Summary
In human vitiligo, cutaneous depigmentation involves cytotoxic activity of autoreactive T cells. It was hypothesized that depigmentation can progress in the absence of regulatory T cells (Treg). The percentage of Treg among skin infiltrating T cells was evaluated by immunoenzymatic double staining for CD3 and FoxP3, revealing drastically reduced numbers of Treg in non-lesional, perilesional and lesional vitiligo skin. Assessment of the circulating Treg pool by FACS analysis of CD4, CD25, CD127 and FoxP3 expression, and mixed lymphocyte reactions in presence and absence of sorted Treg revealed no systemic drop in the abundance or activity of Treg in vitiligo patients. Expression of skin homing receptors CCR4, CCR5, CCR8 and CLA was comparable among circulating vitiligo and control Treg. Treg from either source were equally capable of migrating towards CCR4 ligand and skin homing chemokine CCL22, yet significantly reduced expression of CCL22 in vitiligo skin observed by immunohistochemistry may explain failure of circulating, functional Treg to home to the skin in vitiligo. The paucity of Treg in vitiligo skin is likely crucial for perpetual anti-melanocyte reactivity in progressive disease.
Keywords: autoimmune, T cells, tolerance, depigmentation
Introduction
Vitiligo is characterized by progressive loss of skin pigmentation. This autoimmune disorder strikes approximately 0.5–1% of the world population (Taieb et al., 2007). Depigmentation involves progressive loss of melanocytes from the basal layer of the epidermis (Le Poole et al., 1993). Moreover, depigmentation is associated with inflammatory infiltrates of T cells and macrophages (Van den Wijngaard et al., 2000). In progressive disease, the CD4/CD8 ratio is decreased among skin-infiltrating T cells and CD8+ T cells isolated from vitiligo skin are cytotoxic to melanocytes (Wankowicz-Kalinska et al., 2003). An increased number of Melanoma Antigen Recognized by T cells (MART-1) reactive T cells has been reported among peripheral T cells from patients with active disease, and MART-1 reactivity as well as gp100 reactivity have likewise been demonstrated among skin-infiltrating T cells (Ogg et al., 1998; Oyarbide-Valencia et al., 2006; Wankowicz-Kalinska et al., 2003).
MART-1 and gp100 were first identified as target antigens for T cells infiltrating melanoma tumors. This raised the intriguing question, why the autoimmune response to melanocytes effectively eliminates melanocytes from vitiligo skin whereas T cells infiltrating melanomas fail to clear the tumor. In the past we have proposed that a failure to suppress an ongoing immune response to self antigens may contribute to progressive depigmentation of the skin in vitiligo patients (Das et al., 2001). Regulatory T cells are known to inhibit autoreactivity, explaining why autoreactive T cells are present in the circulation in the absence of autoimmune symptoms (Baecher-Allen and Hafler, 2006). While the mechanism of action for Treg is still not fully understood, TGF-β and IL-10 contribute to Treg mediated immunosuppression. TGF-β is important for imposing a regulatory phenotype to the Treg subset and regulatory activity is dependent on cell-cell contact (Bala and Moudgil, 2006; Joetham et al., 2007; Zhu and Paul, 2008). Markers expressed by Treg include FoxP3, GITR, CTLA-4 and CD25, yet only FoxP3 expression is relatively unique to regulatory T cells (De Boer et al., 2007). This transcription factor affects the expression of many genes (Zheng and Rudensky, 2007), and mutations in FoxP3 can cause severe autoimmune disease as in IPEX (human) and scurfy mice, supporting the importance of Treg to keep autoreactive T cells in check (Lahl et al., 2007). An abundance of Treg in tumor tissues is thought to be the root cause of failing attempts to boost anti-tumor immunity by powerful vaccines, and inclusion of FoxP3 as a target antigen in vaccines was shown to boost anti-tumor immunity (Loddenkemper et al., 2009; Nair et al., 2007). This holds true in particular for melanoma, where self antigens MART-1 and gp100 are among the most immunogenic antigens targeted by the host anti-tumor response (Chakraborty et al., 2004). Among circulating Treg, a large proportion is set to home to the skin (Hirahara et al., 2006). Chemokine CCL1 and its receptor CCR8 are involved in the chemoattractive process that guides Treg to the skin, as are the combinations of CCL5 and CCR5 and of CCL22, CCL17 and CCR4 (Colantonio et al., 2002; Hirahara et al., 2006). The cutaneous lymphocyte antigen (CLA) is a ligand for selectin-like molecules PCAM and ECAM on the endothelial cell surface in a process that determines skin homing of lymphocytes including Treg (Iellem et al., 2003). We have previously reported that CLA is abundantly expressed by CD4+ and CD8+ T cells that infiltrate vitiligo skin (Van den Wijngaard et al., 2000).
Recent advances made in understanding the contribution of regulatory T cells to keep autoimmune responses in check have provided incentive for the current study on the abundance, location and activity of regulatory T cells in vitiligo. Immuno double staining was performed to detect and quantify FoxP3 expressing T cells infiltrating non-lesional, perilesional and lesional vitiligo skin, comparing Treg abundance in diseased and normal control skin. Although vitiligo manifests itself in the skin, an important part of the etiology is defined elsewhere. Failure to clonally delete T cells with high affinity, melanocyte reactive TCR for example, occurs in the thymus (Van den Boorn et al., 2006). Therefore, besides including patient skin samples, the current studies describe skin homing and T cells migrating to skin from the circulation. In lupus eythematosus for example, an intrinsic migratory defect may account for reduced Treg in affected tissues (Lee et al., 2008). Multiple staining procedures were performed to accurately quantify Treg by FACS analysis and to assess their expression of skin homing receptors. Proliferation of CD4+/CD25− cells in the presence of allogeneic dendritic cells, with or without added CD4+/CD25+ Treg maintained with CD3+/CD28+ beads and high dose IL-2 were used to quantify inhibition of proliferation by CD4+/ CD25+ T cells. Immunohistochemistry was followed by image analysis to compare expression of chemoattractants CCL1, CCL17 and CCL22 in vitiligo and control skin. Migration in response to differentially expressed chemokine was compared for control and vitiligo Treg. These studies provide important novel insight into the unique circumstances that allow for progressive cytotoxic activity towards melanocytes by CD8+ T cells in progressive vitiligo.
Results
Paucity of Treg in vitiligo skin tissue samples
Frozen skin sections from 3 mm biopsies double stained for expression of CD3 and FoxP3 were used to evaluate the abundance of Treg in human skin. Treg frequency among infiltrating pan T cells was compared for normal neonatal (n = 5) and adult skin (n = 5), lentigo maligna samples (n = 4) and non-lesional, perilesional, and lesional vitiligo skin (n ≥ 7 each). Cells were quantified in multiple sections of each sample and the Treg fraction among T cells was determined as shown in Figure 1. A significantly reduced percentage of Treg among infiltrating T cells was noted for non-lesional (2.6 ± 3.5%) as well as perilesional (2.0 ± 1.6%) and lesional vitiligo skin (7.3 ± 13.9%) as compared to normal skin from unaffected adults with fluctuating Treg content (46.2 ± 37.8%). The abundance of Treg was not significantly altered when comparing adult to neonatal skin (42.6 ± 16.2%), or adult skin to lentigo maligna samples (57.8 ± 42.4%). In the latter, the overall number of infiltrating T cells was elevated, with similar frequencies of Treg among them. In vitiligo perilesional skin, where the absolute numbers of T cells are increased relative to normal control skin, this increase was not accompanied by a relative increase in Treg.
Figure 1.
Paucity of Treg in vitiligo skin. Treg abundance quantified. (A) Immunostaining of all T cells (blue) and Tregs (red) was light microscopically evaluated by two investigators. (B) The percent Treg among T cells was calculated for 4–8 samples in each group, looking for significant differences to the % Treg found in adult normal skin by Student’s T test, with **P < 0.01, and *P < 0.05.
Circulating Treg quantified
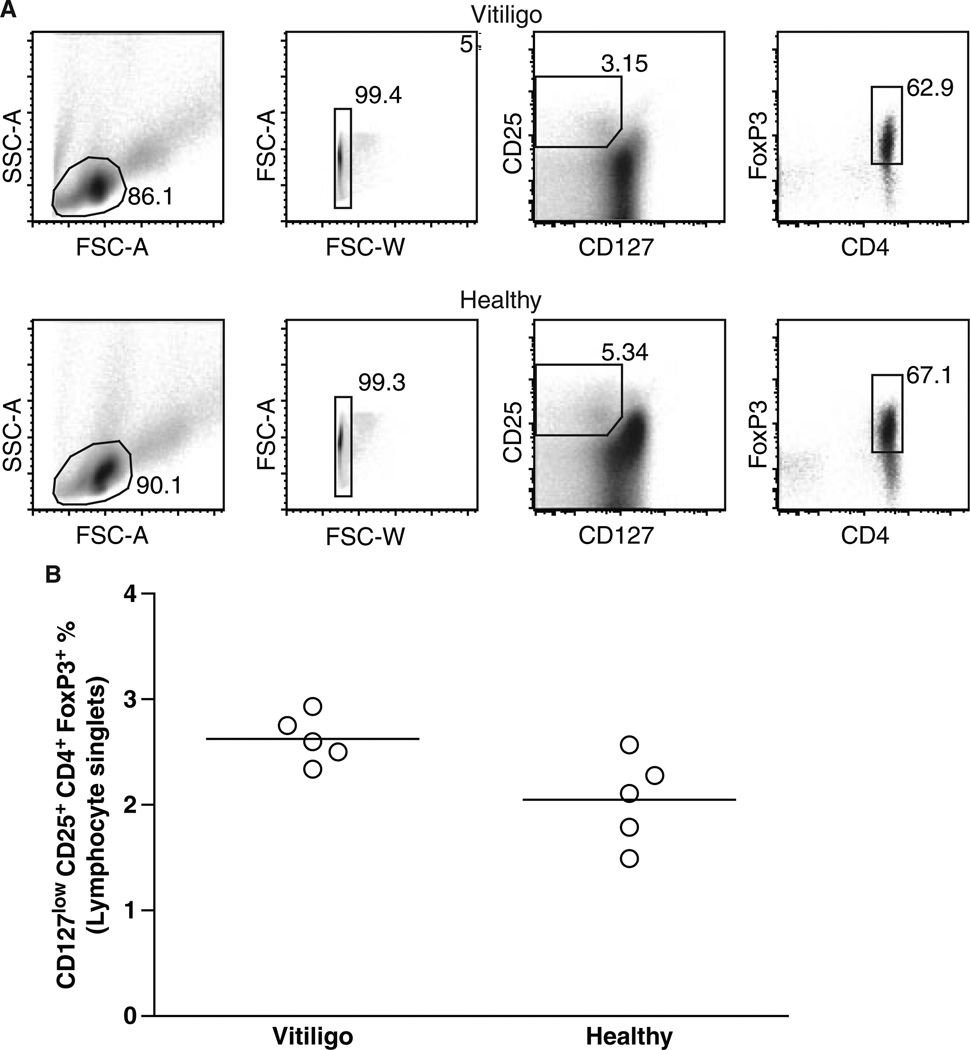
The data presented in Figure 1 initially suggested that Treg may be sparse in vitiligo patients per se, as a reduced frequency was observed not only in involved, but also in unaffected skin. Thus circulating Treg were stained using a combination of surface markers including CD4, and CD25 expression as well as reduced expression of CD127, followed by FoxP3 immunostaining of permeabilized lymphocytes. The data are illustrated in Figure 2(A), and quantified in Figure 2(B), respectively. A trend towards increased expression of CD25 and FoxP3 and decreased expression of CD127 among CD3+CD4+ T cells in patient blood was sometimes observed. These data support the concept that the abundance of circulating Treg is not reduced in vitiligo.
Figure 2.
Abundance of circulating Treg quantified by FACS analysis. (A) Representative FACS plots for a vitiligo and a control blood sample, with CD4+CD25+CD127low and FoxP3+ used to identify Treg. (B) The average percentage of Treg among lymphocytes was quantified and compared among control and vitiligo samples. According to these data, the number of Treg circulating in patients does not support a systemic defect in Treg in vitiligo.
Treg function among PBMC derived cells
The abundance of circulating Treg in vitiligo patients prompted an investigation of regulatory function. Data in Figure 3 represent the % inhibition of proliferation assessed for CD4+CD25+ cells sorted by FACS in a representative experiment among three performed, with sorted Treg stimulated by high dose IL-2 and CD3/CD28 coated beads and subsequently recombined with the CD25− fraction of CD4+ cells in presence of allogeneic DC. The data demonstrating similar suppression of proliferation among CD4 T cells by sorted Treg from patient or control PBMC indicate that there was no demonstrable functional impairment among circulating peripheral Treg in vitiligo patients. Thus, Treg with a functional suppressor profile circulate in vitiligo patients, but these Treg are not found in the skin.
Figure 3.
Inhibition of T cell proliferation quantified. Proliferation of CD4+CD25− lymphocytes from patients and controls was quantified by 3H-thymidine incorporation in the presence and absence of the CD25+ subpopulation of CD4+ cells and allogeneic DC to stimulate mixed lymphocyte responses, showing that circulating Treg from vitiligo patients (n = 3) are equally capable of immunosuppression as Treg from control individuals (n = 3). The data further support that Treg from vitiligo patients do not display a systemic defect to explain their paucity in vitiligo skin.
Differential expression of skin homing receptors
An apparent discrepancy between abundant Treg present in the circulation of vitiligo patients, whereas such Treg are not found in the skin may be explained by a failure of Treg to migrate into the skin. To differentiate circulating Treg destined to home to the skin, FACS staining performed included analysis of CLA expression, as well as expression of CCR4, CCR5 and CCR8 among Treg from control and patient samples. Since the % Treg observed in skin was reduced regardless of depig-mentation status as shown in Figure 1, no differentiation was made between patients with active or stationary disease for these experiments. The data represented in Figure 4, show gating strategies (A) and summarized data (B) representing expression of CCR4, CCR5, CCR8 and CLA among Treg in vitiligo patients and controls (n ≥ 7 as shown). The data demonstrate that expression of receptors previously shown to dictate homing of T cells and specifically Treg to the skin compartment is similar among the patient and control populations, although a slight trend towards increased expression of CCR4 (at 51% of control versus 41% of vitiligo Treg) as well as CCR5 (at 15% of control versus 10% of vitiligo Treg) is present. CLA expression in control and vitiligo skin in double stainings with FoxP3 revealed that cells in vitiligo skin expressing the skin homing receptor are not Treg (not shown).
Figure 4.
Skin homing marker expression among circulating Treg analyzed by FACS. (A) Representative control subject plots show sequential gating strategies for identifying Treg, as well as CD4+FoxP3− and CD4−FoxP3− subsets among CD3+ enriched lymphocytes. These subsets were analyzed for expression of CCR4, CCR5, CCR8 and CLA. Summarized data (B) show a similar percentage of skin homing marker expression among patient and control Treg, and CD4−FoxP3− populations whereas CCR8 expression among non-Treg T cells was significantly higher in vitiligo subjects (not shown). The MFI representing CCR4, CCR5, CCR8 and CLA expression was similar among patient and control Treg.
Differential chemoattraction of Treg populations to the skin in vitiligo
To further investigate whether reduced expression of chemoattractants may have contributed to the paucity of Treg within vitiligo patient skin, experiments were focused on expression of CCL1, CCL17 and CCL22. Expression was assessed by immunostaining for these chemokines in n ≥ 7 samples of control and vitiligo skin each. CCL1 (average 1120 expressing cells/mm2 for all samples) and CCL17 (average 550 expressing cells/mm2 for all samples) expression were similar among immunostained sections of control and vitiligo skin (not shown), yet CCL22 expression (in expressing cells/mm2) was markedly reduced by 43% among vitiligo samples compared to control skin as exemplified in Figure 5(A, B) and quantified in (C), suggesting that Tregs may not be as enticed to extravasate and migrate towards the skin by the chemokine in vitiligo. Vitiligo and control Treg responded equally well to chemoattractant CCL22 in migration assays, as shown in Figure 5(D), suggesting a reduction in the number of CCL22 expressing cells within patient skin is primarily responsible for impaired immigration.
Figure 5.
Skin homing chemokine CCL22 is markedly reduced in vitligo patient skin. Representative images are given for control (A) and non-lesional vitiligo (B) frozen skin immunostained for CCL22. (C) The data reveal a significantly reduced abundance of CCL22 expressing non-lesional and intra-lesional vitiligo skin (P < 0.05). Treg migration in response to CCL22 was also determined in control and vitiligo samples (D). Treg abundance among migrated CD4+ lymphocytes was measured in the absence (spontaneous migration) or presence of CCR4 ligand CCL22. Treg migration data were summarized showing no difference in the % Treg responding to CCL22 among control and vitiligo CD4+ lymphocytes.
Discussion
The presence of regulatory T cells serves to prevent autoimmune reactivity by T cells that express receptors reactive with self-peptides (Danese and Rutella, 2007). Since many anti-tumor vaccines are aimed at boosting reactivity to self-peptides, the regulatory subpopulation of T cells can interfere with anti-tumor efficacy. In fact, depletion of regulatory T cells has been suggested as a means to enhance anti-tumor responses while increasing risk for the development of autoimmunity as demonstrated in human patients and associated mouse models (Dannull et al., 2005; Jacob et al., 2009; Mahnke et al., 2007). In mice vaccinated against melanoma differentiation antigens including gp100 and MART-1, it appears that Treg depletion is accompanied by depigmentation of the pelage (Sutmuller et al., 2001). These data support the concept that in the absence of Treg, T cells reactive to melanocyte differentiation antigens demonstrate an unbridled response to melanocytes.
Research from several groups supports the involvement of autoreactive, cytotoxic T cells in progressive depigmentation of human skin, and cytotoxic T lymphocyte (CTL) activity can induce depigmentation in a mouse model of human vitiligo (Das et al., 2001; Denman et al., 2008). The fact that melanocyte reactive CD8+ T cells are also found in control individuals without vitiligo suggests that autoimmune reactivity is kept in check in the periphery, and that this checkpoint is defective in vitiligo (Visseren et al., 1995). The current data support this hypothesis.
The number of transcription factor FoxP3 expressing T cells was significantly reduced in the skin of vitiligo patients. Such reduction was observed not only in perilesional skin (the site where regulatory activity is needed to suppress the activity of helper and cytotoxic T cells that are actively contributing to depigmentation) but also in non-lesional and in lesional skin. By contrast, the percentage of Treg in lentigo maligna lesions was comparable to control skin. In both lentigo-affected and adult control samples, the percentage of infiltrating Treg was highly variable as can be expected in response to diverse environmental conditions. Depending on the purpose of the study, publications frequently report on the percentage of Treg among CD4 cells instead. Also, different antibodies with reduced sensitivity to FoxP3 may be used. In studies where authors are seeking to identify an increase in Treg abundance, the use of lower avidity antibodies can be fortuitous for that purpose. To allow for a direct data comparison, we have chosen to use the same antibodies in separate studies (see Le Poole et al., 2008). Markedly different percentages of Treg among skin T cells in vitiligo patient and control skin were further supported in a pilot experiment to compare the abundance of Treg among lymphocytes emigrating from skin tissue samples on fibronectin over the course of 2 weeks, at 15.9% from vitiligo and 83.3% from control skin. As CD8+ T cells abundantly infiltrate lentigo maligna samples yet the proportion of Treg is maintained, the marked reduction in the proportion of Treg within vitiligo skin cannot simply be explained by a relative increase in the influx of effector T cells (Le Poole et al., 2002). We have previously reported an influx of (IFN-γ producing) T cells in lentigo maligna, and the absolute number of infiltrating effector cells is generally greater in lentigo maligna than in vitiligo (Le Poole et al., 2002). Such a difference in the number of effector cells between lentigo maligna and vitiligo is perhaps not surprising, since the concentration of target cells for the immune response is greater in lentigo maligna than in vitiligo. In a preliminary experiment (unpublished), quantitative PCR data from perilesional skin of a progressive vitiligo patient compared to normal control skin suggest that reduced infiltration by Treg is accompanied by a 42-fold increase in IFN-γ transcript abundance and a 10% decrease in IL-10 transcript abundance (β-actin transcript abundance serving as the internal reference), favoring a proinflam-matory versus an immunosuppressive environment. Since an influx of effector T cells in vitiligo is not accompanied by an influx of Treg and in fact, resident Treg are reduced in non-lesional (and lesional) vitiligo skin, these data suggest that an ongoing immune response to self antigens as observed in generalized vitiligo is not kept in check by the appropriate immune regulatory mechanisms within the skin.
In vitiligo patients, ongoing immunity is specifically directed against melanocytes and targets melanosomal proteins, and increased numbers of melanocyte-reactive T cells are observed in the circulation. In vitiligo patients, T cells with high affinity for melanosomal antigens escape clonal deletion in primary lymphoid organs, and T cell tuning may allow this process to go forward (Van den Boorn et al., 2006). It follows that in the absence of a regulatory component, these patients will preferentially develop vitiligo, whereas we and others have reported cases of coinciding vitiligo and psoriasis as well (Al-Mutairi and Al-Doukhi, 2009; Hernandez et al., 2008). In bona fide cases of psoriasis however, non-functional Treg present in equal numbers are more commonly held responsible for progressive disease (Goodman et al., 2009). Another inflammatory skin condition involving a T cell component where we have studied the involvement of Treg is condylomata. Here we found the Treg compartment to be significantly more abundant than in control skin, which likely contributes to the lack of CTL efficacy in eliminating existing warts from the skin (Le Poole et al., 2008).
The abundance of circulating Treg was similar among vitiligo patient samples compared to controls. Indeed, if the percentage of Treg among circulating T cells were reduced, one would expect vitiligo patients to suffer from generalized autoimmunity. Whereas vitiligo patients do display an increased incidence of several autoimmune diseases, most notably of Hashimoto’s thyroiditis (Kakourou et al., 2005), the majority of patients are only affected by progressive depigmentation of the skin. Thus it was not surprising to find that vitiligo patients carry similar numbers of Treg in the circulation. Treg circulating in abundance among vitiligo patients compared to control individuals signal an attempt of the immune system to compensate for an effector response to self antigens, accompanying an increased number of effector T cells previously noted for vitiligo patients with active disease (Ogg et al., 1998). Clearly however, these circulating Treg are incapable of keeping the autoimmune response to melanocytes in check, suggesting a possible defect in regulatory function. This prompted our studies to delineate the ability of circulating Treg to control T cell proliferation, comparing Treg isolated from patients with those isolated from control individuals. Our data however demonstrate, that circulating Treg from vitiligo patients- regardless of the activity of their disease- are functional and capable of inhibiting helper T cell proliferation.
Observing a near complete absence of Treg in the skin, whereas functional Treg were abundant in the circulation of vitiligo patients prompted us to investigate the expression of markers associated with Treg skin homing. Cutaneous lymphocyte antigen (CLA), CCR4 and CCR8 have been implicated in this process, and expression of these markers was compared among control and patient samples. In melanoma, we have recently observed significant overexpression of CCR4 and CCR8 measured as both the number of expressing cells and the abundance/cell among circulating Treg, accompanied by marked expression of the ligands within the tumor environment [(Klarquist et al., 2009 (abstract)]. By contrast, the percentage and abundance of expression of CLA, CCR4, CCR5 and CCR8 was comparable among vitiligo patient and control samples although a trend towards decreased expression of CCR4 and CCR5 were observed among vitiligo patient samples. Although outside the scope of the current Treg study, another notable difference was significantly increased expression of CCR8 among non-Treg lymphocytes (both CD4+ and CD8+) at P < 0.01 for each subset, implicating CCR8 in infiltration of proinflammatory T cells into vitiligo skin (not shown).
Markedly differential engagement of chemokine CCL22 was observed, strongly suggesting that impaired skin homing contributes to reduced regulatory activity in the skin environment of vitiligo patients. As patient Treg did not display an intrinsic disability to respond to CCL22 with increased migration, the actual reduction in the number of chemokine expressing cells within patient skin is thought to be primarily responsible for impaired immigration. Remaining expression of CCL22 even among patient skin samples may explain the occasional detection of residual Treg in vitiligo skin. A difference in migration could not be assigned to a lack of CCL1 or CCL17 expression in vitiligo, as patient skin expressed similar levels of the chemokines by immunohistochemistry. It should be noted that the proportion of Treg will be further influenced within the skin environment itself, possibly by differential abundance of IL-6 that can stimulate a pro-inflammatory, Th17-dominated environment as demonstrated for psoriasic lesions (Goodman et al., 2009).
Taken together, an inadequate number of Treg will not be able to suppress an ongoing cytotoxic response in vitiligo skin. Indeed, our data support that a reduced proportion of Treg is retained in the skin of vitiligo patients, and such reduction is accompanied by reduced expression of CCL22. We have previously reported an abundance of CLA expressing T cells in perilesional skin of vitiligo patients with active disease (Van den Wijngaard et al., 2000), and immuno double staining of skin sections with antibodies to FoxP3 and CLA further confirmed that CLA expression in vitiligo skin was not accompanied by expression of regulatory T cell markers (not shown). Taken together, the data can help to explain continued cytotoxic T cell activity contributing to progressive depigmentation. This mechanism may be unique to vitiligo, and differs from other autoimmune disease of the skin, where Treg are abundant in the skin and blood, but appear to display deficient regulatory activity (Sugiyama et al., 2005). The data support a mechanism of reduced homing to the destination site for Treg, allowing an active immune response to melanocytes to proceed despite an abundance of functional Treg circulating in vitiligo patients. The importance of conditions encountered by Treg during priming in peripheral lymph nodes for organ-selective homing (Siewert et al., 2007), as well as reports of temporal changes in Treg function over time (Smyk-Pearson et al., 2008) suggests that opportunities may exist to manipulate the skin-seeking behavior of Treg in vitiligo.
Materials and methods
Patient population
For studies involving blood derived lymphocytes, a total of 30 ml of blood was drawn from patients diagnosed with generalized vitiligo, with either stationary or progressive disease. All patients provided informed consent under IRB approval from Loyola University Chicago. PBMC were isolated by Ficoll gradient, frozen in presence of 10% DMSO in fetal bovine serum (FBS) and stored in liquid nitrogen until use. Control blood was purchased from Life Source in Chicago, IL, USA.
Four mm skin biopsies from vitiligo patients included in this study were obtained under local anesthesia from consenting adults for studies approved by the IRB at Loyola University Chicago. Control skin or tumor samples were obtained as otherwise discarded tissue obtained either during circumcision, abdominoplasty, mamma reduction or during tumor resection, respectively through IRB approved protocols at Loyola University in conjunction with the Departments of Surgery at the University of Chicago (IL) and the University of Cincinnati (OH).
All patient information available and relevant to the study is summarized in Table 1.
Table 1.
(A) Patient skin biopsy samples for Treg quantification by immunohistochemistry. (B) Patient PBMC samples for FACS analysis. (C) Patient PBMC samples involved in proliferation assay. (D) Patients skin biopsy samples included in chemokine immunostaining
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Biopsy site | Patient gender |
Patient age (yr) |
Disease duration (yr) |
Disease activity |
Disease treatment |
Current medication |
1st degree relatives with vitiligo |
| Arm | Male | 31 | 8 | Progressive | N/A | N/A | Yes |
| Arm | Male | 58 | 31 | Progressive | N/A | N/A | Yes |
| Shoulder | Male | 60 | 38 | Progressive | N/A | N/A | Yes |
| Leg | Female | 50 | 11 | Progressive | N/A | Tamoxifen | No |
| Leg | Female | 54 | 29 | Progressive | N/A | N/A | Yes |
| Back | Male | 59 | 54 | Stable | N/A | Naftifin | No |
| Back | Female | 52 | 40 | Stable | N/A | Insulin | Yes |
| Arm | Male | 27 | 5 | Progressive | NB-UVB protopic |
N/A | Yes |
| (B) | |||||||
| Patient gender | Patient age (yr) | Disease duration (yr) | Disease activity | Vitiligo treatment | |||
| Female | 15 | 7 | Progressive | Laser + tacrolimus | |||
| Male | 44 | 5 | Progressive | Laser + tacrolimus | |||
| Female | 24 | 20 | Progressive | Laser + tacrolimus | |||
| Male | 28 | 2 | Stable | Laser + tacrolimus | |||
| Female | 34 | 17 | Progressive | Laser + tacrolimus | |||
| Male | 45 | 18 | Progressive | Laser + tacrolimus | |||
| Female | 55 | 4 | Progressive | Laser + tacrolimus | |||
| Female | 22 | 13 | Stable | Laser + tacrolimus | |||
| Male | 55 | 6 | Regressing | Laser | |||
| Female | 40 | 11 | Regressing | N/A | |||
| Male | 39 | 11 | Regressing | Laser + tacrolimus | |||
| Female | 18 | 9 | Stable | N/A | |||
| Female | 42 | 26 | Stable | N/A | |||
| (C) | |||||||
| Patient gender | Patient age (yr) | Disease duration (yr) | Disease activity | Vitiligo treatment | |||
| Male | 36 | 4 | Progressive | Laser + tacrolimus | |||
| Female | 33 | 30 | Progressive | Laser + tacrolimus | |||
| Female | 48 | 11 | Progressive | Laser + tacrolimus | |||
| (D) | |||||||
| Biopsy site | Patient gender | Patient age (yr) | Disease duration (yr) | Disease activity | Vitiligo treatment | ||
| Thorax | Male | 36 | 6 | Progressive | Laser + tacrolimus | ||
| Elbow | Male | 22 | 3 | Progressive | Laser + tacrolimus | ||
| Buttock | Female | 33 | 16 | Progressive | Laser + tacrolimus | ||
| Elbow | Female | 48 | 22 | Progressive | Laser + tacrolimus | ||
| Hip | Female | 40 | 11 | Progressive | Laser + tacrolimus | ||
| Elbow | Female | 29 | 16 | Progressive | Laser + tacrolimus | ||
| Hip | Female | 22 | 14 | Progressive | Laser + tacrolimus | ||
Monoclonal antibodies
Monoclonal antibodies used for immunostaining of skin specimen, FACS analysis and sorting of peripheral blood samples include antibodies to human antigens CD3 (clone F7.2.38, BD Biosciences, San Jose, CA, USA); unlabeled or FITCCD4 (clone RPA-T4, BD Biosciences) for immunohistology and FACS analysis, respectively; FoxP3 (polyclonal rabbit lgG, Abcam, Cambridge, MA, USA) for immunohistology and PEFoxP3 (clone 206D, Biolegend, San Diego, CA, USA) for FACS analysis; CD25 (clone 2A3, BD Biosciences) for immunohistology and PE-Cy7CD25 (clone M-A251, BD Biosciences), or PECD25 (clone 4E3, MACS Millenyi Biotech, Auburn, CA, USA) for sorting; APC-eFlour780CD127 (clone eBioRDR5, eBioscience, San Diego, CA, USA); PerCP-Cy5.5CCR4 (clone TG6 / CCR4, Biolegend); APCCCR8 (clone FAB1429A, R&D Systems, Minneapolis, MN, USA); CLA (clone HECA452, Rat IgM, BD Biosciences) for immunohistology or bioCLA(HECA452) with Pacific Orangestreptavidin (Invitrogen, Carlsbad, CA, USA) for FACS.
Immunohistology
Single immunostaining procedures were performed essentially as described (Le Poole et al., 2002). Briefly, 8 µm frozen and acetone fixed tissue sections were exposed to an antibody of interest in empirically optimized concentrations, followed by incubations with biotinylated rabbit anti-mouse antiserum 1:200 (DakoCytomation, Glostrup, Denmark) and peroxidase-labeled streptavidin 1:300 (Dako-Cytomation). Color was developed in presence of 250 µg/ml AEC and 0.03% H2O2 and sections were counterstained in Harris modified hematoxilin (Sigma Aldrich, St. Louis, MO) before coverslipping.
Immuno double stainings were similarly performed on frozen sections, essentially as described (Le Poole et al., 2002). Briefly, fixed sections were exposed to a combination of two primary antibodies of different isotypes. Washed sections were then exposed to a combination of isotype-specific secondary antibodies abeled either with peroxidase or alkaline phosphatase (Southern Biotechnologies, Birmingham, AL, USA). Fast Blue BB (Sigma Aldrich) substrate was used to develop blue color by alkaline phosphatase followed by AEC substrate (Sigma Aldrich) to develop red color in presence of peroxidase.
FACS analysis
For Treg quantification and analysis of homing markers, between 2 and 5 million frozen PBMC were thawed and incubated in the presence of primary antibodies labeled as follows: PBMCs were first negatively sorted for T cells on an EasySep magnet using a T cell enrichment antibody cocktail (StemCell Technologies, Vancouver, BC, Canada). Then, antibodies to PerCP-Cy5.5CCR4, FITCCCR5, APCCCR8 and bioCLA were incubated with cells for 20 min at room temp, followed by an additional 40 min at 4°C with V450CD4, PE-Cy7CD25 and APC-eFlour780CD127. Cells were washed twice, then incubated with Pacific OrangeStreptavidin for 25 min at 4°C. Cells were again washed and intracellularly stained with PEFoxP3 according to the Biolegend FoxP3 kit staining protocol. An unstained control, an FMO (FoxP3 excluded), and a fluorescence minus four control (CCR4, CCR5, CCR8 and CLA excluded) were used as negative controls for setting gates. Multicolor analysis of between 200 000 and 1.5 million acquired events of which about 80% were lymphocytes, was performed using the FACSCanto II (BD Biosciences) configured with 405-nm solid state diode, 488-nm solid state, and 633-nm HeNe, lasers and Flowjo analysis software (Tree-Star, Cupertino, CA, USA).
Proliferation assays
Frozen PBMC (approximately 5 × 106/sample) were thawed and immunostained for FAC sorting using directly labeled antibodies to CD4 (FITCCD4) and CD25 (PECD25). T cells were gated based on forward/side scatter patterns. CD4+ cells without or with CD25 expression were individually sorted using our FACSAria (BD Biosciences), equipped with 405, 488 and 633 lasers and is capable of 13 fluorescence detection channels, plus right and forward angle light scatter. Sorted cells were recombined with 10 000 IU/ml of IL-2 (Hoffman-LaRoche Inc., Nutley, NJ, USA) adding 5×104 beads/ml of CD3/CD28 coated dynabeads (Dynal Biotech ASA, Oslo, Norway) in complete media overnight. Complete media consisted of IMDM (Cambex, Walkersville, MD, USA) with 10% inactivated normal human AB serum (Valley Biochemical Inc, Winchester, VA, USA) and penicillin/streptomycin/fungizone 1:100 (Mediatech, Herndon, VA, USA). Equal numbers of CD4+CD25− and CD4+/CD25+ expressing cells were combined in 96 well round bottom plates. CD4+/CD25− and CD4+/CD25+ expressing cells were plated alone or combined in equal cell numbers per well. Cells were co-incubated for 72 h prior to addition of 30 Gy irradiated mature dendritic cells, generated from adherent monocytes cultured in AIMV (Invitrogen) in presence of 100 IU/ml of IL-4 (R&D Systems) and 400 IU/ml of GM-CSF (Berlex Laboratories, Richmond, CA, USA) for 7 days, adding 1000 IU/ml of IFN-γ (R&D systems) for the last 48 h of culture to achieve DC maturation (Pan et al., 2004). At the same time, 2 µCi of 3H–thymidine (25 Ci/mmol specific activity; Amersham Biosciences, Piscataway, NJ, USA) was added per well and cells were co-incubated for an additional 24-h period. In control wells, CD4+/CD25−or CD4+/CD25+ were maintained in the same number as in the co-cultures. Cells exposed to 3HThy were harvested using a Packard Filtermate harvester (Packard Instruments, Meriden, CT, USA) according to manufacturer’s instructions. Dried Unifilter 96 GF/C plates (Packard Bioscience Company, Meriden, CT, USA) were then wet with Microscint 20 (Packard Instruments) and radioactivity was measured by scintillation counting in a Packard Topcount Microplate scintillation counter (Packard Instruments). Incorporation of 3H-Thymidine was quantified as a measure of cell proliferation and the % inhibition of proliferation in presence of Treg was calculated as [cpm (combined Treg and CD25) – cpm background] × 100 / [cpm (Treg) + cpm (CD25−) − cpm background].
Migration assays
Migration assays were performed essentially as described (McFadden et al., 2007) and modified to measure the response of Treg to chemoattractant CCL22. Briefly, 106 control or vitiligo PBMC (n > 5) were added to 5 µm pore size transwell inserts of 24 well plates (Corning Inc. Life Sciences, Lowell, MA, USA), in X-VIVO 20 media (Cambrex, Walkersville, MD, USA). Migration was assessed in duplicate in the presence or absence of 500 ng/ml CCL22 (R&D Systems). After 2-h incubation at 37°C and 5% CO2, samples were individually analyzed for expression of CD3, CD4, CD25 and CD127 expression by FACS to identify the Treg subpopulation among migrated cells. Relative chemoattraction by CCL22 among both donor groups was expressed as % Treg among CD4+ cells migrating in presence of CCL22/% Treg migrating in absence of CCL22.
Evaluation and statistics
Immunostained slides were evaluated for infiltration of Treg by two independent investigators. The number of FoxP3/CD3 double stained cells per section were counted as a percentage of the total number of CD3 stained cells per section. Mean % of Treg (CD3+/FoxP3+) among T cells (CD3+) were compared among nonesional, perilesional and lesional vitiligo patients versus adult as well as neonatal control skin and lentigo maligna samples, 4–8 samples each as indicated in legends. Data were analyzed by Student’s T test using Excel software. In functional assays, the % suppression measured as described above (1 – proliferation of combined helper T cells and regulatory T cells, divided by proliferation of separate helper T cells plus regulatory T cells) was compared among vitiligo patients (n = 3) and controls (n = 3) performing Student’s T test.
The percentage of Treg among T cells in PBMC samples anayzed by FACS was determined in five vitiligo and five control PBMC samples. Levels of expression for skin homing receptors and numbers of migrating Treg were FACS analyzed in terms of percentage of T lymphocytes and the mean and median fluorescence intensity as well as the %. The significance of a difference in Treg percentages was determined in a T test. Finally, the number of chemokine expressing cells per dermal area was estimated by immunohistochemistry and image analysis. Student’s T tests was used to evaluate reduced expression of chemokines their receptors.
Significance.
Progressive depigmentation in vitiligo involves a CTL mediated autoimmune response to melanocytes. Although circulating CTL targeting melanosomal antigens are observed in healthy individuals, their autoimmune impact is limited by the presence of regulatory T cells. In vitiligo patients however, CD8+ T cells are cytotoxic towards melanocytes and Treg apparently fail to keep autoimmunity in check. Studies presented here demonstrate a significant reduction in the abundance of Treg associated with reduced CCL22 expression in patient skin, whereas functional Treg are abundant in the circulation. These studies represent an important step forward in understanding how depigmentation can progress in vitiligo patients.
Acknowledgements
The authors wish to thank pediatric surgeons from the University of Chicago and the University of Cincinnati for their support of these studies by providing normal skin and melanoma samples, and donors for providing samples of their skin and blood to study the etiopathology of vitiligo. These studies were supported in part by NIH grant R01CA109536 to CLP.
References
- Al-Mutairi N, Al-Doukhi A. Familial coexisting and colocalized psoriasis and vitiligo responding to alefacept. J. Cutan. Med. Surg. 2009;13:172–175. doi: 10.2310/7750.2008.08023. [DOI] [PubMed] [Google Scholar]
- Baecher-Allen C, Hafler DA. Human regulatory T cells and their role on autoimmune disease. Immunol. Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- Bala KK, Moudgil KD. Induction and maintenance of self tolerance: the role of CD4+CD25+ regulatory T cells. Arch. Immunol. Ther. Exp. (Warsz) 2006;54:307–321. doi: 10.1007/s00005-006-0035-x. [DOI] [PubMed] [Google Scholar]
- Chakraborty NG, Chattopadhyay S, Mehrotra S, Chhabra A, Mukherji B. Regulatory T-cell reponse and tumor vaccine-induced cytotoxic T lymphocytes in human melanoma. Hum. Immunol. 2004;65:794–802. doi: 10.1016/j.humimm.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Colantonio L, Iellem A, Sinigaglia F, D’Ambrosio D. Skin-homing CLA+ T cells and regulatory CD25+ T cells represent major subsets of human peripheral blood memory T cells migrating in response to CCL1 / I-309. Eur. J. Immunol. 2002;32:3506–3514. doi: 10.1002/1521-4141(200212)32:12<3506::AID-IMMU3506>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Danese S, Rutella S. The Janus face of CD4+CD25+ regulatory T cells in cancer and autoimmunity. Curr. Med. Chem. 2007;14:649–666. doi: 10.2174/092986707780059599. [DOI] [PubMed] [Google Scholar]
- Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 2005;115:3622–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das PK, van den Wijngaard RMJGJ, Wankowicz-Kalinska A, Le Poole IC. A symbiotic concept of autoimmunity and tumour immunity: lessons from vitiligo. Trends Immunol. 2001;22:130–136. doi: 10.1016/s1471-4906(00)01844-5. [DOI] [PubMed] [Google Scholar]
- De Boer OJ, van der Loos CM, Teeling P, van der Wal AC, Teunissen MB. Immunohistochemical analysis of regulatory T cell markers FOXP3 and GITR on CD4+CD25+ T cells in normal skin and inflammatory dermatoses. J. Histochem. Cytochem. 2007;55:891–898. doi: 10.1369/jhc.6A7119.2007. [DOI] [PubMed] [Google Scholar]
- Denman CJ, McCracken J, Hariharan V, Klarquist J, Oyarbide-Valencia K, Guevara-Patiño JA, Le Poole IC. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128:2041–2048. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WA, Levine AD, Massari JV, McCormick TS, Cooper KD. IL-6 signalling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 2009;183:3170–3176. doi: 10.4049/jimmunol.0803721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez C, Reddy SG, Barfuss A, Le Poole IC. Contact leucoderma after application of a compounded phenol cream and narrowband-UVB. Eur. J. Dermatol. 2008;18:593–595. doi: 10.1684/ejd.2008.0481. [DOI] [PubMed] [Google Scholar]
- Hirahara K, Liu L, Clark RA, Yamanaka Y, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25+highFoxp3+ regulatory T cells bear functional skin-homing receptors. J. Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- Iellem A, Colantonio L, D’Ambrosio D. Skin-versus gut-skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+CD25+ suppressor T cells. Eur. J. Immunol. 2003;33:1488–1496. doi: 10.1002/eji.200323658. [DOI] [PubMed] [Google Scholar]
- Jacob JB, Kong YC, Nalbantoglu I, Snower DP, Wei WZ. Tumor regression follwong DNA vaccination and regulatory T cell depletion in neu transgenic mice leads to an increased risk for autoimmunity. J. Immunol. 2009;182:5873–5878. doi: 10.4049/jimmunol.0804074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YN, Dakhama A, Gelfand EW. Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL-10 induction of TGF-β. J. Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- Kakourou T, Kanaka-Gantenbein C, Papadopoulou A, Kaloume-nou E, Chrousos GP. Increased prevalence of chronic autoimmune (Hashimoto’s) thyroiditis in children and adolescents with vitiligo. J. Am. Acad. Dermatol. 2005;53:220–223. doi: 10.1016/j.jaad.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Klarquist J, McKee M, Mehrotra S, Le Poole IC. Markedly enhanced expression of homing receptors by cicultating Treg in melanoma. Pigment Cell Melanoma Res. 2009;22:498. (abstract) [Google Scholar]
- Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3 regulatory T cells induces a scurfy-like disease. J. Exp. Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poole IC, van den Wijngaard RMJGJ, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J. Invest. Dermatol. 1993;100:816–822. doi: 10.1111/1523-1747.ep12476645. [DOI] [PubMed] [Google Scholar]
- Le Poole IC, Riker AI, Quevedo ME, Stennett LS, Wang E, Marincola FM, Kast WM, Robinson JK, Nickoloff BJ. Interferon-c reduces melanosomal antigen expression and recognition of melanoma cells by cytotoxic T cells. Am. J. Pathol. 2002;160:521–528. doi: 10.1016/s0002-9440(10)64871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poole , Denman CJ, Arbiser JL. Immunosuppression may be present within condylomata acuminata. J. Am. Acad. Dermatol. 2008;59:967–974. doi: 10.1016/j.jaad.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Lee HY, Hong YK, Yun HJ, Kim YM, Kim JR, Yoo WH. Altered frequency and migration capacity of CD4+CD25+ regulatory T cells in systemic lupus erythematosus. Rheumotology. 2008;47:789–794. doi: 10.1093/rheumatology/ken108. [DOI] [PubMed] [Google Scholar]
- Loddenkemper C, Hoffmann C, Stanke J, et al. Regulatory (FOXP3+) T cells as target for immune therapy of cervical intraepithelial neoplasia and cervical cancer. Cancer Sci. 2009;100:1112–1117. doi: 10.1111/j.1349-7006.2009.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke K, Scho¨nfeld K, Fondel S, et al. Depletion of CD4(+)CD25(+) human regulatory T cells in vivo Kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int. J. Cancer. 2007;120:2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- McFadden C, Morgan R, Rahangdale S, Green D, Yamasaki H, Center D, Cruikshank W. Preferential migration of T regulatory cells induced by IL-16. J. Immunol. 2007;179:6439–6445. doi: 10.4049/jimmunol.179.10.6439. [DOI] [PubMed] [Google Scholar]
- Nair S, Boczkowski D, Fasnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J. Exp. Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarbide-Valencia K, van den Boorn JG, Denman CJ, Carlson JM, Hernandez C, Nishimura MI, Das PK, Luiten RM, Le Poole IC. Therapeutic implications of autoimmune vitiligo T cells. Autoimmun. Rev. 2006;5:486–492. doi: 10.1016/j.autrev.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Zhang M, Wang J, Wang Q, Xia D, Sun W, Zhang L, Yu H, Liu Y, Cao X. Interferon-gamma is an autocrine mediator for dendritic cell maturation. Immunol. Lett. 2004;94:141–151. doi: 10.1016/j.imlet.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Siewert C, Menning A, Dudda J, Siegmund K, Lauer U, Flo-ess S, Campbell DJ, Hamann A, Huehn J. Induction of organ-selective CD4+ regulatory T cell homing. Eur. J. Immunol. 2007;37:978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- Smyk-Pearson S, Golden-Mason L, Klarquist J, Burton JR, Tester IA, Wang CC, Culbertson N, Vandenbark AA, Rosen HR. Functional suppression by FoxP3+CD4+ CD25(high) regulatory T cells during acute hepatitis C virus infection. J. Infect. Dis. 2008;197:46–57. doi: 10.1086/523651. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J. Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, van Duivenvoorde LM, van Elsas , Schumacher TN, Wildenberg ME, Allison JP, Toes PE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive T lymphocyte responses. J. Exp. Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb A, Picardo M VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27–35. doi: 10.1111/j.1600-0749.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- Van den Boorn JG, Le Poole IC, Luiten RM. T cell avidity and tuning: the flexible connection between tolerance and autoimmunity. Int. Rev. Immunol. 2006;25:235–258. doi: 10.1080/08830180600743081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Wijngaard R, Wankowicz-Kalinska A, Le Poole IC, Tig-ges B, Westerhof W, Das PK. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab. Invest. 2000;80:1299–1309. doi: 10.1038/labinvest.3780138. [DOI] [PubMed] [Google Scholar]
- Visseren MJ, van Elsas A, van der Voort EI, Ressing ME, Kast WM, Schrier PI, Melief CJ. CTL specific for the tyrosinase autoantigen can be induced from healthy donor blood can lyse melanoma cells. J. Immunol. 1995;154:3991–3998. [PubMed] [Google Scholar]
- Wankowicz-Kalinska A, van den Wijngaard RMJGJ, Tigges BJ, Westerhof W, Ogg GS, Cerundolo V, Storkus WJ, Das PK. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab. Invest. 2003;83:683–695. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Rudensky AY. FoxP3 in control of the regulatory T cell lineage. Nat. Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WW. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]