Abstract
The purpose of this study was to investigate the influence of apple pomace (AP) and apple juice concentrate (AC) supplementation on body weight and fat loss as well as lipid metabolism in obese rats fed a high-fat diet. Diet-induced obese rats were assigned to three groups (n=8 for each group): high fat diet (HFD) control, HFD containing 10% (w/w) AP, and HFD containing 10% (w/w) AC. There was also a normal diet group (n=8). After 5 weeks, body weight gain, adipose tissue weight, serum and hepatic lipid profiles, liver morphology, and adipocyte size were measured. Body weight gain, white adipose tissue (WAT) weight, serum total cholesterol, low-density lipoprotein cholesterol and triglyceride concentrations, epididymal adipocyte size, and lesion scores were significantly lower and serum high-density lipoprotein cholesterol concentration and brown adipose tissue weights were significantly higher in the AP and AC groups compared with the HFD group. In addition, atherogenic indices in the AP and AC groups were significantly lower than in the HFD group. These results indicate that supplementing apple products such as AP and AC may help suppress body weight and WAT gain, as well as improve lipid profiles in diet-induced obese rats.
Key Words: apple products, body fat loss, diet-induced obese rats, lipid profile
Introduction
Numerous epidemiological studies have indicated that fruits and vegetable consumption is positively related to a lower prevalence of chronic diseases, including cardiovascular disease (CVD), cancer, and diabetes.1–4 The positive effects of fruits and vegetables are often attributed to dietary fiber (DF) and phenolic compounds. DF and polyphenols induce body weight and fat loss in obese people and improve lipid profiles of CVD patients.5,6 However, the biological mechanisms of these effects are not entirely clear.
In 2007, the consumption of apples and apple products by the Korean population reached ∼8.9 kg per capita, second only to mandarin oranges at 16.8 kg per capita. Apples and their products are popular, partly because they are available year round in a variety of forms (fresh fruit, juice, jam and jellies, etc.).7 Apples contain nutritionally important phytochemicals such as polyphenols, flavonoids, and DF. Although the amount and type of phytochemicals can vary greatly depending on the processing procedure and maturity of the apple, it varies most depending on which part of the apple is consumed. In general, the apple peel is abundant in phytochemicals, whereas apple flesh is not, and unripe apples are richer in polyphenols than are ripe apples. The processing of apples for their juice causes a substantial loss of polyphenols.
Apple juice is obtained from fresh apples by pulping and straight pressing, with the apple pomace (AP) remaining as the residue after the extraction process. AP has a concentration of high polyphenols and flavonoids, including p-coumaric acid, gallic acid, ferulic acid, ptocatehuic acid, epicatechin, and vanillic acids.8 AP is also a rich source of soluble DF (pectin), which is recommended to be the source of ∼30–50% of total DF intake because of its cholesterol-lowering effects.9 Diets high in DF are associated with the prevention and treatment of diverticular diseases, CVD, and colon cancer.10 Hence, dried AP is a potential food source of various bioactive compounds.
Recently, claims have also been made that unsweetened, undiluted apple juice may prevent CVD and some cancers.11,12 A commercial apple juice that has been sweetened and diluted during the manufacturing process has relatively low polyphenols and scarce DF content.10 Apple juice concentrates (ACs) are routinely prepared from apple juice production by concentrating apple juice 3–5 times. Thus, unsweetened, undiluted apple juice is preferred compared with sweetened, diluted apple juice by health-conscious consumers nowadays.
Health benefits of apples are undoubtedly due in part to the presence of DF and/or phytochemicals. More specifically, the positive effects of apples may come from their potential to lower cholesterol: plasma and liver cholesterol levels drop significantly after eating lyophilized apples.6 Moreover, cholesterol excretion increases in the feces of rats fed apples, suggesting that the DF and/or polyphenols found in apples may reduce dietary cholesterol absorption.6 A search of scientific databases revealed no studies on the effect of apples on weight loss or body fat loss. It was shown that processed apple accounts for 67% of whole processed fruit foods,7 and that AP generated during the processing procedure accounts for ∼20% of the entire apple weight.13 Some of the by-products are utilized as compost or feed for cattle, but most of it is discarded. Hence, as the demand for functional foods from natural plant sources is increased, identification of the physiological activity characteristics of AP may provide potential for the development of functional food, expand the range of utilization, reduce the expense of byproduct storage and disposal, and increase the added value of apples in the food industry.
This study investigated the effects of AP and AC on body weight gain, body fat, and lipid metabolism in diet-induced obese rats over 5 weeks.
Materials and Methods
AP, AC and their composition
Apple (Fuji apple, Malus pulmila var. dulcissima KOIDZ.) pomace and AC produced in 2008 were supplied by Gyeongbuk Apple Nonghyup Co. (Daegu, Korea). The AP was a hot-air dried powder, and the AC was in liquid form. The AP and AC were freeze dried for chemical component analysis. General composition, including moisture levels, carbohydrates, protein, fat, fiber, and ash, was measured following the AOAC method.14
The total polyphenol content of AP and AC was measured according to the spectrophotometric Folin–Denis method with some modifications.15 Determination of total polyphenol compounds was carried out in triplicate and calculated from a calibration curve obtained using tannic acid (Yakuri Pure Chemicals Co., Ltd., Tokyo, Japan). This tannic acid calibration curve was used as a standard, and results were expressed as % tannic acid equivalent.
The total flavonoid content of the samples was quantified according to the AOAC method.16 Determination of total flavonoid compounds was carried out in triplicate and calculated from a calibration curve obtained using naringin (Tokyo Kasei Kogyo Co., Ltd., Tokyo, Japan). This naringin calibration curve was used as a standard, and results were expressed as % naringin equivalent.
Animals and experimental protocols
A total of 32 3-week-old male Sprague–Dawley rats, supplied by the Hanlim Experimental Animal Laboratory (Seoul, Korea), were individually housed in stainless steel cages and placed in a room on a 12-h light/dark cycle where the temperature was kept at 21°C±1°C with a relative humidity of 50%±10%. All experimental protocols followed established guidelines for the care and handling of laboratory animals and were approved by the Institutional Animal Ethics Committee of Chung-Ang University, Korea. All rats were fed a pelletized commercial chow diet and deionized water ad libitum for 1 week. After acclimation, the rats were randomly assigned to four experimental groups of eight rats each: the normal diet group (negative control, ND), the high-fat diet group (positive control, HFD), and according to Maria et al.,17 the HFD group containing either 10% AP or AC by feed weight, adjusted for body weight.
The ND was formulated based on the AIN-93G rodent diet composition,18 and the HFD was formulated to provide 15% of the total feed weight from fat by replacing corn starch with lard (8%) and soybean oil (7%) with the same ratio of vitamins and minerals as the ND. In phase I (obesity induction period, 4 weeks), the normal diet control group (ND) was fed the AIN-93G rodent diet, and the other experimental groups were fed the HFD. In phase II (experimental period, 5 weeks), the AP and AC groups were fed two different supplemented diets, while ND and HFD groups maintained their diets. The experimental diets are shown in Table 1. The animals were allowed free access to food and water during the experimental period (Table 2). Food consumption was measured every other day, and body weight was measured weekly. At the end of the experimental period and 12 h after the last feeding, the rats were anesthetized with ketamine, and blood was taken from the orbital venous plexus. Serum was obtained by centrifugation at 1000 g for 15 min at 4°C. Organs, including the liver, kidneys, and testes, as well as interscapular brown adipose tissue (BAT) and three depots of white adipose tissue (WAT; epididymal, perirenal, and visceral) were removed and weighed. Stool was collected during the last 3 days in metabolic cages, and dried stool samples were used to measure total cholesterol (TC) and triglyceride (TG) levels. Serum, liver, and stool samples were stored at −75°C until analysis.
Table 1.
Experimental Diet Composition
| Ingredients | ND | HFD | AP | AC |
|---|---|---|---|---|
| Energy (kcal/100 g) | 394.6 | 434.6 | 426.3 | 408.4 |
| Casein (g) | 20 | 20 | 20 | 20 |
| Sucrose (g) | 10 | 10 | 10 | 10 |
| Maltose dextrin (g) | 13.2 | 13.2 | 13.2 | 13.2 |
| Corn starch (g) | 39.7 | 31.7 | 21.7 | 21.7 |
| Cellulose (g) | 5 | 5 | 5 | 5 |
| Soybean oil (g) | 7 | 7 | 7 | 7 |
| L-cystine (g) | 0.3 | 0.3 | 0.3 | 0.3 |
| Tert-butylhydroquinone (g) | 0.0014 | 0.0014 | 0.0014 | 0.0014 |
| Choline bitartrate (g) | 0.25 | 0.25 | 0.25 | 0.25 |
| Mineral mixture (g) | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mixture (g) | 1 | 1 | 1 | 1 |
| Lard (g) | - | 8 | 8 | 8 |
| AP (g) | - | - | 10 | - |
| AC (g) | - | - | - | 10 |
| Total (g) | 100 | 100 | 100 | 100 |
All diets were based on AIN-93G diet (E-Joeun Pet Feed Co., Ltd., Jeongeup, Korea).
ND, normal diet; HFD, high fat diet; AP, apple pomace; AC, apple juice concentrate.
Table 2.
Composition of Apple Pomace and Apple Juice Concentrate
| Group | Moisture (%) | Carbohydrate (%) | Protein (%) | Fat (%) | Ash (%) | Fiber (%) | Total polyphenols (%TAE) | Total flavonoids (%NE) |
|---|---|---|---|---|---|---|---|---|
| AP | 20.1±1.5 | 74.3±1.8 | 2.0±0.3 | 1.3±0.1 | 2.3±0.1 | 11.0±1.5 | 1538.5±50.5 | 193.1±7.5 |
| AC | 64.3±1.3 | 33.8±1.63 | 0.7±0.11 | 0.0±0.0 | 1.3±0.2 | 1.1±0.4 | 971.8±33.1 | 78.9±4.3 |
Values are expressed as mean±SD of triplicate determinations.
TAE, tannic acid equivalent; NE, naringin equivalent.
Hepatic and adipose tissue analysis
Sections of liver preserved in zinc-buffered formalin were embedded in paraffin, stained with hematoxylin and eosin, viewed with the use of an optical microscope, and photographed at 400× magnification. Hepatic lipid accumulation was measured by using the methods of Rivera et al., with slight modifications.19 Histological characteristics were scored by a pathologist blinded to the study design. The extent of lipid accumulation was assessed according to the following system: 0=no intracellular lipid; 1=intracellular lipid accumulation in at least 50% of the cells in zone 1; 2=intracellular lipid accumulation in at least 50% of the cells in zones 1–2; and 3=panlobular lipid accumulation in at least 50% of the cells.
Small pieces of epididymal and subcutaneous WAT were removed and rinsed with saline. The tissues were fixed with 10% formalin and embedded in paraffin wax. Tissue sections were cut to a thickness of 4 μm on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA), stained with hematoxylin and eosin, and photographed at 200× magnification. Adipocyte area was measured in 40 cells of representative sections using the NIH ImageJ Program (National Institutes of Health, Bethesda, MD, USA) in order to examine the size of the white adipocytes. The mean value was designated as an index of cell size, using methods described by Akagiri et al.20
Measurement of serum, hepatic, and fecal lipid profiles
Serum concentrations of TC, high-density lipoprotein cholesterol (HDL-C), and TG were measured enzymatically using an automatic chemistry analyzer (ADVIA 1650; Bayer, Osaka, Japan) with a reagent kit (Bayer, Pittsburgh, PA, USA). Serum low-density lipoprotein cholesterol (LDL-C) concentrations were calculated using the Friedwald equation, which involves subtracting the HDL-C from the TC concentration.21 Hepatic and fecal lipids were extracted using methods developed by Folch et al.22 Hepatic and fecal concentrations of TC and TG were determined enzymatically, using a commercial kit (Asan Pharmaceuticals Co., Seoul, Korea) based on a modified lipase–glycerol phosphate oxidase method23 and the cholesterol oxidase method,24 respectively.
Statistical analysis
All data were presented as the mean±standard error. Data were evaluated by one-way analysis of variance using SPSS Windows Version 19.0 (SPSS, Inc., Chicago, IL, USA). Mean differences were analyzed by Duncan's multiple range test. Results were considered statistically significant when P<.05.
Results
Body weight, food intake, and feed efficiency ratio
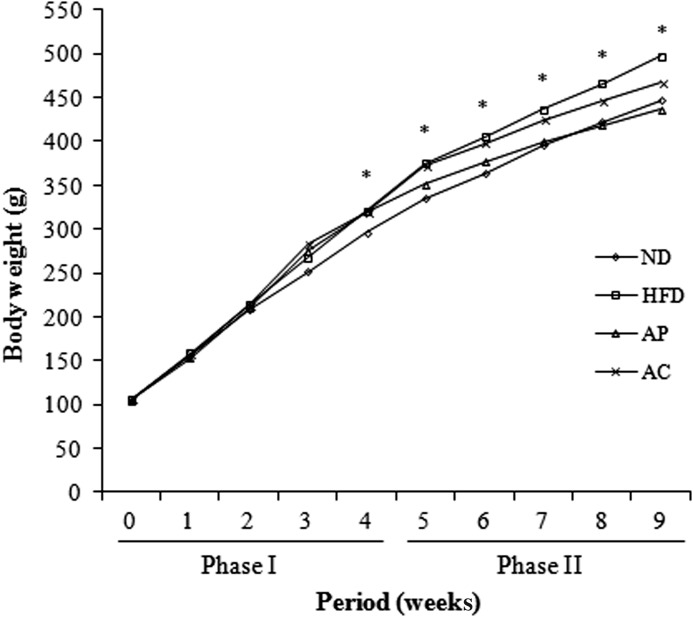
At the end of phase I (no apple supplementation period), body weight gain and feed efficiency ratio (FER) were significantly higher in all HFD groups than in the ND group (Fig. 1 and Table 3). At the end of phase II (apple supplementation period), body weight gain and FER in the AP (116.44±8.99 g, 0.12±0.01), AC (147.33±9.97 g, 0.15±0.01), and ND groups (151.05±6.58 g, 0.15±0.01) were significantly lower than in the HFD group (175.73±2.95 g, 0.18±0.01; P<.05). The lowest body weight gain and FER was observed in the AP group (P<.05), followed by the AC and ND groups. Body weight gain and FER in the AC and ND groups did not significantly differ from each other. This suggests that daily consumption of apple products is effective in curbing weight gain.
FIG. 1.
Body weight changes in rats fed a high-fat diet with AP and AC. Phase I, obesity induction period; Phase II, experimental period. *Significantly different at P<.05 by Duncan's multiple-range test. ND, normal diet; HFD, high-fat diet; AP, apple pomace; AC, apple juice concentrate.
Table 3.
Effects of Apple Pomace– and Apple Juice Concentrate–Supplemented Diets on Weight Gain, Food Intake, and Feed Efficiency Ratio in Rats Fed a High-Fat Diet
| |
No apple (Phase I) |
Apple supplement (Phase II) |
||||
|---|---|---|---|---|---|---|
| Group | Weight gain (g/4 week) | Food intake (g/day) | FER3 | Weight gain (g/5 week) | Food intake (g/day) | FER |
| ND | 191.47±5.65a | 25.24±20.37b | 0.27±0.01a | 151.05±6.58b | 29.11±0.19b | 0.15±0.01b |
| HFD | 215.94±5.76b | 24.78±0.19b | 0.31±0.01b | 175.73±2.95c | 28.62±0.18ab | 0.18±0.00c |
| AP | 215.49±8.57b | 24.09±0.19a | 0.32±0.01b | 116.44±8.99a | 28.23±0.29a | 0.12±0.01a |
| AC | 214.03±8.24b | 23.88±0.07a | 0.32±0.01b | 147.33±9.97b | 28.19±0.12a | 0.15±0.01b |
Values are expressed as mean±SE (n=8). Phase I, diet-induced obese period, 4 weeks; Phase II, experimental period, 5 weeks.
Values in the same column not sharing common superscript letters are significantly different at P<.05.
FER (=weight gain/food intake), feed efficiency ratio; SE, standard error.
Adipose tissue weights
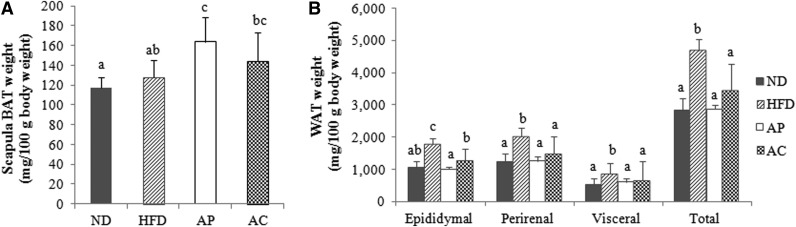
The weight of adipose tissue is presented in Figure 2. BAT weights in the AP group (163.47±25.30 mg/100 g body weight) were significantly higher than in all other groups. BAT weights of the AC group (144.02±29.45 mg/100 g body weight) were higher than that of the ND group (117.56±10.29 mg/100 g body weight), but did not differ from that of the HFD group (127.50±17.53 mg/100 g body weight).
FIG. 2.
Effects of AP- and AC-supplemented diets on adipose tissue in rats fed a high-fat diet. abcResults not sharing common letters are significantly different at P<.05 by Duncan's multiple-range test. (A) BAT, brown adipose tissue; (B) WAT, white adipose tissue.
The weights of epididymal, perirenal, and visceral WAT per body weight were significantly lower in the AP, AC, and ND groups than in the HFD group, and were reflective of the body weight gain. The lowest epididymal weights were observed in the AP group, although those of the ND group were not significantly different from the AP group. There were no significant differences in perirenal, visceral, and total WAT among the ND, AP, and AC groups, with the HFD group being significantly higher, as expected. These results along with the body weight data indicate that the changes in body weight gain were likely due to changes in total WAT caused by the apple products.
Hepatic and adipose tissue analysis
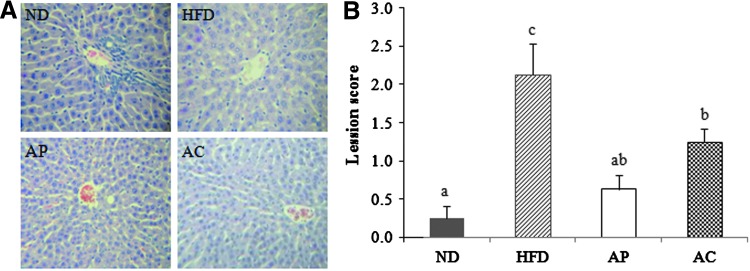
Cytoplasmic lipid accumulation was observed in the portal areas of the liver sections (Fig. 3). The HFD group had considerably more lipid microvacuoles compared with the experimental groups. Although occasional mild periportal microvacuoles were observed in the AP and AC groups, these groups displayed significant inhibition of hepatic lipid accumulation as indicated by the lower hepatic lesion scores. The lowest scores were found in the ND group (0.25±0.46), followed by the AP group (0.63±0.18) and the AC group (1.25±0.16), with the HFD group (2.13±1.13) having the highest score.
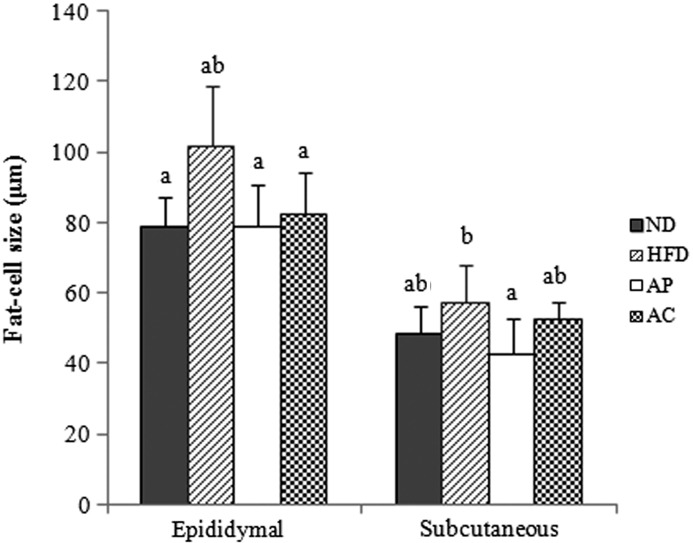
FIG. 3.
Effects of AP- and AC-supplemented diets on fat-cell size in rats fed a high-fat diet. abResults not sharing common letters are significantly different at P<.05 by Duncan's multiple-range test.
Epididymal adipocyte size and subcutaneous fat pads were significantly smaller in the AP, AC, and ND groups than in the HFD group, and subcutaneous adipocyte size in the AP group was the lowest and similar to that in the ND group (Fig. 4).
FIG. 4.
Inhibition of hepatic lipid accumulation by AP and AC supplementation in HFD-induced obese rats. (A) Representative hematoxylin-and-eosin-stained section of liver tissue (original magnification×400). (B) Histological analyses of hepatic lipid accumulation. Pathological scores of hepatic lipid accumulation are as described in Materials and Methods. abcValues not sharing common letters are significantly different at P<.05 by Duncan's multiple-range test. Color images available online at www.liebertpub.com/jmf
Serum and hepatic lipids concentration
To determine whether apple consumption had an impact on circulating lipid levels, a comprehensive serum lipid profile analysis was performed. Serum TC, LDL-C, and TG concentrations were significantly lower in the AP, AC, and ND groups than in the HFD group, with TG concentrations particularly low in the AP group (Table 4). HDL-C concentrations were the highest in the AP group and were significantly lower in the HFD group. HDL-C concentrations in the AP, AC, and ND groups did not differ from each other. The atherogenic index (AI) calculated from these measurements was significantly lower in the AP, AC, and ND groups compared with the HFD group, indicating that apple consumption ameliorated the harmful cardiovascular effects of the HFD feedings. A portion of the liver was analyzed for cholesterol and TG content (Table 4). Hepatic TC concentrations were lowest in the AP group, and significantly different from the HFD group (Table 4). Compared with the HFD rats, both AP and AC as well as ND rats had significantly lower TC and TG in the liver.
Table 4.
Effects of Apple Pomace– and Apple Juice Concentrate–Supplemented Diets on Serum and Hepatic Lipids in Rats Fed a High-Fat Diet
| |
Serum |
Liver |
||||||
|---|---|---|---|---|---|---|---|---|
| Groups | TC (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | TG (mg/dL) | HTR | AI | TC (mg/g) | TG (mg/g) |
| ND | 52.63±1.54a | 19.00±0.50ab | 26.33±1.56a | 75.13±1.53a | 0.36±0.02b | 1.79±0.12a | 2.10±0.04ab | 5.99±0.30a |
| HFD | 78.00±3.77b | 18.00±0.33a | 45.35±3.68b | 73.25±1.60c | 0.23±0.01a | 3.34±0.22b | 2.56±0.06c | 7.86±0.32c |
| AP | 60.13±2.80a | 20.25±0.88b | 29.70±1.92a | 50.88±4.25b | 0.34±0.01b | 1.98±0.10a | 2.08±0.04a | 6.19±0.37a |
| AC | 61.00±3.71a | 19.50±0.89ab | 30.75±3.42a | 53.75±4.59b | 0.33±0.02b | 2.14±0.17a | 2.35±0.06bc | 7.12±0.40b |
Values are expressed as mean±SE (n=8).
Values in the same column not sharing common superscript letters are significantly different at P<.05.
TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; HTR, HDL-C/TC ratio; AI, atherogenic index [(TC) − (HDL-C)]/(HDL-C).
Fecal weight and lipid excretion
Probably due to high fiber content of AP, daily fecal weights in the AP group were significantly higher than in all other groups. Daily fecal weights in the AC and ND groups were similar to each other. Fecal TC concentrations in the AP group were significantly higher than in the other groups, and there were significant differences between the AP, AC, and ND groups as well. Moreover, the AP group had the highest fecal TG concentrations, whereas fecal TG concentrations in the ND, AC, and HFD groups did not differ from each other (Table 5). Thus, AP consumption was clearly superior in terms of excretion of harmful lipids through the stool.
Table 5.
Effects of Apple Pomace– and Apple Juice Concentrate–Supplemented Diets on Fecal Weight and Lipids in Rats Fed a High-Fat Diet
| Group | Fecal weight (g/day) | TC (mg/g feces) | TG (mg/g feces) |
|---|---|---|---|
| ND | 13.62±1.22b | 20.14±0.63a | 58.26±1.44a |
| HFD | 10.30±0.18a | 21.82±0.32ab | 60.06±0.42a |
| AP | 17.05±0.92c | 23.11±0.84b | 70.48±2.25b |
| AC | 13.90±0.47b | 20.97±0.76a | 61.95±2.71a |
Values in the same column not sharing common superscript letters are significantly different at P<.05.
Discussion
Obesity is a major risk factor for CVD, cancer, diabetes, and other chronic diseases. Meta-analyses of prospective studies show that a 5 kg/m2 increase in BMI is associated with increased risk of colon cancer in both men and women.25,26 In this study, body weight gain was significantly repressed in the AP (−59.29 g, −33.74%) and AC groups (−28.40 g, −16.16%) compared with the HFD group in phase II (experimental period, 5 weeks). Food intake did not differ between groups; thus, the FER was lower than other groups. According to Conceicao de Oliveira et al.,27 ingesting apples or pears thrice a day was associated with weight loss in middle-aged hypercholesterolemic overweight women in Brazil. Participants who consumed either of the fruits had a significant weight loss (−1.21 kg) after 12 weeks, whereas those who consumed oat cookies instead of the fruits did not have a significant weight loss. The study claimed that fruit consumption may have decreased energy intake by increasing satiety. Along with insoluble fiber, apples contain high amounts of soluble DF, which increases postmeal satiety.28 Similarly, the significantly repressed weight gain seen in the AP and AC groups of our study might also be attributed to the total fiber content of apples, particularly in the AP group. In this study, crude fiber contents of AP and ACs were 11% and 1.1%, respectively. Thus, AP contains considerably higher crude fiber contents than fresh raw apples or any other apple products. Since 10% corn starch as a carbohydrate source was substituted by the apple products in the AP and AC groups, the AP and AC diets contained the same amount of calories as the HFD group. However, the high DF in the apple products did lead to a reduced energy density, energy intake, and body weight. Energy density affects energy intake independent of macronutrient content or palatability when meals with varying energy density (low, medium, or high) are consumed by humans.29
However, reduced body weight gain was much more pronounced in the AP group than in the AC group, despite the caloric density of the AC group diet (408.4 kcal/100 g) being modestly lower than that of the AP group diet (426.25 kcal/100 g). This might be due to differences in DF content or possibly to phytochemical content, as fiber content and phytochemical content are 10 times and 1.5–2.5 times higher, respectively, in AP than in AC. These findings indicate that DF and phytochemicals in apples may have synergistic effects. Since phytochemicals combined with DF have been found to be more effective in body fat loss,30 it is possible that fiber and phytochemicals acted synergistically in our study for an effective weight and body fat loss.
When adipose tissue weights were compared among the groups in this study, all three types of WAT weights in the AP group were significantly lower than in the HFD group. In spite of this, Nakazato et al.31 reported that polyphenol compounds in apples contribute to a decrease of adipose tissue weight when apple polyphenols (APP) are fed to Wistar rats. Moreover, Murase et al.32 reported that tea catechins reduced retroperitoneal WATs of rats fed catechin containing HFD. APP contains procyanidine as a major component, consisting of (+)-catechin and (−)-epicatechin units similar to tea catechins found in.33–35 Various vitro studies have shown that APP suppresses adipose cell formation.31,36 These results suggest that the lower adipose tissue weights associated with apple product consumption in this study might be influenced by phytochemicals such as polyphenols and flavonoids. Total polyphenols and flavonoids in AP were 1538.5 mg/100 g and 971.8 mg/100 g versus 193.1 mg/100 g and 78.9 mg/100 g, respectively, in ACs. The polyphenol concentration of AP is extremely high, even compared with typical high phytochemical foods such as blueberry (670.9 mg/100 g), dogwood berry (432.0 mg/100 g), and sour cherry (429.5 mg/100 g).
DF is known to increase fecal sterol excretion and decrease blood TC and TG concentrations by shortening transit time, repressing lipolytic enzyme activity, increasing bile acid absorption, enhancing cholesterol biosynthesis, and reducing cholesterol absorption.37–43 Some studies have shown that the soluble DF in apples improves lipid metabolism in the blood and liver of experimental animals.17,30,44,45 There is also evidence that polyphenol enhances fecal cholesterol and bile acid excretion.46–48 In the current study, HDL-C increased; while LDL-C, serum TC and TG, liver lipids, HTR, and AI were significantly decreased in the AC and AP groups when compared with the HFD group. Some of the apple's protective effects against CVD may come from its potential ability to improve lipid profiles. In fact, Aprikian et al.49 reported a significant decrease in plasma cholesterol and liver cholesterols and an increase in HDL-C on supplementing lyophilized apples to cholesterol-fed rats. In addition, they found that cholesterol excretion increased in the feces of rats that were fed apples, implying diminished cholesterol absorption.49 Although interactions between bile acids and apple constituents are complex, pectins or polymerized phenolics of apples may adsorb bile acids, thereby interrupting enterohepatic cycling of bile acids, for which maximal trapping might require a synergism between fibers and phenolic compounds.50
In conclusion, our study showed that consumption of AP and AC in HFD-induced obese rats improved body weight and body fat loss and blood lipid profiles. This occurred by way of modifying lipid metabolism through increased cholesterol, thus decreasing cholesterol absorption. However, further studies are required to elucidate the mechanisms by which specific components of apple products have positive effects on weight control and lipid metabolism.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adebawo O. Salau B. Ezima E, et al. Fruits and vegetables moderate lipid cardiovascular risk factor in hypertensive patients. Lipids Health Dis. 2006;5:14. doi: 10.1186/1476-511X-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leontowicz M. Gorinstein S. Leontowicz H, et al. Apple and pear peel and pulp and their influence on plasma lipids and antioxidant potentials in rats fed with cholesterol containing diets. J Agric Food Chem. 2003;51:5780–5785. doi: 10.1021/jf030137j. [DOI] [PubMed] [Google Scholar]
- 3.Bohm H. Boeing H. Hempel J, et al. Flavonoids, flavones and anthocyanins as natural antioxidants of food and their possible role in the prevention of chronic diseases. Z Ernahrungswiss Suppl. 1998;37:147–163. doi: 10.1007/pl00007376. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Parrilla E. De La Rosa LA. Legarreta P, et al. Daily consumption of apple, pear and orange juice differently affects plasma lipids and antioxidant capacity of smoking and non-smoking adults. Int J Food Sci Nutr. 2010;61:369–380. doi: 10.3109/09637480903514041. [DOI] [PubMed] [Google Scholar]
- 5.Basu A. Rhone M. Lyons TJ. Berries: emerging impact on cardiovascular health. Nutr Rev. 2010;68:168–177. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vafa MR. Haghighatjoo E. Shidfar F, et al. Effects of apple consumption on lipid profile of hyperlipidemic and overweight men. Int J Prev Med. 2011;2:94–100. [PMC free article] [PubMed] [Google Scholar]
- 7.The Principal Statistics Data. MIFAFF; Seoul, Korea: The Ministry for Food, Agriculture, Forestry and Fisheries; pp. 2007pp. 316–321. [Google Scholar]
- 8.Sudha ML. Baskaran V. Leelavathi K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007;104:686–692. [Google Scholar]
- 9.Chandalia M. Garg A. Lutjohann D. Bergmann CV. Grundy SM. Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–1398. doi: 10.1056/NEJM200005113421903. [DOI] [PubMed] [Google Scholar]
- 10.Soyalan B. Minn J. Schmitz HJ, et al. Apple juice intervention modulates expression of ARE-dependent genes in rat colon and liver. Eur J Nutr. 2011;50:135–143. doi: 10.1007/s00394-010-0124-9. [DOI] [PubMed] [Google Scholar]
- 11.Gerhauser C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Med. 2008;74:1608–1624. doi: 10.1055/s-0028-1088300. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JW. Smith BM. Gustafson NJ. Health benefits and practical aspects of high-fiber diets. Am J Clin Nutr. 1994;59:1242S–1247S. doi: 10.1093/ajcn/59.5.1242S. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH. Kim YC. Kim MY. Jung HS. Jung SK. Antioxidative activity and related compounds of apple pomace. Korean J Food Sci Technol. 2000;32:908–913. [Google Scholar]
- 14.USDA. USDA Agriculture Handbook: Composition of Foods. 8th. USDA; Washington, DC: 1975. [Google Scholar]
- 15.Singleton VL. Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 16.AOAC. Official Methods of Analysis. 12th. Association of Official Analytical Chemists; Washington, DC: 1995. [Google Scholar]
- 17.Maria L. Shela G. Elzbieta B. Hanna L. Gustaw K. Simon T. Sugar beet pulp and apple pomace dietary fibers improve lipid metabolism in rats fed cholesterol. Food Chem. 2001;72:73–78. [Google Scholar]
- 18.Reeves PG. Nielsen FH. Fahey GC. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Rivera CA. Abrams SH. Tcharmtchi MH, et al. Feeding a corn oil/sucrose-enriched diet enhances steatohepatitis in sedentary rats. Am J Physiol Gastrointest Liver Physiol. 2006;290:G386–G393. doi: 10.1152/ajpgi.00229.2005. [DOI] [PubMed] [Google Scholar]
- 20.Akagiri S. Naito Y. Ichikawa H, et al. Bofutsushosan, an oriental herbal medicine, attenuates the weight gain of white adipose tissue and the increased size of adipocytes associated with the increase in their expression of uncoupling Protein 1 in High-Fat Diet-Fed Male KK/Ta mice. J Clin Biochem Nutr. 2008;42:158–166. doi: 10.3164/jcbn.2008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedewald WT. Levy RI. Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 22.Folch J. Lees M. Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 23.McGowan MW. Artiss JD. Strandberge DR, et al. A peroxide-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1993;29:538–542. [PubMed] [Google Scholar]
- 24.Allain CC. Poon LS. Chan CSG. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 25.Larsson SC. Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 26.Renehan AG. Tyson M. Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 27.Conceicao de Oliveira M. Sichieri R. Sanchez Moura A. Weight loss associated with a daily intake of three apples or three pears among overweight women. Nutrition. 2003;19:253–256. doi: 10.1016/s0899-9007(02)00850-x. [DOI] [PubMed] [Google Scholar]
- 28.Howarth NC. Saltzman E. Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59:129–139. doi: 10.1111/j.1753-4887.2001.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 29.Bell EA. Castellanos VH. Pelkman CL, et al. Energy density of foods affects energy intake in normal-weight women. Am J Clin Nutr. 1998;67:412–420. doi: 10.1093/ajcn/67.3.412. [DOI] [PubMed] [Google Scholar]
- 30.Aprikian O. Duclos V. Guyot S, et al. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on fecal fermentation and plasma lipids in rats. J Nutr. 2003;133:1860–1865. doi: 10.1093/jn/133.6.1860. [DOI] [PubMed] [Google Scholar]
- 31.Nakazato K. Song H. Waga T. Effects of dietary apple polyphenol on adipose tissues weights in Wistar rats. Exp Anim. 2006;55:383–389. doi: 10.1538/expanim.55.383. [DOI] [PubMed] [Google Scholar]
- 32.Murase T. Nagasawa A. Suzuki J, et al. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 2002;26:1459–1464. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- 33.Kanda T. Akiyama H. Yanagida A, et al. Inhibitory effects of apple polyphenol on induced histamine release from RBL-2H3 cells and rat mast cells. Biosci Biotechnol Biochem. 1998;62:1284–1289. doi: 10.1271/bbb.62.1284. [DOI] [PubMed] [Google Scholar]
- 34.Ohnishi-Kameyama M. Yanagida A. Kanda T, et al. Identification of catechin oligomers from apple (Malus pumila cv. Fuji) in matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and fast-atom bombardment mass spectrometry. Rapid Commun Mass Spectrom. 1997;11:31–36. doi: 10.1002/(SICI)1097-0231(19970115)11:1<31::AID-RCM784>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 35.Dulloo AG. Seydoux J. Girardier L, et al. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord. 2000;24:252–258. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- 36.Shoji T. Kobori M. Shinmoto H, et al. Inhibitory effects of apple polyphenols on differentiation of 3T3-L1 cells into adipocytes. Food Sci Technol Res. 2000;6:119–121. [Google Scholar]
- 37.Yang JL. Suh MJ. Song Y. Effects of dietary fiber on cholesterol metabolism in cholesterol-fed rats. J Korean Soc Food Sci Nutr. 1996;25:392–398. [Google Scholar]
- 38.Garcia-Diez F. Garcia-Mediavilla V. Bayon JE, et al. Pectin feeding influences fecal bile acid excretion, hepatic bile acid and cholesterol synthesis and serum cholesterol in rats. J Nutr. 1996;126:1766–1771. doi: 10.1093/jn/126.7.1766. [DOI] [PubMed] [Google Scholar]
- 39.Arjmandi BH. Ahn J. Nathani S, et al. Dietary soluble fiber and cholesterol affect serum cholesterol concentration, hepatic portal venous short-chain fatty acid concentration and fecal sterol excretion in rats. J Nutr. 1992;122:246–253. doi: 10.1093/jn/122.2.246. [DOI] [PubMed] [Google Scholar]
- 40.Hughes JS. Potential contribution of dry bean dietary fiber to health. Food Technol. 1991;45:122–126. [Google Scholar]
- 41.Lairon D. Dietary fiber, dietary lipids. In: McCleary BV, editor; Prosky L, editor. Advanced Dietary Fiber Technology. Blackwell Science; Oxford: 2001. pp. 177–185. [Google Scholar]
- 42.Marlett JA. Dietary fiber and cardiovascular disease. In: Cho SS, editor; Dreher ML, editor. Handbook of Dietary Fiber. Maecel Dekker; New York: 2001. pp. 17–30. [Google Scholar]
- 43.Uberoi SK. Vadhera S. Soni GL. Role of dietary fiber from pulses and cereals as hypocholesterolemic and hypolipidemic agent. J Food Sci. 1992;29:281–293. [Google Scholar]
- 44.Sable-Amplis R. Sicart R. Bluthe E. Decreased cholesterol ester levels in tissues of hamsters fed with apple fiber enriched diet. Nutr Rep Int. 1983;27:881–889. [Google Scholar]
- 45.Chau CF. Huang YL. Lin CY. Investigation of the cholesterol-lowering action of insoluble fibre derived from the peel of Citrus sinensis L. cv. Liucheng. Food Chem. 2004;87:361–366. [Google Scholar]
- 46.Chisaka T. Matsuda H. Kubomura Y, et al. The effect of crude drugs on experimental hypercholesteremia: mode of action of (−)-epigallocatechin gallate in tea leaves. Chem Pharm Bull. 1998;36:227–233. doi: 10.1248/cpb.36.227. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto N. Okushio K. Hara Y. Effect of black tea polyphenols on plasma lipids in cholesterol-fed rats. J Nutr Sci Vitaminol. 1998;44:337–342. doi: 10.3177/jnsv.44.337. [DOI] [PubMed] [Google Scholar]
- 48.Tebib K. Besancon P. Rouanet JM. Dietary grape seed tannin affect lipoproteins, lipoprotein lipase and tissue lipids in rats fed hypercholesterolemic diets. J Nutr. 1994;124:2451–2457. doi: 10.1093/jn/124.12.451. [DOI] [PubMed] [Google Scholar]
- 49.Aprikian O. Busserolles J. Manach C, et al. Lyophilized apple counteracts the development of hypercholesterolemia, oxidative stress, and renal dysfunction in obese Zucker rats. J Nutr. 2002;132:1969–1976. doi: 10.1093/jn/132.7.1969. [DOI] [PubMed] [Google Scholar]
- 50.Dongowski G. Ehwald R. Binding of water, oil, and bile acids to dietary fibers of the cellan type. Biotechnol Prog. 1999;15:250–258. doi: 10.1021/bp990014c. [DOI] [PubMed] [Google Scholar]