhaled NO was shown to be reduced during the acute phase and returned to normal and beyond during convalescence (40). Infants with RSV bronchiolitis possibly also have increased risk of developing asthma later in life (40). In asthma, the dysregulation of arginine metabolism and modulation of NO homeostasis by increased arginase activity is considered a key aspect of the pathogenesis (41). In the early response, exhaled NO is reduced, which is attributed to reduced NOS3 activity (41). NO production in the lungs is regulated by arginine availability; oral arginine supplementation or arginine inhalation can increase exhaled NO and reduce airway hyperreactivity (41). The previously mentioned studies may indicate that NO synthesis is reduced in infants with acute RSV bronchiolitis because of reduced arginine availability, and there may be a role for arginine supplementation in this population, despite the low systemic inflammation.
The small sample size and lack of healthy controls were limitations of this study but a consequence of ethical considerations. We did not measure arginine kinetics in the fasted state and acute phase because of the burden to subjects and interference with our goal to provide early enteral nutrition. Unfortunately, we were not able to determine de novo arginine synthesis rates in all patients because of a higher than anticipated citrulline appearance, with a consequently lower and more difficult to measure enrichment in the arginine product. However, a reasonable agreement was shown between values of arginine release from protein calculated from arginine appearance and de novo arginine synthesis and values calculated from endogenous phenylalanine appearance. The former calculations had lower values, but arginine release from protein was underestimated because we were not able to correct for splanchnic extraction. It should be noticed that, because of some degree of uncertainty, the de novo arginine synthesis data should only be considered indicative.
In conclusion, arginine appearance and NO synthesis can be increased with an enteral protein-energy–enriched formula in critically ill infants with respiratory failure as a result of viral bronchiolitis, despite not affecting plasma arginine concentrations. The effect of arginine supplementation on outcomes in this population warrants additional study.
Supplementary Material
Acknowledgments
We thank the children who participated in the study and their parents or representatives. We also thank the pediatric intensive care unit nursing and medical staffs for their support in conducting tracer protocols, John Thaden, Joshua Spore, and Marlou Adriaanse for sample analyses, and Dimitris Rizopoulos for statistical consultancy.
The authors’ responsibilities were as follows—NEPD, KFMJ, and DAvW: designed the research; CTIdB and DAvW: conducted the research and had primary responsibility for the final content of the manuscript; CTIdB, NEPD, ACEV, and DAvW: analyzed data; CTIdB, KFMJ, NEPD, ACEV, and DAvW: wrote the manuscript; and all authors: read and approved the final manuscript. Nutricia was not involved in the study design, collection, analysis, and interpretation of data, or the decision to submit the manuscript for publication. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: CRP, C-reactive protein; Fmoc, 9-fluorenylmethoxycarbonyl; I-FABP intestinal fatty acid binding protein; MUMC, Maastricht University Medical Center; NO, nitric oxide; NOS, nitric oxide synthase; NOS3, nitric oxide synthase 3; PE-formula, protein-energy–enriched enteral formula; PE-group, protein-energy–enriched formula–fed infants with viral bronchiolitis; Ra Arg, total arginine appearance from arginine intake, protein breakdown, and de novo arginine synthesis; Ra Cit, citrulline production from glutamine and proline and from arginine as a byproduct of nitric oxide synthesis; RSV, respiratory syncytial virus; S-formula, standard infant formula; S-group, standard formula–fed infants with viral bronchiolitis; TTR, tracer-to-tracee ratio; WbPB, whole-body protein breakdown; WbPS, whole-body protein synthesis; [2H5]Phe, l-[ring-2H5]phenylalanine; [2H2]Tyr, l-[3,3-2H2]tyrosine; [13C]Cit, l-[ureido-13C]citrulline; [13C]Phe, l-[13C]phenylalanine.
REFERENCES
- 1.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J 1998;336:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris SM., Jr Arginine: beyond protein. Am J Clin Nutr. 2006;83:508S–12S [DOI] [PubMed] [Google Scholar]
- 3.Cynober L, Moinard C, De Bandt JP. The 2009 ESPEN Sir David Cuthbertson. Citrulline: a new major signaling molecule or just another player in the pharmaconutrition game? Clin Nutr. 2010;29:545–51 [DOI] [PubMed] [Google Scholar]
- 4.van Waardenburg DA, de Betue CT, Luiking YC, Engel M, Deutz NE. Plasma arginine and citrulline concentrations in critically ill children: strong relation with inflammation. Am J Clin Nutr. 2007;86:1438–44 [DOI] [PubMed] [Google Scholar]
- 5.Argaman Z, Young VR, Noviski N, Castillo-Rosas L, Lu XM, Zurakowski D, Cooper M, Davison C, Tharakan JF, Ajami A, et al. Arginine and nitric oxide metabolism in critically ill septic pediatric patients. Crit Care Med 2003;31:591–7 [DOI] [PubMed] [Google Scholar]
- 6.Kao CC, Bandi V, Guntupalli KK, Wu M, Castillo L, Jahoor F. Arginine, citrulline and nitric oxide metabolism in sepsis. Clin Sci (Lond). 2009;117:23–30. [DOI] [PubMed] [Google Scholar]
- 7.Luiking YC, Poeze M, Ramsay G, Deutz NE. Reduced citrulline production in sepsis is related to diminished de novo arginine and nitric oxide production. Am J Clin Nutr. 2009;89:142–52 [DOI] [PubMed] [Google Scholar]
- 8.Villalpando S, Gopal J, Balasubramanyam A, Bandi VP, Guntupalli K, Jahoor F. In vivo arginine production and intravascular nitric oxide synthesis in hypotensive sepsis. Am J Clin Nutr. 2006;84:197–203 [DOI] [PubMed] [Google Scholar]
- 9.Luiking YC, Poeze M, Dejong CH, Ramsay G, Deutz NE. Sepsis: an arginine deficiency state? Crit Care Med. 2004;32:2135–45 [DOI] [PubMed] [Google Scholar]
- 10.Amin HJ, Zamora SA, McMillan DD, Fick GH, Butzner JD, Parsons HG, Scott RB. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140:425–31 [DOI] [PubMed] [Google Scholar]
- 11.Morris CR, Morris SM, Jr, Hagar W, Van Warmerdam J, Claster S, Kepka-Lenhart D, Machado L, Kuypers FA, Vichinsky EP. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168:63–9 [DOI] [PubMed] [Google Scholar]
- 12.Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, Rice GD, Barr FE, Summar ML. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:58–65 [DOI] [PubMed] [Google Scholar]
- 13.van Waardenburg DA, de Betue CT, Goudoever JB, Zimmermann LJ, Joosten KF. Critically ill infants benefit from early administration of protein and energy-enriched formula: a randomized controlled trial. Clin Nutr. 2009;28:249–55 [DOI] [PubMed] [Google Scholar]
- 14.de Betue CT, van Waardenburg DA, Deutz NE, van Eijk HM, van Goudoever JB, Luiking YC, Zimmermann LJ, Joosten KF. Increased protein-energy intake promotes anabolism in critically ill infants with viral bronchiolitis: a double-blind randomised controlled trial. Arch Dis Child. 2011;96:817–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med 1988;16:1110–6 [DOI] [PubMed] [Google Scholar]
- 16.Lagerwerf FM, Wever RM, van Rijn HJ, Versluis C, Heerma W, Haverkamp J, Koomans HA, Rabelink TJ, Boer P. Assessment of nitric oxide production by measurement of [15N]citrulline enrichment in human plasma using high-performance liquid chromatography-mass spectrometry. Anal Biochem. 1998;257:45–52 [DOI] [PubMed] [Google Scholar]
- 17.Piton G, Manzon C, Cypriani B, Carbonnel F, Capellier G. Acute intestinal failure in critically ill patients: is plasma citrulline the right marker? Intensive Care Med 2011;37:911–7 [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons, 2004 [Google Scholar]
- 19.Vogt JA, Chapman TE, Wagner DA, Young VR, Burke JF. Determination of the isotope enrichment of one or a mixture of two stable labelled tracers of the same compound using the complete isotopomer distribution of an ion fragment; theory and application to in vivo human tracer studies. Biol Mass Spectrom 1993;22:600–12 [DOI] [PubMed] [Google Scholar]
- 20.Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N] arginine-to-[15N]citrulline labeling. Proc Natl Acad Sci USA 1996;93:11460–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo L, Chapman TE, Sanchez M, Yu YM, Burke JF, Ajami AM, Vogt J, Young VR. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA 1993;90:7749–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo L, Yu YM, Marchini JS, Chapman TE, Sanchez M, Young VR, Burke JF. Phenylalanine and tyrosine kinetics in critically ill children with sepsis. Pediatr Res 1994;35:580–8 [PubMed] [Google Scholar]
- 23.Yu YM, Sheridan RL, Burke JF, Chapman TE, Tompkins RG, Young VR. Kinetics of plasma arginine and leucine in pediatric burn patients. Am J Clin Nutr 1996;64:60–6 [DOI] [PubMed] [Google Scholar]
- 24.Poindexter BB, Karn CA, Leitch CA, Liechty EA, Denne SC. Amino acids do not suppress proteolysis in premature neonates. Am J Physiol Endocrinol Metab 2001;281:E472–8 [DOI] [PubMed] [Google Scholar]
- 25.Protein and amino acid requirements in human nutrition: report of a joint FAO/WHO/UNU expert consultation. Geneva, Switzerland: WHO, 2007 [PubMed] [Google Scholar]
- 26.Butte NF. Energy requirements of infants. Public Health Nutr. 2005;8:953–67 [DOI] [PubMed] [Google Scholar]
- 27.Castillo L, Ajami A, Branch S, Chapman TE, Yu YM, Burke JF, Young VR. Plasma arginine kinetics in adult man: response to an arginine-free diet. Metabolism 1994;43:114–22 [DOI] [PubMed] [Google Scholar]
- 28.Castillo L, Sanchez M, Vogt J, Chapman TE, DeRojas-Walker TC, Tannenbaum SR, Ajami AM, Young VR. Plasma arginine, citrulline, and ornithine kinetics in adults, with observations on nitric oxide synthesis. Am J Physiol 1995;268:E360–7 [DOI] [PubMed] [Google Scholar]
- 29.Beaumier L, Castillo L, Ajami AM, Young VR. Urea cycle intermediate kinetics and nitrate excretion at normal and “therapeutic” intakes of arginine in humans. Am J Physiol 1995;269:E884–96 [DOI] [PubMed] [Google Scholar]
- 30.Poeze M, Bruins MJ, Luiking YC, Deutz NE. Reduced caloric intake during endotoxemia reduces arginine availability and metabolism. Am J Clin Nutr. 2010;91:992–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luiking YC, Deutz NE. Isotopic investigation of nitric oxide metabolism in disease. Curr Opin Clin Nutr Metab Care 2003;6:103–8. [DOI] [PubMed] [Google Scholar]
- 32.Luiking YC, Engelen MP, Deutz NE. Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care 2010;13:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gough MS, Morgan MA, Mack CM, Darling DC, Frasier LM, Doolin KP, Apostolakos MJ, Stewart JC, Graves BT, Arning E, et al. The ratio of arginine to dimethylarginines is reduced and predicts outcomes in patients with severe sepsis. Crit Care Med 2011;39:1351–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser M, Vermeulen MA, Richir MC, Teerlink T, Houdijk AP, Kostense PJ, Wisselink W, de Mol BA, van Leeuwen PA, Oudemans-van Straaten HM. Imbalance of arginine and asymmetric dimethylarginine is associated with markers of circulatory failure, organ failure and mortality in shock patients. Br J Nutr. 2012;107:1458–65 [DOI] [PubMed] [Google Scholar]
- 35.Preiser JC, Luiking Y, Deutz N. Arginine and sepsis: a question of the right balance? Crit Care Med. 2011;39:1569–70 [DOI] [PubMed] [Google Scholar]
- 36.Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and Nitric oxide production in disease states. Am J Physiol Endocrinol Metab. 2012. ;303:E1177–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–53 [DOI] [PubMed] [Google Scholar]
- 38.Luiking YC, Deutz NE. Exogenous arginine in sepsis. Crit Care Med. 2007;35(9 suppl):S557–63 [DOI] [PubMed] [Google Scholar]
- 39.Moore ML, Peebles RS., Jr Respiratory syncytial virus disease mechanisms implicated by human, animal model, and in vitro data facilitate vaccine strategies and new therapeutics. Pharmacol Ther. 2006;112:405–24 [DOI] [PubMed] [Google Scholar]
- 40.Gadish T, Soferman R, Merimovitch T, Fireman E, Sivan Y. Exhaled nitric oxide in acute respiratory syncytial virus bronchiolitis. Arch Pediatr Adolesc Med. 2010;164:727–31 [DOI] [PubMed] [Google Scholar]
- 41.Benson RC, Hardy KA, Morris CR. Arginase and arginine dysregulation in asthma. J Allergy (Cairo). 2011;2011:736319 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.