Abstract
Carbonic anhydrase-IX, a hypoxia-induced protein, is expressed in some renal cell carcinomas. In this study, we evaluated its expression in 366 primary and metastatic renal neoplasms with correlation to tumor type and grade.
Carbonic anhydrase-IX immunostaining was performed on one section of tumor from each case. Of the 366 cases, there were 317 primary and 42 metastatic tumors. The distribution of tumors was as follows: 308 renal cell carcinomas (186 clear cell, 52 papillary, 35 chromophobe, 20 unclassified, 15 Xp11.2 translocation), 26 oncocytomas, 2 metanephric adenomas, 1 urothelial carcinoma, 1 mixed epithelial and stromal tumor, 1 angiomyolipoma, 21 unknown and 6 with more than one tumor type.
Variable staining was seen in clear cell, papillary, unclassified and Xp11.2 translocation carcinomas. One chromophobe showed focal, weak staining. No staining was seen with other tumor types. Eleven Xp11.2 carcinomas showed focal expression and the majority (83%) of unclassified carcinomas were low expressors. High expression (>85% positive tumor cells) was observed in 71% of clear cell carcinomas compared to 3% of non-clear cell tumors. There was an association between high expression and tumor type (clear cell versus non-clear cell) when all cases were considered (p<0.01), as well as when primary (p<0.01) cases were analyzed separately. A statistically significant association between carbonic anhydrase-IX expression and grade (p<0.01) in primary clear cell carcinomas was found. The proportion of grade 1, 2, 3, and 4 primary clear cell carcinomas that expressed high carbonic anhydrase-IX was, respectively, 92%, 85%, 76% and 42%. No statistically significant association between carbonic anhydrase-IX and grade of papillary carcinomas (p=0.28) was found.
In conclusion, carbonic anhydrase-IX expression is more common in clear cell renal cell carcinoma than other renal tumor types and is associated with grade. It may be a useful marker to distinguish clear cell carcinoma from chromophobe carcinoma and oncocytoma.
Keywords: Carbonic anhydrase IX, Renal cell carcinoma, Clear cell renal cell carcinoma
Renal cell carcinomas (RCC) account for the majority (90%) of epithelial neoplasms of the kidney, and 3% and 4% of new cancer cases in women and men, respectively.1,2 Of all renal cell carcinomas, the clear cell subtype is the most common and accounts for the majority of renal cell carcinoma metastases.3 At presentation, up to 30% of patients will have metastases, and of patients who undergo nephrectomy for organ-confined disease, approximately 30-50% will later develop metastases.2, 4-6 Approximately, 13,000 patients die of the disease each year in the United States.2 The wide spectrum of histologically different tumor types and limited therapeutic options for systemic disease present distinctive challenges to physicians in terms of proper diagnosis and classification of these neoplasms and management of advanced disease.
Renal epithelial neoplasms are unique in that their morphology is highly variable, in terms of both growth pattern and cytologic features, and there is morphologic overlap amongst the tumor types. Even within individual tumor types, especially clear cell RCC and papillary RCC, the morphology of one tumor to the next can be quite different. Nevertheless, routine hematoxylin and eosin stained sections are usually sufficient to correctly classify renal neoplasms. In some circumstances, however, the proper classification of primary renal tumors and distinguishing metastatic RCC from tumors that arise elsewhere can be problematic. Further compounding the issue are core biopsies, which are not infrequently small and fragmented. These biopsies may be performed on tumors at metastatic sites or on renal tumors of patients who are not surgical candidates.
In recent years, multiple immunohistochemical markers have been studied and offered as tools to distinguish the various renal neoplasms from each other and from morphologically similar non-renal tumors.7-11 No one marker has been found to be entirely specific for RCC in general or for any specific type of RCC. Carbonic anhydrase IX (CAIX) is one such marker that shows expression in RCC.12-14 CAIX is a hypoxia-induced protein that plays a role in regulating intracellular and extracellular pH.14,15 Liao et al13 first reported expression of CAIX in clear cell RCC. Several years later, Bui et al16 found high expression of CAIX in clear cell RCC and, furthermore, reported that decreased levels of expression of CAIX were independently associated with poor outcome in advanced RCC. This latter observation has been refuted by other investigators.17 Atkins, et al, amongst others,16,18 found that CAIX shows promise as a marker for selecting patients with advanced disease who would benefit from certain specific systemic agents, specifically interleukin-2 (IL-2).
We undertook this study to assess the expression of CAIX in a variety of benign and malignant primary renal neoplasms, as well as RCC metastases. We sought to determine if CAIX could be used as an immunohistochemical marker to reliably distinguish amongst different tumor types and if its expression correlated to RCC grade.
Materials and Methods
All research involving human subjects was conducted on anonimized tissues collected from patients during the course of their therapy. This research was approved by the Dana Farber/Harvard Cancer Center (DF/HCC) and the Beth Israel Deaconess Medical Center institutional review boards. The study group consisted of primary and metastatic renal neoplasms mostly retrieved from the surgical pathology files at three institutions including (Brigham and Women’s Hospital (BWH) and the Beth Israel Deaconess Medical Center (BIDMC), Boston, MA and The Johns Hopkins Medical Institution (JH), Baltimore, MD). In addition, tumors resected at other institutions and stored at the DF/HCC Kidney Center SPORE tumor bank were also analyzed.
The classification of the tumors from BWH, BIDMC, and the DF/HCC Kidney Cancer SPORE tumor bank was recorded from review of hematoxylin and eosin-stained slides by two pathologists (EMG and SS) or from pathology reports when all slides were not available for review. The Fuhrman grading system19 was used to grade renal cell carcinomas (RCC). Fifteen cases of Xp11.2 translocation RCC were contributed by a co-investigator from JH.
CAIX immunostaining was performed on one representative section of tumor from each case in a Dako autostainer system (Dako, Carpinteria). Sections were deparaffinized, soaked in alcohol, and after microwave treatment in antigen unmasking solution for ten minutes, incubated in 3% hydrogen peroxide for fifteen minutes to inactivate endogenous peroxidase. Slides were then incubated with the mouse monoclonal antibody MN-75 (1:10,000 dilution) and detection was performed using the Dako LSAB™+ detection kit (Dako, Carpinteria). Semiquantitative assessment of the antibody staining for each slide was performed by two pathologists (EMG and SS) who were blinded to the clinicopathologic variables of each case. Each specimen was scored based on the staining intensity of the cytoplasmic membrane and the percentage of positive cells. As previously described,16,18 specimens in which >85% of tumor cells stained for CAIX were labelled as high CAIX expressing tumors, whereas those in which ≤85% of tumor cells stained for CAIX were labelled as low CAIX expressing tumors. Fisher’s exact tests were used to determine the association between CAIX expression and tumor type and Fuhrman grade. Statistical significance was set at p≤0.05.
Results
Three hundred sixty-six cases (N=366) were available for analysis. Table 1 presents the distribution of the cases by tumor type, grade, stage and CAIX staining. There were 186 (51%) cases of clear cell RCC and the remaining 180 (49%) were either non-clear cell RCC or were unclassified, unknown or more than one tumor type. The distribution of non-clear cell tumor type was as follows: 52 papillary RCC, 35 chromophobe RCC, 20 unclassified RCC, 21 unknown tumor type, 15 Xp11.2 translocation RCC, 26 oncocytomas, 2 metanephric adenomas, 1 urothelial carcinoma, 1 mixed epithelial and stromal tumor, 1 classic angiomyolipoma and 6 with more than one tumor type. There were 317 (87%) primary renal tumors and 42 (11%) metastatic RCC and 7 (2%) cases with unknown site. The distribution of the tumor grades was as follows: 13 grade 1, 96 grade 2, 80 grade 3, and 48 grade 4. Three hundred fifty-six cases had information on CAIX staining and were classified, using a cutoff score of 85%, into high (n=142 or 40%) and low (n=214 or 60%) categories.
Table 1.
Distribution of the Cases by Tumor Type, Grade, Stage and CAIX Status
| N | % | |
|---|---|---|
| Tumor Type | ||
| Clear Cell RCC | 186 | 51 |
| Othera | 180 | 49 |
| Total | 366 | 100 |
| Tumor Stage | ||
| Primary | 317 | 87 |
| Metastatic | 42 | 11 |
| Unknown | 7 | 2 |
| Total | 366 | 100 |
| Tumor Grade | ||
| 1 | 13 | 5 |
| 2 | 96 | 41 |
| 3 | 80 | 34 |
| 4 | 48 | 20 |
| Total | 237 | 100 |
| CAIX Expression | ||
| ≤85 | 214 | 60 |
| >85 | 142 | 40 |
| Total | 356 | 100 |
Other: papillary (n=52), chromophobe (n=35) , oncocytoma (n=26), unclassified (n=20), unknown (n=21), metanephric adenomas (n=2), urothelial carcinoma (n=1), mixed epithelial and stromal (n=1), angiomyolipoma (n=1), more than one tumor type (n=6), XP11.2 translocation tumors (n=15)
Variable cytoplasmic membrane immunoreactivity for CAIX was seen in clear cell RCC, papillary RCC, unclassified RCC, and Xp11.2 translocation RCC (Table 2). One hundred eighty-four (n=184) clear cell RCC demonstrated immunoreactivity for CAIX with most (71%) being high expressing tumors (i.e. >85% positive tumor cells). In contrast, high CAIX expression was seen in only 3% of non-clear cell RCC. The majority (92%) of papillary RCC also expressed some CAIX, however, they were largely low expressing tumors (i.e. ≤85% positive tumor cells) and eleven Xp11.2 translocation RCC had focal (low) expression of CAIX. Some, but not all, of the CAIX positivity in these two RCC subtypes was adjacent to areas of tumor necrosis. One chromophobe RCC showed focal weak staining. While the majority (83%) of unclassified RCC expressed low levels of CAIX, three tumors did demonstrate high levels of CAIX. CAIX staining was not seen with any other tumor type. The distribution of CAIX positive staining was most often diffuse in clear cell RCC compared to the other tumor types that expressed CAIX for which the staining was focal or patchy.
Table 2.
High Versus Low Expression of CAIX in Clear Cell and Non-Clear Cell Renal Neoplasms – Primary and Metastatic Tumors
| Tumor Type | High Expressors (%)a | Low Expressors (%)b |
|---|---|---|
| Clear cell RCCc (n=184) | 131 (71.2) | 53 (28.8) |
| Papillary RCC (n=51) | 4 (8) | 47 (92) |
| Chromophobe RCC (n=35) | 0 (0) | 35 (100) |
| Unclassified RCC (n=18)d | 3 (16.7) | 15 (83.3) |
| Xp11.2 translocation tumor (n=15) | 0 (0) | 15 (100) |
| Other tumor type (n=31)e | 0 (0) | 31 (100) |
High (>85%) expressing tumors
Low (≤ 85%) expressing tumors
RCC, renal cell carcinoma
Unclassified RCC not included in statistical analysis as nature of tumor (clear cell versus non-clear cell) could not be determined
Oncocytomas, metanephric adenomas, urothelial carcinoma, mixed epithelial and stromal tumor, classic angiomyolipoma.
Table 3 presents the distribution of tumor types by CAIX expression categories. A statistically significant correlation between CAIX positivity (high versus low) and tumor type (clear cell RCC versus non-clear cell tumors) was found when all cases were analyzed (p<0.01). Moreover, a significant association was found between CAIX expression and tumor type when the primary tumors were analyzed separately (p<0.01). However, the association between CAIX expression and tumor types did not reach statistical significance when the metastatic cases were analyzed separately (p=1.00)
Table 3.
CAIX Expression in Metastatic and Primary Clear Cell and Non-Clear Cell Carcinomas
| CAIX Expressions (%) |
|||
|---|---|---|---|
| Tumor Type | Higha | Lowb | P-valuec |
|
| |||
| All | <0.001 | ||
| Clear Cell RCCd (n=184) | 131 (71%) | 53 (29) | |
| Non Clear Cell Tumorse (n=132) | 4 (3%) | 128 (97%) | |
|
| |||
| Primary | <0.001 | ||
|
| |||
| Clear Cell RCC (n=158) | 112 (71%) | 46 (29%) | |
|
| |||
| Non Clear Cell RCC (n=131) | 3(2%) | 128 (98%) | |
|
| |||
| Metastatic | 1.00 | ||
|
| |||
| Clear Cell RCC (n=26) | 19 (73%) | 7 (27%) | |
|
| |||
| Non Clear Cell RCC (n=1) | 1 (100%) | 0(0%) | |
High (>85%) expressing tumors
Low (≤ 85%) expressing tumors
Fisher’s exact test p-value.
RCC, renal cell carcinoma
Non-clear cell tumors: papillary, chromophobe , oncocytoma, metanephric adenomas, urothelial carcinoma , mixed epithelial, stromal angiomyolipoma and Xp11.2 translocation tumor
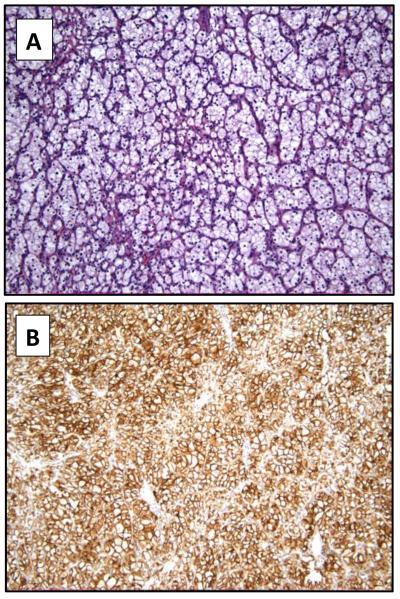
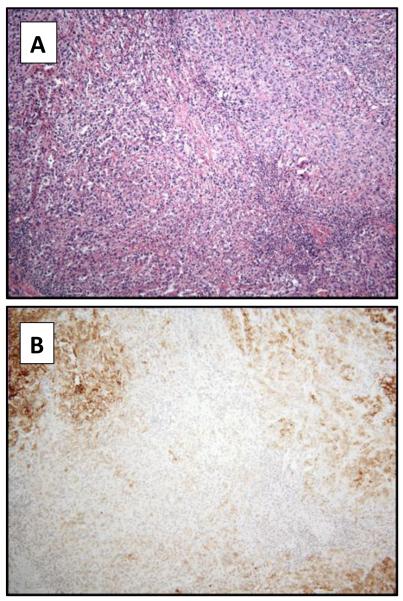
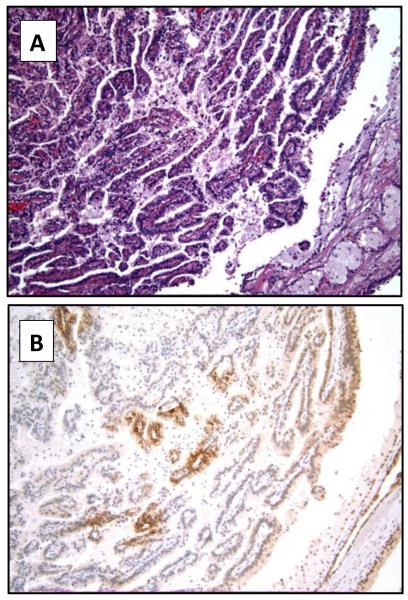
Table 4 presents the association between CAIX expression and tumor grade for primary clear cell and primary papillary RCC. There was a significant association between CAIX expression levels and grade of primary clear cell RCC (p<0.01) with high CAIX expression in the lower grades (Figures 1 and 2). However, no significant correlation was found between CAIX expression and grade of papillary RCC (p=0.28) (Figure 3).
Table 4.
Association of CAIX Expression with Tumor Grade of Primary Clear Cell and Papillary Renal Cell Carcinoma
| CAIX Expressiona | Fuhrman Grade | P-valueb | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Primary Clear Cell RCCc | <0.001 | ||||
| ≤85% (low) | 1 (8%) | 10 (15%) | 10 (24%) | 21 (58%0 | |
| >85% (high) | 11 (92%) | 55 (85%) | 31 (76%) | 15 (42%) | |
| Primary Papillary RCC | 0.28 | ||||
| ≤85% (low) | - | 18 (95%) | 25 (96%) | 3 (75%) | |
| >85% (high) | - | 1 (5%) | 1 (4%) | 1 (25% | |
Percentage of tumor cells positive for CAIX
Fisher’s exact test p-value.
RCC, renal cell carcinoma
Figure 1.
Example of a low-grade clear cell renal cell carcinoma predominantly constituted by cells with clear cytoplasm (hematoxylin and eosin 10x) (A). CAIX expression is detected in the vast majority of tumor cells (10x) (B).
Figure 2.
Example of a high-grade clear cell renal cell carcinoma predominantly constituted by cells with eosinophilic cytoplasm (hematoxylin and eosin 4x) (A). CAIX expression is detected in a small percentage of tumor cells (4x) (B).
Figure 3.
Example of a papillary renal cell carcinoma (hematoxylin and eosin 10x) (A), with focal expression of CAIX (10x) (B).
Discussion
The lack of immunohistochemical markers with fairly high specificity for RCC in general, as well as its specific subtypes, and the lack of effective treatment for systemic disease continue to be diagnostic and therapeutic issues. Renal cell carcinomas as a group are unique in that the morphologies of the tumors are highly variable, and cytogenetic studies have found that many of the tumors have distinctive profiles.20 As such, it would seem improbable that one marker could be used to define all types of RCC. Similarly, finding one therapeutic agent that can target all varieties of RCC seems unlikely. As such, as biomarkers for the various RCC are identified and new classifications of RCC are proposed, it becomes even more crucial to properly classify these tumors.
In most circumstances, the distinction of one renal epithelial neoplasm from another can be accomplished with routine hematoxylin and eosin stained sections. However, even with primary renal neoplasms, there is some morphologic overlap amongst tumor types, and undifferentiated tumors can be altogether impossible to properly classify. Metastatic tumors may also be diagnostically problematic, particularly high grade clear cell RCC, in that the tumors sometimes need to be distinguished from other morphologically similar tumors that are of extra-renal origin. Core biopsies further compound the diagnostic dilemma, as they are frequently small, fragmented, and only sample a small area of a tumor.
Multiple immunohistochemical markers, including EMA, vimentin, C-kit (CD 117), CK 7, CD 10, RCC, TFE-3, p504S (racemase), peanut lectin, ulex lectin, and PAX-2 have been identified as markers to assist in classifying various benign and malignant renal neoplasms.7-11, 20 While no one marker is typically used alone to define a neoplasm, when used in combination as a panel of antibodies, they can often help in classifying diagnostically challenging neoplasms. CAIX, a hypoxia-induced protein, is another marker with reported expression in RCC, predominantly in the clear cell type.12-14 Indeed, most (approximately 60-80%) clear cell RCC cases are characterized by biallelic inactivation of the Von Hippel-Lindau gene, which leads to stabilization of the alpha subunit of Hypoxia Inducible Factor and subsequent induction of various target genes, including CAIX.21,22 Importantly, it has also been shown that CAIX may not only have diagnostic utility but may play a role in the treatment of patients with advanced metastatic disease and be a predictor of outcome.16,18 Several published reports have looked at the expression of CAIX principally in clear cell RCC12, 14, 16 with a few more recent reports describing CAIX immunoreactivity in different types of renal neoplasms.11,13,17
In this study, we evaluated the expression of CAIX in 366 primary and metastatic renal tumors and correlated it with tumor type and grade (of RCC). Biomarker expression can be affected not only by the antibody utilized but also by the area of tumor sampled. Given the variable morphology that can be present within any individual renal neoplasm, expression of molecular markers could also potentially be variable throughout a tumor. The smaller the piece of tissue used for analysis, such as core biopsies or tissue microarrays, the less likely a truly representative section of tumor will be studied. Ideally, although not practical, several areas of a tumor could be sampled. In an effort to evaluate more representative tumor samples, one complete routine section of tumor was available for assessing CAIX expression for all but 9 cases in the current study.
We found that clear cell RCC, papillary RCC, unclassified RCC and Xp11.2 translocation RCC all had some degree of cytoplasmic membrane immunoreactivity for CAIX; however, clear cell RCC more often and more consistently demonstrated high (>85%) expression than any other tumor type. For clear cell RCC, the association with high CAIX expression was not only limited to the tumor type but also correlated to the grade of the neoplasm. As the grade of clear cell RCC increases, the expression of CAIX decreases.
Papillary RCC and Xp11.2 translocation RCC were similar in that immunoreactivity for CAIX, when present, tended to be focal or patchy with overall low expression levels (≤85%). Only one chromophobe RCC demonstrated focal positivity for CAIX but the staining intensity was very weak. Our results for clear cell RCC, papillary RCC, chromophobe RCC, and oncocytomas appear to be similar to those of a recently published study by Gupta et al11 who also evaluated the expression of CAIX in a variety of renal tumors. We found a higher percentage of Xp11.2 translocation RCC had focal or patchy immunoreactivity for CAIX than the study by Gupta et al,11 however, the level of expression was low. The majority of unclassified tumors in our study did not express CAIX, although, three cases showed high expression.
As CAIX is not specific for renal malignancies, having also been found in carcinomas of the lung, breast, cervix, uterus, colon and esophagus, it is not useful, as a solitary marker, for determining site of tumor origin.14,15,22-24 In routine tissue sections, CAIX would be useful in distinguishing clear cell RCC from chromophobe RCC and oncocytoma. CAIX in combination with other immunohistochemical markers, including CK 7, p504s (racemase), and 34βE12, could assist in distinguishing between clear cell RCC and papillary RCC in routine sections, given that CAIX expression in papillary RCC is not as diffuse as in clear cell RCC. However, with core biopsies, this distinction may be more difficult to make because the CAIX positivity in a core biopsy of papillary RCC may appear diffuse. While CAIX reactivity occurs in Xp11.2 translocation RCC, these tumors appear to be low expressors of CAIX and, furthermore, typically do not have diffuse reactivity with EMA or cytokeratin, which allows distinction from clear cell and papillary RCC. These translocation RCC do show reactivity with TFE-3 25; the problem with this latter marker is that it is technically difficult to perform and the results may be difficult to interpret in suboptimally fixed tissue. In the series of Gupta et al 11, they found strong staining in the majority of urothelial carcinomas; the number of urothelial carcinomas in our study is insufficient for comparison. Nevertheless, based on their results, CAIX is not useful for distinguishing clear cell RCC from urothelial carcinoma.
The need to classify renal tumors as accurately as possible is important for several reasons. One of the most important reasons is that the biologic behavior of RCC is variable from one subtype to another, and, therefore, how a tumor is classified will provide information regarding the patient’s potential clinical course.26 Furthermore, as new classifications of RCC are proposed and markers for RCC, in general or its subtypes, are found, the particular subtype that is diagnosed may determine what therapy the patient will receive. CAIX is one such biomarker that is highly expressed in clear cell RCC and potentially may be used to guide patient treatment.
Currently, the mainstay of therapy for primary RCC is surgery. For patients with metastatic disease, treatment with targeted agents has recently shown some success but, unfortunately, not all tumors respond and responses are not durable. To date, the only therapy for advanced RCC that can result in long-term disease free survival is high-dose IL-2.18,27 Unfortunately, long-lasting responses are very rare and high dose IL-2 causes significant side effects.18,27 More recently, interest has been generated in selecting specific patients who would benefit from IL-2 treatment.
Bui et al16 reported that high CAIX staining was associated with a better prognosis for patients who presented with metastatic disease, and those patients with localized high stage, high grade disease had better survival than similar patients with low CAIX staining. The authors concluded that CAIX expression was independently associated with prognosis in advanced RCC. Furthermore, they found that among those patients with metastases who received IL-2, the response rate to IL-2 therapy was higher (27%) in patients with the CAIX high expressing tumors than in patients with the CAIX low expressing tumors (14%). In a case-control study by Atkins et al 18, patient response to IL-2 was also found to be associated with CAIX expression. Specifically, high CAIX expression in tumors was higher in the IL-2 responding patients (78%) than in the IL-2 non-responding patients (51%), and those patients with high CAIX expressing tumors had better survival than those with low expressing tumors. Our study supports other observations that high CAIX expression is much more common in clear cell RCC. Furthermore, we found that CAIX expression was associated with the grade of clear cell RCC. While an analysis of our subset of patients is needed in terms of their response to IL-2, our data suggest that, as a group, patients with lower grade clear cell RCC might respond better to IL-2 than patients with higher grade tumors. The high expression of CAIX in clear cell RCC also raises the possibility that CAIX-targeted therapy could be developed against these tumors. However, given that CAIX is found in some nonneoplastic tissues as well 17, careful controlled studies would be needed.
In summary, CAIX is most often expressed and typically highly expressed in clear cell RCC compared with other RCC subtypes. This marker appears to be diagnostically useful in distinguishing clear cell RCC from chromophobe RCC and oncocytoma and potentially useful, when combined with other markers, in distinguishing clear cell RCC from papillary RCC. CAIX is expressed in both high grade clear cell RCC and urothelial carcinoma and, therefore, is not useful for distinguishing between these tumors. Given that CAIX is expressed in other epithelial malignancies, it is not useful for determining a tumor’s site of origin.
Acknowledgements
This work was supported in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE NCI P50CA101942.
List of abbreviations
- BIDMC
Beth Israel Deaconess Medical Center
- BWH
Brigham and Women’s Hospital
- DF/HCC
Dana Farber/Harvard Cancer Center
- JH
The Johns Hopkins Medical Institution
- CAIX
Carbonic anhydrase IX
- IL-2
Interleukin-2
- RCC
Renal cell carcinoma
Footnotes
Disclosure/Conflict of Interest The authors declare no conflict of interest
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics 2008. CA Cancer J Clin. 2008;58:71–97. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. IARC Press; Lyons: 2004. World Health Organization Classification of Tumors. [Google Scholar]
- 3.Reuter VE. The pathology of renal epithelial neoplasms. Semin Oncol. 2000;33:534–543. doi: 10.1053/j.seminoncol.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Figlin RA. Renal cell carcinoma: management of advanced disease. J Urol. 1999;161:381–386. doi: 10.1016/s0022-5347(01)61897-4. [DOI] [PubMed] [Google Scholar]
- 5.Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30:843–852. doi: 10.1016/s0094-0143(03)00056-9. [DOI] [PubMed] [Google Scholar]
- 6.Lam JS, Pantuck AJ, Bellegrun AS, et al. G250: A carbonic anhydrase IX monoclonal antibody. Curr Oncol Rep. 2005;7:109–115. doi: 10.1007/s11912-005-0036-7. [DOI] [PubMed] [Google Scholar]
- 7.Skinnider BF, Amin MB. An immunohistochemical approach to the differential diagnosis of renal tumors. Semin Diagn Pathol. 2005;22:51–68. doi: 10.1053/j.semdp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhou M, Roma A, Magi-Galluzzi C. The usefulness of immunohistochemical markers in the differential diagnosis of renal neoplasms. Clin Lab Med. 2005;25:247–257. doi: 10.1016/j.cll.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang HY, Mills SF. KIT and RCC are useful in distinguishing chromophobe renal cell carcinoma from the granular variant of clear cell renal cell carcinoma. Am J Surg Pathol. 2005;29:640–646. doi: 10.1097/01.pas.0000157943.33903.92. [DOI] [PubMed] [Google Scholar]
- 10.Memeo L, Jhang J, Assaad AM, et al. Immunohistochemical analysis for cytokeratin 7, KIT, and PAX2: value in the differential diagnosis of chromophobe renal cell carcinoma. Am J Clin Pathol. 2007;127:225–229. doi: 10.1309/9KWEA4W9Y94D1AEE. [DOI] [PubMed] [Google Scholar]
- 11.Gupta R, Balzer B, Picken M, et al. Diagnostic implications of transcription factor PAX2 protein and transmembrane enzyme complex carbonic anhydrase IX immunoreactivity in adult renal epithelial neoplasms. Am J Surg Pathol. 2009;33:241–247. doi: 10.1097/PAS.0b013e318181b828. [DOI] [PubMed] [Google Scholar]
- 12.Oosterwijk E, Ruiter OJ, Hoedemaeker PJ, et al. Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer. 1986;38:489–494. doi: 10.1002/ijc.2910380406. [DOI] [PubMed] [Google Scholar]
- 13.Liao SY, Aurelio ON, Jan K, et al. Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res. 1997;57:2827–2831. [PubMed] [Google Scholar]
- 14.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potter CP, Harris AL. Diagnostic, prognostic and therapeutic implications of carbonic anhydrases in cancer. Br J Cancer. 2003;89:2–7. doi: 10.1038/sj.bjc.6600936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bui MHT, Seligson D, Han K-r, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 17.Leibovch BC, Sheinin Y, Lohse CM, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25:4757–4764. doi: 10.1200/JCO.2007.12.1087. [DOI] [PubMed] [Google Scholar]
- 18.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Reuter VE, Tickoo SK. Adult renal tumors. In: Mills SE, Carter D, Greenson JK, Oberman HA, Reuter V, Stoler MH, editors. Sternberg’s Diagnostic Surgical Pathology. Fourth edition Lippincott Williams & Wilkins; Philadelphia, PA: 2004. pp. 1955–1999. [Google Scholar]
- 21.Linehan WM, Lerman MI, Zbar B. Identification of the von Hippel-Lindau (VHL) gene. Its role in renal disease. JAMA. 1995;273:564–570. [PubMed] [Google Scholar]
- 22.Kaelin WG., Jr The von Hippel-Lindau tumor suppressor gene and kidney cancer. Clin Cancer Res. 2004;10:6290–6295. doi: 10.1158/1078-0432.CCR-sup-040025. [DOI] [PubMed] [Google Scholar]
- 23.Leppilampi M, Saarnio J, Karttunen TJ, et al. Carbonic anhydrase isozymes IX and XII in gastric tumors. World J Gastroenterol. 2003;9:1398–1403. doi: 10.3748/wjg.v9.i7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynninen P, Vaskivuo L, Saarnio J, et al. Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumors. Histopathology. 2006;49:594–602. doi: 10.1111/j.1365-2559.2006.02523.x. [DOI] [PubMed] [Google Scholar]
- 25.Argani P, Lal P, Hutchinson B, et al. Aberrant nuclear immunoreactivity for TFE3 in neoplasm with TFE3 gene fusions: a sensitive and specific immunohistochemical assay. Am J Surg Pathol. 2003;27:750761. doi: 10.1097/00000478-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Amin MB, Tamboli P, Javidan J, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–291. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Atkins MB. Treatment selection for patients with metastatic renal cell carcinoma: identification of features favoring upfront IL-2-based immunotherapy. Med Oncol. 2009;26:S18–S22. doi: 10.1007/s12032-008-9148-x. [DOI] [PubMed] [Google Scholar]