Abstract
Objective
The purpose of this study was to describe the safety, tolerability, and efficacy of quetiapine monotherapy continued for up to 26-weeks in youth with schizophrenia or bipolar I disorder.
Methods
Medically healthy boys and girls with a baseline Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV-TR) diagnosis of schizophrenia (ages 13–17 years) or a manic episode of bipolar I disorder (ages 10–17 years) who participated in one of two acute, double-blind, placebo-controlled studies of immediate-release quetiapine were potentially eligible to enroll in a 26-week, open-label study. During the open-label study, quetiapine was flexibly dosed at 400–800 mg/day, with options to reduce dosing to 200 mg/day based on tolerability. Safety and tolerability outcomes assessed from open-label baseline to week 26 included adverse events (AEs), metabolic/laboratory parameters, extrapyramidal symptoms, suicidality, and vital signs.
Results
Of 381 patients enrolled in the open-label study (n=176, schizophrenia; n=205, bipolar disorder diagnosis), 237 patients (62.2%) completed the 26-week study period (71.0%, schizophrenia; 54.6%, bipolar disorder). The most common AEs reported during the study included somnolence, headache, sedation, weight increase, and vomiting. A total of 14.9% of patients experienced a shift to potentially clinically significant low levels of high-density lipoprotein cholesterol and 10.2% of patients experienced a shift to potentially clinically significant high triglyceride levels. Weight gain ≥7% was reported in 35.6% of patients between open-label baseline and final visit. After adjustment for normal growth, 18.3% of study participants experienced clinically significant weight gain (i.e., increase in body mass index ≥0.5 standard deviations from baseline).
Conclusions
In this 26-week study, quetiapine flexibly dosed at 400–800 mg/day, with options to reduce dosing based on tolerability, was generally safe and well tolerated in youth. Clinicians should monitor lipid profiles and weight gain in youth with schizophrenia or bipolar disorder during treatment with quetiapine.
Clinical trial registration information
Quetiapine Fumarate (Seroquel) in the Treatment of Adolescent Patients With Schizophrenia and Bipolar I Disorder (ANCHOR 150). Available at: http://clinicaltrials.gov/ct2/show/NCT00227305
Introduction
Early-onset schizophrenia and bipolar disorder are chronic and debilitating psychiatric disorders. Between 20 and 40% of patients with schizophrenia receive a diagnosis before the age of 20 years, whereas up to two-thirds of patients with bipolar disorder experience onset of symptoms during childhood or adolescence (Loranger 1984; Lish et al. 1994; Perlis et al. 2004).
Onset of schizophrenia or bipolar disorder during youth is associated with poor outcomes, as patients fail to achieve their full educational potential and suffer impaired social functioning that often persists into adulthood (Birmaher et al. 2006; Geller et al. 2006; Reichert et al. 2008). Early-onset schizophrenia is associated with high rates of recurrent psychotic symptoms, depression, and suicidality (Lay et al. 2000; Fleischhaker et al. 2005; Goldstein et al. 2005; Reichert et al. 2008). Early onset bipolar disorder is similarly associated with high rates of recurrence, increased symptom severity, and greater risk of suicidality when compared with onset later in life (Strober et al. 1995; Post et al. 2001; Geller et al. 2006). Evidence is emerging that the response to treatment may decline if bipolar disorder is allowed to go untreated, with more severe mood episodes, increased rates of rapid cycling, increased risk of suicidality, and adverse effects on cognitive functioning in later life (Post et al. 1996; Tsai et al. 2007; Findling 2009; Berk et al. 2010). For all these reasons, effective, safe, and well-tolerated treatments are needed for youth with schizophrenia and bipolar disorder.
Pharmacotherapy, frequently with atypical antipsychotics, is a mainstay of treatment for schizophrenia and bipolar disorder in adults. Until recently, there has been a paucity of controlled data on atypical antipsychotic use during childhood and adolescence. However, data are now emerging on the efficacy, safety, and tolerability of atypical antipsychotics, primarily as an acute treatment, in children and adolescents with schizophrenia (Findling et al. 2008; Jensen et al. 2008; Kumra et al. 2008; Sikich et al. 2008; Haas et al. 2009b; Kryzhanovskaya et al. 2009; Findling et al. 2010) and those with a manic episode associated with bipolar disorder (Tohen et al. 2007; Findling et al. 2009; Haas et al. 2009a; Zeni et al. 2009; Correll et al. 2009; Fraguas et al. 2010; Pavuluri et al. 2010).
Two recent multicenter, placebo-controlled trials investigated the acute efficacy and safety of immediate-release quetiapine for psychotic symptoms in adolescents with schizophrenia at a dose of 400 or 800 mg/day (Findling et al. 2012) and for manic episodes in children and adolescents with bipolar I disorder at a dose of 400 or 600 mg/day (Pathak et al. 2013). Based in part on the results of these trials, immediate-release quetiapine is approved by the United States Food and Drug Administration (FDA) for the treatment of schizophrenia in adolescents ages 13–17 years, and for the acute treatment of manic episodes of bipolar disorder in children and adolescents ages 10–17 years.
As schizophrenia and bipolar disorder in youth frequently require continued pharmacological management, this open-label study investigated the safety, tolerability, and efficacy of immediate-release quetiapine monotherapy for up to 26-weeks in patients who participated in the two acute studies described.
Methods
This 26-week, open-label study was conducted at 59 centers in Asia, Central and Eastern Europe, South Africa, and the United States from August 2004 to January 2008 (Study D144C00150; ClinicalTrials.gov identifier: NCT00227305).
Study population
Medically healthy boys and girls with a baseline Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR; American Psychiatric Association 2000) diagnosis of schizophrenia (ages 13–17 years) or a manic episode associated with bipolar I disorder (ages 10–17 years), who had completed or discontinued participation in one of two acute, double-blind, placebo-controlled studies of quetiapine monotherapy (ClinicalTrials.gov identifiers: NCT00090324 and NCT00090311; Findling et al. 2012; Pathak et al. 2013), were potentially eligible to participate in the current open-label study. Diagnoses in the acute studies were confirmed by the Schedule of Affective Disorders and Schizophrenia for School-Aged Children–Present and Lifetime Version (K-SADS-PL; Kaufman et al. 1997). Details on the inclusion and exclusion criteria in the acute studies are reported by Findling et al. (2012) and Pathak et al. (2013).
Patients and their parents or legal guardians who indicated a willingness to enroll in the open-label study were screened for eligibility. Criteria for exclusion from the open-label study included DSM-IV-TR Axis I diagnoses of schizophreniform disorder, schizoaffective disorder, psychotic disorder not otherwise specified (NOS), bipolar II disorder, and bipolar disorder NOS. Patients with psychosis judged to be the direct consequence of a medical condition or treatment, at current or prior suicidal or homicidal risk, with substance abuse or dependence, or with unstable physical or metabolic disease that could, in the opinion of the investigator, be negatively affected by study medication, were excluded from the open-label study (as in the acute studies). The interval between the last double-blind study visit and day 1 of the open-label study could not exceed 7 days. Patients were required to express a willingness to adhere to the schedule of assessments. Written assent from the patient and informed consent from the parent or legal guardian were obtained prior to study procedures.
Study treatments
Eligible enrolled patients received open-label quetiapine initiated at a dose of 50 mg on the evening of day 1 and escalated to 400 mg by day 5. Thereafter, the quetiapine dose of 400 mg/day (administered two or three times daily) was maintained or increased to a maximum of 800 mg/day at the investigator's discretion, with options to reduce dosing to 200 mg/day based on tolerability.
Concomitant medications considered necessary to the patient's safety and well-being were permitted to be initiated or continued at the discretion of the investigator, with the exception of other antipsychotic medications, potent CYP3A4 inhibitors (e.g., ketoconazole) and inducers (e.g., carbamazepine, phenytoin), fluoxetine, monoamine oxidase inhibitors, or atomoxetine. Mood stabilizers and antidepressants, psychostimulants and benzodiazepines (for acute anxiety/agitation), nonprophylactic benztropine (for extrapyramidal symptoms [EPS]), and diphenhydramine (for insomnia) were permitted during the study if clinically indicated.
Study end-points
The primary study objective was evaluation of the safety and tolerability of quetiapine monotherapy from open-label baseline to week 26. Assessments included the incidence and severity of adverse events (AEs) reported at any time during the open-label study using Medical Dictionary for Regulatory Activities (MedDRA) coding, withdrawals caused by AEs, changes in weight and body mass index (BMI), clinical laboratory tests performed under fasting conditions (i.e., absence of food or liquids, other than water, for ≥8 hours), vital signs, changes in 12 lead electrocardiogram (ECG), and physical examination. AEs of special interest were identified by physicians during evaluation of the safety data after study completion, including marked hematological and other laboratory abnormalities and events (other than those classified as serious) that led to intervention, dose reduction, or significant additional treatment. Emergent EPS were assessed by AEs; by the Simpson–Angus Scale (SAS; Simpson and Angus 1970), Barnes Akathisia Rating Scale (BARS; Barnes 1989), and Abnormal Involuntary Movement Scale (AIMS; Guy 1976) scores; and by anticholinergic use. Assessments were conducted at baseline and on study visits at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 26, with ECG assessments performed at baseline, week 12, and final study visit.
Suicide-related behaviors were assessed as AEs during the study. Suicidality analyses were also conducted retrospectively for each patient by independent trained physicians using standardized classifications similar to those in the Columbia Suicidality Classification Project; that is, suicidal behavior, suicidal ideation, and possible suicide events (including self-injurious behavior with unknown intent, not enough information, and not fatal) (Posner et al. 2007, 2011). Additional safety assessments included physical development measured by Tanner staging (Tanner 1962; Morris and Udry 1980) and initiation or changes in menses, both assessed by the investigating physician.
Exploratory efficacy end-points in patients with schizophrenia included the Positive and Negative Syndrome Scale (PANSS; Kay et al. 1987) and the Clinical Global Impressions (CGI) Improvement and Severity of Illness scale (Guy and Bonato 1970). The Young Mania Rating Scale (YMRS; Young et al. 1978) and the CGI-Bipolar Disorder (CGI-BP) Improvement and Severity of Illness scale (Spearing et al. 1997) were used to assess efficacy in patients with bipolar disorder. Functioning was measured in all patients by the Children's Global Assessment Scale (CGAS; Shaffer et al. 1983). PANSS and YMRS were assessed at open-label baseline and weeks 4, 8, 16, and 26; CGI measures were recorded at all study visits; and CGAS was assessed at open-label baseline and week 26.
The burden experienced by parents or guardians was measured by the Caregiver Strain Questionnaire (CGSQ), using a scoring convention that calculates a global score from the subscale scores: internalized subjective strain, externalized subjective strain, and objective strain; higher scores indicate increased burden (Brannan et al. 1997). CGSQ was assessed at open-label baseline and week 26.
Statistical analyses
Descriptive statistics are presented throughout. Analyses of safety and tolerability were performed on the safety population, which consisted of all patients who took at least one dose of open-label study medication. AEs were reported that occurred at any time during the open-label study, including the development of new medical conditions or the deterioration of pre-existing medical conditions. Changes in weight, BMI, and laboratory parameters were assessed from baseline of the open-label study to final visit. Changes in EPS rating scale scores were analyzed from baseline of the open-label study to week 26 using a last observation carried forward (LOCF) approach. Analyses of efficacy were based on observed cases or using an LOCF approach in the safety population.
Results
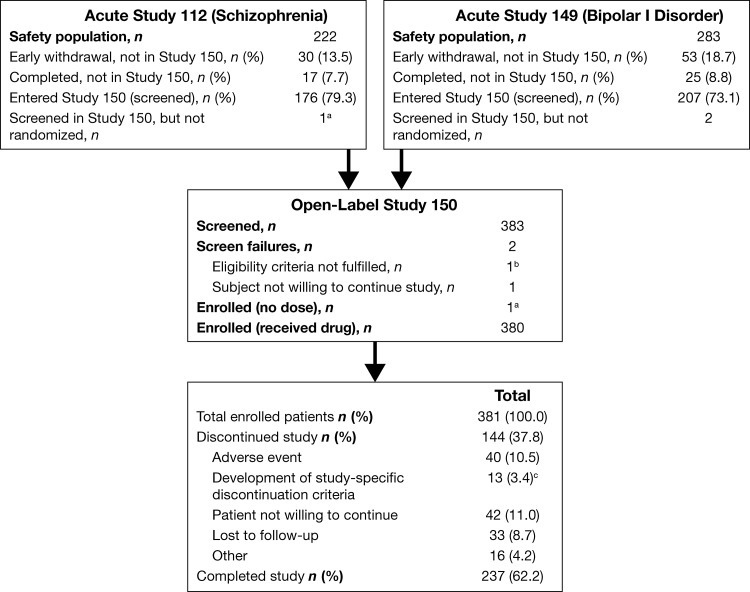
The safety populations of the two acute, double-blind, placebo-controlled trials of quetiapine included 505 patients (n=222, schizophrenia; n=283, bipolar disorder) (Findling et al. 2012; Pathak et al. 2013). Of these, 383 patients were screened for the open-label study and 381 were enrolled (n=176, schizophrenia; n=205, bipolar disorder); 2 patients with bipolar disorder were screen failures, because of unwillingness to adhere to the schedule of assessments and unwillingness to continue the study, respectively (Fig. 1). An additional patient with schizophrenia was enrolled but discontinued before receiving study medication because of unwillingness to continue the study (n=380, safety population). Of the 175 patients with schizophrenia in the open-label safety population, 113 had been treated with quetiapine (400 or 800 mg/day) and 62 with placebo in the acute 6 week study. Of the 205 patients with bipolar disorder in the open-label safety population, 138 were treated with quetiapine (400 or 600 mg/day) and 67 with placebo in the acute 3 week study.
FIG. 1.
Patient disposition. aDiscontinued the study before receiving any study drug and not included in the safety population. bUnwillingness to adhere to schedule of assessments. cVoluntary discontinuation by patient or parent/guardian, safety reasons assessed by investigator, Clinical Global Impressions (CGI) Global Improvement (schizophrenia) or CGI-BP Global Improvement (bipolar I disorder) score of 6 (or 5 at two consecutive visits), severe noncompliance, clinically significant adverse event (AE) not consistent with study continuation, absolute neutrophil count <1.0×109/L, unable to tolerate at least 200 mg/day quetiapine, unable to comply with restrictions on use of concomitant medications, pregnancy, or imminent risk of suicide.
Demographic and clinical characteristics of patients who comprised the open-label safety population are summarized in Table 1, together with the characteristics, for comparison, of patients who participated in the acute studies. Patients were excluded from the open-label safety population (n=47, schizophrenia; n=78, bipolar disorder) primarily because of early withdrawal from the acute studies (Fig. 1). Patients who chose not to enter the open-label study (n=42) had similar demographic and clinical characteristics at last assessment to the open-label safety population at baseline. In the schizophrenia subgroup that chose not to enroll (n=17), mean (standard deviation [SD]) age was 15.2 (1.4) years, 7 were female, 9 were white, and 5 were black, and the mean (SD) CGAS score was 51.9 (10.2), whereas the bipolar subgroup that chose not to enroll (n=25) had a mean age of 13.5 (2.0) years, 12 were female, 11 were white, and 8 were black, and the mean (SD) CGAS score was 55.4 (9.5).
Table 1.
Demographic and Clinical Characteristics of Patients at Baseline of Open-Label Study Versus Baseline of Acute Studies (Safety Populations)
| |
Acute study baseline |
Open-label study baseline |
|||
|---|---|---|---|---|---|
| Category | Schizophrenia population (n=222)a | Bipolar population (n=283)a | Combined population (n=380) | Schizophrenia subgroup (n=175) | Bipolar subgroup (n=205) |
| Demographic characteristics | |||||
| Age, years, mean (SD) | 15.4 (1.3) | 13.2 (2.2) | 14.4 (2.2) | 15.7 (1.4) | 13.3 (2.1) |
| Gender, n (%) | |||||
| Female | 91 (41.0) | 123 (43.5) | 154 (40.5) | 69 (39.4) | 85 (41.5) |
| Male | 129 (59.0) | 160 (56.5) | 226 (59.5) | 106 (60.6) | 120 (58.5) |
| Weight, (kg), mean (SD) | 61.7 (16.1) | 60.9 (18.3) | 62.1 (17.6) | 62.4 (16.0) | 61.8 (18.9) |
| BMI (kg/m2), mean (SD) | 22.3 (5.0) | 23.7 (5.2) | 23.3 (5.4) | 22.5 (5.5) | 24.1 (5.3) |
| Race, n (%) | |||||
| White | 137 (61.7) | 216 (76.3) | 268 (70.5) | 105 (60.0) | 163 (79.5) |
| Black | 27 (12.2) | 40 (14.1) | 45 (11.8) | 18 (10.3) | 27 (13.2) |
| Asian | 40 (18.0) | 1 (0.4) | 38 (10.0) | 38 (21.7) | 0 (0) |
| Other | 18 (8.1) | 26 (9.2) | 29 (7.6) | 14 (8.0) | 15 (7.3) |
| Clinical characteristics | Schizophrenia population (n=175)b | Bipolar population (n=205)b | Combined population (n=380) | Schizophrenia subgroup (n=175) | Bipolar subgroup (n=205) |
|---|---|---|---|---|---|
| CGAS score, mean (SD) | 42.7 (10.9) | 45.7(10.2) | 56.9 (14.7) | 54.7 (15.1) | 58.7 (14.1) |
| CGSQ score, mean (SD) | 5.7 (2.4) | 7.1 (2.3) | 5.5 (2.5) | 5.7 (2.4) | 5.2 (2.4) |
| PANSS total score, mean (SD) | 96.9 (16.8) | - | - | 73.1 (22.1) | - |
| CGI-S, mean (SD) | 4.6 (0.7) | - | 3.6 (1.2) | - | |
| YMRS total score, mean (SD) | - | 29.7 (5.9) | - | - | 16.3 (10.3) |
| CGI-BP-S, mean (SD) | - | 4.7 (0.7) | - | - | 3.1 (1.5) |
All patients in acute study safety populations.
Patients from acute studies who entered the open-label study
BMI, body mass index; CGAS, Children's Global Assessment Scale; CGSQ, Caregiver Strain Questionnaire; PANSS, Positive and Negative Syndrome Scale; CGI-S, Clinical Global Impressions – Severity; YMRS, Young Mania Rating Scale; CGI-BP-S, Clinical Global Impressions – Bipolar – Severity.
The planned treatment duration of 26-weeks was completed by 237 (62.2%) of the 381 enrolled patients (71.0%, schizophrenia; 54.6%, bipolar disorder). Reasons for study discontinuation were most commonly “unwilling to continue” and “adverse events” (each approximating 11% of the total population).
The mean (SD) daily dose of quetiapine during open-label treatment was 599 (157) mg, over a mean duration of 145.6 (60.4) days. Mean daily quetiapine dose and duration of treatment were 632 mg and 156 days in the schizophrenia subgroup and 571 mg and 137 days in the bipolar disorder subgroup. Treatment compliance, calculated as the number of tablets taken (dispensed – returned) divided by the number of tablets prescribed, was high in both diagnostic subgroups (110%, schizophrenia; 104%, bipolar disorder). A figure>100% is assumed to result from a failure of patients to return the tablets prescribed as contingency stock to maintain access to medication in the event of delay, cancellation, or rescheduling of a study visit.
Concomitant medications were taken by 250 patients (65.7%) during the study (53.7%, schizophrenia; 76.1%, bipolar disorder). The most common concomitant medications in the total population were acetaminophen (20.3%), ibuprofen (15.3%), lorazepam (7.9%), and methylphenidate hydrochloride (7.4%). There were no notable subgroup differences in the use of antidepressants (n=3 patients, each diagnostic subgroup) or mood stabilizers (n=2, schizophrenia; n=7, bipolar subgroup). Psychostimulant use was more frequent in the bipolar disorder (n=29, 14.1%) than in the schizophrenia (n=3, 1.7%) subgroup.
Tolerability
AEs were reported by 84.5% of patients in the safety population during the open-label study (78.3%, schizophrenia; 89.8% bipolar disorder). The most common AEs in both diagnostic subgroups were somnolence, headache, sedation, weight increase, and vomiting (Table 2).
Table 2.
Common (>5% in Any Group) Adverse Events of Any Severity During Open-Label Study (Safety Population)
| Adverse event, n (%) | All patients(n=380) | Schizophrenia subgroup(n=175) | Bipolar disorder subgroup(n=205) |
|---|---|---|---|
| Somnolence | 87 (22.9) | 42 (24.0) | 45 (22.0) |
| Headache | 71 (18.7) | 22 (12.6) | 49 (23.9) |
| Sedation | 54 (14.2) | 12 (6.9) | 42 (20.5) |
| Weight increase | 51 (13.4) | 16 (9.1) | 35 (17.1) |
| Vomiting | 41 (10.8) | 18 (10.3) | 23 (11.2) |
| Nausea | 36 (9.5) | 11 (6.3) | 25 (12.2) |
| Dizziness | 33 (8.7) | 12 (6.9) | 21 (10.2) |
| Fatigue | 31 (8.2) | 7 (4.0) | 24 (11.7) |
| Insomnia | 31 (8.2) | 13 (7.4) | 18 (8.8) |
| Increased appetite | 27 (7.1) | 9 (5.1) | 18 (8.8) |
| Upper respiratory tract infection | 26 (6.8) | 12 (6.9) | 14 (6.8) |
| Agitation | 20 (5.3) | 13 (7.4) | 7 (3.4) |
| Irritability | 19 (5.0) | 8 (4.6) | 11 (5.4) |
| Tachycardia | 19 (5.0) | 9 (5.1) | 10 (4.9) |
| Upper abdominal pain | 17 (4.5) | 4 (2.3) | 13 (6.3) |
| Pyrexia | 17 (4.5) | 6 (3.4) | 11 (5.4) |
| Nasal congestion | 15 (3.9) | 4 (2.3) | 11 (5.4) |
| Bipolar disorder | 11 (2.9) | 0 | 11 (5.4) |
| Anxiety | 10 (2.6) | 9 (5.1) | 1 (0.5) |
| Schizophrenia | 9 (2.4) | 9 (5.1) | 0 |
AEs were responsible for study discontinuation in 10.5% of the enrolled population (6.3%, schizophrenia; 14.1%, bipolar disorder). Of the 62 AEs associated with discontinuation, 33 (53.2%) were considered treatment related. Irritability (1.6%) was the most frequently reported AE leading to discontinuation in both diagnostic subgroups; other AEs associated with discontinuation occurred in<1% of patients. Most AEs were mild or moderate in intensity. Sixty AEs in 11.3% of patients were rated serious, including exacerbation of bipolar disorder (2.9% of patients), schizophrenia (1.8%), and aggression (0.8%). Serious AEs of appendicitis, overdose, and psychotic disorder each occurred in 0.5% of patients. AEs of special interest identified by physicians included events potentially associated with neutropenia (0.6% schizophrenia; 2.0%, bipolar), diabetes mellitus (1.1%; 1.5%), QT prolongation ≥500 msec (1.7%; 1.0%), and syncope (0.6%; 1.0%).
The incidence of any AEs and the incidence of serious AEs in the bipolar disorder subgroup were similar between children ages 10–12 years (88.5% and 9.2%, respectively) and adolescents ages 13–18 years (90.7% and 9.3%, respectively). AEs during open-label study were reported in 85.7% of patients who were treated with quetiapine (80.5%, schizophrenia; 89.9%, bipolar disorder) and in 82.2% who were treated with placebo (74.2%, schizophrenia; 89.6%, bipolar disorder) in the acute studies. The most common AEs occurring during the open-label study in patients categorized by prior treatment in the acute studies were somnolence (23.9%, quetiapine; 20.9%, placebo), headache (19.5%; 17.1%), sedation (12.4%; 17.8%), dizziness (6.8%; 12.4%), nausea (9.2%; 10.1%), weight increase (10.8%; 18.6%), and increased appetite (5.2%; 10.9%).
Metabolic/laboratory parameters
Mean changes in metabolic and laboratory parameters between open-label baseline and final visit are summarized in Table 3. Shifts from normal to predefined values of potential clinical importance at any time during the study for fasting glucose, HbA1c, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, and prolactin concentrations are reported in Table 4.
Table 3.
Mean (SD) Changes in Metabolic and Laboratory Parameters, Vital Signs, and ECG Measures from Open-Label Baseline to Final Visit (Safety Population)
| Parameter | All patients | n | Schizophrenia subgroup | n | Bipolar disorder subgroup | n |
|---|---|---|---|---|---|---|
| Weight (kg) | 3.7 (7.3) | 374 | 3.3 (9.1) | 175 | 4.0 (5.2) | 199 |
| BMI (kg/m2) | 0.9 (3.3) | 371 | 0.8 (4.2) | 175 | 0.9 (2.1) | 196 |
| Fasting glucose (mg/dL) | 2.6 (20.0) | 334 | 5.3 (25.2) | 161 | 0.1 (13.1) | 173 |
| Insulin (μIU/mL) | 0.1 (31.5) | 317 | 2.0 (25.1) | 158 | −1.8 (36.7) | 159 |
| HbA1c (%) | 0.1 (0.5) | 332 | 0.1 (0.7) | 160 | 0.0 (0.2) | 172 |
| Total cholesterol (mg/dL) | −2.5 (25.7) | 334 | −0.5 (28.2) | 161 | −4.4 (23.1) | 173 |
| LDL cholesterol (mg/dL) | −1.3 (22.3) | 333 | −0.2 (23.6) | 160 | −2.4 (21.1) | 173 |
| HDL cholesterol (mg/dL) | −1.7 (8.5) | 334 | −0.6 (8.6) | 161 | −2.7 (8.2) | 173 |
| Triglycerides (mg/dL) | 1.8 (69.0) | 334 | −0.1 (68.0) | 161 | 3.6 (70.1) | 173 |
| ALT (IU/L) | −2.7 (20.9) | 332 | −2.8 (24.2) | 160 | −2.6 (17.2) | 172 |
| Prolactin (ng/mL) | −1.0 (12.4) | 334 | 0.5 (13.8) | 161 | −2.2 (10.8) | 173 |
| TSH (μIU/mL) | 0.2 (1.3) | 327 | 0.3 (1.2) | 157 | 0.0 (1.3) | 170 |
| Total thyroxine (μg/mL) | −0.1 (1.7) | 333 | −0.1 (1.7) | 160 | −0.0 (1.6) | 173 |
| Supine pulse (bpm) | 0.8 (14.7) | 375 | 1.0 (12.9) | 175 | 0.7 (16.2) | 200 |
| ECG heart rate (bpm) | 0.2 (16.36) | 233 | −0.06 (16.3) | 118 | 0.5 (16.5) | 115 |
| ECG QT interval (msec) | −2.1 (27.4) | 233 | −0.3 (25.2) | 118 | −4.1 (29.4) | 115 |
BMI, body mass index; HbA1c, glycated hemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein; ALT, alanine transaminase; TSH, thyroid stimulating hormone; ECG, electrocardiogram.
Table 4.
Potentially Clinically Significant Shifts in Selected Metabolic, Laboratory, and Vital Sign Parameters from Open-Label Baseline to Final Visit (Safety Population)
| |
Shift to low, n (%) |
Shift to high, n (%) |
||||
|---|---|---|---|---|---|---|
| Parameter (shift criteria) | All patients | Bipolar I disorder subgroup | Schizophrenia subgroup | All patients | Bipolar disorder subgroup | Schizophrenia subgroup |
| Fasting glucose (≥126 mg/dL)a | 0 | 0 | 0 | 7 (2.1) | 1 (0.6) | 6 (3.8) |
| HbA1c (>7.5%) | NA | NA | NA | 2 (0.6) | 0 | 2 (1.3) |
| Total cholesterol (≥240 mg/dL) | NA | NA | NA | 1 (0.3) | 0 | 1 (0.6)) |
| LDL cholesterol (≥160 mg/dL) | NA | NA | NA | 1 (0.3) | 1 (0.6) | 0 |
| HDL cholesterol (≤40 mg/dL) | 40 (14.9) | 19 (13.4) | 21 (16.5) | NA | NA | NA |
| Triglycerides (≥200 mg/dL) | NA | NA | NA | 31 (10.2) | 18 (11.9) | 13 (8.4) |
| Prolactin (>26 μg/L females, >20 μg/L males) | NA | NA | NA | 15 (8.5) | 5 (6.0) | 10 (10.8) |
| Blood pressure (mm Hg) | ||||||
| Supine systolic (increase or decrease ≥20 mmHg) | 11 (3.3) | 3 (1.7) | 8 (5.1) | 16 (4.8) | 12 (6.7) | 4 (2.6) |
| Supine diastolic (increase ≥30 mmHg or decrease ≥20 mmHg | 9 (2.6) | 5 (2.7) | 4 (2.5) | 21 (6.1) | 12 (6.5) | 9 (5.5) |
| Standing systolic (increase or decrease ≥20 mmHg) | 13 (4.0) | 9 (5.4) | 4 (2.6) | 17 (5.3) | 9 (5.4) | 8 (5.2) |
| Standing diastolic (increase ≥30 mmHg or decrease ≥20 mmHg) | 5 (1.6) | 3 (1.9) | 2 (3.3) | 44 (14.0) | 31 (19.5) | 13 (8.3) |
Patient-reported fasting ≥8 hours between time of last meal and time of blood draw.
HbA1c, glycated hemoglobin; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Body weight and BMI
The mean change in body weight from open-label baseline to final visit was 3.7 (SD 7.3) kg (Table 3). Weight gain ≥7% was recorded in 134 patients (35.6%) in the total population. Weight gain ≥7% occurred in 29.1% of the schizophrenia subgroup (28.3% and 30.6% in the prior quetiapine and placebo groups, respectively) and in 41.3% of the bipolar disorder subgroup (38.2% and 47.7% in the prior quetiapine and placebo groups, respectively).
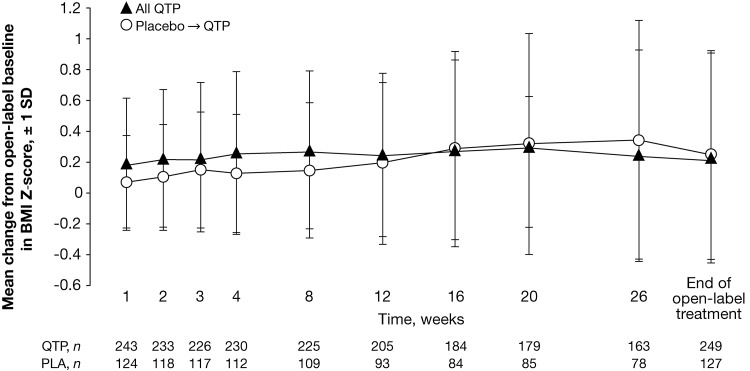
The mean change in BMI from open-label baseline to final visit was 0.9 kg/m2 (0.8 kg/m2, schizophrenia; 0.9 kg/m2, bipolar disorder). To adjust for normal growth over 26-weeks, a predetermined increase in BMI of at least 0.5 SD from baseline was used as a measure of clinically significant change (Centers for Disease Control and Prevention 2000). By this criterion, 18.3% of patients experienced a clinically significant gain in weight during 26-weeks of treatment. Changes in BMI z score, adjusted for age and gender, are compared in patient groups categorized by prior treatment (i.e., quetiapine or placebo) in the acute studies in Figure 2.
FIG. 2.
Mean change in body mass index (BMI) z score, adjusted for age and gender, from open-label baseline to final visit (safety population; observed cases). PLA, placebo; QTP, quetiapine.
EPS
AEs potentially associated with EPS were reported in 10.0% (n=38) of the safety population during open-label study (11.4%, schizophrenia; 8.8%, bipolar disorder). The most common EPS-related AEs were akathisia (3.7%) and restlessness (2.1%), with other potentially related events (i.e., extrapyramidal disorder, muscle rigidity, and tremor) occurring at a frequency of<2%. Dyskinesia occurred in 0.5% of patients. The majority of EPS-related events were rated mild or moderate in intensity.
Mean (SD) changes in SAS total score from open-label baseline to week 26 were −0.2 (1.49) in the total population, −0.2 (1.86) in the schizophrenia subgroup, and −0.1 (1.05) in the bipolar disorder subgroup (LOCF). Equivalent mean changes in BARS score were −0.1 (0.48), −0.1 (0.40), and −0.1 (0.54), and changes in AIMS-7 score were 0.0 (1.24), −0.1 (1.40) and 0.1 (1.08), respectively. Most patients experienced either no change or an improvement in SAS (90.9%), BARS (97.0%), and AIMS-7 (94.1%) scores. Anticholinergics were used to treat EPS in 4.2% of patients (2.9%, schizophrenia; 5.4% bipolar disorder).
Suicidality
AEs potentially associated with suicidality during the open-label study consisted of two cases of suicidal ideation and one suicidal attempt in the schizophrenia subgroup and one case each of self-mutilation and intentional self-injury in the bipolar disorder subgroup.
Retrospective analyses similar to those used in the Columbia Suicidality Classification Project (Posner et al. 2007, 2011) identified one case of suicidal behavior, two cases of suicidal ideation, and two possible suicide events in the schizophrenia subgroup, and three cases of suicidal ideation and seven possible suicide events in the bipolar disorder subgroup.
Vital signs
Mean changes in pulse and ECG parameters are reported in Table 3. Shifts of potential clinical importance in pulse and heart rate (i.e., an increase or decrease of ≥15 beats per minute from baseline) occurred in<10% of the total population during the study. Shifts to potentially clinically significant high or low systolic and diastolic blood pressures are described in Table 4.
ECG-related AEs that were reported by more than one patient included tachycardia (n=19 patients), sinus tachycardia (n=5), bundle branch block (n=3), and prolonged QT ≥500 msec (n=5). All patients with prolonged QT had normal QTc (Fridericia) intervals below the 450 msec cutoff (range 412–445 msec).
Additional safety variables
Assessment of the Tanner stage and menstruation status identified no untoward events relating to growth and development or to menstruation status during continued quetiapine treatment.
Exploratory efficacy outcomes
Between open-label baseline and week 26, the mean (SD) PANSS total score decreased by 9.8 (18.1) points in the schizophrenia subgroup (−6.1 [17.3] and −17.0 [17.9] in the prior quetiapine and placebo groups, respectively) (LOCF). The mean YMRS total score decreased by 3.5 (10.6) points in the bipolar subgroup (−1.4 [10.4] and −8.0 [9.7] in the prior quetiapine and placebo groups, respectively) (Table 5). Overall mean score changes from the acute study baseline to the end of the open-label study were reductions of 34 (21.9) points in the schizophrenia subgroup and 17 (9.5) points in the bipolar subgroup (LOCF).
Table 5.
Efficacy Rating Scale Scores from Open-Label Baseline to Week 26, Categorized by Diagnosis and Prior Treatment During Acute Studies (Safety Population)
| |
|
|
Prior treatment group |
|||
|---|---|---|---|---|---|---|
| |
Total safety population |
Quetiapine |
Placebo |
|||
| Rating scale | Open-label baseline (mean, SD) | Change at week 26 (mean, SD) | Open-label baseline (mean, SD) | Change at week 26 (mean, SD) | Open-label baseline (mean, SD) | Change at week 26 (mean, SD) |
| Schizophrenia subgroup | ||||||
| PANSSa | 73.1 (22.1) | −9.8 (18.1) | 69.4 (18.9) | −6.1 (17.3) | 79.8 (25.9) | −17.0 (17.9) |
| CGI-Sa | 3.6 (1.2) | −0.5 (1.2) | 3.4 (1.0) | −0.3 (1.1) | 4.0 (1.4) | −1.0 (1.3) |
| CGSQb | 5.7 (2.4) | −0.6 (1.8) | 5.5 (2.4) | −0.4 (1.7) | 6.1 (2.4) | −0.9 (1.9) |
| CGASb | 54.7 (15.1) | +8.2 (12.7) | 56.8 (12.8) | +6.6 (11.9) | 51.0 (18.0) | +11.3 (13.9) |
| Bipolar I subgroup | ||||||
| YMRSa | 16.3 (10.3) | −3.5 (10.6) | 14.2 (9.6) | −1.4 (10.4) | 20.6 (10.5) | −8.0 (9.7) |
| CGI-BP-Sa | 3.1 (1.5) | −0.6 (1.5) | 2.9 (1.4) | −0.4 (1.6) | 3.6 (1.5) | −1.1 (1.3) |
| CGSQb | 5.2 (2.5) | −0.4 (2.5) | 5.0 (2.6) | −0.3 (2.5) | 5.6 (2.3) | −0.5 (2.6) |
| CGASb | 58.7 (14.1) | +6.1 (14.9) | 60.8 (13.7) | +4.0 (14.9) | 54.4 (13.8) | +10.7 (14.1) |
Last observation carried forward.
Observed cases.
PANSS, Positive and Negative Syndrome Scale; CGI-S, Clinical Global Impressions – Severity; CGSQ, Caregiver Strain Questionnaire; CGAS, Children's Global Assessment Scale; YMRS,Young Mania Rating Scale; CGI-BP-S,Clinical Global Impressions – Bipolar – Severity.
A response in the schizophrenia subgroup (i.e., defined as a ≥30% PANSS score reduction from open-label baseline) was reported in 17.4% of patients at week 26 (observed cases). A response in the bipolar disorder subgroup (i.e., YMRS score reduction ≥50%) was reported in 30.9% of patients, whereas remission (i.e., YMRS score ≤12 points) was recorded in a greater proportion (56.7%), a difference in rates that may be explained by the floor effect caused by previous quetiapine treatment (observed cases).
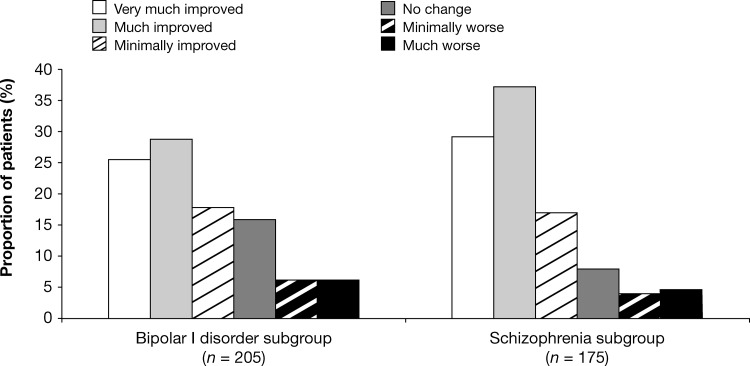
Mean (SD) CGI Severity of Illness scores improved during open-label study by 0.5 (1.2) points in the schizophrenia subgroup (−0.3 [1.12] and −1.0 [1.32] in the prior quetiapine and placebo groups, respectively) and by 0.7 (1.47) points in the bipolar subgroup (−0.5 [1.53] and −1.1 [1.27] in the prior quetiapine and placebo groups, respectively) (LOCF). The majority of patients were “much improved” (37.1%) or “very much improved” (29.1%) on the CGI-Global Improvement scale at week 26 relative to open-label baseline in the schizophrenia subgroup (36.3% and 27.4% respectively in the prior quetiapine group; 38.7% and 32.3% respectively in the prior placebo group) (LOCF). Most patients were also “much improved” (28.9%) or “very much improved” (25.4%) on the CGI-BP Global Improvement scale in the bipolar disorder subgroup, relative to open-label baseline (28.7% and 25.7%, respectively in the prior quetiapine group; 29.2% and 24.6%, respectively in the prior placebo group) (Fig. 3).
FIG. 3.
Clinical Global Impressions (CGI)-Global Improvement ratings at week 26 versus open-label baseline (safety population; last observation carried forward [LOCF]).
Mean (SD) changes in CGAS total score from open-label baseline to week 26 were 8.2 (12.7) in the schizophrenia subgroup (6.6 [11.9] in the prior quetiapine group and 11.3 [13.9] in the prior placebo group) and 6.1 (14.9) in the bipolar subgroup (4.0 [14.9] in the prior quetiapine group and 10.7 [14.1] in the prior placebo group) (observed cases). As for the other efficacy variables assessed, CGAS score improvements during the open-label study were greater in patients treated previously with placebo than in those treated with quetiapine during the acute studies (Table 5).
Mean (SD) CGSQ score changes from open-label baseline to week 26 were −0.6 (1.8) in the schizophrenia subgroup and −0.4 (2.5) in the bipolar disorder subgroup (observed cases) (Table 5).
Discussion
In this 26-week, open-label study, quetiapine monotherapy flexibly dosed at 400–800 mg/day, with options to reduce dosing based on tolerability, was generally safe and well tolerated in youth with schizophrenia and bipolar I disorder. The safety profile of long-term quetiapine in these children and adolescents was generally consistent with the long-term safety of quetiapine reported in adults (e.g., Weisler et al. 2011) and with previous short-term prospective observations in youth, including the acute studies that preceded the current open-label study (McConville et al. 2000; Shaw et al. 2001; DelBello and Kowatch 2006; DelBello et al. 2007; Schimmelmann et al. 2007; DelBello et al. 2009; Findling et al. 2012; Pathak et al. 2013). The most common AEs in the current trial included somnolence, headache, sedation, and vomiting, similar to the profile reported in adults with bipolar disorder (Weisler et al. 2011), whereas weight increase and increased appetite were additional common AEs in this pediatric population. A number of common AEs, including sedation, dizziness, weight increase, and increased appetite, were more common in patients from the prior-placebo than prior-quetiapine group in the acute studies. This observation may suggest that AEs are more often seen at the time of initiation of treatment. It also suggests that tolerability improves with continued treatment, as evidenced by the lower AE rates observed in those previously treated with quetiapine.
In the current study, 18.3% of patients experienced a clinically significant weight gain after adjustment for normal growth. Clinical study data suggest that weight gain is frequent among youth treated with agents in the atypical antipsychotic class, and that youth are more vulnerable than adults to this weight gain (Correll et al. 2009; Mattai et al. 2010; Maayan and Correll 2011). Therefore, physicians should be alert to weight gain among youth receiving atypical antipsychotic therapy and consider options for weight loss programs in suitable cases (Townsend and Findling 2010).
Mean changes in clinical chemistry parameters from open-label baseline to final visit were generally small. Shifts to potentially clinically significant high levels of fasting serum glucose occurred in 2.1% of patients. Shifts to potentially clinically significant low levels of high-density lipoprotein cholesterol occurred in 14.9% of patients, whereas shifts to potentially significant high levels of triglycerides occurred in 10.2%.
A shift in standing diastolic blood pressure to potentially clinically significant high levels occurred in 14% of patients, whereas shifts to potentially significant high supine systolic and diastolic blood pressures occurred at lower rates. Observations of elevated blood pressure have been reported previously in youth treated with atypical antipsychotics, including the acute studies of quetiapine in pediatric patients (McIntyre and Jerrell 2008; Findling et al. 2012; Pathak et al. 2013). The mechanism of blood pressure change in youth is not clearly understood; potential explanations include a genuine increase in blood pressure or an overly robust orthostatic response. It is recommended that blood pressure be measured at the beginning and periodically during treatment with quetiapine in children and adolescents (Seroquel® prescribing Information 2012).
There are few study data on potential differences in safety profile between patients with schizophrenia and bipolar disorder during atypical antipsychotic therapy. One study found no differences in weight gain or metabolic changes between youth diagnosed with bipolar disorder, other psychotic disorders, or other nonpsychotic disorders following 3 months of atypical antipsychotic treatment (Moreno et al. 2010). In the current study, differences in safety profile between the diagnostic subgroups included higher frequencies of AEs and occurrences of weight gain ≥7% in youth with bipolar disorder compared with schizophrenia. Differences in the frequency of use and type of permitted concomitant medications (in 76.1% of the bipolar disorder vs. 53.7% of the schizophrenia subgroup, including psychostimulant use in 14.1% and 1.7%, respectively) may have contributed to observed differences between the two groups. The profile of commonly reported AEs was comparable between the schizophrenia and bipolar disorder subgroups.
Suicide represents a risk of death in youth with schizophrenia and bipolar disorder (Gould et al. 2003; Fleischhaker et al. 2005; Goldstein et al. 2005; Reichert et al. 2008). Suicidality analyses in the current study were performed using a methodology similar to the Columbia Suicidality Classification Project (Posner et al 2007, 2011). No patients completed a suicide attempt and the overall frequency of suicidal ideation and behaviors was low. Although no relationship between suicidality and quetiapine treatment has been established in clinical studies, the FDA has issued a boxed warning for an increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants (including quetiapine) for psychiatric disorders including schizophrenia and bipolar disorder.
Patients treated with quetiapine during the acute studies that preceded the current open-label study experienced significant improvements in efficacy measures (Findling et al. 2012; Pathak et al. 2013). In these patients, symptoms and functioning continued to improve during 26-weeks of open-label quetiapine treatment, while caregiver burden diminished. As may be predicted, improvements in symptoms and functioning during open-label treatment were generally greater in patients who were treated with placebo than in patients treated with quetiapine in the acute studies.
The results presented here should be considered within the context of an observational, open-label, nonrandomized, parallel-group study design, in which the patients were drawn from participants in two previous acute, placebo-controlled studies. In all, 380 patients participated in the 26-week open-label study, from a safety population of 505 patients in the two acute studies. No differences in demographics or clinical characteristics were identified between enrolled and non-enrolled patients in the open-label study that might potentially influence the validity of the findings. The current study was not designed to provide a comparison of the safety of quetiapine in the two diagnostic subgroups. Whereas differences were observed in the overall frequency of AEs between the schizophrenia and bipolar disorder subgroups, clinical and treatment factors (e.g., differences in quetiapine dose, concomitant medications) may have impacted the observations reported.
Conclusions
In conclusion, the results of this open-label study show that quetiapine flexibly dosed at 400–800 mg/day for 26-weeks is safe and generally well tolerated in youth with schizophrenia or bipolar I disorder over this time course of treatment.
Clinical Significance
Few medications are approved for the treatment of youth with schizophrenia or bipolar disorder. Data are also limited to inform the long-term use of approved agents for these indications in pediatric patients. The safety and tolerability data presented for quetiapine in the current 26-week, open-label study may inform clinical decision making, and may additionally assist in providing insights into appropriate monitoring during continued treatment.
Acknowledgment
We thank Dr. Tracey Lonergan, from PAREXEL, who provided medical writing support funded by AstraZeneca Pharmaceuticals LP.
Disclosures
Dr. Findling receives or has received research support from, acted as a consultant for, received royalties from, and/or served on a speaker's bureau for Abbott, Addrenex, Alexza, American Psychiatric Press, AstraZeneca, Biovail, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson & Johnson, KemPharm Lilly, Lundbeck, Merck, National Institutes of Health, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Physicians Postgraduate Press, Rhodes Pharmaceuticals, Roche, Sage, Sanofi-Aventis, Schering-Plough, Seaside Therapeutics, Sepracor, Shionogi, Shire, Solvay, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, WebMD, and Wyeth. Dr. DelBello receives or has received research support from, acted as a consultant, and/or served on a speaker's bureau for AstraZeneca, Brain Behavior and Research Foundation (previously NARSAD), Bristol-Myers Squibb, Eli Lilly, France Foundation, GlaxoSmithKline, Janssen, Johnson & Johnson, Kappa Clinical, Martek, Merck & Co., National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Drug Abuse (NIDA), National Institute of Mental Health (NIMH), Pfizer, Repligen, Shire, Schering-Plough, Somerset, and the Thrasher Foundation. Drs. Liu and Pathak are employees, and Dr. Earley is a former employee, of AstraZeneca Pharmaceuticals LP, USA.
The Trial 150 Study Investigators
Alexey Agarkov, Si Mental Health Research Institute, Tomsk, Russia; Daisy Ann Artuz, Brokenshire Hospital, Davao City, Philippines; Sarah Atkinson, Finger Lakes Clinical Research, Rochester, NY, USA; Deborah Bergen, Cientifica Inc. at Prairie View Inc., Newton, KS, USA; Valeriy Bitenskyy, Odessa State Medical University, Odessa, Ukraine; Jeffrey Blumer, University Hospitals of Cleveland, Cleveland, OH, USA; David Spiegel Brighton, Research Group, LLC, Virginia Beach, VA, USA; Rajinder Dhillon Brighton, Research Group, LLC, Virginia Beach, VA, USA; Ann Childress, Center for Psychiatry and Behavioral Medicine, Inc., Las Vegas, NV, USA; Miroslaw Dabkowski, Wojewodzki Ostrodek Lecznictwa, Torun, Poland; Melissa DelBello, University of Cincinnati Medical Center, Cincinnati, OH, USA; Vladislav Demchenko, Kiev City Psychoneurological Hospital #2, Kiev, Ukraine; Vladimir Diligenski, Clinical-Hospital Center Dr Dragisa, Belgrade, Serbia; Joseph Fanelli, Midwest Center for Neurobehavioral Medicine, Oakbrook Terrace, IL, USA; Robert Findling, University Hospitals of Cleveland, Cleveland, OH, USA; Donald Garcia, Jr, FutureSearch Trials, Austin, TX, USA; Thomas Gazda, Meadowbrook Research, Inc., Scottsdale, AZ, USA; John Gilliam, International Clinical Research Associates, LLC, Richmond, VA, USA; Lawrence Ginsberg, Red Oak Psychiatry Associates, PA, Houston, TX, USA; Georgina M. Gozo-Oliver, Veterans Memorial Medical Center, Diliman Quezon City, Philippines; Barbara Gracious, University of Rochester Medical Center, Rochester, NY, USA; Harinder Grewal, World Wide Research Centers, Inc., Fallbrook, CA, USA; Nelson Handal Harmonex, Dothan, AL, USA; Robert Hendren, University of California, Davis, Sacramento, CA, USA; Willis Holloway, Jr, Cutting Edge Research Group, Oklahoma City, OK, USA; Malgorzata Janas-Kozik, Centrum Pediatrii Oddziai Psychiatrii Wicku, Sosnowies, Poland; Gregory Kaczenski, K & S Professional Research Services, LLC, Little Rock, AR, USA; Ali Kashfi, Altamonte Springs, FL, USA; Arifulla Khan, Northwest Clinical Research Center, Bellevue, WA, USA; Saaid Khojasteh, Saaid Khojasteh and Associates, Inc., St Charles, MO, USA; James Knutson, MD, Kirkland, WA, USA; Irina A. Kozlova, Mental Health Research Center, Moscow, Russia; Valeriy Krasnov, Moscow Research Institute of Psychiatry, Moscow, Russia; David Krefetz, CNS Research Institute, PC, Clementon, NJ, USA; Chandra Krishnasastry, Clinical Research Services at Tennessee Christian Medical Center, Madison, TN, USA; Aneta Lakic, University of Belgrade, Belgrade, Serbia; David Linden, Linden Research Consultants, LLC, Oklahoma City, OK, USA; Donna Londino, Medical College of Georgia, Augusta, GA, USA; Ramesh Kumar Mahendru, Mahendru Psychiatric Centre, Kanpur, India; Kathleen McKenna, Children's Memorial Hospital, Chicago, IL, USA; Dragan Mitrovic, Institute of Psychiatry, Novi Sad, Serbia; Aida L. Muncada, National Center for Mental Health, Mandaluyong City, Philippines; William Murphy, Psychiatric Associates, Overbrook Park, KS, USA; Syed Mustafa, Kirkland, WA, USA; Americo Padilla, Miami Children's Hospital, Miami, FL, USA; Anjali Pathak, Ten Broeck Hospital Jacksonville, Jacksonville, FL, USA; Theodore Petti, UMDNJ, Robert Wood Johnson Medical School, Piscataway, NJ, USA; Steven Pliszka, UTHSCSA, San Antonio, TX, USA; Yuriy Popov, Bekhterev Psychoneurology Research Institute, St. Petersburg, Russia; Smiljka Popovic, Deusic Institute for Mental Health, Belgrade, Serbia; Humberto Quintana, LSU Health Sciences Center, New Orleans, LA, USA; Joachim Raese, Behavioral Health 2000, LLC, Riverside, CA, USA; Andrzej Rajewski, Klinika Psychiatrii Dzieci I Mlodziey, Poznan, Poland; Cynthia Ramos-Leynes, UP College of Medicine, Manila, Philippines; Rakesh Ranjan, Rakesh Ranjan, MD & Associates, Inc., Lyndhurst, OH, USA; Karl Rickels, University of Pennsylvania, Philadelphia, PA, USA; Moira A. Rynn, University of Pennsylvania, Philadelphia, PA, USA; Russell Scheffer, Children's Health System, Milwaukee, WI, USA; Jon Shaw, University of Miami, Miami, FL, USA; Franco Sicuro, St Louis, MO, USA; Linmarie Sikich, UNC Hospitals, Chapel Hill, NC, USA; Ahmad Hatum Sulaiman, University of Malaya Medical Centre, Kuala Lumpur, Malaysia; Ivana Timotijevic Markovic, Institute for Mental Health, Belgrade, Serbia; Chin Lee Toh, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia; Jitendra Kumar Trivedi, King George Medical University GM and Associated Hospitals, Lucknow, India; Madeleine Valencerina, Clinical Pharmacological Studies, Inc., Cerritos, CA, USA; Roger Vogelfanger, Research Strategies of Memphis, LLC, Memphis, TN, USA; Petro Voloshyn, Academy of Medical Sciences, Kharkiv, Ukraine; Karen Wagner, University of Texas Medical Branch, Galveston, TX, USA; Herman Walter, Pretorius Weskoppies Hospital, Pretoria, South Africa; Marianne Wamboldt, The Children's Hospital, Denver, CO, USA; Elizabeth Weller, CHOP Behavioral Health Center, Philadelphia, PA, USA; Johnny Williamson, Community Mental Health Council, Inc., Chicago, IL, USA; Michel Woodbury-Farina, Clinic of M. Woodbury-Farina, San Juan, PR, USA; Tony Yang, UCSD Healthcare Child and Adolescent Psychiatry Service, San Diego, CA, USA; Jill Zweig, Meadowbrook Research, Inc., Scottsdale, AZ, USA.
References
- American Psychiatric Association. Text Revision. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Berk M. Hallam K. Malhi GS. Henry L. Hasty M. Macneil C. Yucel M. Pantelis C. Murphy B. Vieta E. Dodd S. McGorry PD. Evidence and implications for early intervention in bipolar disorder. J Ment Health. 2010;19:113–126. doi: 10.3109/09638230903469111. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Axelson D. Strober M. Gill MK. Valeri S. Chiappetta L. Ryan N. Leonard H. Hunt J. Iyengar S. Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan AM. Heflinger CA. Bickman L. The caregiver strain questionnaire: Measuring the impact on the family of living with a child with serious emotional disturbance. J Emot Behav Disord. 1997;5:212–222. [Google Scholar]
- Centers for Disease Control and Prevention. Growth charts. 2000. http://www.cdc.gov/growthcharts/ [Feb 5;2013 ]. http://www.cdc.gov/growthcharts/
- Correll CU. Manu P. Olshanskiy V. Napolitano B. Kane JM. Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use on children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBello MP. Adler CM. Whitsel RM. Stanford KE. Strakowski SM. A 12-week single-blind trial of quetiapine for the treatment of mood symptoms in adolescents at high risk for developing bipolar I disorder. J Clin Psychiatry. 2007;68:789–795. doi: 10.4088/jcp.v68n0520. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Chang K. Welge JA. Adler CM. Rana M. Howe M. Bryan H. Vogel D. Sampang S. Delgado SV. Sorter M. Strakowski SM. A double-blind, placebo-controlled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disord. 2009;11:483–493. doi: 10.1111/j.1399-5618.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Kowatch RA. Pharmacological interventions for bipolar youth: Developmental considerations. Dev Psychopathol. 2006;18:1231–1246. doi: 10.1017/S0954579406060597. [DOI] [PubMed] [Google Scholar]
- Findling R. McKenna K. Earley W. Stankowski J. Pathak S. Efficacy and safety of quetiapine in adolescents with schizophrenia investigated in a 6-week, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2012;22:327–342. doi: 10.1089/cap.2011.0092. [DOI] [PubMed] [Google Scholar]
- Findling RA. Johnson JL. McClellan J. Frazier JA. Vitiello B. Hamer RM. Lieberman JA. Ritz L. McNamara NK. Lingler J. Hlastala S. Pierson L. Puglia M. Maloney AE. Kaufman EM. Noyes N. Sikich L. Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) study. J Am Acad Child Adolesc Psychiatry. 2010;49:583–594. doi: 10.1016/j.jaac.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL. Diagnosis, treatment of bipolar disorder in young patients. J Clin Psychiatry. 2009;70:e45. doi: 10.4088/JCP.8125cc10c. [DOI] [PubMed] [Google Scholar]
- Findling RL. Nyilas M. Forbes RA. McQuade RD. Jin N. Iwamoto T. Ivanova S. Carson WH. Chang K. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: A randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2009;70:1441–1451. doi: 10.4088/JCP.09m05164yel. [DOI] [PubMed] [Google Scholar]
- Findling RL. Robb A. Nyilas M. Forbes RA. Jin N. Ivanova S. Marcus R. McQuade RD. Iwamoto T. Carson WH. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165:1432–1441. doi: 10.1176/appi.ajp.2008.07061035. [DOI] [PubMed] [Google Scholar]
- Fleischhaker C. Schulz E. Tepper K. Martin M. Hennighausen K. Remschmidt H. Long–term course of adolescent schizophrenia. Schizophr Bull. 2005;31:769–780. doi: 10.1093/schbul/sbi014. [DOI] [PubMed] [Google Scholar]
- Fraguas D. Correll CU. Merchan–Naranjo J. Rapado–Castro M. Parellada M. Moreno C. Arango C. Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: Comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol. 2010;21:621–645. doi: 10.1016/j.euroneuro.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Geller B. Tillman R. Bolhofner K. Zimerman B. Strauss NA. Kaufmann P. Controlled, blindly rated, direct-interview family study of a prepubertal and early-adolescent bipolar I disorder phenotype: Morbid risk, age at onset, and comorbidity. Arch Gen Psychiatry. 2006;63:1130–1138. doi: 10.1001/archpsyc.63.10.1130. [DOI] [PubMed] [Google Scholar]
- Goldstein TR. Birmaher B. Axelson D. Ryan ND. Strober MA. Gill MK. Valeri S. Chiappetta L. Leonard H. Hunt J. Bridge JA. Brent DA. Keller M. History of suicide attempts in pediatric bipolar disorder: Factors associated with increased risk. Bipolar Disord. 2005;7:525–535. doi: 10.1111/j.1399-5618.2005.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould MS. Greenberg T. Velting DM. Shaffer D. Youth suicide risk and preventive 23 interventions: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2003;42:386–405. doi: 10.1097/01.CHI.0000046821.95464.CF. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: U.S. Department of Health, Education, and Welfare; 1976. Abnormal Involuntary Movement Scale; pp. 534–537. [Google Scholar]
- Guy W. Bonato RR. Manual for the ECDEU Assessment Battery. 2nd. Chevy Chase, MD: U.S. Department of Health, Education and Welfare; 1970. CGI clinical global impression; pp. 12–1–12–6. [Google Scholar]
- Haas M. DelBello MP. Pandina G. Kushner S. Van Hove I. Augustyns I. Quiroz J. Kusumakar V. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: A randomized, double-blind, placebo-controlled study. Bipolar Disord. 2009a;11:687–700. doi: 10.1111/j.1399-5618.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- Haas M. Unis AS. Aementeros J. Copenhaver MD. Quiroz JA. Kushner SF. A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol. 2009b;19:611–621. doi: 10.1089/cap.2008.0144. [DOI] [PubMed] [Google Scholar]
- Jensen JB. Kumra S. Leitten W. Oberstar J. Anjum A. White T. Wozniak J. Lee SS. Schulz SC. A comparative pilot study of second-generation antipsychotics in children and adolescents with schizophrenia-spectrum disorders. J Child Adolesc Psychopharmacol. 2008;18:317–326. doi: 10.1089/cap.2007.0123. [DOI] [PubMed] [Google Scholar]
- Kay SR. Fiszbein A. Opler LA. The positive and negative syndrome scale (PANSS) Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K SADSPL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kryzhanovskaya L. Schulz SC. McDougle C. Frazier J. Dittmann R. Robertson–Plouch C. Bauer T. Xu W. Wang W. Carlson J. Tohen M. Olanzapine versus placebo in adolescents with schizophrenia: A 6-week, randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48:60–70. doi: 10.1097/CHI.0b013e3181900404. [DOI] [PubMed] [Google Scholar]
- Kumra S. Kranzler H. Gerbino-Rosen G. Kester HM. De Thomas C. Kafantaris V. Correll CU. Kane JM. Clozapine and “high-dose” olanzapine in refractory early-onset schizophrenia: A 12-week randomized and double-blind comparison. Biol Psychiatry. 2008;63:524–529. doi: 10.1016/j.biopsych.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Lay B. Blanz B. Hartmann M. Schmidt M. The psychosocial outcome of adolescent schizophrenia: A 12-year follow-up. Schizophr Bull. 2000;26:801–816. doi: 10.1093/oxfordjournals.schbul.a033495. [DOI] [PubMed] [Google Scholar]
- Lish JD. Dime–Meenan S. Whybrow PC. Price RA. Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–294. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Loranger AW. Sex difference in age of onset of schizophrenia. Arch Gen Psychiatry. 1984;41:157–161. doi: 10.1001/archpsyc.1984.01790130053007. [DOI] [PubMed] [Google Scholar]
- Maayan L. Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol. 2011;21:517–535. doi: 10.1089/cap.2011.0015. [DOI] [PubMed] [Google Scholar]
- Mattai AK. Hill JL. Lenroot RK. Treatment of early onset schizophrenia. Curr Opin Psychiatry. 2010;23:304–310. doi: 10.1097/YCO.0b013e32833b027e. [DOI] [PubMed] [Google Scholar]
- McConville BJ. Arvanitis LA. Thyrun PT. Yeh C. Wilkinson LA. Chaney RO. Foster KD. Sorter MT. Friedman LM. Brown KL. Heubi JE. Pharmacokinetics, tolerability, and clinical effectiveness of quetiapine fumarate: An open-label trial in adolescents with psychotic disorders. J Clin Psychiatry. 2000;61:252–260. doi: 10.4088/jcp.v61n0403. [DOI] [PubMed] [Google Scholar]
- McIntyre RS. Jerrell JM. Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adolesc Med. 2008;162:929–935. doi: 10.1001/archpedi.162.10.929. [DOI] [PubMed] [Google Scholar]
- Moreno C. Merchan–Naranjo J. Alvarez M. Baeza I. Alda JA. Martínez-Cantarero C. Parellada M. Sánchez B. de la Serna E. Giráldez M. Arango C. Metabolic effects of second-generation antipsychotics in bipolar youth: Comparison with other psychotic and nonpsychotic diagnoses. Bipolar Disord. 2010;12:172–184. doi: 10.1111/j.1399-5618.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- Morris NM. Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Pathak S. Findling RL. Earley W. Acevedo LD. Stankowski J. DelBello MP. Efficacy and safety of quetiapine in children and adolescents with mania associated with bipolar I disorder: A 3-week, double-blind, placebo-controlled trial. J Clin Psychiatry. 2013;74:e100–e109. doi: 10.4088/JCP.11m07424. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Henry DB. Findling RL. Parnes S. Carbray JA. Mohammed T. Janicak PG. Sweeney JA. Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder. Bipolar Disord. 2010;12:593–605. doi: 10.1111/j.1399-5618.2010.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH. Miyahara S. Marangell LB. Wisniewski SR. Ostacher M. DelBello MP. Bowden CL. Sachs GS. Nierenberg AA STEP-BD Investigators. Long-term implications of early onset in bipolar disorder: Data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Posner K. Brown GK. Stanley B. Brent DA. Yershova KV. Oquendo MA. Currier GW. Melvin GA. Greenhill L. Shen S. Mann JJ. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K. Oquendo MA. Gould M. Stanley B. Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM. Leverich GS. Xing G. Weiss RB. Developmental vulnerabilities to the onset and course of bipolar disorder. Dev Psychopathol. 2001;13:581–598. doi: 10.1017/s0954579401003091. [DOI] [PubMed] [Google Scholar]
- Post RM. Weiss RB. Leverich GS. George MS. Frye M. Ketter TA. Developmental psychobiology of cyclic affective illness: Implications for early therapeutic intervention. Dev Psychopathol. 1996;8:273–305. [Google Scholar]
- Reichert A. Kreiker S. Mehler–Wex C. Warnke A. The psychopathological, psychosocial outcome of early-onset schizophrenia: Preliminary data of a 13-year follow-up. Child Adolesc Psychiatry Ment Health. 2008;2:6. doi: 10.1186/1753-2000-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmelmann BG. Mehler–Wex C. Lambert M. Schulze-zur-Wiesch C. Koch E. Flechtner HH. Gierow B. Maier J. Meyer E. Schulte–Markwort M. A prospective 12-week study of quetiapine in adolescents with schizophrenia spectrum disorders. J Child Adolesc Psychopharmcol. 2007;17:768–778. doi: 10.1089/cap.2007.0048. [DOI] [PubMed] [Google Scholar]
- Seroquel® (quetiapine fumarate) prescribing Information. 2012. http://www1.astrazeneca-us.com/pi/Seroquel.pdf. [Feb 5;2013 ]. http://www1.astrazeneca-us.com/pi/Seroquel.pdf
- Shaffer D. Gould MS. Brasic J. Ambrosini P. Fisher P. Bird H. Aluwahlia S. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shaw JA. Lewis JE. Pascal S. Sharma RK. Rodriguez RA. Guillen R. Pupo–Guillen M. A study of quetiapine: Efficacy and tolerability in psychotic adolescents. J Child Adolesc Psychopharmacol. 2001;11:415–424. doi: 10.1089/104454601317261591. [DOI] [PubMed] [Google Scholar]
- Sikich L. Frazier JA. McClellan J. Findling RL. Vitiello B. Ritz L. Ambler D. Puglia M. Maloney AE. Michael E. De Jong S. Slifka K. Noyes N. Hlastala S. Pierson L. McNamara NK. Delporto–Bedoya D. Anderson R. Hamer RM. Lieberman JA. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: Findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PubMed] [Google Scholar]
- Simpson GM. Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212(Suppl 44):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Spearing MK. Post RM. Leverich GS. Brandt D. Nolen W. Modification of the Clinical Global Impressions (CGI) scale for use in bipolar illness (BP): The CGI-BP. Psychiatr Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Strober M. Schmidt–Lackner S. Freeman R. Bower S. Lampert C. DeAntonio M. Recovery and relapse in adolescents with bipolar affective illness: A five-year naturalistic, prospective followup. J Am Acad Child Adolesc Psychiatry. 1995;34:724–731. doi: 10.1097/00004583-199506000-00012. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at Adolescence. Oxford: Blackwell Scientific Publications; 1962. [Google Scholar]
- Tohen M. Kryzhanovskaya L. Carlson G. DelBello M. Wozniak J. Kowatch R. Wagner K. Findling R. Lin D. Robertson–Plouch C. Xu W. Dittmann RW. Biederman J. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry. 2007;164:1547–1556. doi: 10.1176/appi.ajp.2007.06111932. [DOI] [PubMed] [Google Scholar]
- Townsend L. Findling RL. Modifying the risk of atypical antipsychotics in the treatment of juvenile-onset schizophrenia. Expert Opin Pharmacother. 2010;11:195–205. doi: 10.1517/14656560903473165. [DOI] [PubMed] [Google Scholar]
- Tsai SY. Lee HC. Chen CC. Huang YL. Cognitive impairment in later life in patients with early-onset bipolar disorder. Bipolar Disord. 2007;9:868–875. doi: 10.1111/j.1399-5618.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- Weisler RH. Nolen WA. Neijber A. Hellqvist A. Paulsson B for the Trial 144 Study Investigators. Continuation of quetiapine versus switching to placebo or lithium for maintenance treatment of bipolar I disorder (trial 144: A randomized controlled study) J Clin Psychiatry. 2011;72:1452–1464. doi: 10.4088/JCP.11m06878. [DOI] [PubMed] [Google Scholar]
- Young RC. Biggs JT. Ziegler VE. Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zeni CP. Tramontina S. Ketzer CR. Pheula GF. Rohde LA. Methylphenidate combined with aripiprazole in children and adolescents with bipolar disorder: A randomized crossover trial. J Child Adolesc Psychopharmacol. 2009;19:553–561. doi: 10.1089/cap.2009.0037. [DOI] [PubMed] [Google Scholar]