Abstract
Lake Magic is one of the most extreme of hundreds of ephemeral acid-saline lakes in southern Western Australia. It has pH as low as 1.7, salinity as high as 32% total dissolved solids, temperatures ranging from 0°C to 50°C, and an unusually complex aqueous composition. Optical petrography, UV-vis petrography, and laser Raman spectrometry were used to detect microorganisms and organic compounds within primary fluid inclusions in modern bedded halite from Lake Magic. Rare prokaryotes appear as 1–3 μm, bright cocci that fluoresce green with UV-vis illumination. Dimpled, 5–7 μm yellow spherules that fluoresce blue with UV-vis illumination are interpreted as Dunaliella algae. Yellow-orange beta-carotene crystals, globules, and coatings are characterized by orange-red fluorescence and three distinct Raman peaks. Because acid saline lakes are good Mars analogues, the documentation of prokaryotes, eukaryotes, and organic compounds preserved in the halite here has implications for the search for life on Mars. Missions to Mars should incorporate such in situ optical and chemical examination of martian evaporites for possible microorganisms and/or organic compounds in fluid inclusions. Key Words: Acid—Extremophiles—Western Australia—Fluid inclusions—Lake Magic—Dunaliella. Astrobiology 13, 850–860.
1. Introduction
1.1. Life in extreme acid brines
Acid saline ephemeral lakes in southern Western Australia are among the most extreme environments on Earth. Although the geochemistry, mineralogy, and sedimentology of these rare natural systems have been recently described (Benison et al., 2007; Bowen and Benison, 2009), little is known about life there. Field observations have shown evidence of microbial life in the form of foams, slimes, and oily sheens (Benison, 2008). Limited molecular characterization of select lakes in Western Australia has documented diverse communities, composed of many novel prokaryotes (Mormile et al., 2009). However, the study focused only on prokaryotes, and the most extreme lakes were not sampled. In addition, preliminary isolation, enrichment, and culturing of some of the most extreme lake waters have resulted in fungal growth (J. Romanowski, personal communication), further suggesting biodiversity in acid brines.
Brines and acids present challenges to traditional microbiological identification methods. For example, DNA extraction from brines is notoriously difficult, as is reproducing exact synthetic acid brines for culturing (M. Mormile, personal communication). However, in situ optical and chemical methods may be used as an alternate method to gather some microbiological data. The goal of this study was to characterize and document microorganisms and organic compounds in fluid inclusions from halite precipitated in Lake Magic.
1.2. Geological setting
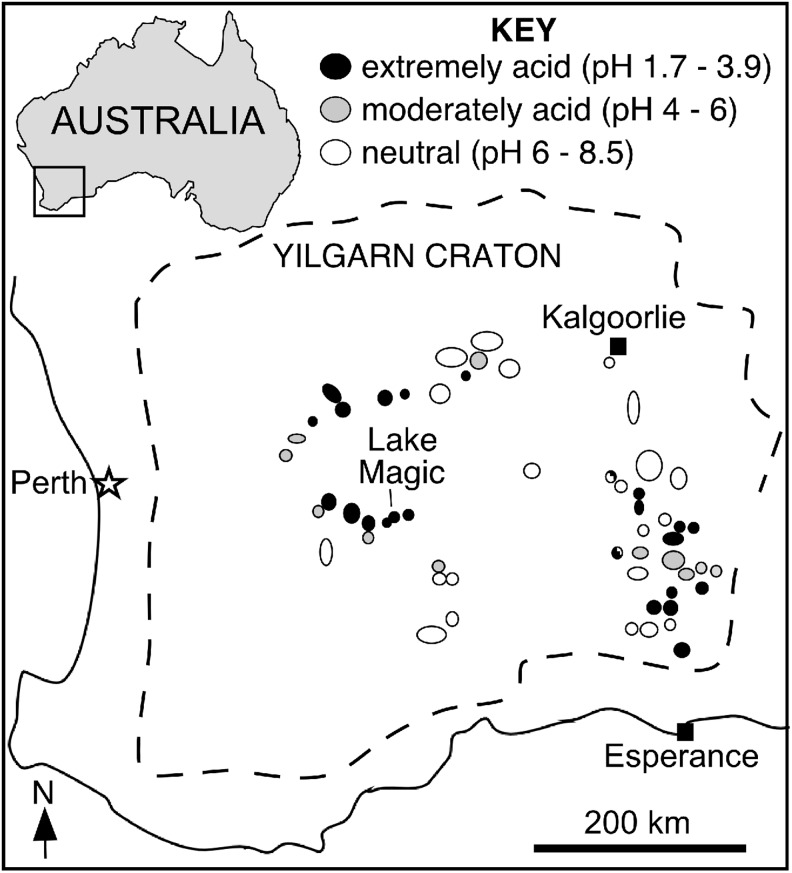
Lake Magic is a small (∼1 km diameter), round, ephemeral acid saline lake near the town of Hyden in southern Western Australia (Fig. 1). Located on the Yilgarn Craton, the lake is hosted by Archean granite, which comprises the nearby Wave Rock and Hippo's Yawn tourist sites. Locally, there is thin modern sediment cover, mostly composed of quartz, feldspar, and gypsum sands.
FIG. 1.
Map of southern Western Australia and Lake Magic.
Lake Magic has the most extreme lake water chemistry of any of the dozens of acid saline lakes documented in Western Australia (Bowen and Benison, 2009). Lake water pH has been measured as low as 1.7, and salinity has been documented as high as 32% total dissolved solids (TDS; Table 1). Lake Magic consists of Na-Mg-K-Ca-Cl-SO4-H2SO4 waters that are highly complex and temporally variable. Of note are unusually high aluminum (up to 1774 mg/mL), silica (up to 510 mg/mL), and iron (up to 331 mg/mL).
Table 1.
Selected Environmental Conditions at Lake Magic during Five Field Trips
| Time | Lake stage | Water color | Minerals forming | pH | Salinity (% TDS) | SO4 (mg/mL) | Al (mg/mL) | Microbial suspects in the field |
|---|---|---|---|---|---|---|---|---|
| July 1, 2005 | flooded–starting evapoconcentration | clear | gypsum | 2.5 | 25 | 11,903 | 927 | none detected |
| Jan. 11–12, 2006 | evapoconcentration–nearing desiccation | bright yellow | halite, gypsum | 1.7–1.9 | 28–29 | 35,169 | 1,774 | none detected |
| Jan. 18, 2008 | evapoconcentration–nearing desiccation | pale yellow | halite, gypsum | 2.3 | 30–32 | n.d. | n.d. | pale yellow foam |
| Jan. 14, 2009 | flooded | clear | none | 3.5 | 7 | n.d. | n.d. | none detected |
| Sept. 17–18, 2011 | flooded–starting evapoconcentration | pale green-yellow | gypsum | 2.9–3.1 | 20 | n.d. | n.d. | yellow foam, yellow slime |
Flooding, evapoconcentration, and desiccation of the lake are driven by local rain, hailstorms, and droughts and not simply by seasons (Benison et al., 2007). During intense rainstorms, and for weeks to months after, the lake is flooded to depths of ∼1–2 m. Arid periods promote evapoconcentration, which results in increasingly shallower, more acid, and more saline waters, as well as precipitation of halite and gypsum. Eventually, Lake Magic sometimes undergoes total desiccation (Fig. 2). Our field observations at Lake Magic and similar ephemeral acid saline lakes in southern Western Australia made over an 11-year period suggest that desiccation periods may last from months to decades (Benison et al., 2007).
FIG. 2.
Lake Magic during various stages. (A) Evapoconcentration stage in January, 2006; white halite crust covered with centimeter-deep yellow acid brine (pH 1.7–1.9, TDS 28–29%, 1774 mg/mL Al). (B) Nearing desiccation stage in January, 2008; white halite and gypsum crust with isolated patches of shallow surface acid brine (pH 2.3, TDS 30–32%). (C) Flooding stage in January, 2009, two months after large rain- and hailstorm; no halite or gypsum present, lake water ∼1 m deep (pH 3.5, TDS 7%). All photos taken from approximately the same location. Note double hills of Archean granitic Wave Rock in background.
Halite and gypsum crystals grow rapidly during favorable conditions, mainly during evapoconcentration stages. For example, cumulate halite crystals can be observed growing increasingly larger at the brine-air interface within a span of a few minutes. Over a period of 6 months (from July 2005 until January 2006), at least 48 cm of bedded halite formed at Lake Magic (Benison et al., 2007). During precipitation of halite and gypsum from saline lakes, fluid inclusions are trapped along growth bands, preserving the various components of the system—the lake waters, rare atmospheric bubbles, some crystals, and microorganisms (Goldstein, 2001; Lowenstein, 2008). At the time that this halite was sampled (January 2006), groundwater below the halite was at depths greater than 48 cm, suggesting that desiccation was extensive.
1.3. Previous work on biology of fluid inclusions
For decades, microbiologists and geologists have realized that archaea and bacteria are capable of existing in fluid inclusions in halite (Reiser and Tasch, 1960; Dombrowski, 1963, 1966; Norton and Grant, 1988; Fredrickson et al., 1997; Grant et al., 1998; Stan-Lotter et al., 1999, 2002; McGenity et al., 2000; Fish et al., 2002; Fendrihan and Stan-Lotter, 2004; Adamski et al., 2006; Fendrihan et al., 2006; Vreeland et al., 2007; Lowenstein, 2008). For example, Mormile et al. (2003) isolated a bacterium from fluid inclusions in 97,000-year-old halite from Death Valley. Vreeland et al. (2000) claimed to have identified two strains of viable bacteria from a large, isolated fluid inclusion in the Permian Salado Formation halite from New Mexico. These studies have focused on using traditional microbiological methods to identify prokaryotes in halite from neutral saline environments. In contrast, the fluid inclusions and parent lake brines from Lake Magic, the focus of this manuscript, have extremely low pH values.
Optical study of microorganisms and organic compounds in halite is new and has included in situ UV-vis fluorescent imaging. Mormile and Storrie-Lombardi (2005) used this technique to detect bacterial colonies in halite crystals. Both transmitted plane light and UV-vis petrography were employed to confirm organic morphologies and composition of “hairy blobs” in halite and gypsum from modern acid saline lakes in Western Australia, as well as in Permian halite from the subsurface of Kansas and North Dakota (Benison et al., 2008).
Most recently, a series of studies have employed a combination of petrographic observations with traditional molecular methods to document microorganisms in halite up to ∼100,000 years old from Death Valley and up to ∼150,000 years old from Saline Valley, California, USA (Schubert et al., 2009a, 2009b, 2010a, 2010b; Lowenstein et al., 2011; Winters et al., 2012). Prokaryotes, eukaryotes, and associated organic compounds have been described in these fluid inclusions in modern halite.
Until now, no detailed examination of biological components in fluid inclusions from acid-precipitated halite has been conducted. Here, we present an optical and chemical microbiological study of fluid inclusions in bedded halite from acid saline Lake Magic, Western Australia.
2. Materials and Methods
2.1. Sample collection, preparation, and storage
Fieldwork was conducted at Lake Magic during five visits since 2005 (2005 and 2006 field data are documented in Benison et al., 2007, and geochemical methods and results are described in Bowen and Benison, 2009). Each field trip consisted of measuring lake and groundwater depths, pH, salinity, and temperature, as well as air temperature. Physical conditions at the lake were documented with photographs. Microbial suspects, such as foam and filamentous structures, were noted in the field. Lake water and sediment samples were collected during each field trip. Evaporite mineral samples were collected when they were present.
Lake water and halite used in this study were collected during the January, 2006, and January, 2008, field trips, at times when Lake Magic was shallow (less than 10 cm deep) and geochemistry was most extreme (pH 1.7–1.9, salinity up to 32% TDS, and bright yellow water; Table 1). Water samples were collected and stored in sterile HDPE bottles. Waters were not acidified further (nor treated in any other way) and were stored at room temperature. Halite samples were stored in airtight plastic bags and transported back to Central Michigan University.
Razor blades were used to cleave small chips of halite from the samples returned from Australia. Cleaved halite chips are 2.5–5.0 mm in diameter and ∼0.5 mm thick. Cleaved halite chips were stored in glass vials, some with 1–2 beads of calcium chloride desiccant.
2.2. Petrography
Cleaved chips of halite, as well as 2006 lake water samples, were examined with plane-transmitted, reflected, and polarized light with Olympus SZ12 stereo microscopes and Olympus BX511R research petrographic microscopes at Central Michigan University and West Virginia University. Research microscopes have a magnification range up to 2000 times and are equipped with a UV-vis light source, which emits combined 330 nm UV and 385 nm visible light. Long-working distance microscope objectives of 40×, 50×, 60×, and 100× provide high-quality optics for viewing fluid inclusions. Microscopes are equipped with digital imaging systems.
2.3. Laser Raman spectrometry
Laser Raman spectrometry was performed with a Kaiser Optical System, Inc., Holoprobe laser microprobe. The microprobe is equipped with a 532 nm green argon laser (with a spectral range of ∼100 to ∼4275 cm−1) and a 785 nm near-IR laser (with a spectral range of ∼200 to ∼3200 cm−1) with dedicated microscope. Microscope objectives used included 40×, 50×, and 100× long-working distance objectives. Halite chips were placed on glass slides for Raman analysis. Various laser powers and exposure times were used. Power output settings were adjusted according to microscope objectives. Effective power settings were 1.40–1.52 mW for use with the 10× microscope objective, 0.84–0.94 mW for use with the 50× microscope objective, and 0.50–0.64 mW for use with the 100× microscope objective. Exposure and accumulation times ranged from 1 to 5 s and 5 to 50 accumulations. Higher laser and longer exposure times sometimes caused fluorescence to be emitted during Raman analyses and overprint Raman spectra, especially when excited with the 532 nm laser. Most Raman analyses were conducted after petrographic observations, including exposure to UV light.
3. Results
3.1. Petrography
The majority of halite collected at Lake Magic in 2006 and 2008, and used in this study, was millimeter-scale cumulate crystals that formed at the brine-air interface and within the water column. These cubic crystals are randomly oriented in thin beds (Fig. 3A). They are characterized by dark, fluid-inclusion-rich patches that have straight edges parallel to crystal faces and form 90° angles (Fig. 3B). Clear halite in cumulates has few fluid inclusions and indicates slower growth.
FIG. 3.
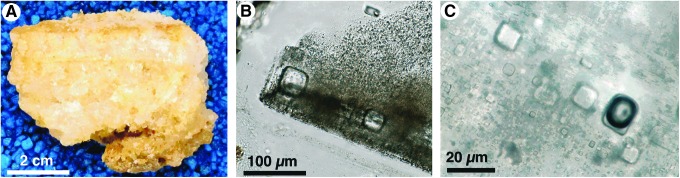
Lake Magic halite. (A) Bedded halite composed of cumulate crystals. (B) Dark, fluid inclusion–rich growth bands. Note yellow algae in inclusion in upper right. (C) Primary fluid inclusions along growth bands. Many inclusions are all-liquid. Note rare large black air bubble in inclusion near lower right. (B) and (C) are illuminated with transmitted plane light.
Rare chevron halite crystals are centimeter-scale and have corners oriented upward from the lake bed. They are defined by alternating millimeter-scale dark, fluid inclusion-rich and clear, fluid inclusion-poor growth bands.
Fluid inclusions in cumulates and chevrons from Lake Magic are considered primary because they are oriented parallel to crystal faces, have negative crystal shapes, and are hosted by halite less than 10 years old (Fig. 3B). No secondary or pseudosecondary fluid inclusions were observed in Lake Magic halite. Primary fluid inclusions in this halite are very abundant. They range in size from ∼1 to ∼100 μm, but the majority are between ∼3 and 30 μm. Most have cubic–rectangular shapes. The most common phase is liquid, and most inclusions are all-liquid inclusions (Fig. 3C). Large dark vapor bubbles exist in rare fluid inclusions (Fig. 3C); these liquid–vapor inclusions are typically more than ∼50 μm in size, and the vapor bubble occupies the majority of the inclusion. Some mineral crystals were observed in fluid inclusions. The most common minerals include both single blades and star-shaped clusters of gypsum, identified partly by their birefringence. Less common clear and red amorphous minerals exist in some primary fluid inclusions (Jagniecki and Benison, 2010).
Bright, high-relief, 1–3 μm cocci were observed in ∼1% of the primary fluid inclusions. In some otherwise all-liquid inclusions, single cocci are found (Fig. 4A). Some inclusions with spherules also contain 1–2 cocci. In inclusions with star-shaped gypsum crystal clusters, several (up to 12) cocci were observed.
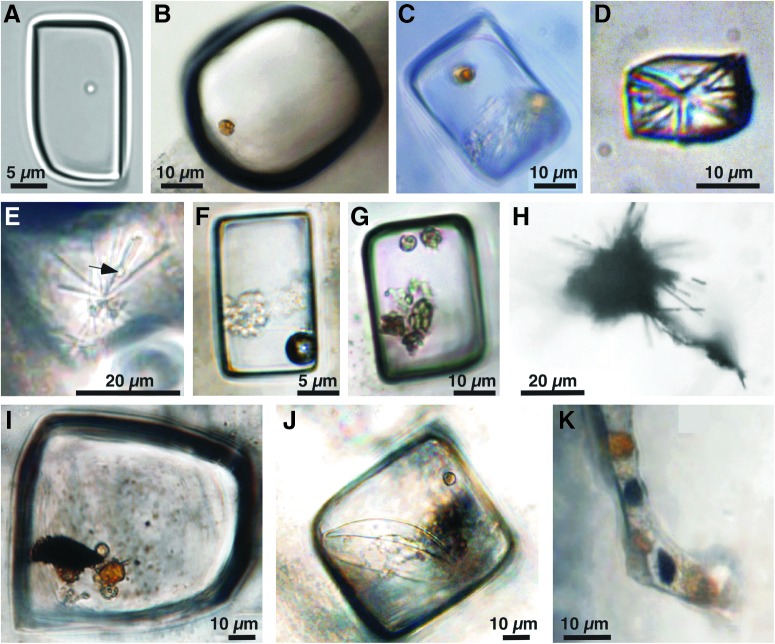
FIG. 4.
Individual fluid inclusions, all photos illuminated with transmitted plane light. (A) Single prokaryotic cell in liquid inclusion. (B) Single Dunaliella algal cell in liquid inclusion. (C) Two yellow algal cells, dark orange beta-carotene globule, gel, and liquid in inclusion. (D) Gypsum crystal cluster and liquid in inclusion. (E) Gypsum crystal cluster and prokaryote (arrow) in liquid in inclusion. (F) Inclusion containing liquid, vapor bubble, and denatured algal cell. (G) Inclusion containing liquid and several algal cells. (H) “Hairy blob” filling and extending from cubic inclusion. (I) Inclusion with liquid, algal cells, and dark “hairy blob.” Laser-elicited fluorescence during laser Raman analysis. (J) Inclusion with liquid, algal cells, and dark “hairy blob.” Laser-elicited fluorescence during laser Raman analysis. (K) Atypical inclusion shape containing liquid, yellow and dark orange algal cells, and dark blobs that may be iron oxides.
Dimpled, 5–7 μm spherules were documented in ∼20% of the primary fluid inclusions. These vary in color and appearance from pale yellow and transparent to deeper yellow to orange and opaque (Figs. 4, 5, 6). Some spherules are surrounded by a clear and colorless gel-like envelope (Fig. 5). A second type of orange substance with different appearance than the 5–7 μm spherules is present within some fluid inclusions and appears as dark orange oily-textured globules (Fig. 4C, Fig. 5).
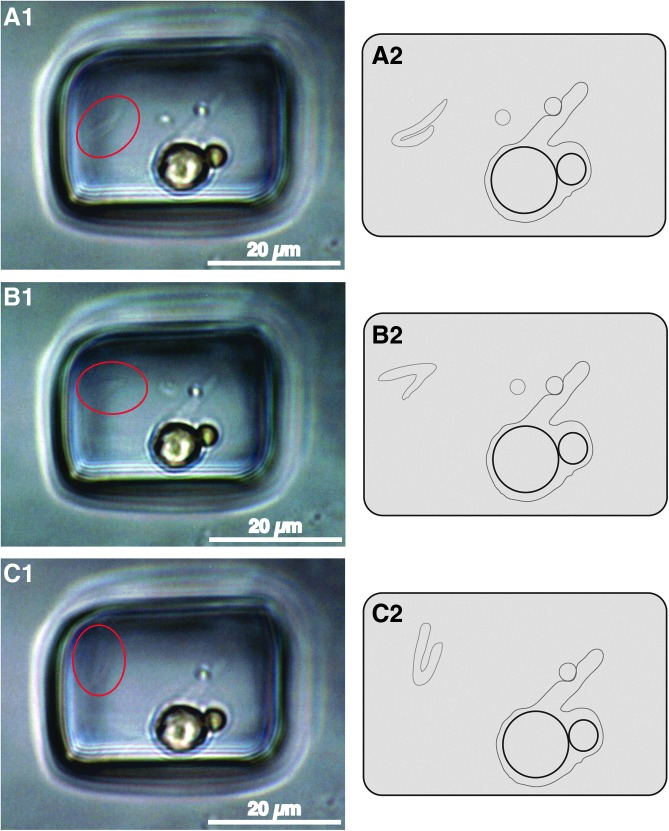
FIG. 5.
Paired photographs (A1, B1, C1) and sketches (A2, B2, C2) of primary fluid inclusion characterized by moving mass. Fluid inclusion in Lake Magic halite with liquid, large yellow Dunaliella algal cell adjacent to smaller yellow beta-carotene globule (lower center part of inclusion), two prokaryote cells (both seen best in A1), clear gel envelope encompassing algal cell, globule, and one prokaryote. Within red circle is moving bent tubular shape suspected to be flagellum.
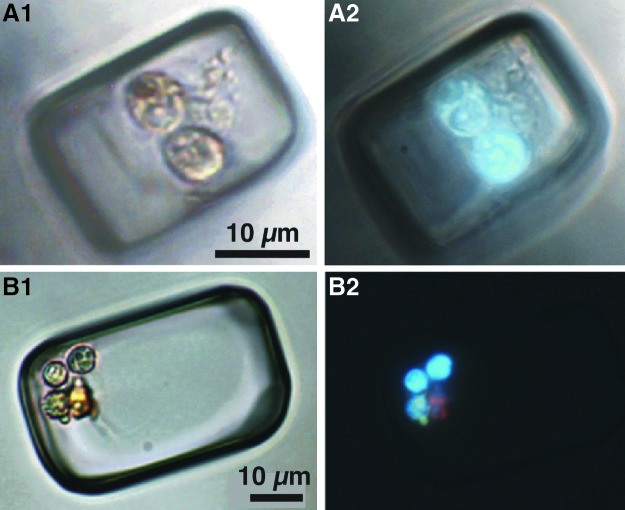
FIG. 6.
Images of microorganisms in two primary fluid inclusions as seen with plane-transmitted light (A1 and B1) and corresponding UV-vis epi-illumination (A2 and B2). (A1) Two pale yellow Dunaliella algal cells. (A2) Two Dunaliella algal cells fluoresce blue. (B1) Three pale yellow Dunaliella algal cells, one clear, bright cocci, and one yellow beta-carotene globule. (B2) Three algal cells fluoresce blue, cocci fluoresce green, and beta-carotene globule fluoresces pink.
“Hairy blobs,” reflective masses of black radiating “hairs,” were recognized in Lake Magic halite (Fig. 4H, 4I, 4J). Some are solid inclusions, while others exist within and extending out of fluid inclusions. The Lake Magic “hairy blobs” are consistent in appearance with those from other Western Australian acid saline lake halite and gypsum (Benison et al., 2008).
Lake water collected in 2006 was also examined microscopically. Individual cocci and yellow spherules matching the description of those observed in the fluid inclusions were abundant in the waters. There were also clusters composed of both cocci and yellow spherules, as well as less abundant diatoms, pollen, and insect legs in the lake water. Lake water from September, 2011, a time when the lake was under different conditions (flooding stage with pH 2.9–3.1, TDS 20%), was also examined microscopically and was rich in yellow fungal cells. Fungal cells, if present, were not obvious in microscopic examination in the 2006 lake waters.
3.2. UV-vis petrography
Host halite, inclusion waters, air bubbles in inclusions, and most minerals within fluid inclusions have no response to the combined UV-vis 330 nm and 385 nm illumination. In contrast, three components have a strong response. Cocci fluoresce green (Fig. 6). Pale yellow spherules fluoresce blue, and dark yellow and orange spherules fluoresce pale yellow to orange-red. Orange oily-textured globules fluoresce dark orange (Fig. 6).
3.3. Laser Raman spectrometry
Laser Raman analyses of inclusion waters with the 532 nm green laser yielded strong peaks at 3760–3824 cm−1. Inclusion waters also commonly had peaks at ∼986 cm−1 and rarely had peaks at ∼1054 cm−1. Solids within fluid inclusions showed Raman spectra characteristic of gypsum, unknown hydrated sulfates (Jagniecki and Benison, 2010), and possible hydrated chlorides. Air bubbles gave no Raman spectra.
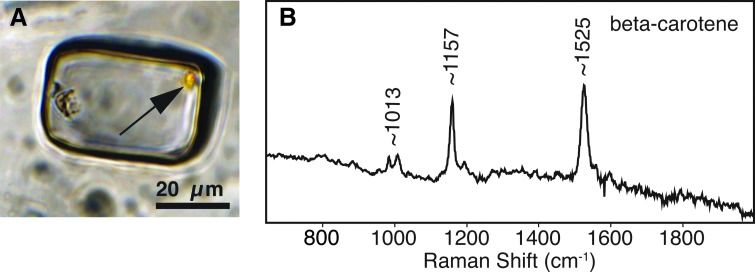
Raman analyses of spherules and oily globules produced three distinct Raman peaks. Analyses with the 785 nm red laser resulted in peaks at 1013, 1157, and 1525 cm−1 (Fig. 7). Analyses with the 532 nm green laser yielded the same approximate three peaks, but in ranges of 982–1013, 1156–1178, and 1521–1552 cm−1 (Fig. 8), as well as a broad peak at 3809–3821 cm−1.
FIG. 7.
Yellow alga with corresponding Raman spectra resulting from 532 nm excitation. (A) Fluid inclusion containing yellow alga (see arrow), illuminated with transmitted plane light. (B) Raman spectra of yellow alga in (A) show three peaks at 1013, 1157, and 1525 cm−1, indicating beta-carotene.
FIG. 8.
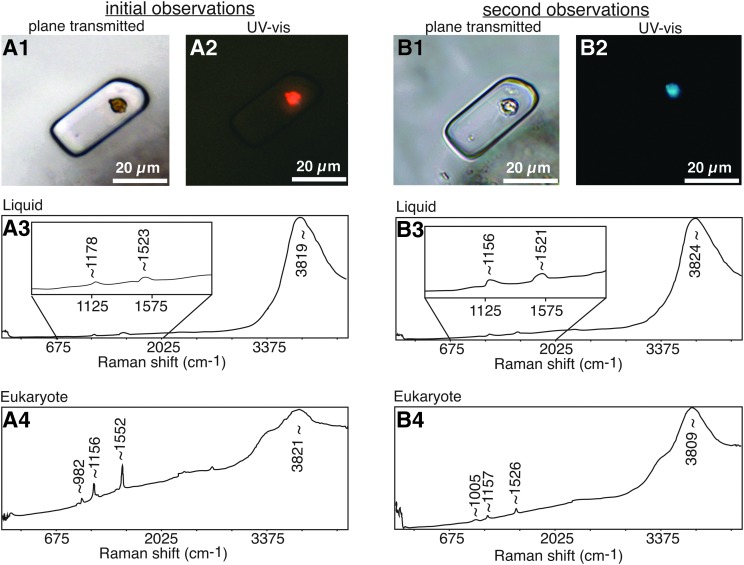
Effects of exposure to laser on fluid inclusion in Lake Magic halite as documented by plane light petrography, UV-vis epi-illumination with combined 330 nm UV and 385 nm visible light, and laser Raman spectra resulting from 532 nm excitation. (A) Initial observations: (A1) primary fluid inclusion with dark orange alga, as seen in plane-transmitted light; (A2) red fluorescent response of same inclusion to UV-vis light. (A3) Raman spectra of liquid in same inclusion and (A4) alga in same inclusion. (B) Second round of analyses after exposure to laser during initial laser Raman analyses. (B1) primary fluid inclusion with alga, as seen in plane-transmitted light; note that alga is now pale yellow; (B2) blue fluorescent response of same inclusion to UV-vis light. (B3) Raman spectra of liquid in same inclusion and (B4) alga in same inclusion.
Some fluid inclusions with solids elicit fluorescence when exposed to the laser. This fluorescent response masked the Raman spectra for those fluid inclusions. In addition, the 532 nm laser altered the appearance of the organic compounds and minerals when laser power output was higher than 1.52 mW, exposure times were longer than 5 seconds, and the accumulations were greater than 50. Prolonged exposure, accumulations, and high power settings resulted in reduction of pigmentation of orange spherules and in some cases rendered them clear and colorless (Fig. 8).
4. Discussion
4.1. Interpretation of results
Plane-transmitted light petrography, UV-vis fluorescence, and laser Raman spectroscopy indicated that there are prokaryotes, eukaryotes, and organic compounds within primary fluid inclusions in Lake Magic halite.
Cocci size, shape, and UV fluorescent response suggest all cocci are bacteria or archaea. The 1–3 μm size, spherical shape, high relief, and green fluorescent response to UV-vis epi-illumination are consistent with prokaryotes (Mormile and Storrie-Lombardi, 2005; Nadeau et al., 2008; Schubert et al., 2009a; Dartnell et al., 2010; Storrie-Lombardi et al., 2011). Although prokaryotes are present in Lake Magic fluid inclusions, they are rare. We estimate that approximately 1% of primary fluid inclusions host prokaryotes.
Abundant dimpled spherules are interpreted as algae. More specifically, the 5–7 μm size, yellow color, dimpled texture, and blue fluorescent response are characteristic of Dunaliella algae, a common algal genus in brines (Oren, 2002; Schubert et al., 2010a). We estimate that approximately 20% of primary fluid inclusions contain at least one eukaryote.
Beta-carotene is confirmed in Lake Magic fluid inclusions by its laser Raman signature. Peaks in some samples at 1013, 1157, and 1525 cm−1 are consistent with pure beta-carotene (Vitek et al., 2009; Osterrothová and Jehlička, 2011). Other samples with a small range of these three Raman peaks (982–1013, 1156–1178, and 1521–1552 cm−1) are also interpreted as beta-carotene, but perhaps as slightly different variations of the carotenoid. The beta-carotene Raman signature was detected for the spherules and the oily orange globules, showing that the beta-carotene exists in both solid and liquid form in the fluid inclusions. In addition, the UV-vis orange fluorescence is another indicator of beta-carotene.
Another biological pigment, scytonemin, has Raman peaks at 1518, 1155, and 1000 cm−1 (Vitek et al., 2010). Scytonemin, produced by some cyanobacteria to serve as a sunscreen, is a realistic chemical component of Lake Magic. However, the samples have a closer optical and Raman match with beta-carotene, so there is no definitive identification of scytonemin in the Lake Magic samples.
The beta-carotene Raman signature and UV-vis orange fluorescence that results from analyses of the spherules suggest that beta-carotene coats some Dunaliella algal cells. The Raman peaks for beta-carotene are more intense for the darkest orange algae and are weaker for the pale yellow algae. Therefore, there seems to be a relationship between the orange color and the amount of beta-carotene present. Furthermore, darker orange and pale yellow algae have slightly different degrees of dimpled texture. One possible explanation is that all algae are dimpled but that the coating of beta-carotene fills the dimples and slightly smoothes the spherule surface. Another possible explanation is that the algae without the beta-carotene may be transitioning into a collapsed “survival mode” cyst stage (Schubert et al., 2010a).
Exposure to the laser during laser Raman spectrometry likely caused some phase changes of the beta-carotene. This was detected in subsequent plane light and UV-vis microscopy and laser Raman analyses conducted after the laser was initially used on some fluid inclusions. As a rule, laser Raman spectroscopy was conducted last, after plane-transmitted light petrography, UV petrography, and photodocumentation were complete. However, a test was performed to determine whether exposure to the laser affected the fluid inclusion composition. Figure 8 shows the results of that test. Figure 8A documents the initial petrographic, UV, and Raman analyses of an inclusion. The alga was originally dark orange in plane light (Fig. 8A1), had orange-red fluorescence (A2), and had strong Raman peaks for beta-carotene (A4), but the inclusion liquid had subdued Raman peaks for beta-carotene (A3). Figure 8B shows the same types of analyses performed on the same inclusion after the initial laser exposure. These subsequent analyses resulted in pale yellow alga (B1) with blue fluorescence (B2). The second laser Raman analyses on this inclusion resulted in less intense beta-carotene peaks for the alga (B4) and more intense beta-carotene peaks for the inclusion liquid (B3). We hypothesize that the energy from the laser disrupted the beta-carotene, resulting in the beta-carotene envelope around the alga being dispersed in the inclusion liquid.
The strong Raman peak in the range of ∼3760 to ∼3824 cm−1 for inclusion liquids and in the range of ∼3809 to ∼3821 cm−1 for algae has not been identified (Fig. 8). It is not considered to be a peak for the O-H stretching vibrational mode of water because that water peak is typically found at ∼3500 cm−1 and because there is no peak at ∼1645 cm−1 to indicate the associated H-O-H bending vibrational mode for water (see Benison et al., 1998, for two water peaks for acid saline fluid inclusions in acid halite). Most Raman studies do not include the spectrum in this high wavenumber range, so relatively few compounds with peaks in this range have been documented. Mori et al. (2001) noted a peak at ∼3820 cm−1 attributed to H2 in the presence of high Si. This may be a reasonable explanation for strong peaks ∼3820 cm−1 due to the unusually high concentrations of Si and abundant H ions due to the low acidity in these inclusions. Raman peaks at ∼986 cm−1 are due to aqueous sulfate, and rare peaks at ∼1054 cm−1 are attributed to high concentrations of bisulfate, a hallmark of acid waters (Benison et al., 1998). More work is needed to identify the strong Raman peak at ∼3820 cm−1 and to better understand the Raman spectra of acid brines.
4.2. Benefits, challenges, and complications
There are benefits to using optical and chemical methods in lieu of traditional molecular methods to document microorganisms and organic compounds in fluid inclusions in halite. Petrography is the foundation of any fluid inclusion study and offers a relatively quick way to detect microorganisms. In situ, relatively nondestructive analyses of individual inclusions allows for avoidance of contamination, application of multiple analytical methods, and confirmation that samples are representative of parent lake waters. In contrast, the high salinity, low pH, and great chemical complexity of natural extreme acid brines have posed challenges to traditional molecular methodology (Mormile et al., 2009). Therefore, in situ optical and chemical techniques allow for an alternate pathway to detect microorganisms and organic compounds trapped in salts.
Optical and chemical methods for investigating biological materials in fluid inclusions, such as the plane light and UV petrography and laser Raman spectrometry used in this study, do not identify microorganisms at the species level (and perhaps also pose challenges to identifying many microorganisms at the genus and family levels).
Fluorescence encountered during laser Raman analyses was problematic with regard to inclusions, particularly those that contained or were near metals, such as iron oxide solid inclusions. In addition, iron oxides can also absorb the laser light, causing sample heating and decreasing the number of photons available for target excitation (Storrie-Lombardi, personal communication). The fluorescence can mask Raman analyses, and the laser can alter sample material.
We suggest that the most ideal way to investigate biological materials in fluid inclusions is with a multidisciplinary approach. The benefits of the combined petrographic and laser Raman techniques employed in this study are their nondestructive and noncontaminating nature, as well as the minimal sample preparation. For these reasons, optical and UV-vis petrography and laser Raman analyses are appropriate for preliminary investigation of samples prior to traditional molecular and DNA investigations. A combination of optical petrography, UV-vis petrography, and laser Raman spectrometry, followed by traditional molecular and DNA methods, would allow for full characterization of appearance, composition, identification, and preservation style of both microorganisms and organic compounds from modern and past saline environments.
4.3. Comparison to studies of microorganisms in fluid inclusions in neutral halite
Recent advances in the understanding and methodology of biological materials in fluid inclusions in halite have been made (Schubert et al., 2009a, 2009b, 2010a, 2010b; Lowenstein et al., 2011; Winters et al., 2012). These studies have used a combination of optical, UV, and molecular methods to determine microorganisms and organic compounds in modern neutral brines, fluid inclusions in modern halite, and fluid inclusions in ancient counterpart halite from Death Valley and Saline Valley. Dunaliella cells, prokaryotes, and carotenoids have been documented in both Death Valley and Saline Valley modern brines and fluid inclusions in halite.
There are similarities and differences between the halite from neutral Death Valley and Saline Valley and the halite from acid Lake Magic. Both the neutral and acid inclusions contain prokaryotes, Dunaliella algae, and beta-carotene. Fluid inclusions in Lake Magic halite have much fewer prokaryotes compared to Death Valley and Saline Valley halite. In addition, the Lake Magic inclusions seem to have more consistency in the occurrence and abundance of the algae. The Death Valley and Saline Valley halite may record past algal blooms, during which several algal cells are trapped within fluid inclusions, as well as times when algae was less abundant. Lake Magic halite has fewer algal cells in individual inclusions, but the presence of algae in fluid inclusions appears to be more consistent across growth bands.
Lake Magic halite preserves some different biological components due to its low pH and highly complex chemical composition compared to the saline neutral lakes in California. Lake Magic contains “hairy blobs,” which are unique to modern and ancient acid-precipitated halite (Benison et al., 2008). It is likely that some of the prokaryotes in Lake Magic halite represent different bacteria and archaea than do the neutral halite counterparts. In addition, the Dunaliella algae in Lake Magic fluid inclusions may belong to a previously unknown acidophilic species (Juergen Polle, personal communication).
4.4. Extreme conditions for microorganisms at Lake Magic
Microorganisms at Lake Magic are extremophiles in several different aspects. First, they withstand host lake waters with up to 32% TDS and pH down to at least 1.7, so they are halophilic and acidophilic. The lake waters also have among the highest known concentrations of dissolved aluminum and silica in the world (Bowen and Benison, 2009), as well as high sulfate, chloride, sodium, and high and varying metals. Solar radiation is high in southern Western Australia, averaging 15 MJ/m2 (Australian Government Bureau of Meteorology, 2013). Lake water and air temperatures fluctuate from 0°C to 50°C; commonly, there is a diurnal temperature range of 25–30°C (Benison et al., 2007). Finally, there are dynamic changes in physical and chemical conditions over varying time spans, driven by flooding, evapoconcentration, and desiccation.
4.5. Environmental conditions
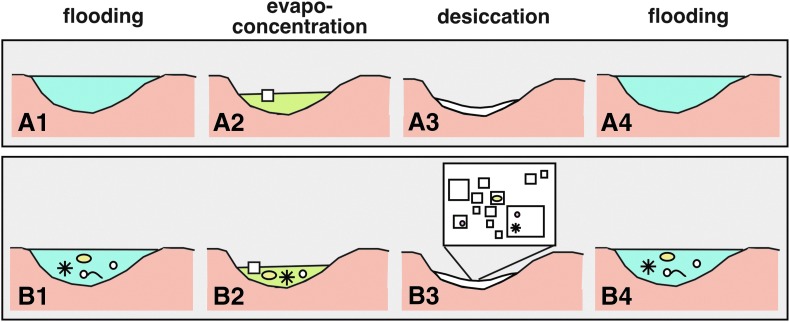
The flooding-evapoconcentration-desiccation cycles at Lake Magic, which cause rapid changes in lake water depth, pH, and salinity, also promote halite precipitation and dissolution (Fig. 9). Algae and prokaryotes may thrive in some environmental conditions at Lake Magic and then go into a slowed or dormant “survival” mode to withstand other conditions. It is possible that they remain viable within fluid inclusions during desiccation stages, which may last for years or decades, and then thrive again when flooding dissolves their host halite and releases them back into lake water. In this way, the microorganisms and organic compounds are recycled repeatedly through time (Fig. 9).
FIG. 9.
Schematic diagram depicting relationship between physiochemical lake changes (A) and microbiological communities (B) at Lake Magic. (A1) Flooding results in moderately acid, moderately saline, and relatively deep (∼1 m) lake water. (A2) Evaporation lowers pH, increases salinity, lowers water depth to centimeter-scale, and promotes precipitation of halite and gypsum. (A3) Desiccation leaves dry salt crust. (A4) Flooding dissolves halite and gypsum and freshens lake. (B1) Community of microorganisms that live in flooded lakes. (B2) Life that thrives in extreme acid lake brine. (B3) Microorganisms and organic compounds trapped, likely in dormant stage, within fluid inclusions in halite. (B4) Dissolution of halite liberates microorganisms and organic compounds into flooded lake.
4.6. Implications for life on Mars
Modern and Permian acid saline lakes and associated environments on Earth, including Lake Magic, are analogues for Mars sedimentary deposits (Benison and LaClair, 2003; Benison, 2006; Benison and Bowen, 2006). Mineralogy and chemistry, sedimentary textures and sedimentary structures, and early diagenetic features on Mars are consistent with those throughout southern Western Australia. Chloride and sulfate minerals, including acid-indicating minerals such as jarosite, as well as iron oxides and clays, have been detected in acid saline lakes in Western Australia and on Mars (i.e., Osterloo et al., 2008; King and McLennan, 2010). The chloride and sulfate minerals on Mars likely contain fluid inclusions trapped during their precipitation from a liquid. It is possible that any existing microorganisms and/or organic compounds in past martian liquids may have been trapped in those fluid inclusions. Future Mars sample return missions should plan to analyze fluid inclusions in martian halite, gypsum, and other evaporites for signs of past life on Mars (Farmer et al., 2009). Optical and chemical methods, such as plane-transmitted light petrography and laser Raman spectrometry (Vitek et al., 2012) could be attempted both with returned martian samples and by future rovers.
5. Conclusions
Rare prokaryotes, common Dunaliella algae, and beta-carotene are trapped in fluid inclusions from extremely acid and saline Lake Magic in Western Australia. Optical petrography, UV-vis petrography, and laser Raman spectrometry are effective for detecting biological materials trapped in salt and should serve as preliminary methods prior to the undertaking of traditional microbiological lab techniques. Implications for this study of microorganisms and organic compounds trapped in Lake Magic halite include the understanding of life in extreme terrestrial environments and suggested planning for instrumentation and detailed methodology for the search for life on Mars.
Acknowledgments
Funding to A.J.C. was granted through a Michigan Space Grant Consortium Fellowship and a Central Michigan University Summer Scholar Award. Fieldwork and sample collection was funded by National Science Foundation grants EAR-0433040 and EAR-0719822 and a National Geographic Society Research and Exploration Committee grant to K.C.B. We thank Carlos Sanchez Botero, Brenda Beitler Bowen, Jeremy Conner, Mercedes Gonzalez, Elliot Jagniecki, Jonathan Knapp, Tim Lowenstein, Melanie Mormile, Francisca Oboh-Ikuenobe, Brian Schubert, Mona Sirbescu, Stacy Story, Michael Storrie-Lombardi, James Student, Mary Tecklenburg, Reed Wicander, Yaicha Winters, and James Zambito for their help in the field, in the lab, and in sharing their expertise. We also thank editor Sherry Cady and two anonymous reviewers for their helpful suggestions.
Author Disclosure Statement
No competing financial interests exist.
Abbreviation
TDS, total dissolved solids.
References
- Adamski J.A. Roberts J.R. Goldstein R.H. Entrapment of bacteria in fluid inclusions in laboratory-grown halite. Astrobiology. 2006;6:552–562. doi: 10.1089/ast.2006.6.552. [DOI] [PubMed] [Google Scholar]
- Australian Government Bureau of Meteorology. Daily solar exposure for Australia. 2013. http://www.bom.gov.au/jsp/awap/solar/index.jsp http://www.bom.gov.au/jsp/awap/solar/index.jsp
- Benison K.C. A martian analog in Kansas: comparing martian strata with Permian acid saline lake deposits. Geology. 2006;34:385–388. [Google Scholar]
- Benison K.C. Life and death around acid saline lakes. Palaios. 2008;23:571–573. [Google Scholar]
- Benison K.C. Bowen B.B. Acid saline lake systems give clues about past environments and the search for life on Mars. Icarus. 2006;183:225–229. [Google Scholar]
- Benison K.C. LaClair D.A. Modern and ancient extremely acid saline deposits: terrestrial analogs for martian environments? Astrobiology. 2003;3:609–618. doi: 10.1089/153110703322610690. [DOI] [PubMed] [Google Scholar]
- Benison K.C. Goldstein R.H. Wopenka B. Burruss R.C. Pasteris J.D. Extremely acid Permian lakes and groundwaters in North America. Nature. 1998;392:911–914. [Google Scholar]
- Benison K.C. Bowen B.B. Oboh-Ikuenobe F.E. Jagniecki E.A. LaClair D.A. Story S.L. Mormile M.R. Hong B.Y. Sedimentology of acid saline lakes in southern Western Australia: newly described processes and products of an extreme environment. Journal of Sedimentary Research. 2007;77:366–388. [Google Scholar]
- Benison K.C. Jagniecki E.A. Edwards T.B. Mormile M.C. Storrie-Lombardi M.C. “Hairy blobs”: Microbial suspects from modern and ancient ephemeral acid saline evaporites. Astrobiology. 2008;8:807–821. doi: 10.1089/ast.2006.0034. [DOI] [PubMed] [Google Scholar]
- Bowen B.B. Benison K.C. Geochemical characteristics of naturally acid and alkaline saline lakes in southern Western Australia. Appl Geochem. 2009;24:268–284. [Google Scholar]
- Dartnell L.R. Storrie-Lombardi M.C. Ward J.M. Complete fluorescent fingerprints of extremophilic and photosynthetic microbes. International Journal of Astrobiology. 2010;9:245–257. [Google Scholar]
- Dombrowski H. Bacteria from Paleozoic salt deposits. Ann NY Acad Sci. 1963;108:453–460. doi: 10.1111/j.1749-6632.1963.tb13400.x. [DOI] [PubMed] [Google Scholar]
- Dombrowski H.J. Geological problems in the question of living bacteria in Paleozoic salt deposits. In: Rau J., editor. Second Symposium on Salt. Northern Ohio Geological Society; Cleveland, OH: 1966. pp. 215–220. [Google Scholar]
- Farmer J.D. Bell J.F., III Benison K.C. Boynton W.V. Cady S.L. Ferris F.G. MacPherson D. Race M.S. Thiemens M.H. Wadhwa M. Return Missions, Space Studies Board, National Research Council, National Academy Press; Washington, DC: 2009. Assessment of Planetary Protection Requirements for Mars Sample. [Google Scholar]
- Fendrihan S. Stan-Lotter H. Survival of halobacteria in fluid inclusions as a model of possible biotic survival in martian halite. In: Teodorescu H., editor; Griebel H., editor. Mars and Planetary Science and Technology. Performantica Press; Iasi, Romania: 2004. pp. 9–18. [Google Scholar]
- Fendrihan S. Legat A. Pfaffenhuemer M. Gruber C. Weidler G. Gerbl F. Stan-Lotter H. Extremely halophilic archaea and the issue of long-term microbial survival. Rev Environ Sci Biotechnol. 2006;5:203–218. doi: 10.1007/s11157-006-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish S.A. Shepherd T.J. McGenity T.J. Grant W.D. Recovery of 16S ribosomal RNA gene fragments from ancient halite. Nature. 2002;417:432–436. doi: 10.1038/417432a. [DOI] [PubMed] [Google Scholar]
- Fredrickson J.K. Chandler D.P. Onstott T.C. Potential for preservation of Halobacteria and their macromolecular constituents in brine inclusions from bedded salt deposits. Proc SPIE. 1997;3111:318–329. [Google Scholar]
- Goldstein R.H. Paleoenvironment: clues from fluid inclusions. Science. 2001;294:1009–1011. doi: 10.1126/science.1066322. [DOI] [PubMed] [Google Scholar]
- Grant W.D. Gemmell R.T. McGenity T.J. Halobacteria: the evidence for longevity. Extremophiles. 1998;2:279–287. doi: 10.1007/s007920050070. [DOI] [PubMed] [Google Scholar]
- Jagniecki E.A. Benison K.C. Criteria for the recognition of acid-precipitated halite. Sedimentology. 2010;57:273–292. [Google Scholar]
- King P.L. McLennan M. Sulfur on Mars. Elements. 2010;6:107–112. [Google Scholar]
- Lowenstein T.K. Ancient microorganisms in salt. In: Weil J., editor; Blumel D., editor; Malmoli S., editor; Netting J., editor. McGraw-Hill Yearbook of Science and Technology. McGraw-Hill; New York: 2008. pp. 13–15. [Google Scholar]
- Lowenstein T.K. Schubert B.A. Timofeeff M.N. Microbial communities in fluid inclusions and long-term survival in halite. GSA Today. 2011;21:4–9. [Google Scholar]
- McGenity T.R. Gemmell T. Grant W.D. Stan-Lotter H. Origins of halophilic microorganisms in ancient salt deposits. Environ Microbiol. 2000;2:243–250. doi: 10.1046/j.1462-2920.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- Mori T. Otsuka K. Umehara N. Ishioka K. Kitajima M. Hishita S. Murakami K. Multivacancies trapping hydrogen molecules. Physica B Condens Matter. 2001;308–310:171–173. [Google Scholar]
- Mormile M.R. Storrie-Lombardi M.C. The use of ultraviolet excitation of native fluorescence for identifying biomarkers in halite crystals. Proc SPIE. 2005;5906:246–253. [Google Scholar]
- Mormile M.R. Biesen M.A. Gutierrez M.C. Ventosa A. Pavlovich J.B. Onstott T.C. Fredrickson J.K. Isolation of Halobacterium salinarum retrieved directly from halite brine inclusions. Environ Microbiol. 2003;5:1094–1102. doi: 10.1046/j.1462-2920.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- Mormile M.R. Hong B. Benison K.C. Molecular analysis of the microbial communities of Mars analog lakes in Western Australia. Astrobiology. 2009;9:919–930. doi: 10.1089/ast.2008.0293. [DOI] [PubMed] [Google Scholar]
- Nadeau J.L. Perreault N.N. Niederberger T.D. Whyte L.G. Sun H.J. Leon R. Fluorescence microscopy as a tool for in situ life detection. Astrobiology. 2008;8:859–874. doi: 10.1089/ast.2007.0043. [DOI] [PubMed] [Google Scholar]
- Norton C. Grant W.D. Survival of halobacteria within fluid inclusions in salt crystals. J Gen Microbiol. 1988;134:1365–1373. [Google Scholar]
- Oren A. Halophilic Microorganisms and Their Environments. Kluwer Academic; Dordrecht, the Netherlands; 2002. [Google Scholar]
- Osterloo M.M. Hamilton V.E. Bandfield J.L. Glotch T.D. Baldridge A.M. Christensen P.R. Tornabene L.L. Anderson F.S. Chloride-bearing materials in the southern highlands of Mars. Science. 2008;319:1651–1654. doi: 10.1126/science.1150690. [DOI] [PubMed] [Google Scholar]
- Osterrothová K. Jehlička J. Investigation of biomolecules trapped in fluid inclusions inside halite crystals by Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2011;83:288–296. doi: 10.1016/j.saa.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Reiser R. Tasch P. Investigation of the viability of osmophilic bacteria of great age. Trans Kans Acad Sci. 1960;63:31–34. [PubMed] [Google Scholar]
- Schubert B.A. Lowenstein T.K. Timofeeff M.N. Microscopic identification of prokaryotes in modern and ancient halite, Saline Valley and Death Valley, California. Astrobiology. 2009a;9:467–482. doi: 10.1089/ast.2008.0282. [DOI] [PubMed] [Google Scholar]
- Schubert B.A. Lowenstein T.K. Timofeeff M.N. Parker M.A. How can prokaryotes survive in fluid inclusions in halite for 30,000 years? Geology. 2009b;37:1059–1062. [Google Scholar]
- Schubert B.A. Timofeeff M.N. Polle J.E.W. Lowenstein T.K. Dunaliella cells in fluid inclusions in halite: significance for long-term survival of prokaryotes. Geomicrobiol J. 2010a;27:61–75. [Google Scholar]
- Schubert B.A. Lowenstein T.K. Timofeeff M.N. Parker M.A. Halophilic archaea cultured from ancient halite, Death Valley, California. Environ Microbiol. 2010b;12:440–454. doi: 10.1111/j.1462-2920.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H. McGenity T.J. Legat A. Denner E.B.M. Glaser K. Stetter K.O. Wanner G. Very similar strains of Halococcus salifodinae are found in geographically separated Permo-Triassic salt deposits. Microbiology. 1999;145:3565–3574. doi: 10.1099/00221287-145-12-3565. [DOI] [PubMed] [Google Scholar]
- Stan-Lotter H. Radax C. Gruber C. Legat A. Pfaffenhuemer M. Wieland H. Leuko S. Weidler G. Komle N. Kargi G. Astrobiology with haloarchaea from Permo-Triassic rock salt. International Journal of Astrobiology. 2002;1:271–284. [Google Scholar]
- Storrie-Lombardi M.C. Hall A.P. Hang S. Lyzenga G.A. Clark C.M. Sattler B.I. Bej A.K. Hoover R.B. Spectral profiling and imaging (SPI): extending L.I.F.E. technology for the remote exploration of life in ice caves (R.E.L.I.C.) on Earth and Mars. Proc SPIE. 2011;8152:2–12. [Google Scholar]
- Vitek P. Osterrothová K. Jehlička J. Beta-carotene: a possible biomarker in the martian evaporitic environment: Raman micro-spectroscopic study. Planet Space Sci. 2009;57:454–459. [Google Scholar]
- Vitek P. Edwards H.G.M. Jehlička J. Ascaso C. de los Rios A. Valea S. Jorge-Villar S.E. Davila A.F. Wierzchos J. Microbial colonization of halite from the hyper-arid Atacama Desert studied by Raman spectroscopy. Philos Transact A Math Phys Eng Sci. 2010;368:3205–3221. doi: 10.1098/rsta.2010.0059. [DOI] [PubMed] [Google Scholar]
- Vitek P. Jehlička J. Edwards H.G.M. Hutchinson I. Ascaso C. Wierzchos J. The miniaturized Raman system and detection of traces of life in halite from the Atacama Desert: some considerations for the search for life signatures on Mars. Astrobiology. 2012;12:1095–1099. doi: 10.1089/ast.2012.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeland R.H. Rosenzweig W.D. Powers D.W. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature. 2000;407:897–900. doi: 10.1038/35038060. [DOI] [PubMed] [Google Scholar]
- Vreeland R.H. Jones J. Monson A. Rosenzweig W.D. Lowenstein T.K. Timofeeff M. Satterfield C. Cho B.C. Park J.S. Wallace A. Grant W.D. Isolation of live Cretaceous (121–112 million years old) halophilic archaea from primary salt crystals. Geomicrobiol J. 2007;24:275–282. [Google Scholar]
- Winters Y.D. Lowenstein T.K. Timofeeff M.N. Identification of carotenoids in ancient salt from Death Valley, Saline Valley, and Searles Lake, California using laser Raman spectroscopy. Geological Society of America Abstracts with Programs. 2012;44:74. doi: 10.1089/ast.2012.0952. [DOI] [PubMed] [Google Scholar]