Abstract
Aims
Mutant protein aggregation (PA) cardiomyopathy (MPAC) is characterized by reductive stress (RS), PA (of chaperones and cytoskeletal components), and ventricular dysfunction in transgenic mice expressing human mutant CryAB (hmCryAB). Sustained activation of nuclear erythroid-2 like factor-2 (Nrf2) causes RS, which contributes to proteotoxic cardiac disease. The goals of this pre-clinical study were to (i) investigate whether disrupting Nrf2-antioxidant signalling prevents RS and rescues redox homeostasis in hearts expressing the mutant chaperone and (ii) elucidate mechanisms that could delay proteotoxic cardiac disease.
Methods and results
Non-transgenic (NTG), transgenic (TG) with MPAC and MPAC-TG:Nrf2-deficient (Nrf2-def) mice were used in this study. The effects of Nrf2 diminution (Nrf2±) on RS mediated MPAC in TG mice were assessed at 6–7 and 10 months of age. The diminution of Nrf2 prevented RS and prolonged the survival of TG mice (∼50 weeks) by an additional 20–25 weeks. The TG:Nrf2-def mice did not exhibit cardiac hypertrophy at even 60 weeks, while the MPAC-TG mice developed pathological hypertrophy and heart failure starting at 24–28 weeks of age. Aggregation of cardiac proteins was significantly reduced in TG:Nrf2-def when compared with TG mice at 7 months. Preventing RS and maintaining redox homeostasis in the TG:Nrf2-def mice ameliorated PA, leading to decreased ubiquitination of proteins.
Conclusion
Nrf2 deficiency rescues redox homeostasis, which reduces aggregation of mutant proteins, thereby delaying the proteotoxic pathological cardiac remodelling caused by RS and toxic protein aggregates.
Keywords: Nrf2, reductive stress, protein aggregation, cardiomyopathy, antioxidants
1. Introduction
Heart failure is a leading health problem with over 550 000 new cases and 275 000 deaths annually in the USA.1,2 Cardiac diseases caused by mutations in contractile, cytoskeletal proteins and molecular chaperones account for ∼30% of inheritable cardiomyopathies.3–5 A shift in the redox equilibrium towards increased reactive oxygen species (ROS) and oxidative stress is involved in the pathophysiology of many cardiovascular diseases including hypertrophic cardiomyopathy.6–9 Recently, we demonstrated that reductive stress (RS) and protein aggregation (PA) are associated with hypertrophic cardiomyopathy and heart failure in transgenic mice expressing human mutant CryAB (hR120GCryAB-TG).10
Inherited mutations in cytoskeletal proteins contribute to the pathogenesis of heart failure in part via redox signalling mechanisms. Glutathione (GSH) is an ubiquitous, non-protein thiol antioxidant that is crucial for detoxification and scavenging of toxic molecules generated in various pathogenic processes.11,12 Mutant PA cardiomyopathy (MPAC) is characterized by chronic RS, PA (of chaperones and cytoskeletal components), and ventricular dysfunction in transgenic mice expressing human mutant CryAB.10,13 Prior to the onset of chronic RS, sustained activation of nuclear erythroid-2 like factor-2 (Nrf2) signalling was evident in MPAC hearts.13 Thus, the diminution of Nrf2-signalling is expected to prevent RS and restore redox homeostasis, thereby ameliorating aggregation of pathological proteins and preventing proteotoxic cardiac disease. Our observations in the MPAC-TG mice indicate that increased Nrf2 signalling occurs in two stages. The initial induction is through ROS generation (at 3 months) and the latter is due to Keap1 dysfunction (at 6 months).13
Nrf2, a basic leucine zipper protein, is a redox-sensitive, master transcriptional regulator of several hundred cytoprotective and antioxidant genes that acts by binding to the antioxidant response element in the promoters.14,15 We recently identified the Nrf2-Keap1 pathway as a critical regulator of the cardiac antioxidant defences and demonstrated its role in RS and MPAC.13 Another study suggested a critical patho-physiological role for Nrf2 in the heart.16 Although RS has been implicated in mutant PA, cardiac hypertrophy,10,13 and protein misfolding in neurodegeneration,17 the underlying molecular mechanistic role for chronic RS is unknown. In the current study, using genetically modified Nrf2-deficient (Nrf2-def) hR120GCryAB (TG) mouse models, we investigated whether and by what mechanism(s) attenuation of Nrf2 signalling prevents RS and rescues MPAC.
2. Methods
2.1. Animals
MPAC-TG:Nrf2-def (TG:Nrf2± or TG:Nrf2-def) were generated by crossing αMHC-R120GCryAB-TG (generously provided by Dr Ivor Benjamin, Medical College of Wisconsin, Milwaukee, WI, USA) with Nrf2-knockout (Nrf2−/−) mice on a C57/BL6 background. All experimental protocols conducted on the mice were approved by the Institutional Animal Care and Use Committee (IACUC#08-07006) at the University of Utah in accordance with the standards established by the US Animal Welfare Act.
2.2. Genotyping for hR120G-CryAB and Nrf2 knockout
The litters from reciprocal breeding between the hR120GCryAB and Nrf2± mice were genotyped for transgenic (hR120GCryAB) and Nrf2 deficiency (Nrf2±) using tail DNA (RedExtract-N-amp PCR kit; Sigma-Aldrich).
2.3. Myocardial GSH redox state
The redox state of GSH (GSH/GSSG) was determined in heart tissue extracts from NTG, TG, and TG:Nrf2-def mice at 6–7 months of age as previously described.10,13,18,19
2.4. Echocardiography analysis of cardiac function by ultrasound
Mice were examined by high-resolution ultrasound using a VisualSonics Vevo2100 ultrasound with 40 MHz transducer (MS550D) and inhalational anaesthesia (isofluorane 1–3%).20,21
2.5. RNA isolation, reverse transcription, and gene expression using quantitative real-time PCR
Heart tissues were harvested from NTG, TG, and TG:Nrf2-def mice at 6–7 months of age after in situ perfusion with 10 ml of RNase-free PBS and 10 ml of RNA later reagent. The total RNA was extracted from ∼20 mg of ventricular tissue using an RNAeasy kit (Qiagen#74106) and the messenger RNA (mRNA) expression levels for all samples were normalized to the level of Arbp1 or GAPDH.10,13,18,19,22,23
2.6. Preparation of heart tissue homogenates and immunoblotting
Heart tissue homogenates from control and TG mice at 6–7 and 10 months of age were prepared. About 30–40 μg of both cytosolic and nuclear proteins were resolved on the appropriate (8–12%) SDS–PAGE. The blots were probed using specific antibodies.10,13,18,19
2.7. Immunofluorescence analysis of antioxidants
Frozen heart (OCT; Tissue-Tek#4583) sections at 5μm thickness from NTG, TG, and TG:Nrf2-def mice at 6–7 months were immuno-stained with specific primary antibodies.13,18 Protein aggregates were identified using anti-CryAB (1:50) and anti-Hsp25 (1:100; Assay design #SPA-801) antibodies.
2.8. Statistical analysis
Statistical analyses were performed using SPSS. The results are expressed as mean ± SD. Student's t-test and analysis of variance (ANOVA) were used to compare the groups. Statistical significance was considered at P < 0.05.
3. Results
3.1. Deficiency of Nrf2 rescues myocardial redox homeostasis and prevents RS in MPAC mice
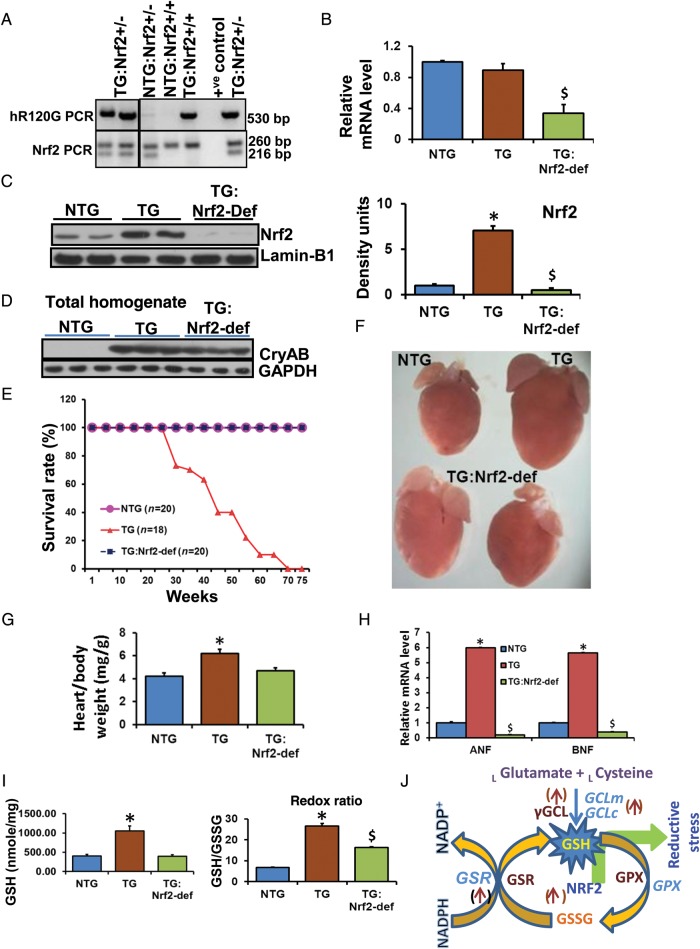
We identified Nrf2 as a causal factor for RS in MPAC.13 Disruption of Nrf2 is known to down-regulate antioxidative pathways including GSH biosynthesis.15,24 Therefore, to reduce Nrf2 mediated RS in MPAC-TG, we generated MPAC-TG with Nrf2 deficiency (TG:Nrf2-def) and confirmed by genotyping (Figure 1A). Q-PCR analysis revealed that the levels of Nrf2 mRNA were similar between NTG and TG hearts, but were significantly down-regulated in the TG:Nrf2-def mice hearts (Figure 1B). Immunoblots using nuclear extracts from ventricles showed significant decreases of Nrf2 protein in TG:Nrf2-def relative to NTG or TG mice (Figure 1C). Interestingly, the TG:Nrf2+/+ (TG) hearts had >6.0-fold higher levels of nuclear Nrf2 protein when compared with NTG mice. These results indicate the activation of Nrf2 in TG hearts and would be predicted to induce transcription of Nrf2-dependent genes. At 2 months of age, the hmCryAB levels were similar among TG and TG:Nrf2-def hearts suggesting comparable amounts of protein aggregates in the TG groups (Figure 1D).
Figure 1.
Nrf2 deficiency rescues redox homeostasis, prevents sudden cardiac death, and extends survival of the MPAC-TG mice. (A) Genotyping for human mutant R120GCryAB (TG) and Nrf2 deficiency (nrf2±) using specific primers sets. (B) Q-PCR determination of myocardial Nrf2-mRNA levels in NTG, MPAC-TG, and TG:Nrf2-def mice (n = 4–6/gp). (C) Immunoblots for the Nrf2 protein in the nuclear extracts of hearts from the above mouse models exhibiting either abundant (in MPAC-TG) or reduced Nrf2 (in TG:Nrf2-def) expression. (D) Immunoblots showing CryAB expression in the total homogenates of mouse hearts at 2 months of age. No significant difference was observed between TG vs. TG:Nrf2-def mice (n = 3/group). (E) Kaplan–Meier Survival plot (n ≥ 10 mice/group). (F) Representative images of hearts showing the gross morphology and reduced hypertrophy in Nrf2-def mice at 6–7 months of age. Images were obtained at 4× using a light microscope. (G) Heart-to-body weight ratios at 6–7 months of age showing significant hypertrophy in TG mice. (H) Quantitative PCR analysis of cardiac hypertrophy markers (n = 4–6/group). *NTG vs. TG; $NTG vs. TG:Nrf2-def; P < 0.05. (I) Myocardial GSH state determined by enzyme-kinetic assays (n = 4–6/group). (J) Scheme representing the transcriptional role of Nrf2 on antioxidants and GSH metabolism. *NTG vs. TG; $NTG vs. TG:Nrf2-def; P < 0.05.
Myocardial GSH levels were doubled in TG relative to NTG mice, but the TG:Nrf2-def mice showed comparable GSH levels as NTG and had significantly reduced GSH content and the redox (GSH/GSSG) ratio when compared with TG mice (Figure 1I), reflecting that the increase of reducing power was blunted in these hearts. Activation of Nrf2 increased (i) GSH biosynthesis and (ii) recycling of oxidized GSH (GSSG) to the reduced form (GSH) by inducing rate-limiting enzymes in the GSH cycle (Figure 1J). To elucidate the physiological roles of Nrf2 in basal conditions, we analysed heart morphology and cardiac hypertrophy in WT, and Nrf2−/− young (2 months) and old (>20 months) mice (Supplementary material online, Figure S1). Although Nrf2 is protective against transient stress, its ablation under basal conditions is not deleterious in that the cardiac phenotype of Nrf2−/− mice is normal (Supplementary material online, Figure S1).
3.2. Deficiency of Nrf2 prevents premature/sudden death and extends the survival of MPAC mice
Survival curves showed no mortality in the TG:Nrf2-def mice, while 50 and >90% of the MPAC-TG mice died as a consequence of pathological hypertrophy and cardiomyopathy/heart failure at 25–30 and 50–60 weeks of age, respectively (Figure 1E). Intriguingly, the TG:Nrf2-def mice exhibited prolonged survival beyond 70 weeks with no observed cardiac dysfunction, suggesting that the Nrf2 deficiency can permanently quench RS and maintain redox homeostasis, which we hypothesized would prevent PA. We analysed cardiac hypertrophy/cardiomyopathy by (i) autopsy–morphology and heart-to-body weight (HW/BW) ratios (Figure 1F and G), (ii) hypertrophy markers (Figure 1H), and (iii) echocardiography (Figure 2) and found no evidence for cardiac dysfunction in the TG:Nrf2-def mice while the MPAC-TG mice had severe hypertrophy at 6 months of age. The HW/BW ratio analysis revealed no signs of cardiac hypertrophy in the TG:Nrf2-def mice up to ∼15 months of age, while the TG mice developed significant cardiac hypertrophy at 6–7 months of age (Figure 1F). While a significant increase of ANF and BNP mRNA levels were recorded in MPAC-TG compared with NTG mice, suppression of these markers in the TG:Nrf2-def mice (Figure 1H) suggests prevention or delayed onset of cardiac hypertrophy.
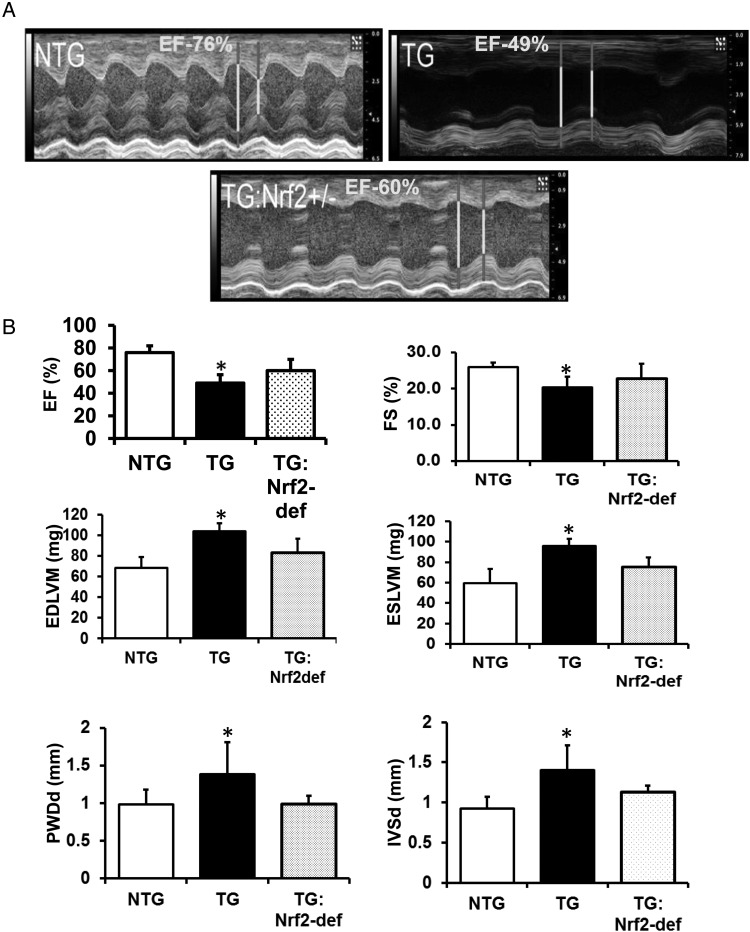
Figure 2.
Deficiency of Nrf2 preserves the cardiac function in the MPAC-TG mice. (A) M-mode images from echocardiographic analysis (Vevo2100, VisualSonics) with high-resolution (40 MHz) ultrasound to evaluate the systolic function. The LV cavity (white) and wall thickness (grey) are displayed in diastole and systole for each genotype at 6–7 months of age (n = 5 mice/group). (B) Quantitative analysis of echocardiography for systolic function (FS, ejection fraction) and hypertrophy (the end-diastolic and end-systolic LV mass from 2D imaging, and diastolic dimensions of the interventricular septal, and posterior walls from M-mode imaging). *NTG vs. TG; P < 0.05.
3.3. Diminution of Nrf2 preserves cardiac function in the MPAC-TG mice
Echocardiographic analysis of NTG, TG, and TG:Nrf2-def mice at 6–7 months of age demonstrated that TG mice had significantly lower fractional shortening (FS), lower ejection fraction (EF), and greater hypertrophy (end diastolic left ventricular mass, end systolic left ventricular mass, posterior wall diastolic diameter, and inter ventricular septum diameter) when compared with NTG (Figure 2A and B). Most of the echocardiograph parameters were identical between the NTG and TG:Nrf2-def mice, and these results are in line with heart morphology and remodelling (Figure 1).
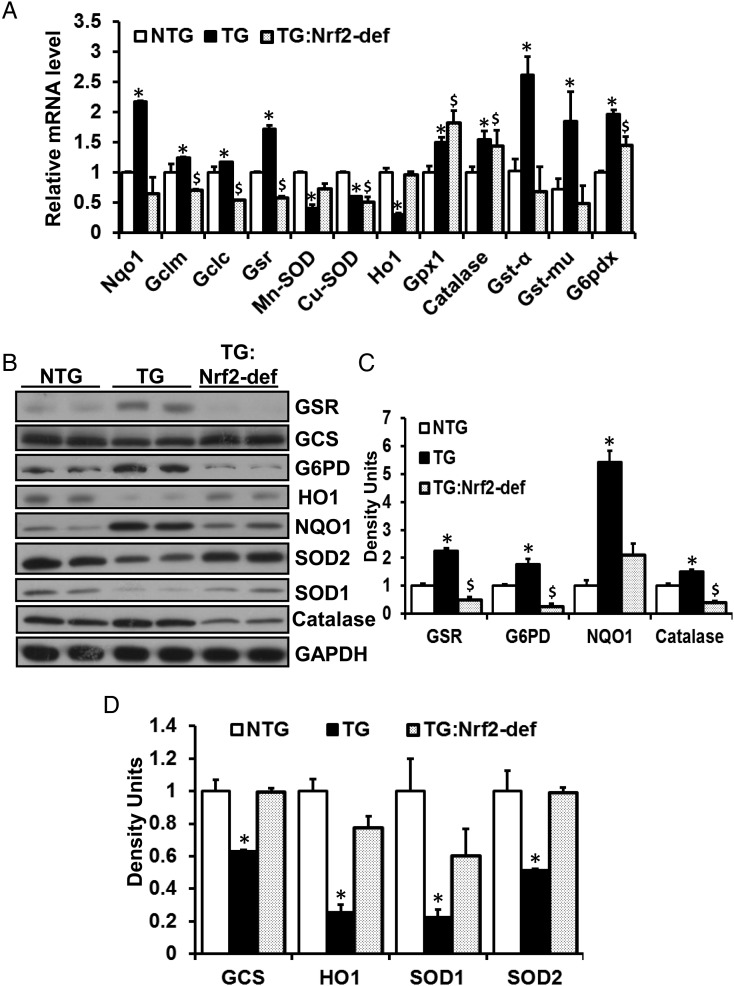
3.4. Nrf2 deficiency suppresses the RS response signature in the MPAC heart
A focused gene expression array analysis revealed a profile of suppression of Nrf2-regulated genes in the TG:Nrf2-def myocardium, while the TG mice exhibited significant induction of these genes contributing to RS (Figure 3A). To characterize whether the Nrf2 deficiency, in part, was responsible for the decreased GSH levels and prevention of RS in the MPAC-TG mouse hearts, we performed immunoblotting of antioxidative proteins. We observed significant increase of expression of these genes in the TG mice along with sustained activation of Nrf2. In contrast, a reduced expression of NQO1, catalase, G6PD, and GSR was observed with deficiency of Nrf2 in the TG mice (Figure 3B and D, Supplementary material online, Figure S3). Thus activation of Nrf2 in the TG mice (Figure 1C, Supplementary material online, Figure S2) is directly associated with up-regulation of GSH metabolic and antioxidative enzymes as compensatory mechanisms against MPAC pathogenesis. Interestingly, the mRNA and protein levels for HO1, Cu/Zn-SOD (SOD1), and Mn-SOD (SOD2) were significantly decreased in TG mice due to RS, but these enzymes were restored in the TG:Nrf2-def mice indicating that these proteins are highly sensitive to redox changes (Figure 3B and D).
Figure 3.
(A) Disruption of Nrf2 down-regulates antioxidants transcript levels in the MPAC-TG mice. QPCR analysis showing the transcript levels of antioxidant enzymes at 6–7 months of age. The mRNA data were normalized to Arbp1 or Gapdh of the respective samples and fold changes were calculated by comparing with the NTG group, (n = 4–6/group). *NTG vs. TG; $NTG vs. TG:Nrf2-def; P < 0.05. (B–D) The effect of Nrf2 deficiency on antioxidant enzymes in the MPAC-TG mice. (B) Immunoblots of glutathione-S-reductase (GSR), γ-glutamyl cysteine synthetase (γ-GCS), glucose-6-phosphate dehydrogenase (G6PD), hemoxygenase-1 (HO1), NADH-quinone oxidase (NQO1), superoxide dismutase-1 (Cu/Zn-SOD), superoxide dismutase-2 (Mn-SOD), and catalase in the heart tissues from NTG, TG, and TG:Nrf2-def mice at the age of 6–7 months. (C) and (D) Densitometry analysis of the immunoblots were performed from three sets of experiments and expressed as mean average ± SD for 4–6 animals/group. *NTG vs. TG; $NTG vs. TG:Nrf2-def; P < 0.05.
Further, immunohistochemistry of cardiac tissue confirmed the suppression of catalase, G6PD, and NQO1 proteins in the hearts of the TG:Nrf2-def mice relative to NTG or MPAC-TG mice (Supplementary material online, Figure S3). Immunofluorescence (IF) analysis confirmed the immunoblot results that signals for HO1 were significantly lower in TG or TG:Nrf2-def relative to NTG hearts (Supplementary material online, Figure S3). Sustained activity of Nrf2 likely led to overcompensation and shifted the homeostatic physiological redox environment towards pathological RS.13 The results indicate that the disruption of Nrf2 prevents overcompensated activation of GSH metabolizing genes and thereby quenches chronic RS.
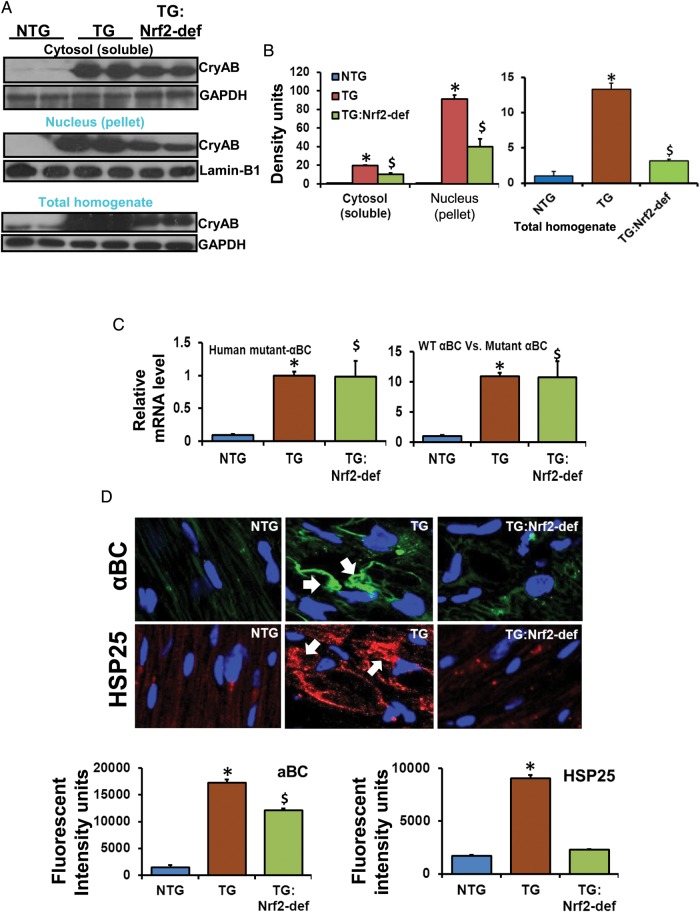
3.5. Decreased aggregation of proteins in the MPAC-TG:Nrf2-def mice
A redox homeostatic environment in cardiomyocytes is expected to delay/prevent the process of mutant PA in TG/Nrf2-def mice. Indeed, ∼50% decreased mutant CryAB protein in the soluble (cytosol) and insoluble (pellet) fractions, and the total homogenates of the TG:Nrf2-def myocardium was observed when compared with the MPAC-TG mice (Figure 4A and B). These results suggest the reduction of PA under homeostatic or pro-oxidative redox conditions caused by Nrf2 deficiency.
Figure 4.
Rescue of redox homeostasis prevents or delays PA in the MPAC-TG mice. (A) Representative western blots from soluble and insoluble fractions, and total homogenates of hearts (n = 4–6). Signals probed against anti-CryAB antibody showing soluble and insoluble aggregates in TG and TG:Nrf2-def mice. (B) Respective densitometry analysis from three independent experiments/animals. (C) Q-PCR analysis for human-mutant CryAB gene expression (n = 3–4 animals/group). (D) IF analysis for protein aggregates. Cryo-sections from heart tissues were fixed and processed with anti-CryAB or anti-Hsp25 antibodies and imaged by confocal microscopy. Green, protein aggregates; blue, DAPI nuclear staining; arrows indicate aggregates around the nucleus. Fluorescence was quantified by appropriate densitometry analysis using the Simple PCI 6 Imaging Software (Hamamatsu Corporation, Sewickley, PA). *NTG vs. TG; $NTG vs. TG:Nrf2-def; P < 0.05.
To compare the transcript levels for mutant CryAB in TG and TG:Nrf2-def hearts, we performed qPCR using WT and mutant CryAB primers (Figure 4C). The analyses using human mutant CryAB primers showed 10–12 fold higher mRNA levels in MPAC-TG or TG:Nrf2-def relative to NTG (normalized to mCryAB), indicating similar levels of human mutant CryAB gene expression in all the TGs, but significant differences in the levels of protein accumulation/aggregation due to the widely varying redox conditions.
IF analysis of PA was performed using anti-CryAB and anti-Hsp25 antibodies (Figure 4D). While the MPAC-TG had prominent protein aggregates (green) around the nuclei, these were significantly reduced in the TG:Nrf2-def mice. Staining for anti-Hsp25, a surrogate partner that co-localizes with hmCryAB, showed very minimal interactions for Hsp25 with PA in TG:Nrf2-def (Figure 4D). These results demonstrate that deficiency of Nrf2 is critical for maintaining redox homeostasis and preventing PA in the model.
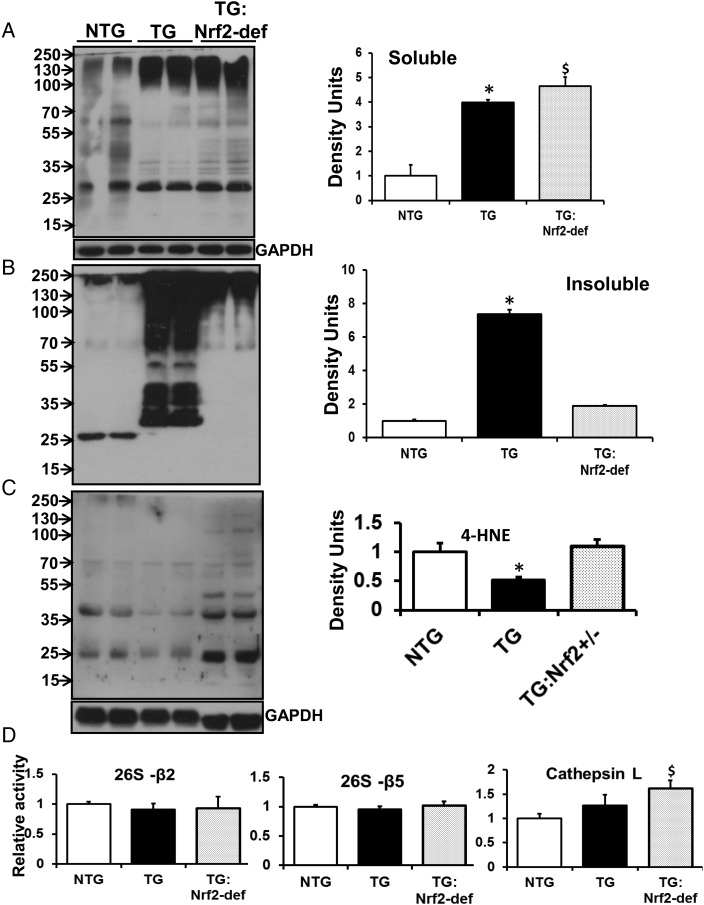
3.6. Diminution of Nrf2 and abrogation of RS prevents ubiquitination in MPAC-TG hearts
We observed increased ubiquitination of proteins under conditions of chronic RS (Figure 5). In MPAC-TG mouse hearts, insoluble pellet fractions showed increased ubiquitination of multiple protein species across a wide range of molecular weights (Figure 5B) when compared with NTG or TG:Nrf2-def mice. The soluble fraction from TG:Nrf2-def had higher levels of ubiquitination when compared with the MPAC-TG hearts, whereas the insoluble fraction showed significantly decreased ubiquitination when compared with TG hearts, suggesting that the diminution of Nrf2 might be sufficient to prevent PA (Figure 5A). Of note, we observed increased ubiquitination in soluble and decreased ubiquitination in insoluble fractions from TG:Nrf2-def when compared with TG hearts, which resulted in insignificant levels of protein aggregates as shown in densitometry analysis.
Figure 5.
Rescue of redox homeostasis prevents ubiquitination and favours basal oxidation of proteins in MPAC-TG mice. Representative western blots for poly-ubiquitination from (A) soluble and (B) insoluble fractions of myocardial proteins. A 12% poly-acrylamide gel was used to separate proteins, which were then probed with mono- and poly-ubiquitin antibodies to detect ubiquitinated proteins. Densitometry analyses of immunoblots indicate increased protein ubiquitination in the insoluble fraction of TG mice. (C) Representative western blot from the total homogenate probed for anti-4-HNE antibody showing oxidized proteins. Densitometry indicates decreased protein oxidation in TG. Data are expressed as mean ± SE (n = 4–6) mice. (D) Effects of Nrf2 deficiency on proteasome and cathepsin L function. Cathepsin L activity is significantly increased in TG:Nrf2-def hearts (n = 3/group). *NTG vs. TG; $NTG vs. TG:Nrf2-def; P < 0.05.
We also examined the role of RS on oxidation of proteins by determining the expression of 4-hydroxy-nonenol (4-HNE) adduct-positive proteins. Under conditions of RS, we observed significantly lower levels of 4-HNE-positive proteins in MPAC-TG when compared with NTG littermates (Figure 5C), suggesting that the RS can prevent signals that are required for basal redox regulation of proteins. Such an absence of redox regulation of proteins is suspected to be more detrimental than oxidative stress. The TG:Nrf2-def hearts showed significantly increased and comparable levels of 4-HNE-positive proteins when compared with MPAC-TG mice.
Since the ubiquitin proteasome system (UPS) has been shown to be the major proteolytic system involved in generating and removing ubiquitinated proteins,25 we determined the effects of Nrf2-def on proteasome function. Although both beta 2 (trypsin-like) and beta 5 (chymotrypsin-like) proteasome activity were similar among the groups, cathepsin-L activity was significantly increased in TG:Nrf2-def hearts when compared with other groups (Figure 5D). Therefore, cathepsin-L (lysosomal) activity could account for the degradation of ubiquitinated proteins in TG:Nrf2-def hearts.
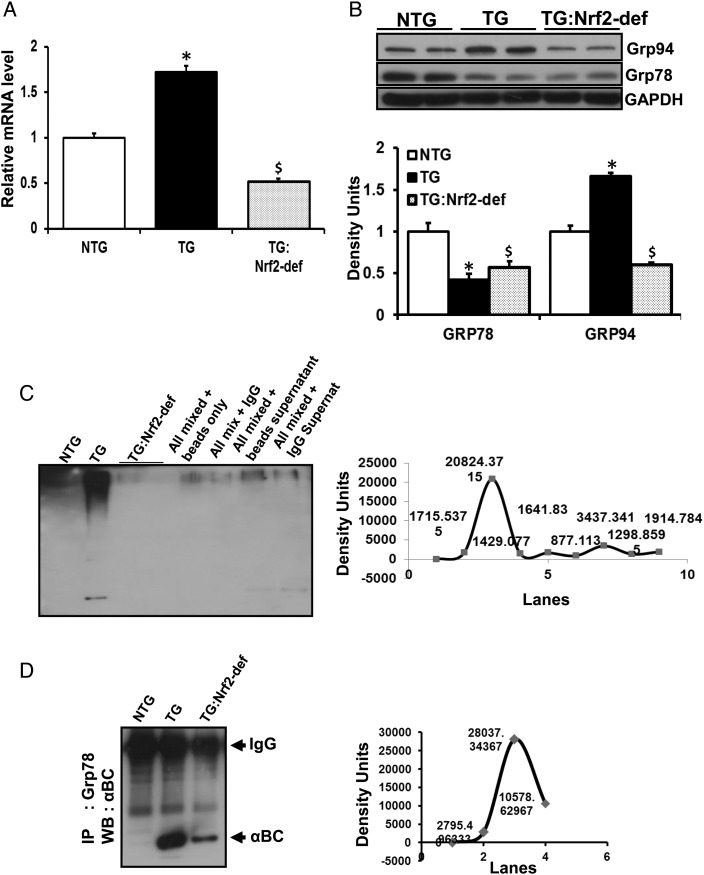
3.7. Rescue of redox homeostasis partially prevented endoplasmic reticulum stress in the MPAC mice
To further investigate whether Nrf2-dependent mechanisms are crucial for endoplasmic reticulum (ER) function, we assessed ER stress response pathways. Remarkably, ER-resident chaperones were significantly altered in the MPAC-TG mice bearing chronic RS (Figure 6). Q-PCR analysis showed significantly increased mRNA levels for Grp94 in TG relative to NTG or TG:Nrf2-def mice (Figure 6A). Immunoblots for Grp94 showed significantly increased protein, while Grp78 was decreased in TG compared with either NTG or TG:Nrf2-def mice hearts (Figure 6B). Immunoprecipitation (IP) followed by immunoblotting analysis revealed sequestration of Grp78 in protein aggregates, which is expected to affect the chaperone activity of Grp78 in MPAC-TG hearts (Figure 6C). Further, IP with anti-Grp78 and immunoblotting with anti-ubiquitin-ab showed high levels of ubiquitinated Grp78 in MPAC-TG hearts when compared with other groups (Figure 6D). These results suggest that either sequestration or ubiquitination of ER-resident chaperones such as Grp78 might permanently compromise ER function, and thereby lead to the accumulation of misfolded/unfolded proteins in RS hearts. Partial rescue of ER stress is evident from decreased RNA and protein levels for Grp94 in the TG:Nrf2-def mice. However, the Grp78 levels were not back to NTG values, but comparable among the TG and TG:Nrf2-def mice.
Figure 6.
Rescue of redox homeostasis ameliorates ER stress in the MPAC-TG mice. (A) Q-PCR analysis showing Grp94 RNA levels from NTG, TG, and TG:Nrf2-def mice. (B) Immunoblots for Grp94 and Grp78 indicating ER stress in TG. This was partially rescued by Nrf2 deficiency. (C) IP and Co-IP with αBC or Grp78 antibodies followed by immunoblotting showing sequestration of Grp78 in TG mice. Data are mean ± SD for 3–4 animals/group. *NTG vs. TG; $NTG vs. TG:Nrf2-def; P < 0.05.
3.8. Effects of Nrf2 deficiency on PA and antioxidant status in TG mice at 10 months of age
To determine the time-dependent effects of RS on the progression of cardiomyopathy, we determined the degree of PA and the state of selective antioxidants in TG and TG:Nrf2-def mice at the age of 10 months. In heart homogenates, an over 40% decrease of protein aggregates was observed in the TG:Nrf2-def mice (Supplementary material online, Figure S4A). Furthermore, the increased antioxidant expression (NQO1, catalase, and G6PD) in TG hearts was absent in the TG:Nrf2-def mice at 10 months (Supplementary material online, Figure S4B). Similarly, significantly reduced SOD1 and SOD2 protein levels in TG were partially rescued in the TG:Nrf2-def mice at the age of 10 months (Supplementary material online, Figure S4B). Taken together, most of these parameters exhibited similar trend at 6–7 and 10 months of age (Figure 3, Supplementary material online, Figure S4A and B). Similarly, changes in 4-HNE levels were comparable between these age groups (Supplementary material online, Figure S4C and Figure 5C).
3.9. Effect of RS in vitro
To address whether RS in vitro could modulate PA, we transfected HL1 cardiomyocytes with human mutant CryAB plasmids and analysed the levels of Nrf2, glutathione reductase (GSR), protein aggregates, ubiquitination, and protein oxidation (Supplementary material online, Figure S5A and B). Dose-dependent increases in Nrf2 and GSR levels were associated with increased PA (Supplementary material online, Figure S5A). Significantly decreased 4-HNE (Supplementary material online, Figure S5B), but increased ubiquitination (Supplementary material online, Figure S5C) of proteins in HL1 cardiomyocytes transfected with hmCryAB were observed. These results suggest that an increased reductive environment (Nrf2 and GSR) promotes PA in association with impaired oxidation and ubiquitination of proteins.
4. Discussion
In the present study, we describe a novel mechanism for chronic RS-mediated PA cardiac disease. Chronic RS increases PA resulting in hypertrophic cardiomyopathy and heart failure. Restoration of redox homeostasis reverses excessive ubiquitination and ER stress and prevents pathological PA. Unlike other cells, cardiomyocytes have very limited capacity for regeneration or proliferation upon stress or cell death, but they initially withstand such conditions through physiological hypertrophy.26–29 However, chronic stress results in transition to pathological hypertrophy, which then causes contractile dysfunction in cardiomyocytes leading to cardiomyopathy/heart failure. As evident in the MPAC-TG mice, sustained activation of Nrf2 contributes to RS-dependent pathology in the heart.10 Using genetic manipulation we generated Nrf2 deficiency in the MPAC-TG mice and determined whether decreasing chronic RS by dampening Nrf2-dependent trans-activation of antioxidative enzymes prevents cardiac hypertrophy/heart failure. Indeed, the decreased RS in the TG:Nrf2-def mice is associated with prevention of cardiac hypertrophy. However, to understand the underlying mechanisms we have addressed the following key questions: (i) does Nrf2 deficiency result in transcriptional down-regulation of antioxidants? (ii) does down-regulation of antioxidants prevent ‘reductive overcompensation’ and thereby rescue ‘redox homeostasis’ in the TG myocardium? (iii) does Nrf2-dependent quenching of RS have a positive (therapeutic) impact on ubiquitination and oxidation of mutant proteins? and (iv) does redox homeostasis reduce levels of protein aggregates?
4.1. Rescue of redox homeostasis prevents or delays the process of PA and cardiac hypertrophy
Increased intracellular reducing power causes RS in the MPAC-TG mice.10,13 Nrf2 deficiency is associated with significant GSH depletion in vivo and in vitro.15,24,30,31 The diminution of Nrf2 is expected to down-regulate transcription of GSH metabolizing (biosynthesis and recycling) enzymes and thereby deplete GSH levels, which in turn will prevent RS in the TG myocardium. Under basal conditions, abrogation of Nrf2 does not affect the structure or function of the heart, but the Nrf2−/− mice are susceptible to oxidative19 or pressure overload stress.16 In general, transient activation of Nrf2 upon stress is protective, whereas sustained activation could be detrimental, especially for cardiomyocytes with their limited regenerative capacity. As evident in the MPAC-TG mice, sustained activation of Nrf2 promotes RS and pathological processes in the heart.13 By maintaining a more homeostatic redox milieu via disrupting Nrf2 expression, we found that there is a delay in the process of PA. Western blot analyses showed a ∼50% decrease in levels of protein aggregates (cytosol and nuclear pellet) in the TG:Nrf2-def when compared with MPAC-TG mice, suggesting either (i) prevention of aggregation and/or (ii) effective clearance of aggregated proteins by the UPS or lysosome dependent pathways. Thus, while acute RS can be a compensatory mechanism to protect cells from PA diseases, such stress for chronic periods can result in pathological hypertrophy of cardiomyocytes/heart. In addition to our studies demonstrating the role of RS in MPAC,10,13 it appears that increased ‘reducing power’ induces ER stress/unfolded protein response and PA in several organ systems.17,32–34 Previous studies reported elevated sulfhydryls, G6PD, and protein aggregates in the brains of patients with Alzheimer's disease.17 Further, a 1.5-fold increase of reduced GSH causes unfolded protein response in lower eukaryotes.32
4.2. RS increases ubiquitination, but decreases oxidation of proteins, in the MPAC mouse heart
Numerous studies demonstrate that increased oxidative stress is coupled with subsequent increases in ubiquitination of proteins.35,36 However, we observed that the MPAC-TG hearts have significantly increased levels of ubiquitinated proteins, but significantly decreased oxidation of proteins when compared with NTG mice. These observations suggest that a highly reducing environment promotes ubiquitination of proteins and prevents basal levels of protein oxidation that are necessary and crucial for normal physiological processes. Such uncontrolled ubiquitination of proteins in a chronic reductive environment is expected to impair the unfolded protein response and lead to the accumulation of protein aggregates in cardiomyocytes. Although the level of ubiquitinated proteins was comparable among soluble fractions of both TG groups, it is likely that the efficiency of subsequent protein degradation may vary between the TG and TG:Nrf2-def mice. An effective ubiquitination process is expected to degrade the damaged proteins (aggregates) more efficiently in the TG:Nrf2-def than in TG mice. This possibility is supported by our finding of increased cathepsin-L-dependent degradation of proteins in the TG:Nrf2-def mice. Furthermore, it is possible that quenching of RS and maintaining redox homeostasis in the TG:Nrf2-def mice delay and/or suppress PA.
Since chronic RS is a causal mechanism for MPAC, we expected that the degree of PA might be diminished or prevented under a homeostatic redox state in the TG/Nrf2-def mouse. IF analysis revealed the presence of prominent protein aggregates in cardiomyocytes of TG mice, but the TG:Nrf2-def mice had significantly reduced levels and smaller protein aggregates. These observations suggest that either decreased aggregate formation or an effective degradation of oligomeric proteins might be occurring under a homeostatic redox state. It is noteworthy that continued synthesis of mutant proteins challenges the intracellular environment and activates compensatory pathways including Nrf2-Keap1 signalling in the hearts of the MPAC-TG mice. Our previous studies suggest that mutant protein expression is able to activate Nrf2 to a greater degree than that which occurs under more physiological conditions.13
4.3. Nrf2 deficiency partially prevented ER stress in the MPAC mouse
The ER molecular environment and oxidative redox state (GSH/GSSG = −250) are ideal for folding of secretory proteins.37,38 Under RS conditions in the TG mice, there is likely a shift in the redox state of ER towards a more reductive environment, resulting in an increased misfolded/unfolded protein response. Indeed, we observed that the deficiency of Nrf2 in the MPAC-TG mice partially prevented the ER stress and its dysfunction by maintaining redox homeostasis. Our findings relating to ER stress chaperones/proteins indicate possible sequestration of Grp78 into protein aggregates, while Grp94 was intact and up-regulated, due to RS in the TG mice. Future studies will investigate the age at which the ER stress occurs and how it contributes to the progression of hypertrophic cardiomyopathy.
5. Conclusions and future directions
Accumulation of aggregates in response to chronic RS results in excessive ubiquitination, which induces pathological hypertrophy and remodelling of the TG heart. Conversely, a significant decrease of protein oxidation in association with chronic RS in the TG hearts might compromise the de-ubiquitination and downstream protein degradation pathways. Intracellular oxidative modification of proteins is a key event required for proper ubiquitination and protein degradation.39,40 Therefore, prevention of PA and pathological cardiac remodelling through quenching chronic RS has potential clinical relevance in the recovery of redox homeostasis and PA dependent hypertrophic cardiomyopathy. Taken together, our results support the conclusion that chronic RS exacerbates PA and causes pathological remodelling, and that a homeostatic redox milieu is necessary and sufficient to prevent pathological PA.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by grants from the American Heart Association (AHA-BGIA # 0865015F) and NIH-NIA (R03 # 1R03AG042860-01) to Namakkal S. Rajasekaran and research funds from the divisions of Cardiology and Pulmonary in the Department of Internal Medicine, University of Utah. Echocardiograph analysis was performed at the University of Utah Small Animal Ultrasound Core Laboratory supported by NIH instrumentation funds (S10RR027506-01A1).
Supplementary Material
Acknowledgements
We thank Dr Robert Paine III and Dr John Michael for discussion and suggestions about the project. Mr Subanna assisted with scanning of blots, densitometry analyses, and XL graphs. The authors thank Dr Srinvas S. Somanchi (Pediatrics, UTMDACC, TX), Mrs Jessica Kieper and Ms Jennifer Hammond for editorial assistance on the manuscript.
Conflict of interest: none declared.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N Engl J Med. 1997;336:775–785. doi: 10.1056/NEJM199703133361107. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the cardia study. Coronary artery risk development in (young) adults. Circulation. 1995;92:785–789. doi: 10.1161/01.CIR.92.4.785. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Pelliccia A, Spirito P. Cardiac disease in young trained athletes. Insights into methods for distinguishing athlete's heart from structural heart disease, with particular emphasis on hypertrophic cardiomyopathy. Circulation. 1995;91:1596–1601. doi: 10.1161/01.CIR.91.5.1596. [DOI] [PubMed] [Google Scholar]
- 6.Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, et al. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. 2005;179:43–49. doi: 10.1016/j.atherosclerosis.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajasekaran NS, Sathyanarayanan S, Devaraj NS, Devaraj H. Chronic depletion of glutathione (gsh) and minimal modification of ldl in vivo: Its prevention by glutathione mono ester (gme) therapy. Biochim Biophys Acta. 2005;1741:103–112. doi: 10.1016/j.bbadis.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 10.Rajasekaran NS, Connell P, Christians ES, Yan LJ, Taylor RP, Orosz A, et al. Human alpha b-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martensson J, Jain A, Stole E, Frayer W, Auld PA, Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci USA. 1991;88:9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meister A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol Ther. 1991;51:155–194. doi: 10.1016/0163-7258(91)90076-X. [DOI] [PubMed] [Google Scholar]
- 13.Rajasekaran NS, Varadharaj S, Khanderao GD, Davidson CJ, Kannan S, Firpo MA, et al. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid Redox Signal. 2011;14:957–971. doi: 10.1089/ars.2010.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copple IM, Goldring CE, Kitteringham NR, Park BK. The keap1-nrf2 cellular defense pathway: mechanisms of regulation and role in protection against drug-induced toxicity. Handb Exp Pharmacol. 2010:233–266. doi: 10.1007/978-3-642-00663-0_9. [DOI] [PubMed] [Google Scholar]
- 15.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, et al. Genetic ablation of nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 17.Russell RL, Siedlak SL, Raina AK, Bautista JM, Smith MA, Perry G. Increased neuronal glucose-6-phosphate dehydrogenase and sulfhydryl levels indicate reductive compensation to oxidative stress in alzheimer disease. Arch Biochem Biophys. 1999;370:236–239. doi: 10.1006/abbi.1999.1404. [DOI] [PubMed] [Google Scholar]
- 18.Gounder SS, Kannan S, Devadoss D, Miller CJ, Whitehead KS, Odelberg SJ, et al. Impaired transcriptional activity of nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One. 2012;7:e45697. doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthusamy VR, Kannan S, Sadhaasivam K, Gounder SS, Davidson CJ, Boeheme C, et al. Acute exercise stress activates nrf2/are signaling and promotes antioxidant mechanisms in the myocardium. Free Radic Biol Med. 2012;52:366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bader FM, Islam N, Mehta NA, Worthen N, Ishihara S, Stehlik J, et al. Noninvasive diagnosis of cardiac allograft rejection using echocardiography indices of systolic and diastolic function. Transplant Proc. 2011;43:3877–3881. doi: 10.1016/j.transproceed.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Symons JD, Hu P, Yang Y, Wang X, Zhang QJ, Wende AR, et al. Knockout of insulin receptors in cardiomyocytes attenuates coronary arterial dysfunction induced by pressure overload. Am J Physiol Heart Circ Physiol. 2011;300:H374–H381. doi: 10.1152/ajpheart.01200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller CJ, Gounder SS, Kannan S, Goutam K, Muthusamy VR, Firpo MA, et al. Disruption of nrf2/are signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim Biophys Acta. 2012;1822:1038–1050. doi: 10.1016/j.bbadis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Rajasekaran NS, Firpo MA, Milash BA, Weiss RB, Benjamin IJ. Global expression profiling identifies a novel biosignature for protein aggregation r120gcryab cardiomyopathy in mice. Physiol Genomics. 2008;35:165–172. doi: 10.1152/physiolgenomics.00297.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, et al. Disruption of nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Ann Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abel ED, Doenst T. Mitochondrial adaptations to physiological vs. pathological cardiac hypertrophy. Cardiovasc Res. 2011;90:234–242. doi: 10.1093/cvr/cvr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Drozdov I, Tsoka S, Ouzounis CA, Shah AM. Genome-wide expression patterns in physiological cardiac hypertrophy. BMC Genom. 2010;11:557. doi: 10.1186/1471-2164-11-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song HK, Hong SE, Kim T, Kim do H. Deep RNA sequencing reveals novel cardiac transcriptomic signatures for physiological and pathological hypertrophy. PloS One. 2012;7:e35552. doi: 10.1371/journal.pone.0035552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii T, Itoh K, Yamamoto M. Roles of nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002;348:182–190. doi: 10.1016/s0076-6879(02)48637-5. [DOI] [PubMed] [Google Scholar]
- 31.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keap1 regulates both cytoplasmic–nuclear shuttling and degradation of nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 32.Trotter EW, Grant CM. Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol Microbiol. 2002;46:869–878. doi: 10.1046/j.1365-2958.2002.03216.x. [DOI] [PubMed] [Google Scholar]
- 33.Lipinski B. Evidence in support of a concept of reductive stress. Br J Nutr. 2002;87:93–94. doi: 10.1079/BJN2001435. [DOI] [PubMed] [Google Scholar]
- 34.Dimmeler S, Zeiher AM. A ‘reductionist’ view of cardiomyopathy. Cell. 2007;130:401–402. doi: 10.1016/j.cell.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 35.Hermann J, Gulati R, Napoli C, Woodrum JE, et al. Oxidative stress-related increase in ubiquitination in early coronary atherogenesis. FASEB J. 2003;17:1730–1732. doi: 10.1096/fj.02-0841fje. [DOI] [PubMed] [Google Scholar]
- 36.Gu Z, Nakamura T, Yao D, Shi ZQ, Lipton SA. Nitrosative and oxidative stress links dysfunctional ubiquitination to Parkinson's disease. Cell Death Diff. 2005;12:1202–1204. doi: 10.1038/sj.cdd.4401705. [DOI] [PubMed] [Google Scholar]
- 37.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 38.Appenzeller-Herzog C. Glutathione- and non-glutathione-based oxidant control in the endoplasmic reticulum. J Cell Sci. 2011;124:847–855. doi: 10.1242/jcs.080895. [DOI] [PubMed] [Google Scholar]
- 39.Jung T, Bader N, Grune T. Oxidized proteins: intracellular distribution and recognition by the proteasome. Arch Biochem Biophys. 2007;462:231–237. doi: 10.1016/j.abb.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 40.Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Sent to destroy: The ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 2010;106:463–478. doi: 10.1161/CIRCRESAHA.109.208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.