Abstract
Objective
This study was conducted to assess the long-term effect of methylphenidate (MPH) or atomoxetine (ATX) on growth in attention-deficit/hyperactivity disorder (ADHD) drug-naïve children.
Design
The study was an observational, post-marketing, fourth phase study.
Methods
Data on height and weight were collected at baseline and every 6 months up to 24 months.
Results
Both ATX and MPH lead to decreased height gain (assessed by means of z-scores); the effect was significantly higher for ATX than for MPH. At any time, height z-score decrease in the ATX group was higher than the corresponding decrease observed in the MPH group, but the difference was significantly relevant only during the first year of treatment. An increment of average weight was observed both in patients treated with MPH and in those treated with ATX. However, using Tanner's percentile, a subset of patients showed a degree of growth lower than expected. This negative effect was significantly higher for ATX than for MPH.
Conclusions
We conclude that ADHD drugs show a negative effect on linear growth in children in middle term. Such effect appears more evident for ATX than for MPH.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common behavioral disorders in children and adolescents (Skounti et al. 2007). Pharmacological treatment may reduce ADHD symptom severity (MTA Cooperative Group 1999; Biederman and Faraone 2005). Methylphenidate (MPH) and other psychostimulants are recommended as first-choice drugs for ADHD (Schachter et al 2001). Atomoxetine (ATX), a selective norepinephrine reuptake inhibitor, is considered as a second choice (Cheng et al. 2007). Adverse events may occur both with psychostimulants and ATX. Available evidence suggests that children and adolescents are at higher risk than adults for adverse events during treatment with psychotropic drugs (Greenhill et al. 2003).
According to a systematic review (Faraone et al. 2008), ∼38% of included studies showed a growth slowdown in children treated with ADHD drugs. The effect, although attenuated, persisted over time for 4 years (Mattes and Gittelman 1983). However, discontinuation of treatment with stimulants showed a compensatory growth spurt (Mattes and Gittelman 1983; Klein et al. 1988; Klein and Mannuzza 1988).
As for ATX, meta-analytic evidence shows a slight weight decrease (∼1 kg) in the short term (2–3 months) (Cheng et al. 2007). Two additional meta-analyses assessed reported the effect of long-term use of ATX on height and weight. The first showed a decrease in weight (average 2.5 kg) and in height (average 2.7 cm) after 2 years of treatment with ATX in 6–7-year-old children in relation to baseline percentiles (Kratochvil et al. 2006). The second meta-analysis reported a less evident effect on weight and height, 0.87 kg and 0.44 cm, respectively (Spencer et al. 2005).
The Italian ADHD National Registry was activated in April 2007. It is managed by the Italian National Institute of Health (Istituto Superiore di Sanità, ISS) and supervised by a national panel of experts with the aim of implementing an active pharmacovigilance, and to assess the risk/benefit ratio of ADHD drugs (Panei et al. 2004). According to Italian regulation, children can receive pharmacological treatment for ADHD only after registration with the ADHD National Registry. Care providers choose the treatment based on their own experience, and on current clinical practice. Italian law requires close monitoring of drugs for 2 years after registration, in order to assess safety in current clinical practice. An observational post-marketing study of pharmacovigilance is mandatory for every new drug approved for ADHD.
The objective of this study was to assess the effect on growth during 2 years of treatment with MPH or ATX in ADHD children and adolescents enrolled in the Italian ADHD National Registry.
Patients and Methods
Subjects
This observational prospective study included 1758 children and adolescents (6–18 years of age) with ADHD, who were consecutively recruited from 87 centers accredited for the management of ADHD in Italy between June 2007 and June 2010. All subjects treated with ADHD drugs were included in this study and were drug naïve.
Participants were either referred by their child neuropsychiatrists or self-referred to a reference center for a suspicion of ADHD.
ADHD was diagnosed according to American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) (American Psychiatric Association 1994) criteria for ADHD based on clinical history and confirmed by a structured interview. Accordingly, to be diagnosed with ADHD, subjects had to present with a significant functional impairment and symptoms had to: 1) Be present, at least in part, before the age of 7 years, 2) persist for at least 6 months, and 3) be present in more than one setting (e.g., at home, and/or at school, and/or in another setting). All subjects were screened for other mental disorders, and participants with an autism spectrum disorder were excluded, as per DSM-IV criteria. Subjects with follow-up or compliance problems were also excluded.
All subjects who accepted the pharmacological treatment signed an informed consent explaining the aim of the study and the tests to be performed in order to evaluate the primary parameters (i.e., effect on height growth).
The study was approved by the Ethical Committee of the Istituto Superiore di Sanità.
Treatments
Two study groups were defined according to the pharmacological treatment, and the choice of treatment was based on current clinical practice by child neuropsychiatrists.
Group A
This group consisted of subjects treated with MPH plus behavioral treatment. The drug compound was methylphenidate chlorhydrate 10 mg tablet (Ritalin®, Novartis Pharma, Italia). MPH was administered orally (0.3–0.6 mg/kg/dose/day).
A methylphenidate test dose of 0.3 mg/kg was administered first. The dosage could be increased up to 0.6 mg/kg/dose depending upon the subject's clinical response and tolerability. The total dose could be administered in two or three doses/day. The duration of the renewable prescription was 1 month.
Group B
This group consisted of subjects treated with ATX plus behavioral treatment. Atomoxetine chlorhydrate (5 mg, 10 mg, 18 mg, 25 mg, 40 mg, or 60 mg tablets; Strattera®, Lilly) was used. Route of administration was oral, with the following schedule: Beginning with 0.5 mg/kg/day once a day, at least for 7 days, then increase the dose up 1.2 mg/kg/day, related to the subject's clinical response and tolerability. Duration of the renewable prescription was 1 month.
Data collection and management
All relevant information was collected by standard procedures. The clinical assessment was performed monthly, and included measurement of height in centimeters, and of weight in kilograms. Height and weight measurements were collected in according Tanner's standard procedure (Tanner et al. 1966). Each measure of weight and height was also computed in percentiles.
Clinical monitoring of the register included regular checking via the Internet. All clinical data, relative to recruitment and follow-up of each enrolled child, were entered in an electronic Case Report Form (eCRF), that was located in a restricted area (https://www.farmaco-iss.org/cgi-bin/adhd/index_gen) of the web site www.iss.it/adhd. Centers, child psychiatrist services, and pediatricians could access this restricted area through user i.d. and password.
The database of the register was based at Istituto Superiore di Sanità, Rome, which was responsible for its protection and management. The data management was designed by an infrastructure named “Advanced Multicenter Research developed by Consorzio Inter-Universitario per il Calcolo Automatico dell'Italia nord-orientale.” This program application allowed the checking of any informative flow, the data input, the monitoring of information, and the analysis of results.
Determination of sample size
The required sample size was estimated with respect to the 1 year variation in height z-score (the primary outcome of the study), based on the paired Student's t test (comparison of the mean value between baseline and 12 months within the treatment group), two tailed (we were interested in demonstrating differences in height z-score variation in whatever direction).
From previous studies, the standard deviation of the 1 year variation in height z-score in the overall group of subjects, apart from sex and age, was estimated at 0.4.
Moreover, we considered as clinically relevant a z-score difference from baseline to 12 months height z-score variation ≥0.1 (corresponding to a Cohen's d=0.25, i.e., a small-to-medium effect size according to Cohen, 1988). Finally, considering a type I error probability α=0.05 and a power 1-β=0.80, the minimal sample size required for the study was 133 subjects in each treatment group.
This sample size also allowed for detection of a difference between MPH and ATX groups in the height z-score 1 year variation ≥0.15 (corresponding to a Cohen's d=0.375, i.e., a small-to-medium effect size according to Cohen, 1988), based on a two tailed Student's t test for independent samples, with a type I error probability α=0.05 and a power 1-β=0.85.
Statistical analysis
Categorical variables (i.e., sex, treatment, and height and weight percentile changes) are shown as absolute and percent frequencies, whereas quantitative variables (age, height, weight) are summarized as means±standard deviations.
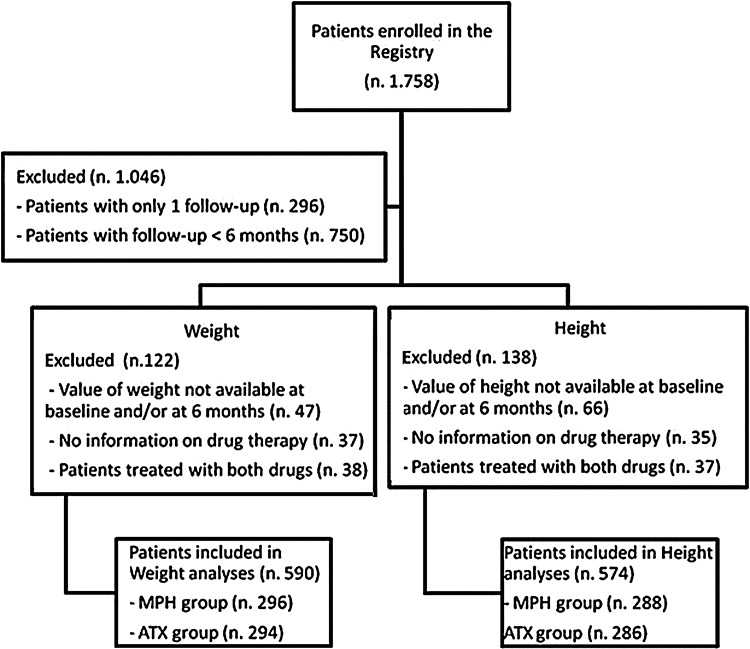
In order to maximize the number of subjects included in the statistical analyses, data were separately analyzed according to three reference periods: From enrollment (time 0) to 6 months, from 0 to 12 months, and from 0 to 24 months of follow-up. With respect to treatment, subjects were divided into four groups according to drug(s) received during the reference period under examination: MPH-treated (MPH group), ATX-treated (ATX group), both drugs-treated (MIXED group), and those not treated with either MPH or ATX, but receiving other psychotropic drugs. The last two groups were excluded from the analysis (Fig. 1).
FIG. 1.
Flow chart of patients at 6 months of follow-up. The figure reports the number of patients included in weight and height analysis, stratified for each group of treatment (methylphenidate group, atomoxetine group). The reasons for which the patients were excluded from analysis were also reported.
For any reference period, only subjects with data at baseline and at the end of the period were included in the statistical analyses.
To assess the difference between observed and expected changes in height and weight during each reference periods, heights and weights were categorized in percentile classes (from 0 to 3rd, from >3rd to 10th, from >10th to 25th, from >25th to 50th, from >50th to 75th, from >75th to 90th, from >90th to 97th, and >97th percentile) based on Tanner's age- and sex-specific data. Subsequently, each subject was classified as passing to a lower percentile (percentile decreased), remaining in the same percentile (percentile unchanged), or passing to a higher percentile (percentile increased) from baseline to the end of the reference period. The frequency of subjects shifting to a lower percentile class was compared with that of those moving to a higher class using the binomial test. Moreover, the distribution of subjects according to the percentile variation from baseline to the end of the period was compared between the two treatment groups, using the χ2 test.
To take into account the effect of sex and age, height was also transformed in z-score, according to the formula
 |
where heighti=height of the subject at the time of assessment, height mean=mean of sex- and age-specific height, height standard deviation=standard deviation of sex- and age-specific height, using sex- and age-specific height means and standard deviations taken from Tanner's tables on cross-sectional-type standards for height attained (Tanner et al. 1966).
Because of asymmetry in the variable distribution, weight could not be transformed in z-scores, and, therefore, was analyzed and presented as raw data.
Comparisons within the MPH or ATX groups with respect to height and weight data were performed by paired Student's t test to compare measurements taken at baseline and at the end of the specific reference period within each treatment group. The differences between the MPH and ATX groups for height and weight changes, occurring during the period, were tested using Student's t test for independent samples. Nonparametric tests (Wilcoxon and Mann–Whitney U tests for paired data and independent samples, respectively) were also performed on weight data to validate results of parametric tests. As the results were concordant, only parametric tests were reported.
Results
Through June 30 2010, 1758 children and adolescents with ADHD were recruited from the Italian ADHD National Registry. Of these, 1558 (88.6%) were males. Analyzing age classes, subjects <11 years were the most represented (991 subjects) and accounted for ∼57% of the entire population.
Stratified by type of treatment, 840 (47.8%) subjects were treated with MPH and 918 (52.2%) were treated with ATX.
MPH was prescribed at average daily dose equal to 0.48 mg/kg (SD±0.22) and with total average daily dose of 18.8 mg (SD±10.7). ATX was prescribed at average daily dose of 38.7 mg (SD±20.5).
Subjects in the MPH or ATX groups in relation to the three different periods (0 vs. 6 months, 0 vs. 12 months, 0 vs. 24 months) were compared with respect to age at baseline and sex distribution. No significant differences were found between MPH and ATX groups, except for sex when comparing the two groups of treatment in relation to the period 0 versus 24 months, when a lower proportion of females was observed in the ATX group (46 males and 9 females in the MPH group vs. 34 males and only 1 female in the ATX group, p=0.047).
During the study, monitoring of height and weight was recommended monthly. The mean number of height measures per subject was 6.11, ranging from 1 to 33, whereas the mean number of weight measures per subject was 6.14, ranging from 1 to 33.
For primary analysis, we used follow-up data at 6, 12, and 24 months. One thousand and sixty-four (60.5%) subjects dropped out of the study. Reasons for dropping out are reported in Figure 1.
Weight evaluation
Five hundred and ninety subjects were included in the analysis (Table 1). The comparison for age, sex, subtype of ADHD and comorbidity showed no significant differences between the subjects included in the analysis and those excluded, except for the depression. Two hundred and ninety-six out of 590 (50.2%) were treated with MPH and 294 (49.8%) were treated with Atomoxetine.
Table 1.
Demographic and Clinical Characteristics of Subjects Stratified by groups Included and not Included in the Weight Analysis
| Variables | Included in the analysis 590 subjects | Not included in the analysis 1168 subjects |
|---|---|---|
| Gender, n (%) | ||
| Male | 514 (87.1) | 1044 (89.4) |
| Female | 76 (12.9) | 124 (10.6) |
| Age class, n (%) | ||
| <11 years | 341 (57.8) | 662 (56.8) |
| 11 -<15 years | 207 (35.1) | 406 (34.9) |
| ≥15 years | 42 (7.1) | 97 (8.3) |
| Type of ADHD, n (%) | ||
| ADHD – I | 33 (5.6) | 61 (5.2) |
| ADHD – H | 26 (4.4) | 60 (5.1) |
| ADHD – C | 531 (90.0) | 1028 (88.0) |
| Presence of comorbidity | ||
| Oppositional defiant disorder | 247 (41.9) | 463 (39.6) |
| Conduct disorder | 32 (5.4) | 79 (6.8) |
| Depression | 32 (5.4) | 82 (7.0) |
| Anxiety | 73 (12.4) | 190 (16.3) |
| Learning disorder | 269 (45.6) | 479 (41.0) |
ADHD, attention-deficit/hyperactivity disorder; ADHD-I, Subtype Inattentive ADHD; ADHD-H, Subtype Hyperactive ADHD; ADHD-C, Subtype Combined ADHD.
Percentile variations are shown in Table 2. As can be seen, in all reference periods, the proportion of subjects shifting to a lower percentile class was larger than that of those moving to a higher percentile class. The difference between these two groups has always been significant, except for MPH subjects in the reference period 0–24 months. The difference was stronger in ATX- than in MPH-treated subjects; however, the difference between the two groups was significant only for the reference periods 0–6 months and 0–12 months.
Table 2.
Percentile Changes of Patients' Weight at Different Reference Periods Stratified by Type of Drugs
| |
From 0 to 6 months(n 296 MPH; n 294 ATX)Percentile |
From 0 to 12 months(n 184 MPH; n 159 ATX)Percentile |
From 0 to 24 months(n 55 MPH; n 28 ATX)Percentile |
||||||
|---|---|---|---|---|---|---|---|---|---|
| decreased | unchanged | increased | decreased | unchanged | increased | decreased | unchanged | increased | |
| Methylphenidate (MPH) | 83 (28.1%) | 178 (60.1%) | 35 (11.8%) | 58 (31.5%) | 92 (50.0%) | 34 (18.5%) | 23 (41.8%) | 17 (30.9%) | 15 (27.3%) |
| Binomial | test | p<0.001 | Binomial | test | p=0.018 | Binomial | test | p=0.267 | |
| Atomoxetine (ATX) | 112 (38.1%) | 158 (53.7%) | 24 (8.2%) | 73 (45.9%) | 63 (39.6%) | 23 (14.5%) | 15 (53.6%) | 9 (32.1%) | 4 (14.3%) |
| Binomial | test | p<0.001 | Binomial | test | p<0.001 | Binomial | test | p=0.010 | |
| MPH vs ATX | χ2=7.43 | df=2 | p=0.024 | χ2=7.48 | df=2 | p=0.024 | χ2=1.94 | df=2 | p=0.380 |
The binomial test refers to the comparison between frequencies of percentile decreased and increased subjects, based on the null hypothesis of equal distribution between the two categories.
The χ2 test refers to the comparison between the distributions of MPH- and ATX-treated subjects in the percentile change categories.
df, degrees of freedom.
We calculated the mean value of weight before the treatment and after 6, 12, and 24 months. For MPH, at each time point, a statistically significant weight increase was detected. The mean difference from baseline was +1.21 (SD 2.66) kg at 6 months, +3.21 (SD 3.63) kg at 1 year, and +8.44 (SD 5.18) kg at 24 months (p<0.001 for all periods). Similarly, a statistically significant difference was observed for ATX. The mean difference from baseline was +0.38 (SD 3.03) kg at 6 months, +2.03 (SD 4.11) kg at 1 year, and +5.65 (SD 5.44) kg at 24 months (p=0.031, p<0.001, and p<0.001, respectively). These findings are summarized in Table 3. The comparison between MPH- and ATX-treated patients with respect to weight change at 6, 12, and 24 months shows a statistically significant difference (p<0.001, p=0.005 and p=0.025, respectively).
Table 3.
Weight of Patients Stratified by Type of Drugs at Different Times from Enrolment
| MPH n | Mean±SD (kg) | ATX n | Mean±SD (kg) | MPH vs ATX t testa | |
|---|---|---|---|---|---|
| 0 months | 296 | 38.70±13.40 | 294 | 41.03±15.84 | |
| 6 months | 39.91±13.75 | 41.41±15.76 | |||
| 0 vs 6 mo. t testb | t295=7.85 | t293=2.17 | t588=3.53 | ||
| p<0.001 | p<0.031 | p<0.001 | |||
| 0 months | 184 | 38.38±13.26 | 159 | 40.55±13.93 | |
| 12 months | 41.58±13.72 | 42.58±14.42 | |||
| 0 vs 12 mo. t testb | t183=11.98 | t158=6.23 | t341=2.81 | ||
| p<0.001 | p<0.001 | p<0.005 | |||
| 0 months | 55 | 38.36±11.31 | 28 | 43.46±17.35 | |
| 24 months | 46.80±13.41 | 49.11±18.33 | |||
| 0 vs 24 mo. t testb | t54=12.08 | t27=5.48 | t81=2.28 | ||
| p<0.001 | p<0.001 | p<0.025 |
Weights are reported as means±standard deviations in kg.
Unpaired Student's t test: Comparisons between MPH- and ATX-treated subjects regarding weight changes between baseline and the end of the specific reference period.
Paired Student's t test: Comparisons between measurements taken at baseline and at the end of the specific reference period, within each treatment group.
MPH, methylphenidate; ATX, atomoxetine; df, degrees of freedom.
Height evaluation
Five hundred and seventy-four subjects were included in the analyses (Table 4). The comparison for age, sex, subtype of ADHD, and comorbidity between the group included in the analysis and the one excluded, showed a statistically significant difference only for anxiety. Two hundred and eighty-eight out of 574 (50.2%) were treated with MPH and 286 (49.8%) were treated with ATX.
Table 4.
Demographic and Clinical Characteristics of Subjects Stratified by Groups Included and not Included in the Height Analysis
| Variables | Included in the analysis 574 subjects | Not included in the analysis 1184 subjects |
|---|---|---|
| Gender, n (%) | ||
| Male | 500 (87.1) | 1058 (89.3) |
| Female | 74 (12.9) | 126 (10.7) |
| Age class, n (%) | ||
| <11 years | 340 (59.3) | 663 (56.0) |
| 11 to<15 years | 200 (34.8) | 413 (35.0) |
| ≥15 years | 34 (5.9) | 105 (9.0) |
| Type of ADHD, n (%) | ||
| ADHD – I | 33 (5.7) | 61 (5.1) |
| ADHD – H | 27 (4.7) | 59 (4.9) |
| ADHD – C | 514 (89.6) | 1045 (90.0) |
| Presence of comorbidity | ||
| Oppositional defiant disorder | 243 (42.3) | 467 (39.4) |
| Conduct disorder | 33 (5.7) | 78 (6.6) |
| Depression | 33 (5.7) | 81 (6.8) |
| Anxiety | 69 (12.0) | 194 (16.4) |
| Learning disorder | 265 (46.2) | 483 (40.1) |
ADHD, attention-deficit/hyperactivity disorder; ADHD-I, Subtype Inattentive ADHD; ADHD-H, Subtype Hyperactive ADHD; ADHD-C, Subtype Combined ADHD.
Percentile variations are shown in Table 5. The proportion of subjects shifting to a lower percentile class was higher than that of those moving to a higher percentile class, but the difference was significant only for ATX subjects in the reference periods 0–12 months and 0–24 months. The difference was slightly stronger in ATX- than in MPH-treated subjects; however, the difference between the two groups never reached statistical significance.
Table 5.
Percentile Changes of Patients' Height at Different Reference Periods Stratified by Type of Drugs
| |
From 0 to 6 months(n 288 MPH; n 286 ATX)Percentile |
From 0 to 12 months(n 167 MPH; n 139 ATX)Percentile |
From 0 to 24 months(n 55 MPH; n 35 ATX)Percentile |
||||||
|---|---|---|---|---|---|---|---|---|---|
| decreased | unchanged | increased | decreased | unchanged | increased | decreased | unchanged | increased | |
| Methylphenidate (MPH) | 54 (18.8%) | 185 (64.2%) | 49 (17.0%) | 46 (27.5%) | 91 (54.5%) | 30 (18.0%) | 17 (30.9%) | 25 (45.5%) | 13 (23.6%) |
| Binomial | test | p=1.000 | Binomial | test | p=0.099 | Binomial | test | p=0.292 | |
| Atomoxetine (ATX) | 63 (22.0%) | 178 (62.2%) | 45 (15.7%) | 48 (34.5%) | 75 (54.0%) | 16 (11.5%) | 14 (40.0%) | 16 (45.7%) | 5 (14.3%) |
| Binomial | test | p=0.138 | Binomial | test | p<0.001 | Binomial | test | p=0.032 | |
| MPH vs ATX | χ2=0.99 | df=2 | p=0.609 | χ2=3.31 | df=2 | p=0.191 | χ2=1.45 | df=2 | p=0.485 |
The binomial test refers to the comparison between frequencies of percentile decreased and increased subjects, based on the null hypothesis of equal distribution between the two categories.
The χ2 test refers to the comparison between the distributions of MPH- and ATX-treated subjects in the percentile change categories.
We analyzed the mean value of height before the treatment and after 6, 12, and 24 months (Table 6). A statistically significant increase of height was detected in the MPH group. The mean difference from baseline was +2.92 (SD 2.32) cm at 6 months, +5.01 (SD 2.77) cm at 1 year, and +10.48 (SD 4.83) cm at 24 months (p<0.001 for all periods). For ATX, the difference from baseline was +2. 64 (SD 2.50) cm at 6 months, +4.09 (SD 2.80) cm at 1 year, and +8.31 (SD 5.31) cm at 24 months (p<0.001 for all periods). At any period, height increase in the ATX group was lower than the corresponding increase observed in the MPH group, but the difference was significant only after ≥1 year of treatment (p=0.176, p=0.004, and p=0.050 for 6, 12, and 24 months, respectively).
Table 6.
Height of Patients Stratified by Type of Drugs at Different Times from Enrolment
| MPH n | Mean±SD (cm) | ATX n | Mean±SD (cm) | MPH vs ATX t testa | |
|---|---|---|---|---|---|
| 0 months | 288 | 140.90±15.12 | 286 | 143.02±17.01 | |
| 6 months | 143.82±15.22 | 145.66±17.04 | |||
| 0 vs 6 mo. t testb | t287=21.36 | t285=17.87 | t572=1.36 | ||
| p<0.001 | p<0.001 | p=0.176 | |||
| 0 months | 167 | 141.22±15.50 | 139 | 144.01±16.89 | |
| 12 months | 146.23±15.73 | 148.10±17.00 | |||
| 0 vs 12 mo. t testb | t166=23.34 | t138=17.25 | t304=2.87 | ||
| p<0.001 | p<0.001 | p=0.004 | |||
| 0 months | 55 | 140.45±14.49 | 35 | 145.49±17.57 | |
| 24 months | 150.93±15.04 | 153.80±18.18 | |||
| 0 vs 24 mo. t testb | t54=16.08 | t34=9.26 | t88=1.99 | ||
| p<0.001 | p<0.001 | p=0.050 |
Heights are reported as means±standard deviations in cm.
Unpaired Student's t test: Comparisons between MPH and ATX-treated subjects regarding weight changes between baseline and the end of the specific reference period.
Paired Student's t test: Comparisons between measurements taken at baseline and at the end of the specific reference period, within each treatment group.
MPH, methylphenidate; ATX, atomoxetine; df, degrees of freedom.
Z-score for height
When considering height z-scores, the mean difference from baseline for MPH was −0.001 (SD 0.334) at 6 months, −0.104 (SD 0.381) at 12 months, and −0.175 (SD 0.660) at 24 months (p=0.961, p<0.001, and p=0.055 for 6, 12, and 24 months, respectively). For ATX, the difference from baseline was −0.037 (SD 0.375) at 6 months, −0.229 (SD 0.399) at 12 months, and −0.441 (SD 0.734) at 24 months (p=0.093, p<0.001, p=0.001 for 6, 12, and 24 months, respectively). At any period, height z-score decrease in the ATX group was higher than the corresponding decrease observed in the MPH group, but the difference was significant only at 1 year of treatment (p=0.220, p=0.006, and p=0.203 for 6, 12, and 24 months, respectively). The results are summarized in Table 7.
Table 7.
Height z-Scores of Patients Stratified by Type of Drugs at Different Times from Enrolment
| MPH n | Mean±SD | ATX n | Mean±SD | MPH vs ATX t testa | |
|---|---|---|---|---|---|
| 0 months | 288 | 0.35±1.19 | 286 | 0.32±1.27 | |
| 6 months | 0.35±1.16 | 0.27±1.20 | |||
| 0 vs 6 mo. t testb | t287=0.05 | t285=1.68 | t572=1.23 | ||
| p<0.961 | p<0.093 | p=0.220 | |||
| 0 months | 167 | 0.37±1.22 | 139 | 0.55±1.21 | |
| 12 months | 0.27±1.19 | 0.32±1.16 | |||
| 0 vs 12 mo. t testb | t166=3.54 | t138=6.77 | t304=2.79 | ||
| p<0.001 | p<0.001 | p=0.006 | |||
| 0 months | 55 | 0.36±1.21 | 35 | 0.84±1.22 | |
| 24 months | 0.18±1.12 | 0.40±1.30 | |||
| 0 vs 24 mo. t testb | t54=1.96 | t34=3.55 | t88=1.27 | ||
| p<0.055 | p<0.001 | p=0.203 |
Unpaired Student's t test: Comparisons between MPH and ATX-treated subjects regarding weight changes between baseline and the end of the specific reference period.
Paired Student's t test: Comparisons between measurements taken at baseline and at the end of the specific reference period, within each treatment group.
MPH, methylphenidate; ATX, atomoxetine; df, degrees of freedom.
Discussion
This observational study assessed the impact of MPH and ATX on growth. The study showed a different growth rate between the two drugs, with ATX-treated patients growing significantly more slowly than MPH-treated patients.
With regard to weight, there was a significant trend for weight increase for both drugs. For the effect on height we observed a statistically significant decrease, evaluated in percentiles, for both drugs. However, comparing the effect between the two drugs on height decrease, a statistically significant difference were observed only at 12 months.
Although the two variables, weight and height, are both important in the assessment of growth, height is more important because, once growth stops at the end of adolescence, height cannot increase any more, whereas weight changes throughout life.
Therefore, in order to more accurately assess growth with respect to height, we used the z-score that correlates with chronological age and gender.
The z-score values after 12 and 24 months of therapy were more reduced in the ATX group than in the MPH one, but a significant difference was detected only at 12 months. At 24 months of follow-up, a greater difference in z-score between the two groups was observed, but it is not statistically significant, because the number of subjects included in this subanalysis, in the two groups, was lower. Therefore, it is possible to state that, in the first year of treatment, ATX causes a significantly greater growth delay than MPH. Additionally, both drugs showed a cumulative effect over time. After 24 months, the z-score had halved for both groups. Our results are in accordance with other studies (Spencer et al. 2005; Charach et al. 2006), in which a slowdown of growth rate in long-term treated patients was observed. Although this finding is also confirmed by a recent review (Faraone 2008), a recent naturalistic study did not support any association between deficits in growth process and psychostimulant treatment in ADHD patients (Villarreal et al. 2010).
As for ATX, a meta-analysis of long-term studies of ATX in children showed that the stronger negative effect occurred after 18 months of treatment, and that then this effect decreased with time (Spencer et al. 2005). A recent randomized, double-blind, placebo-controlled study of a Japanese pediatric population showed that the mean height increases in the ATX group were lower than those in the placebo group (Takahashi et al. 2009). On the other hand, one placebo-controlled trial did not show clinically significant effects on growth rate with ATX (Donnelly et al. 2009).
Our results should be considered in the light of study limitations. First, it is not clear whether the observed slowdown in growth is a transient effect or a permanent potential reduction for individual growth with respect to the final height. As our observation time was only 24 months of follow-up, we were not able to evaluate if the negative effect on growth persisted after 24 months of treatment. Second, we could not assess if the negative effect observed on height would persist after permanent discontinuation of drugs (Safer et al. 1975). Unfortunately, our study did not include a specific follow-up for subjects with permanent discontinuation of treatment. Third, ∼60% of subjects could not be included in the statistical analyses. To understand the relative impact of this issue on the results, we compared “population included” versus “population not included” in the statistical analysis, following some factors, such as age classes, sex, subtype of ADHD, and comorbidity. Statistically significant differences were observed only for depression in the weight analysis and for anxiety in the height analysis. Therefore, we believe that the population included in the analysis was representative of the whole population enrolled in the Italian ADHD National Registry.
Conclusions
The long-term effects of therapies for chronic diseases represent one of the most important issues in the evaluation of the profile benefit/risk. Our study highlights that the use of MPH and ATX in children and adolescents with ADHD seems not to be the cause of permanent growth effects. Both drugs cause a moderate slowdown in the height velocity highlighted by the values of the z-score. However, this effect does not seem to be permanent, and there is no significant difference between ATX and MPH. On the other hand, both drugs cause an increase in the average weight in pharmacologically treated patients. After 2 years of pharmacological treatment, we have observed an average weight about +5 SD from the 50th percentile. This finding should be confirmed by a randomized controlled study with two active drug arms and one control group.
Clinical Significance
Regular monitoring of growth parameters (parent's height, height, and weight measurements) is recommended for all patients, but it should be strongly recommended for subjects treated with ADHD drugs. So far, attention has been focused on the effect of ADHD medications on height growth. In view of our findings, it is necessary to devote the same attention to the risk of onset of obesity in patients treated with these drugs.
Contributor Information
Collaborators: the Italian ADHD Regional Reference Centers
Acknowledgments
We thank all participating regional reference centers: Region Liguria (Dr. Barbara Bobba, Dr. Edvige Veneselli, Dr. Maria Josè Baldizzone, Dr. Gianni De Nobili), region Lazio (Dr Marco Marcelli, Prof. Maria Giulia Torrioli, Dr. Stefano Vicari, Dr. Sandro Bartolomeo, Prof. Paolo Curatolo, Prof. Anna Fabrizi, Dr. Renato Donfrancesco), region Emilia-Romagna (Dr Roberto Parisi, Dr. Dora Suglia, Dr. Modena Nicoletta, Dr. Paolo Stagi, Dr. Flaviana Murru, Dr. Andrea Tullini, Dr. Simona Chiodo, Dr. Antonio Pirisi), region Veneto (Dr. Ettore Morbin, Dr Luca Milantoni, Prof. Bernardo Dalla Bernardina, Dr. Dino Maschietto, Dr.ssa Cristina Mambelli, Prof. Antonio Condini, Dr. Maurizio Brighenti, Dr. Piergiorgio Miottello, Dr. Roberto Tombolato, Dr Lenio Rizzo, Dr. Andrea Gemma), region Sicilia (Dr. Sebastiano Musumeci, Dr. Francesca Vanadia, Dr. Giancarlo Costanza, Dr. Donatella Ragusa, Dott. Filippo Calamoneri, Prof. Domenico Mazzone), region Friuli Venezia Giulia (Dr. Ferruccio Giaccherini, Dr. Marco Carrozzi, Dr. Silvana Cremaschi), region Lombardia (Dr. Alberto Ottolini, Dr. Daniele Arisi, Dr. Alessandra Tiberti, Dr. Maria L. Terragni, Dr. Paola Morosini, Dr. Corrado Meraviglia, Prof. Carlo Lenti, Dr. Marco Pezzani, Prof. Umberto Balottin, Prof. Paolo Piccinelli, Dr. Giuseppe Chiarenza, Dr. Emilio Brunati, Dr. Vincenzo Montrasio, Dr. Massimo Molteni, Dr. Francesco Rinaldi, Dr. Giorgio Rossi, Dr. Roberto Segala), region Piemonte (Dr. Flavio Guccione, Dr. Paolo Bailo, Dr. Dante Besana, Dr. Bianca Bassi, Dr. Marco Rolando, Dr. Laura Jarre, Dr. Francesca Ragazzo), region Sardegna (Prof. Alessandro Zuddas, Prof. Massimo Tondi), province Alto Adige (Dr. Gianluca Casara, Dr. Donatella Arcangeli, Dr Ingo Stermann), Province Trento (Dr. Costanza Giannelli), region Val D'Aosta (Dr. Giovanni Voltolin), region Abruzzo (Dr. Maria Pia Legge, Prof. Enzo Sechi, Dr. Elena Gennaro), region Calabria (Dr. Giovanna Campolo, Dr. Antonio La Vitola, Dr. Annalisa Mingolla), region Puglia (Dr. Angelo Spina, Prof. Lucia Margari, Dr. Angelo Massagli), region Campania (Dr. Carmela Bravaccio, Dr. Rosario Granato, Dott.ssa Giampina Grimaldi), region Umbria (Prof. Giovanni Mazzotta), region Toscana (Dr. Gabriele Masi, Prof. Giovanni Cioni), and region Marche (Dr. Maurizio Pincherle, Dr. Cardinali Cesare, Dr. Vera Stoppioni, Dr. Tasca Rosolino). We also thank Mrs. Federica Maria Regini for editorial assistance and Dr. Samuele Cortese, Postdoctoral Fellow at New York University Child Study Center, for suggestions.
Disclosures
No competing financial interests exist.
References
- American Psychiatric Association: Diagnostic, Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Biederman J. Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Charach A. Figueroa M. Chen S. Ickowicz A. Schachar R. Stimulant treatment over 5 years: Effect on growth. J Am Acad Child Adolesc Psychiatry. 2006;45:415–421. doi: 10.1097/01.chi.0000199026.91699.20. [DOI] [PubMed] [Google Scholar]
- Cheng JY. Chen RY. Ko JS. Ng EM. Efficacy and safety of Atomoxetine for attention-deficit hyperactivity disorder in children and adolescents – meta-analysis and meta-regression analysis. Psychopharmacology. 2007;194:197–209. doi: 10.1007/s00213-007-0840-x. [DOI] [PubMed] [Google Scholar]
- Cohen I. Statistical Power Analysis for the Behavioural Sciences. Hilsdale, NI: Lawrence Erlbaum Associates Inc.; 1988. [Google Scholar]
- Donnelly C. Bangs M. Trzepacz P. Jin L. Zhang S. Witte MM. Ball SG. Spencer TJ. Safety and tolerability of Atomoxetine over 3 to 4 years in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2009;48:176–185. doi: 10.1097/CHI.0b013e318193060e. [DOI] [PubMed] [Google Scholar]
- Faraone SV. Biederman J. Morley CP. Spencer TJ. Effect of stimulants on height and weight: A review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:994–1009. doi: 10.1097/CHI.ObO13e31817eOea7. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Vitiello B. Riddle MA. Fisher P. Shockey E. March JS. Levine J. Fried J. Abikoff H. Zito JM. McCracken JT. Findling RL. Robinson J. Cooper TB. Davies M. Varipatis E. Labellarte MJ. Scahill L. Walkup JT. Capasso L. Rosengarten J. Review of safety assessment methods used in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2003;42:627–633. doi: 10.1097/01.CHI.0000046841.56865.37. [DOI] [PubMed] [Google Scholar]
- Klein RG. Landa B. Mattes JA. Klein DF. Methylphenidate and growth in hyperactive children. A controlled withdrawal study. Arch Gen Psychiatry. 1988;45:1127–1130. doi: 10.1001/archpsyc.1988.01800360075011. [DOI] [PubMed] [Google Scholar]
- Klein RG. Mannuzza S. Hyperactive boy almost grown up. III. Methylphenidate effect on ultimate height. Arch Gen Psychiatry. 1988;45:1131–1134. doi: 10.1001/archpsyc.1988.01800360079012. [DOI] [PubMed] [Google Scholar]
- Kratochvil CJ. Wilens TE. Greenhill LL. Gao H. Baker KD. Feldman PD. Gelowitz DL. Effect of long-term Atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:919–927. doi: 10.1097/01.chi.0000222788.34229.68. [DOI] [PubMed] [Google Scholar]
- Mattes JA. Gittelman R. Growth of hyperactive children on maintenance regimen of Methylphenidate. Arch Gen Psychiatry. 1983;40:317–321. doi: 10.1001/archpsyc.1983.01790030087011. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14-month randomised clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Panei P. Arcieri R. Vella S. Bonati M. Martini N. Zuddas A. Italian attention-deficit/hyperactivity disorder registry. Pediatrics. 2004;114:514. doi: 10.1542/peds.114.2.514. [DOI] [PubMed] [Google Scholar]
- Safer DJ. Allen RP. Barr E. Growth rebound after termination of stimulant drugs. J Pediatr. 1975;86:113–116. doi: 10.1016/s0022-3476(75)80720-7. [DOI] [PubMed] [Google Scholar]
- Schachter HM. Pham B. King J. Langford S. Moher D. How efficacious and safe is short-acting Methylphenidate for treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165:1475–1488. [PMC free article] [PubMed] [Google Scholar]
- Skounti M. Philalithis A. Galanakis E. Variations in prevalence of attention deficit hyperactivity disorder worldwide. Eur J Pediatr. 2007;166:117–123. doi: 10.1007/s00431-006-0299-5. [DOI] [PubMed] [Google Scholar]
- Spencer TJ. Newcorn JH. Kratochvil CJ. Ruff D. Michelson D. Biederman J. Effect of Atomoxetine on growth after 2-year treatment among pediatric patients with attention-deficit/hyperactivity disorder. Pediatrics. 2005;116:e74–e80. doi: 10.1542/peds.2004-0624. [DOI] [PubMed] [Google Scholar]
- Takahashi M. Takita Y. Yamazaki K. Hayashi T. Ichikawa H. Kambayashi Y. Koeda T. Oki J. Saito K. Takeshita K. Allen AJ. A randomized, double-blind, placebo-controlled study of Atomoxetine in Japanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:341–350. doi: 10.1089/cap.2008.0154. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Whitehouse RH. Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children 1965. Arch Dis Child. 1966;41:454–471. doi: 10.1136/adc.41.219.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal JM. Hernández–Lucas I. Gil F. Calderón IL. Calva E. Saavedra CP. A naturalistic 10-year prospective study of height and weight in children with attention deficit/hyperactivity disorder grown up: sex and treatment effects. J Pediatr. 2010;157:636–640. doi: 10.1016/j.jpeds.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]