Abstract
TP63 is required for preservation of epithelial regenerative stasis and regulates the activity of diverse genetic pathways; however, specific effector pathways are poorly understood. Data presented here indicate that reciprocal regulatory interactions between hedgehog signaling and TP63 mediate stage-specific effects on proliferation and clonigenicity of separable enriched mammary stem and progenitor fractions. Analysis of ΔN-p63 and TA-p63 indicates segregated expression in mammary stem and progenitor fractions, respectively, demonstrating that differential TP63 promoter selection occurs during elaboration of mammary progenitors by mammary stem cells. This segregation underlies mammary progenitor-specific expression of Indian Hedgehog, identifying it as a binary transcriptional target of TP63. Hedgehog activation in vivo enhances elaboration of mammary progenitors and decreases label retention within mammary stem cell-enriched fractions, suggesting that hedgehog exerts a mitogenic effect on mammary stem cells. Hedgehog signaling promotes differential TP63 promoter usage via disruption of Gli3 or Gli3R accumulation, and shRNA-mediated disruption of Gli3 expression was sufficient to alter TP63 promoter usage and enhance clonigenicity of mammary stem cells. Finally, hedgehog signaling is enhanced during pregnancy, where it contributes to expansion of the mammary progenitor compartment. These studies support a model in which hedgehog activates elaboration and differentiation of mammary progenitors via differential TP63 promoter selection and forfeiture of self-renewing capacity.
Keywords: Mammary stem cells, Mammary progenitors, Quiescence, TP63, Hedgehog, Gli3
Introduction
Regenerative stasis requires a cellular hierarchy in which the mitotic offspring of adult stem cells undergo cell-fate decisions governing preservation or forfeiture of self-renewal. This decision is influenced by diverse physiologic states, such as embryonic development [1], tissue regeneration [2], wound healing [3], aging [4], and oncogenesis [5]. Prolonged tissue stasis requires a subset that preserves self-renewal, whereas physiologic functions require a subset to forfeit self-renewal and commit to terminal differentiation. Factors influencing this decision have important implications for regenerative medicine and cancer initiation.
TP63 is required for maintenance of diverse epithelial and apocrine structures [6–8]. TP63-deficient mice display multiple epithelial hypoplasias that have been attributed to nonregenerative differentiation [8] or the failure of simple ectoderm to commit to stratified lineages [6]. Mutations in the coding region of TP63 underlie a broad spectrum of human syndromes, which have in common deficiencies in epithelial stasis [9]. The mechanisms by which TP63 influences epithelial stasis are confounded by usage of two distinct promoters encoding isoforms possessing (TA-p63) or lacking (ΔN-p63) an amino-terminal transactivation domain and alternative splicing to generate C-terminal diversity [10]. Expression studies in epithelial structures [11, 12] and functional analysis of multiple isoforms [10] support a model in which ΔN-p63 preserves self-renewal by opposing the transcriptional activity of p53 family members. In support of this model, targeted ablation of TA-p63 causes no defects in epithelial stasis, demonstrating that ΔN-p63 isoforms mediate preservation of self-renewal and epithelial stasis [13]. This model predicts that forfeiture of self-renewal involves suppression of ΔN-p63 and is consistent with studies of ΔN-p63-α expression in the corneal epithelia [14]. In addition, ΔN-p63 isoforms mediate positive transcriptional responses [15, 16] that regulate genetic pathways underlying stem cell renewal and tissue stasis [17, 18]. Although they are not mutually exclusive, these models suggest that functional interactions between ΔN-p63 and other p53 family members underlie complex patterns of bidirectional target gene regulation, which is consistent with recent profiling of ΔN-p63-α occupancy and target gene expression in the human genome [19].
Mammalian hedgehog signaling regulates embryonic patterning, cell fate specification, and regenerative stasis. This functional diversity is achieved by expression of three distinct hedgehog ligands, sonic hedgehog (Shh), Indian hedgehog (Ihh), and desert hedgehog (Dhh); two distinct Patched genes, Ptch1 and Ptch2; and three orthologs of Cubitus interruptus, Gli1, Gli2, and Gli3. Multiple studies support a model in which Gli1, Gli2, and Gli3 confer positive transcriptional regulation [20–23] and can be proteolytically processed into transcriptional repressors [24–26]. Within this model, hedgehog regulates the ratio of full-length to processed Gli proteins, thereby affecting target genes in complex and context-dependent patterns.
Genetic analysis of hedgehog components has identified stromal/epithelial interactions critical for mammary gland development. Activation of hedgehog signaling via Ptch1 heterozygosity results in developmental dysplasia and occlusion of the ductal lumen [27]. Tissue recombination studies indicate that Ptch1 heterozygosity in the surrounding stroma underlies these effects [27]. Similarly, dysplasias resulting from Gli2 ablation were not observed following transplantation of Gli2-deficient MECs into cleared fat pads of wild-type recipients [28]. Two recent studies indicate that mutations in Gli3 lead to loss of the third and fifth mammary placode. In the first, Gli3 expression in underlying somites regulates expression of Fgf10 in primitive ectoderm. Gli3 mutation or perturbation of Fgf10 gradients resulted in displacement of the embryonic mammary line and impaired elaboration of specific mammary placodes [29]. In the second, transcriptional repression by Gli3 contributes to formation and localization of mammary placodes. These effects were mediated by the ability of Gli3 to repress expression of Gli1 in the surrounding stroma [30]. These studies indicate that many of the effects of hedgehog on mammary gland development are mediated by stromal/epithelial interactions.
In addition to stromal/epithelial interactions, recent studies indicate that intraepithelial hedgehog activity contributes to mammary epithelial stasis. Hedgehog activation in normal and malignant human MECs in vitro enhanced clonigenicity, suggesting that hedgehog stimulates self-renewal in a subset of MECs [31]. This study also demonstrated active hedgehog signaling in enriched populations of breast cancer stem cells, defined as Lin−/CD44+/CD24−/low, suggesting a role for hedgehog deregulation in breast cancer initiation. In addition, ectopic expression of a constitutively activated allele of Smoothened (Smo) in MECs leads to increased mammosphere-forming efficiency but decreased mammary regenerative capacity [32]. Finally, there is evidence of hedgehog activation in breast cancers [33]. These studies implicate hedgehog in regulation of mammary epithelial stasis independent of the stromal/ epithelial interactions that regulate mammary development.
Abundant evidence implicates diverse genetic pathways [17, 19] as mediators of the effects of ΔN-p63. Advances in isolation of adult stem cells from diverse tissues represent an opportunity to dissect the relationship between TP63 and genetic pathways governing self-renewal. We report a reciprocal regulatory relationship between TP63 and hedgehog in the mammary regenerative compartment. Results support a model in which hedgehog promotes elaboration of mammary progenitors by mammary stem cells and mediates differential TP63 promoter use. Analysis of separable fractions of murine mammary stem and progenitor cells indicates segregated expression of ΔN-p63 and TA-p63, respectively, suggesting that forfeiture of self-renewal is reflected in differential TP63 promoter usage. In vivo hedgehog activation enhances elaboration of mammary progenitors via increased mitotic activity in mammary stem cells and reduces the clonigenicity of mammary progenitors. These effects are mediated by hedgehog-dependent disruption of Gli3 and Gli3R accumulation, indicating a role for Gli3 in preservation of self-renewal. In addition, Ihh expression in mammary progenitors requires TA-p63, and repression of Ihh in mammary stem cells is mediated by ΔN-p63-α. Finally, we demonstrate that Gli3 and Gli3R accumulation is disrupted during pregnancy, suggesting that hedgehog signaling contributes to expansion of the mammary gland. These studies indicate that hedgehog signaling promotes elaboration and differentiation of mammary progenitors and support the assertion that hedgehog signaling mediates stage-specific cellular responses within the mammary regenerative hierarchy.
Materials and Methods
Cell Culture
Establishment and maintenance of the immortalized mammary epithelial cell (IMEC) line has been previously described [34]. Stable transfectants expressing mouse Ihh were generated by cotransfection of an Ihh expression plasmid and pcDNA3.1 (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) and selection in 100 µg/ml G418. Sonic hedgehog-conditioned medium (Shh-cm) was prepared by culturing a HEK-293 derivative programmed to over-express Shh in MEGM (Lonza, Walkersville, MD, http://www.lonza.com) for 20 hours. All experiments used Shh-cm diluted 1:1 with MEGM. Hedgehog neutralization with 5E1 was done at a final concentration of 1 µg/ml for 2 hours prior to use.
Mice
B6/129 wild-type (wt) and Ptch1−/+mice (Jackson Laboratories, Bar Harbor, ME, http://www.jax.org) were bred and maintained according to institutional guidelines. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Dartmouth Medical School.
Plasmid, siRNA, and shRNA
Adenoviruses expressing shRNAs directed against p63 have been described previously [35]. Lentiviruses expressing shRNAs directed against Ihh and Gli3 were purchased from Openbiosystems (Huntsville, AL, http://www.openbiosystems.com) and packaged according to the manufacturer’s protocol. The Ihh expression plasmid pCMV-Sport6-mIhh was obtained from Openbiosystems. Specific siRNA sequences for Gli3 and ΔN-p63 appear in the supplemental online Methods.
Western Blot
Cell lysates were prepared in NETN (100 mM Tris-C1 [pH 7.8], 1 mM EDTA, 100 mM NaCl, and 0.1% Triton X-100). Western blotting was done as previously described [36]. Specific antibodies used are described in the supplemental online methods.
Quantitative Reverse Transcription-Polymerase Chain Reaction
RNA was isolated using RNeasy mini kit (Qiagen, Hilden, Germany, http://www1.qiagen.com) per the manufacturer’s protocol and reverse transcribed using random hexamers and Superscript III (Invitrogen). Quantitative polymerase chain reaction (Q-PCR) was conducted using the 2× SYBR Green master mix (Bio-Rad, Hercules, CA, http://www.bio-rad.com). Specific Q-PCR primer sequences appear in the supplemental online Methods. For quantification of gene expression changes, the 2−ΔΔCT method was used to calculate relative changes normalized to glyceraldehyde-3-phos-phate dehydrogenase.
Bromodeoxyuridine Incorporation
Mice were injected with 50 mg/kg bromodeoxyuridine (BrdU) i.p. daily for 8 days prior to long-term retention studies or once 3 hours prior to harvest for short-term proliferation studies. Following sacrifice, BrdU was detected by immunohistochemistry or immunocyto-fluorescence of cell fractions samples applied to a glass slide.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation (ChIP) assay was performed using the ChIP-IT system (Active Motif, Carlsbad, CA, http://www.activemotif.com) according to the manufacturer’s protocol. The antibodies used were mouse IgG (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com), p63 antibody (4A4) (BD Pharmingen, San Diego, http://www.bdbiosciences.com/index_us.shtml), and Myc antibody 9E10 (Covance, Princeton, NJ, http://www.covance.com). IMECs were plated at 25% confluence on 15-cm dishes and trans-fected with empty vector or TAp63γ-myc plasmid with FuGene (Roche Diagnostics, Basel, Switzerland, http://www.roche-applied-science.com). Cells were refed with fresh culture medium the following day and assayed at 48 hours post-transfection. Primer sequences used for amplification of retained DNA fractions appear in the supplemental online methods.
Acini Culture
Isolated murine epithelial fractions were resuspended in chilled 100% Matrigel (BD Biosciences, San Diego, http://www.bdbiosciences.com). Gels were allowed to solidify at 37°C in an incubator for 10 minutes and then covered with MEGM plus 5% fetal bovine serum as previously described [37]. Addition of 5 µg/ml prolactin (Sigma-Aldrich) in MEGM was used to induce differentiation. Conditioned medium was prepared as described above and immunodepleted with 0.1 µg/ml of the hedgehog-neutralizing monoclonal antibody 5E1 or nonspecific mouse IgG.
Immunofluorescence Microscopy and Imaging
Immunofluorescent analysis of acini was done as previously described [37], with slight modifications (supplemental online Methods).
Statistical Methods
Quantitative data statistical analysis consisted of calculating the mean of triplicate points and the SEM. Data are presented as mean values, and error bars represent the SEM. p values were calculated using Student’s t test.
Results
Segregated Expression of TP63 Isoforms and Ihh in Mammary Stem and Progenitor Fractions
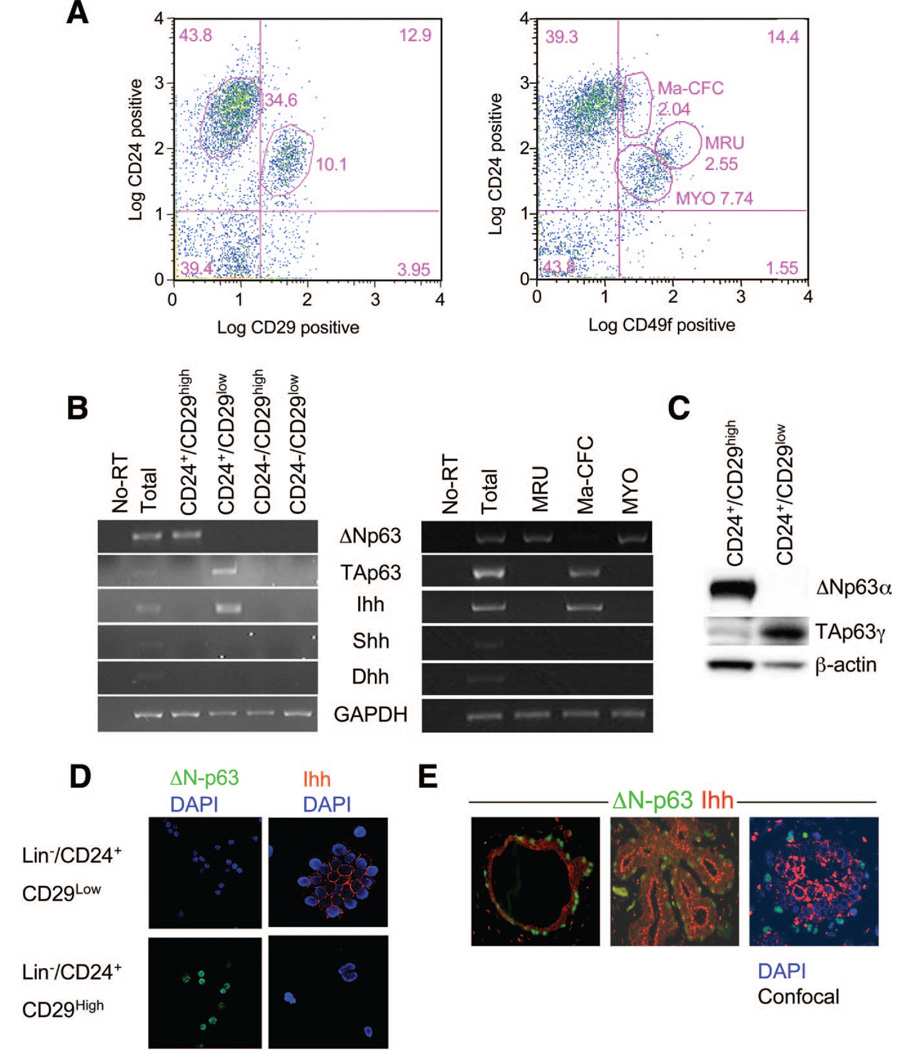
Recently, two distinct sorting strategies led to identification of highly enriched fractions of mouse mammary stem and progenitor cells, suggesting a regenerative hierarchy in which mammary stem cells give rise to bipotent mammary progenitors [38, 39]. In each of these studies, limiting-dilution transplantation indicated that single cells within the enriched mammary stem cell fraction could reconstitute mammary morphogenesis and self-renewal. To better understand the actions of TP63 and hedgehog signaling in mammary regenerative stasis, separable enriched fractions of mammary stem and progenitor cells were isolated (Fig. 1A), and expression of ΔN-p63, TA-p63, Shh, Ihh, and Dhh was analyzed by reverse transcription (RT)-PCR. Results (Fig. 1B) indicate that enriched mammary stem cell fractions, previously described as either Lin−/CD24+/CD29high or Lin−/CD24+/CD49fhigh, express ΔN-p63 and do not express TA-p63 or hedgehog ligands. By contrast, enriched mammary progenitor fractions, previously described as either Lin−/ CD24+/CD29low or Lin−/CD24+/CD49f low, exclusively express TA-p63 and Ihh. Western blot analysis of Lin−/CD24+/ CD29high and Lin−/CD24+/CD29low using antisera specific for TA-p63 [36] or ΔN-p63 [11] confirmed the segregation of TP63 isoforms (Fig. 1C), and immunofluorescent analysis of Lin−/CD24+/CD29high and Lin−/CD24+/CD29low fractions confirmed the segregation of ΔN-p63 and Ihh, respectively (Fig. 1D). The uniform expression of ΔN-p63 in Lin−/CD24+/CD29high cells, coupled to the finding that myoepithelia copurify with mammary stem cells [38, 39] in this fraction, indicates that ΔN-p63 is expressed in myoepithelia and mammary stem cells. This finding is consistent with reports identifying p63 as a marker of myoepithelia [40] and also with genetic analysis indicating that ΔN-p63 is required for preservation of self-renewal in epithelial structures [7, 8]. To determine whether the segregation of ΔN-p63 and Ihh is observed in human mammary gland, two-color immunofluorescent detection of ΔN-p63 and Ihh was performed on formalin-fixed, paraffin-embedded tissue derived from reduction mammoplasty. Results (Fig. 1E) indicate that ΔN-p63 and Ihh are differentially expressed in the human mammary gland. These results indicate that TP63 promoter selection and differential Ihh expression occur during elaboration of mammary progenitors by mammary stem cells.
Figure 1.
Segregated expression of ΔN-p63, TA-p63, and Ihh in the mammary regenerative hierarchy. (A): Fluorescence-activated cell sorting of mouse MECs on the basis of CD24/CD29 (left) and CD24/CD49f (right). Nomenclature used in the right panel is as previously described [2]. (B): Reverse transcription-polymerase chain reaction analysis indicates segregation of ΔN-p63 from TA-p63 and Ihh in mammary epithelial fractions derived from CD24/CD29-based sorting (left) and CD24/CD49f–based sorting (right). (C): Western blot analysis of Lin−/CD24+/CD29high and Lin−/CD24+/ CD29low with antibodies specific against ΔN-p63, TA-p63, and β-actin confirms the segregated expression of TP63 isoforms. (D): Immunocytofluorescent analysis of ΔN-p63 and Ihh in Lin−/CD24+/CD29high and Lin−/CD24+/CD29low confirms the segregated expression of ΔN-p63 and Ihh. (E): Two-color immunofluorescence demonstrates segregated expression of ΔN-p63 and Ihh in human primary mammary ducts (left) and smaller ducts and lobules (right). The image at the right was captured by confocal microscopy and includes nuclear staining with DAPI. Abbreviations: DAPI, 4,6-di-amidino-2-phenylindole; Dhh, desert hedgehog; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Ihh, Indian hedgehog; Ma-CFC, mammary colony-forming cells; MRU, mammary regenerative units; MYO, myoepithelia; No-RT, no reverse transcription; Shh, sonic hedgehog.
Ihh Is a Bidirectional Transcriptional Target of TP63
The binary relationship between Ihh expression and TP63 promoter usage suggested that expression of Ihh may be differentially regulated by ΔN-p63 and TA-p63. To test this, Lin−/CD24+/CD29high and Lin/CD24+/CD29low fractions were infected with adenoviruses expressing shRNAs directed against the α-specific carboxy terminus of TP63, the DNA-binding domain of TP63, or nonspecific shRNAs [35], and Ihh mRNA levels were measured by Q-PCR. Results indicate that ablation of ΔN-p63 in Lin−/CD24+/CD29high enhanced expression of Ihh (Fig. 2A) and that ablation of TA-p63 in Lin−/CD24+/CD29low suppressed Ihh expression (Fig. 2B). To determine whether TA-p63-γ is sufficient to activate expression of Ihh, an IMEC line with properties of basal epithelia [34] was trans-fected with ΔN-p63α, TA-p63-γ, orp53, and expression of Ihh was measured by Q-PCR. TA-p63-γ was sufficient to activate expression of Ihh to a greater extent than p53, whereas ΔN-p63-α was unable to activate Ihh expression (Fig. 2C). ChIP indicated that ectopic myc-tagged TA-p63-γ occupies a putative p63-binding element identified in the Ihh promoter, implying that the effects of TA-p63-γ on Ihh expression are direct (Fig. 2C, center and right). These observations suggest that Ihh may be directly repressed by ΔN-p63-α in IMECs. To test this, ΔN-p63-specific siRNA or a scrambled control was transfected into IMECs and RNA was isolated for Q-PCR analysis. Results (Fig. 2D) indicate that ablation of ΔN-p63 activated expression of Ihh. In addition, ChIP analysis indicates that endogenous ΔN-p63-α occupies the same region of the Ihh promoter as ectopic TA-p63-γ. These results indicate that Ihh is a direct transcriptional target of positive and negative regulation byTA-p63 and ΔN-p63, respectively.
Figure 2.
Ihh is a direct bidirectional target of ΔN-p63 and TA-p63. (A): shRNA-mediated suppression of ΔN-p63 (right) in Lin−/CD24+/CD29high cells leads to increased Ihh mRNA levels (left). p63-directed shRNAs were targeted to the DBD (p63DBD) or the α-specific carboxy terminus (p63-α). (B): shRNA-mediated suppression of TA-p63 (right) in Lin−/CD24+/CD29low cells leads to decreased Ihh mRNA levels (left). (C): Ectopic myc-tagged TA-p63-γ activates expression of Ihh in IMECs. Left panel shows the fold activation of Ihh relative to GAPDH, and Western blot (below) confirms the expression of ectopic TA-p63-γ, ΔN-p63-α, and p53. Center panel shows chromatin immunoprecipitation (ChIP) indicating binding of myc-tagged TA-p63-γ to a putative p63-binding element located at −1,392 to −1,412 relative to the initiator ATG of Ihh. Right panel shows the sequence of the putative p63-binding element above the consensus sequence. Bases shown in red represent divergence from the canonical p53/p63-binding element. (D): siRNA-mediated ablation of ΔN-p63-α (middle) in IMECs leads to enhanced expression of Ihh (left). ChIP indicates direct binding of endogenous ΔN-p63-α to a region of the Ihh promoter located at −1,392 to −1,412 relative to the initiator ATG of the human Ihh gene (right). Error bars represent the SEM derived from triplicate experimental data. Throughout the figure, p values <.001 are indicated by ***p values <.01 are indicated by **, and p values <.05 are indicated by *. Abbreviations: DBD, DNA binding domain; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Ihh, Indian hedgehog; IMEC, immortalized mammary epithelial cell; NS, nonspecific; scr, scrambled.
Hedgehog Activation Enhances Elaboration of Mammary Progenitors In Vivo
Heterozygosity of the negative hedgehog regulator, Ptch1, has revealed an important role for hedgehog in mammary development [27]. In vivo labeling experiments have identified a population of cells capable of long-term label retention in diverse tissues. Label-retaining capacity is viewed as evidence of quiescence. Other reports have ascribed it to a unique ability to asymmetrically retain template chromatids while segregating de novo synthesized chromatids [41]. Several studies indicate that label-retaining cells (LRCs) are enriched in cellular fractions possessing mammary regenerative capacity [42, 43]. To determine the effects of hedgehog activation on mammary epithelial stasis, wt and Ptch1−/+littermates were labeled with BrdU, and Lin−/CD24+/CD29low and Lin−/CD24+/CD29high fractions were isolated either 3 hours or 8 weeks after labeling to measure rates of proliferation and label retention, respectively. Flow cytometric analysis indicated expansion of the Lin−/CD24+/CD29low fraction in Ptch1−/+mice relative to wild-type littermates (Fig. 3A), suggesting that hedgehog activation promotes elaboration of mammary progenitors. Consistent with wild-type mice, Ptch1−/+mice displayed complete segregation of ΔN-p63 from TA-p63 and Ihh expression in Lin−/CD24+/CD29high and Lin−/CD24+/ CD29low fractions, respectively (Fig. 3B). These observations indicate that Ptch1 heterozygosity may result in increased elaboration of committed progenitors by mammary stem cells. Analysis of BrdU incorporation from mice sacrificed 3 hours after labeling indicated that Ptch1−/+ mice displayed higher rates of BrdU incorporation than wild-type littermates (Fig. 3C). In situ analysis of BrdU incorporation and Ki-67 expression indicate greater rates of proliferation in Ptch1−/+mice in both Lin−/CD24+/CD29low and Lin−/CD24+/CD29high fractions (Fig. 3D). These observations support the conclusion that hedgehog activation enhances proliferation with the mammary regenerative compartment.
Figure 3.
Activation of hedgehog signaling leads to enhanced elaboration of MPs by MSCs. (A): CD24/CD29-based sorting of mouse MECs from wild-type and Ptch1−/+mice indicates an expansion of the Lin−/CD24+CD29low population in Ptch1−/+mice. (B): Reverse transcription-polymerase chain reaction (PCR) confirms the segregated expression of ΔN-p63 from TA-p63 and Ihh in Lin−/CD24+CD29high and Lin−/CD24+/CD29low cells from Ptch1−/mice. (C): Quantification of BrdU uptake following a 3-hour labeling indicates enhanced proliferation in Lin−/CD24+/CD29low and Lin−/CD24+/CD29high in Ptch−/+mice relative to wild-type littermates. Data represent quantification of 10 microscopic fields per mouse and three mice. Error bars represent the SEM. (D): Enhanced BrdU uptake and Ki-67 expression in Ptch−/+ mice. The first two columns are representative images of Lin−/CD24+/ CD29high and Lin−/CD24+/CD29low fractions that were fixed to glass and stained with anti-BrdU (green) and DAPI (blue). The third column is in situ detection of BrdU in wt and Ptch1−/+ virgin mice. The last two columns are representative images of Lin−/CD24+/CD29high and Lin−/CD24+/CD29low fractions that were fixed to glass and stained with anti-Ki-67. (E): Quantification of BrdU retention following a 10-week chase indicates that Lin−/CD24+/CD29high cells from wild-type but not Ptch1−/+ are able to retain BrdU. Data represent quantification of 10 microscopic fields per mouse and four mice. Error bars represent the SEM. Images at right are representative of enrichment of BrdU retaining cells (white arrowheads) in the Lin−/CD24+/CD29high fraction of wild-type but not Ptch1−/+ mice. (F): Semiquantitative PCR analysis of GATA3 indicates that expression of GATA3 is enhanced in the stem cell-enriched fractions from Ptch1−/+. Throughout the figure, p values <.001 are indicated by ***, p values <.01 are indicated by **, and p values <.05 are indicated by *. Abbreviations: BrdU, bromodeoxyuridine; DAPI, 4,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Ihh, Indian hedgehog; MG, mammary gland; MP, mammary progenitor; MSC, mammary stem cell; WT, wild-type; wt, wild-type.
The observation that hedgehog activation increases mitotic activity in the Lin−/CD24+/CD29high fraction predicts that label retention may be compromised in Ptch1−/+ mice. Analysis of BrdU retention from mice harvested 8 weeks after labeling shows that LRCs were highly enriched in the Lin−/CD24+/CD29high fraction of wild-type mice and not in Lin−/CD24/CD29high fractions of Ptch1−/+mice (Fig. 3E). These data are consistent with reports indicating that this fraction contains mammary stem cells. They also support a model in which hedgehog activation enhances elaboration of mammary progenitors, causing expansion of the Lin−/CD24+/CD29low fraction and forfeiture of label retention within the Lin−/CD24+/CD29high fraction. In addition, the absence of label retention in the Lin−/CD24+CD29high from Ptch1−/+ mice, coupled to the observed expression of Ki-67 in this fraction, implies that activation of hedgehog signaling results in a quiescence defect in the mammary stem cell-enriched fraction. Recently, two reports have shown that GATA3 is required for terminal differentiation of mammary progenitors to luminal epithelia [45, 46]. This finding, coupled to the observation that hedgehog activation results in aberrant elaboration of mammary progenitors, suggested that expression of GATA3 may be enhanced in the mammary regenerative compartment of Ptch1−/+mice. Consistent with the two previous reports, RT-PCR data indicate that GATA3 expression is higher in the Lin−/CD24+/CD29low cells than in the Lin−/CD24+/CD29high cells of wt mice; however, in the Ptch1−/+mice, expression of GATA3 was elevated in the Lin−/CD24+/CD29high fraction to levels that were similar to those of the Lin−/CD24+/CD29low cells (Fig. 3F). This observation is consistent with our finding that hedgehog activation leads to enhanced elaboration of mammary stem cells and suggests that expression of GATA3 in the Lin −/CD24+/ CD29high cells reflects the activated state of mammary stem cells associated with Ptch1 heterozygosity (described in Summary).
Hedgehog Alters TP63 Promoter Selection via Disruption of Gli3 and Gli3R Accumulation
The finding that hedgehog activation enhances elaboration of mammary progenitors coupled to segregated expression of ΔN-p63 and TA-p63 in the mammary regenerative hierarchy suggests that hedgehog activation may mediate differential TP63 promoter usage. We therefore sought to determine whether and by what mechanism hedgehog signaling affected TP63 promoter use in IMECs and Lin−/CD24+CD29high cells. Treatment of IMECs with an Shh-cm reduced proliferation and expression of ΔN-p63-α (supplemental online Fig. 1). Under these conditions, expression and accumulation of Gli3 and Gli3R was disrupted in manner that was sensitive to the hedgehog-neutralizing antibody 5E1 (Fig. 4A), and expression levels of Gli1, Ptch1, and Ptch2 were unaffected (supplemental online Fig. 2). To confirm these results, IMECs were stably transfected with an expression vector encoding Ihh or an empty vector control, and expression of ΔN-p63-α, Gli3 and Gli3R was evaluated. Results (Fig. 4B) indicate that ectopic Ihh reduces levels of ΔN-p63-α, Gli3, and Gli3R. The observed segregation of ΔN-p63 and TA-p63 in mammary stem and progenitor fractions and the finding that hedgehog activation enhances elaboration of mammary progenitors predicted that overexpression of Ihh in IMECs would alter expression of ΔN-p63 and TA-p63 in IMECs. Q-PCR analysis of ΔN-p63 and TA-p63 indicates that Ihh repressed ΔN-p63 expression and activated TA-p63 expression (Fig. 4C), suggesting that hedgehog signaling is sufficient to cause selective TP63 promoter usage during elaboration of mammary progenitors. To determine whether these effects were mediated by disruption of expression and accumulation of Gli3 and Gli3R, siRNAs directed against Gli3, and scrambled controls, were transfected into IMECs, and relative levels of TA-p63 mRNA and ΔN-p63 mRNA were evaluated. Results (Fig. 4D) indicate that suppression of Gli3 expression was sufficient to promote differential TP63 promoter selection. These results demonstrate that the ability of hedgehog to limit expression and accumulation of Gli3 and Gli3R underlies the effects on TP63 promoter usage in IMECs. To determine whether this mechanism regulates expression of ΔN-p63 and TA-p63 in the mammary regenerative compartment, cells from the Lin−/CD24+/CD29high fraction were infected with a lentivirus encoding an shRNA directed against Gli3 or a nonspecific control. RT-PCR analysis indicated that disruption of Gli3 expression led to silencing of ΔN-p63 and activation of TA-p63 and Ihh expression (Fig. 4E). Together these data support the assertion that hedgehog-mediated repression of Gli3 and Gli3R expression accounts for differential TP63 promoter usage during elaboration of mammary stem cells.
Figure 4.
Hedgehog activation causes differential TP63 promoter usage via disruption of Gli3R accumulation. (A): Western blot analysis of Gli3 and Gli3R expression in response to Shh-cm in IMECs indicates that hedgehog activation disrupts Gli3R accumulation in a manner that is sensitive to 5E1. (B): Ectopic expression of Ihh in IMECs diminishes expression of ΔN-p63-α and Gli3R. (C): Ectopic expression of Ihh causes differential TP63 promoter selection in IMECs. RNA from IMEC-EV, IMEC-Ihh1, and IMEC-Ihh2 was harvested at 24, 48, and 72 h after plating, and expression of ΔN-p63 and TA-p63 was evaluated by quantitative polymerase chain reaction (Q-PCR). Data represent the mean of triplicate points, and error bars represent the SEM. (D): siRNA-mediated ablation of Gli3 leads to differential TP63 promoter usage in IMECs. RNA was harvested at 24, 48, and 72 h following transfection with a scr or Gli3-directed siRNA, and expression of ΔN-p63 and TA-p63 was measured by Q-PCR. Data represent the mean of triplicate points and error bars represent the SEM. Western blot at right demonstrates the efficacy of the Gli3-directed siRNA. (E): Ablation of Gli3 from Lin−/CD24+/CD29high leads to differential TP63 promoter usage and expression of Ihh. Cells were isolated and plated on Bio-Coat prior to infection with lentiviruses expressing a nonspecific shRNA or a Gli3-directed shRNA. RNA was harvested 48 h after infection, and expression of ΔN-p63, TA-p63, and Ihh was measured by reverse transcription-polymerase chain reaction. Western blot at right demonstrates the efficacy of the Gli3-directed shRNA in IMECs. Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; h, hours; Ihh, Indian hedgehog; IMEC, immortalized mammary epithelial cell; scr, scrambled; Shh-cm, sonic hedgehog-conditioned medium.
Differential Effects of Hedgehog in Mammary Stem and Progenitor Cells
Expansion of Lin−/CD24+/CD29low cells in Ptch1−/+ mice may be due to enhanced elaboration of mammary progenitors or increased mitotic activity within the Lin−/CD24+/CD29low fraction. The ability of primary or cultured MECs to form acinar structures, in a three-dimensional extracellular matrix (ECM) culture system, has been interpreted as evidence of clonigenicity and developmental potency [31, 37, 44]. To determine the effects of hedgehog activation on clonigenicity of the Lin−/CD24+/CD29low fraction, cells were cultured in ECM in the presence or absence of Shh-cm with or without 5E1 or a nonspecific antibody, and acini were biochemically characterized and quantified. Results indicate that in the absence of Shh-cm, Lin−/CD24+/CD29low cells efficiently developed uniformly round acini composed of two biochemically distinct cell layers (Fig. 5A). The outer layer expressed p63, whereas the inner layer could be induced to express milk proteins upon treatment with prolactin, indicating a degree of cellular differentiation during formation of acinar structures. Under these conditions, Shh-cm abolished acini formation capacity of Lin−/CD24+/CD29low cells in a manner that was sensitive to 5E1 but not nonspecific mouse IgG (Fig. 5B), indicating that hedgehog activation has an antiproliferative effect on mammary progenitors that is distinct from the proliferative effect on mammary stem cells. Coupled to the enhanced proliferation the of Lin−/ CD24+/CD29high fraction of Ptch1−/+, these data support the assertion that expansion of Lin−/CD24+/CD29low cells in Ptch1−/+mice is the result of enhanced elaboration of mammary progenitors by mammary stem cells.
Figure 5.
Differential effects of hedgehog on clonigenicity of enriched mammary stem and progenitor fractions. (A): Lin−/CD24+/CD29low cells form bilayered acinar structures with distinct patterns of expression in specific cell layers. Following 10 days of growth, acini were stimulated with prolactin, fixed, and stained with anti-milk, anti-p63α, and DAPI. (B): Hedgehog ablates acini formation by Lin−/CD24+/CD29low cells. Cells were isolated and cultured under acini-forming conditions in the presence of Shh-cm −/+ 5E1 or a nonspecific mouse IgG. After 10 days, acini were counted. Data represent the mean of four experiments, and error bars represent the SEM. (C): Cells from the Lin−/CD24+/CD29high fraction form complex multilobular structures. Top row shows expression of basal/myoepithelial markers CK14 and p63 merged with phase images of complex acini. Bottom row shows a single lobe from one of the complex acini expressing the luminal marker CK19 and the basal/myoepithelial marker p63 in two distinct cell layers. (D): Lin−/CD24+/CD29low and Lin−/CD24+/CD29high cells were cocultured under acini-forming conditions in the presence or absence of 5E1, and complex acini were counted. Data represent the mean of four experiments, and error bars represent the SEM. (E): Ablation of Ihh from Lin−/CD24+/CD29low cells restricts their ability to stimulate complex acini formation by Lin−/CD24+/CD29high cells. Isolated Lin−/CD24+/CD29low cells were infected with lentiviruses encoding nonspecific shRNA or Ihh-directed shRNA. Following infection, cells were cocultured under acini-forming conditions with Lin−/CD24+/CD29high cells, and complex acini were counted. Data represent the mean of three distinct experiments, and error bars represent the SEM. Immunofluorescence of Lin−/CD24+/CD29low cells (right) demonstrates the efficacy of the Ihh-directed shRNA. (F): Disruption of Gli3R accumulation in Lin−/CD24+/CD29high cells promotes complex acini formation. Lin−/CD24+/CD29high cells were infected with lentiviruses encoding a nonspecific shRNA or a Gli3-directed shRNA. Following infection, cells were cultured under acini-forming conditions, and complex acini were counted. Data represent the mean of three distinct experiments, and error bars represent SEM. Throughout the figure, p values <.001 are indicated by ***, p values <.01 are indicated by **, and p values <.05 are indicated by *. Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; Ihh, Indian hedgehog; Shh, sonic hedgehog.
In contrast to Lin−/CD24+/CD29low, acini formation by Lin−/CD24+/CD29high cells is highly inefficient and resulted in formation of complex multilobed structures (referred to here as complex acini). Biochemical analysis of these structures indicated that individual lobes were composed of two distinct cellular layers expressing basal/myoepithelial markers p63 and CK14 and the luminal epithelial marker CK19 (Fig. 5C). This suggested that complex acini formation may involve production of mammary progenitors that give rise to individual lobes that are biochemically similar to progenitor-derived acini. This observation and the finding that hedgehog activation enhanced elaboration of mammary progenitors suggested that stimulation of Lin−/CD24+/CD29high cells with hedgehog would enhance acini formation. Lin−/CD24+/CD29low and Lin−/CD24+/ CD29high cells were cocultured in ECM in the presence or absence of 5E1 or a nonspecific IgG, and complex acini formation was quantified. Results (Fig. 5D) indicated that coculture of Lin−/CD24+/CD29low with Lin−/CD24+/CD29High cells increased complex acini formation in a manner that was sensitive to 5E1. To confirm that Lin−/CD24+/CD29low-derived Ihh was stimulating complex acini formation, Lin−/CD24+/CD29low cells were infected with a lentivirus programmed to express an shRNA against Ihh or a nonspecific control. Infected cells were extensively washed to remove excess virus and cocultured with Lin−/CD24+/CD29high cells and complex acini were counted. Results (Fig. 5E) indicate that the ability of Lin−/CD24+/ CD29low cells to stimulate complex acini formation by Lin−/CD24+/CD29high cells depended on Ihh. These results indicate that hedgehog stimulation of Lin−/CD24+/CD29high cells enhanced clonigenicity and are consistent with the observation that hedgehog activation enhances elaboration of mammary progenitors. This result, coupled to the finding that hedgehog stimulates differential TP63 promoter usage by restricting accumulation of Gli3 and Gli3R, suggested that disruption of Gli3 expression in Lin−/CD24+/CD29high cells would enhance complex acini formation. Lin−/CD24+/CD29high cells were infected with a lentivirus programmed to express an shRNAs against Gli3 or a nonspecific control, and complex acini forming capacity was measured. Results (Fig. 5F) indicate that disruption of Gli3 expression enhanced complex acini formation by Lin−/ CD24+/CD29high cells. Together, these results indicate that, in contrast to Lin−/CD24+/CD29low cells, hedgehog signaling has a mitogenic effect on Lin−/CD24+/CD29high cells, and they are consistent with enhanced BrdU uptake and Ki-67 expression in Ptch1−/+mice. These results support the conclusion that hedgehog has proliferative and antiproliferative effects on Lin−/CD24+/CD29low and Lin−/CD24+/CD29high cells, respectively.
Enhanced Hedgehog Activity During Pregnancy-Associated Mammary Gland Expansion
The observation that Ihh-expressing Lin−/CD24+/CD29low cells stimulate acini formation of Lin−/CD24+/CD29high cells, coupled to biochemical similarities observed between progenitor-derived acini and lobes of stem cell-derived acini, suggests a positive feedback system in which elaboration of mammary progenitors becomes self-promoting during the proliferative phase of the mammary regenerative cycle. To determine whether this feedback system underlies expansion of the mammary gland during pregnancy, the distribution of Lin−/CD24+/CD49fhigh and Lin−/CD24+/CD49flow cells in virgin mice and mice at day 14 of pregnancy (P14) was compared. This sorting strategy was selected because it improves resolution of myoepithelia and mammary regenerative units (MRUs) [39]. Sorting by these criteria indicated that the number of MRUs (Lin−/CD24+/CD49f high) remained constant, whereas fractions representing myoepithelia and mammary colony-forming cells (Ma-CFCs) expanded during pregnancy (Fig. 6A). Under these conditions, we observed an approximately fourfold increase in the Lin−/CD24+/CD49flow fraction (Ma-CFCs). This result suggests that during pregnancy, the number of mammary stem cells remains stable, whereas the relative number of mammary progenitors increases, which is consistent with increased elaboration of mammary progenitors by mammary stem cells observed in Ptch1−/+mice. To determine whether expansion of mammary progenitors correlates with hedgehog activation in mammary stem cells, levels of Gli3R were measured in Lin−/CD24+/CD29high fractions from virgin mice and P14 mice. Results (Fig. 6B) indicate lower levels of Gli3R in P14 stem cell-enriched fractions, suggesting enhanced hedgehog signaling in mammary stem cells during the proliferative phase of the mammary regenerative cycle. This observation suggests a role for hedgehog signaling in pregnancy-associated mammary gland expansion.
Figure 6.
Hedgehog signaling is active during pregnancy-associated expansion of the mammary gland. (A): Sorting of MECs from virgin and P14 mice on the basis of CD24/CD49f indicated that the MRU-containing fraction remained constant while the MYO fraction was expanded. Results also indicate expansion of the Ma-CFC fraction. (B): Western blot analysis of Gli3 and Gli3R indicated reduced levels of Gli3R in the Lin−/CD24+/CD29High fraction of P14 mice relative to virgin mice. Abbreviations: Ma-CFC, mammary colony-forming cells; MRU, mammary regenerative units; MYO, myoepithelial; P14, progeny day 14.
Discussion
Data presented here support a model (Fig. 7) in which a reciprocal regulatory relationship between TP63 and hedgehog regulates initiation and progression of the mammary regenerative cycle. Our data indicate that hedgehog exerts a mitogenic effect on mammary stem cells, via disruption of Gli3 and Gli3R accumulation, thereby enhancing elaboration of mammary progenitors, and an antiproliferative effect on mammary progenitors, leading to forfeiture of clonigenicity. In Ptch1−/+mice, increased proliferation of Lin−/CD24+/CD29low cells was observed relative to wt counterparts, suggesting that hedgehog activation alone is insufficient to cause the growth arrest of mammary progenitors. Together, these data may suggest that hedgehog signaling initiates elaboration of mammary progenitors and that other factors, which were not present in the acini formation assay, support continued proliferation of mammary progenitors. We present evidence that hedgehog signaling, via disruption of Gli3 and Gli3R accumulation, causes differential TP63 promoter selection, which underlies activation of Ihh expression in mammary progenitors. Taken together, these results support the assertion that hedgehog ligands act as morpho-gens in the mammary gland by exerting stage-specific effects within the mammary regenerative hierarchy. Additional studies involving disruption of various components of this pathway in the Lin−/CD24+/CD29high or Lin−/CD24+/CD49fhigh fractions followed by transplantation into the cleared fat pads of recipient mice will be necessary to validate this model and the physiologic contributions of the relationship between TP63 and Ihh expression to mammary epithelial stasis.
Figure 7.
Proposed model for the role of reciprocal interactions between TP63 and hedgehog signaling in the mammary regenerative hierarchy. Hedgehog stimulation of mammary stem cells is mitogenic and promotes elaboration of mammary progenitors and differential TP63 promoter use. Differential TP63 promoter usage in mammary stem and progenitor cells underlies the progenitor-specific expression of Ihh, which feeds back upon mammary stem cells to promote further elaboration of mammary progenitors. Hedgehog activation of Lin−/CD24+/CD29Low cells restricts their proliferative capacity and clonigenicity, suggesting a role in directing terminal differentiation of mammary progenitors. Abbreviation: HH, hedgehog; Ihh, Indian hedgehog.
In this article, we report distinctly different proliferative states of enriched fractions of mammary stem cells from wt and Ptch1−/+ mice. Our findings indicate that mammary stem cells in wt mice do not express measurable levels of Ki-67, incorporate BrdU at a low rate, and retain BrdU through 10 weeks. In contrast, enriched mammary stem cell fractions from Ptch1−/+ mice express Ki-67, incorporate BrdU rapidly, and fail to retain BrdU through 10 weeks. Together these findings suggest a quiescence defect in the mammary stem cells of the Ptch1−/+mice and may suggest that mammary stem cells exist in distinct states of quiescence or activation. Given the role of ΔN-p63 in preserving the regenerative and replicative capacity of epithelial stem cells and the observation that p63 isoforms are phosphorylated, it will be interesting to determine whether the phosphorylation status of p63 isoforms differs in activated and quiescent mammary stem cells.
Two recent reports have identified GATA3 as a critical regulator of luminal epithelial differentiation by showing that conditional loss of GATA3 resulted in the accumulation of mammary progenitor cells and incomplete luminal epithelial differentiation [45, 46]. Here we present data indicating that in the enriched mammary stem cell fraction of the Ptch1−/+ model, GATA3 expression was increased to levels comparable to those of the progenitor fraction. These results suggest that mammary stem cells that are actively elaborating progenitors express higher levels of GATA3, which is consistent with the critical regulation of GATA3 in the mammary developmental hierarchy. This interpretation is also consistent with the proposed two-state model of quiescence and activation in mammary stem cells. In addition, we present data indicating that individual cells from the enriched progenitor fraction (Lin−/CD24+/CD29low) are able to produce acinar structures that contain p63-positive basal/myoepithelia, suggesting that a degree of bipotency remains within the luminal progenitors. This observation indicates a degree of developmental plasticity within the progenitors that may enable them to sense and respond to aberrant conditions of cellular stasis, such as those under which the acini formation assay is typically conducted. Further study will be necessary to determine the degree of plasticity of luminal progenitors, the role of GATA3 in regulating this plasticity, and the overall biological significance of these observations.
An important question raised by this study pertains to the presence of ΔN-p63 in the myoepithelia. Recent studies have shown that deletion of myocardin-related transcription factor A results in a mammary gland phenotype in which the organization and stasis of the ductal tree appears to be intact, but lactating mice fail to expel milk due to the severe depletion of myoepithelia [47]. This finding indicates that mammary epithelia are able to undergo regenerative cycles in the absence of myoepithelia, suggesting that expression of ΔN-p63 in these cells does not account for the regenerative capacity of the mammary gland. Other studies have shown that ΔN-p63 is required for expression of a genetic program that promotes cellular adhesion, which raises the possibility that ΔN-p63 may function in myoepithelia to promote their adhesion to the surrounding basement membrane. In addition, several studies have linked ΔN-p63 deficiency with premature aging phenotypes [48–50] in mice and have suggested that ΔN-p63 may preserve adult epithelial stem cells by limiting their proliferation and evading telomeric erosion. It is possible that in myoepithelia, ΔN-p63 similarly restricts proliferation while preserving proliferative potential, which would enable myoepithelia to reenter the cell cycle during mammary gland expansion. Additional experimentation is necessary to determine whether either of these two hypotheses regarding the activity of ΔN-p63 in myoepithelia can be proven.
Although the precise identity of mammary stem cells remains elusive, two distinct sorting strategies have identified enriched, separable fractions of mammary stem and progenitor cells [38, 39]. Data presented here indicate that both sorting strategies yielded fractions with identical patterns of ΔN-p63, TA-p63, and Ihh expression. In addition, we provide evidence indicating that LRCs are enriched only in the Lin−/CD24+/CD29high and that these cells are clonigenic and multipotent. These observations are consistent with the presence of mammary stem cells in Lin−/CD24+/CD29high and Lin−/CD24+/CD49fhigh fractions.
The observation that siRNA-mediated disruption of Gli3 expression enhances clonigenicity and alters TP63 promoter usage in Lin−/CD24+/CD29high cells is consistent with recent reports indicating an important role for Gli3 in mammary development [29, 30]. These studies clearly implicate Gli3 in embryonic mammary development, whereas data presented here imply that disruption of Gli3 or Gli3R accumulation mediates hedgehog signaling during mammary stasis. A previous report indicates that deletion of Smo from skin epithelium resulted in development of structures resembling mammary epithelia [51]. These findings are consistent with the differential requirements of Shh during development of hair follicles and mammary gland [52]. These findings, coupled to the important roles of Gli3 and Gli3R in elaboration of mammary progenitors and in mammary gland development [29, 30], suggest that loss of Smoothened in skin may result in enhanced expression of Gli3 and Gli3R.
Although this report demonstrates that Ihh production by mammary progenitors leads to increased clonigenicity of mammary stem cells, it does not rule out other sources of hedgehog ligands that may contribute to or account for activation of mammary stem cells. The observation that complex-acini formation is enhanced by mammary progenitor-derived Ihh may suggest a positive feedback mechanism through which elaboration of mammary progenitors becomes self-promoting. Although it is intriguing to consider this possibility during periods of mammary epithelial expansion, it is equally possible that hedgehog stimulation of mammary stem cells is the result of a stromal/epithelial interaction, as is the case during mammary development [27–30]. In addition, it would be of significant interest to determine whether estrogen signaling, which is an important regulator of the proliferative phase of the mammary regenerative cycle, interacts with hedgehog signaling. Although a compelling case has been made for stromal/epithelial hedgehog signaling during mammary development, data presented here suggest that contributions of hedgehog signaling to mammary regenerative stasis may also be mediated via intraepithelial interactions. Finally, it should be pointed out that although the ability to form heterogeneous acinar structures in a three-dimensional matrix indicates clonigenicity and developmental potency, it is not necessarily reflective of self-renewing capacity.
Previous analysis of label retention in the mammary gland has suggested that long label-retaining cells retain template chromatids via asymmetric segregation [41]. Data presented here indicate that hedgehog activation via Ptch1 heterozygosity caused forfeiture of label retention in the Lin−/CD24+/CD29high fraction. Together with the observations that MECs from Ptch1−/+mice are robustly labeled in short-term labeling experiments and that hedgehog signaling enhances the clonigenicity of Lin−/CD24+/CD29high cells, a likely explanation for forfeiture of label retention observed in the Ptch1−/+is enhanced proliferative capacity in the absence of asymmetric segregation of sister chromatids. These data support our observation of a quiescence defect in the Ptch1−/+mammary stem cell population and are consistent with a recent study indicating that hematopoietic stem cells do not asymmetrically segregate sister chromatids [53]. This further supports the conclusion that hedgehog signaling is mitogenic in this fraction of cells and is consistent with the Ki-67 staining patterns observed. Several possible explanations might account for this inconsistency. It is possible that Ptch1 heterozygosity restricts the ability of mammary stem cells to asymmetrically segregate template and daughter chromatids. A second potential explanation is the fact that the previous study [41] provided estrogenic stimulation during the chase period on the basis of a report that labeling efficiency is maximal during estrus or metestrus [54]. No such treatment was included in the studies presented here. These two possible explanations suggest that either hedgehog activation disrupts asymmetric segregation of sister chromatids or that superphysiologic exposure to estrogen promotes asymmetric segregation; however, no evidence of asymmetric segregation was noted in these studies. Further analysis will be necessary to determine whether either of these explanations accounts for the forfeiture of label retention observed in the Ptch1−/+mice.
Supplementary Material
Acknowledgments
We gratefully acknowledge Dr. John Stingl for helpful analysis of cell-sorting data, Gary Ward for assistance with fluorescence-activated cell sorting, and Ann Levanway for assistance with confocal microscopy. This work was funded by a grant from the National Cancer Institute (5RO1CA108539-04) (to J.D.) and by a grant from the Susan G. Komen Breast Cancer Foundation (BCTR0600556) (to J.D.).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tataria M, Perryman SV, Sylvester KG. Stem cells: Tissue regeneration and cancer. Semin Pediatr Surg. 2006;15:284–292. doi: 10.1053/j.sempedsurg.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 4.Krtolica A. Stem cell: Balancing aging and cancer. Int J Biochem Cell Biol. 2005;37:935–941. doi: 10.1016/j.biocel.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 6.Mills AA, Zheng B, Wang XJ, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 7.Senoo M, Pinto F, Crum CP, et al. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 9.van Bokhoven H, McKeon F. Mutations in the p53 homolog p63: Allele-specific developmental syndromes in humans. Trends Mol Med. 2002;8:133–139. doi: 10.1016/s1471-4914(01)02260-2. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, Kaghad M, Wang Y, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Cherukuri P, Li N, et al. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 2007;67:501–510. doi: 10.1158/0008-5472.CAN-05-4571. [DOI] [PubMed] [Google Scholar]
- 12.Nylander K, Vojtesek B, Nenutil R, et al. Differential expression of p63 isoforms in normal tissues and neoplastic cells. J Pathol. 2002;198:417–427. doi: 10.1002/path.1231. [DOI] [PubMed] [Google Scholar]
- 13.Suh EK, Yang A, Kettenbach A, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dohn M, Zhang S, Chen X. p63alpha and DeltaNp63alpha can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene. 2001;20:3193–3205. doi: 10.1038/sj.onc.1204427. [DOI] [PubMed] [Google Scholar]
- 16.Wu G, Nomoto S, Hoque MO, et al. DeltaNp63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res. 2003;63:2351–2357. [PubMed] [Google Scholar]
- 17.Laurikkala J, Mikkola ML, James M, et al. p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development. 2006;133:1553–1563. doi: 10.1242/dev.02325. [DOI] [PubMed] [Google Scholar]
- 18.Osada M, Park HL, Nagakawa Y, et al. A novel response element confers p63- and p73-specific activation of the WNT4 promoter. Biochem Bio-phys Res Commun. 2006;339:1120–1128. doi: 10.1016/j.bbrc.2005.11.118. [DOI] [PubMed] [Google Scholar]
- 19.Yang A, Zhu Z, Kapranov P, et al. Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Mol Cell. 2006;24:593–602. doi: 10.1016/j.molcel.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Bai CB, Auerbach W, Lee JS, et al. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129:4753–4761. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- 21.Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128:5161–5172. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- 22.Kasper M, Regl G, Frischauf AM, et al. GLI transcription factors: Mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Pan Y, Wang B. A hypermorphic mouse Gli3 allele results in a polydactylous limb phenotype. Dev Dyn. 2007;236:769–776. doi: 10.1002/dvdy.21082. [DOI] [PubMed] [Google Scholar]
- 24.Aza-Blanc P, Lin HY, Ruiz i Altaba A, et al. Expression of the vertebrate Gli proteins in Drosophila reveals a distribution of activator and repressor activities. Development. 2000;127:4293–4301. doi: 10.1242/dev.127.19.4293. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Zhang H, Choi SC, et al. Sonic hedgehog signaling regulates Gli3 processing, mesenchymal proliferation, and differentiation during mouse lung organogenesis. Dev Biol. 2004;270:214–231. doi: 10.1016/j.ydbio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 27.Lewis MT, Ross S, Strickland PA, et al. Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development. 1999;126:5181–5193. doi: 10.1242/dev.126.22.5181. [DOI] [PubMed] [Google Scholar]
- 28.Lewis MT, Ross S, Strickland PA, et al. The Gli2 transcription factor is required for normal mouse mammary gland development. Dev Biol. 2001;238:133–144. doi: 10.1006/dbio.2001.0410. [DOI] [PubMed] [Google Scholar]
- 29.Veltmaat JM, Relaix F, Le LT, et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development. 2006;133:2325–2335. doi: 10.1242/dev.02394. [DOI] [PubMed] [Google Scholar]
- 30.Hatsell SJ, Cowin P. Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development. 2006;133:3661–3670. doi: 10.1242/dev.02542. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moraes RC, Zhang X, Harrington N, et al. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development. 2007;134:1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- 33.Kubo M, Nakamura M, Tasaki A, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64:6071–6074. doi: 10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 34.DiRenzo J, Signoretti S, Nakamura N, et al. Growth factor requirements and basal phenotype of an immortalized mammary epithelial cell line. Cancer Res. 2002;62:89–98. [PubMed] [Google Scholar]
- 35.Rocco JW, Leong CO, Kuperwasser N, et al. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell. 2006;9:45–56. doi: 10.1016/j.ccr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Li H, Cherukuri P, et al. TA-p63-gamma regulates expression of DeltaN-p63 in a manner that is sensitive to p53. Oncogene. 2005 doi: 10.1038/sj.onc.1209270. [DOI] [PubMed] [Google Scholar]
- 37.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 38.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 39.Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 40.Barbareschi M, Pecciarini L, Cangi MG, et al. p63, a p53 homologue, is a selective nuclear marker of myoepithelial cells of the human breast. Am J Surg Pathol. 2001;25:1054–1060. doi: 10.1097/00000478-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–687. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- 42.Welm B, Behbod F, Goodell MA, et al. Isolation and characterization of functional mammary gland stem cells. Cell Prolif. 2003;36(suppl 1):17–32. doi: 10.1046/j.1365-2184.36.s.1.3.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodward WA, Chen MS, Behbod F, et al. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- 44.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kouros-Mehr H, Slorach EM, Sternlicht MD, et al. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 47.Li S, Chang S, Qi X, et al. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol Cell Biol. 2006;26:5797–5808. doi: 10.1128/MCB.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holder-Espinasse M, Martin-Coignard D, Escande F, et al. A new mutation in TP63 is associated with age-related pathology. Eur J Hum Genet. 2007;15:1115–1120. doi: 10.1038/sj.ejhg.5201888. [DOI] [PubMed] [Google Scholar]
- 49.Keyes WM, Wu Y, Vogel H, et al. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev. 2005;19:1986–1999. doi: 10.1101/gad.342305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sommer M, Poliak N, Upadhyay S, et al. DeltaNp63alpha overexpression induces downregulation of Sirt1 and an accelerated aging phenotype in the mouse. Cell Cycle. 2006;5:2005–2011. doi: 10.4161/cc.5.17.3194. [DOI] [PubMed] [Google Scholar]
- 51.Gritli-Linde A, Hallberg K, Harfe BD, et al. Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling. Dev Cell. 2007;12:99–112. doi: 10.1016/j.devcel.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallego MI, Beachy PA, Hennighausen L, et al. Differential requirements for shh in mammary tissue and hair follicle morphogenesis. Dev Biol. 2002;249:131–139. doi: 10.1006/dbio.2002.0761. [DOI] [PubMed] [Google Scholar]
- 53.Kiel MJ, He S, Ashkenazi R, et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature. 2007;449:238–242. doi: 10.1038/nature06115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeps N, Dawkins HJ, Papadimitriou JM, et al. Detection of a population of long-lived cells in mammary epithelium of the mouse. Cell Tissue Res. 1996;286:525–536. doi: 10.1007/s004410050722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.