Abstract
Aims
Emerging evidence indicates a critical role for junctophilin-2 (JP2) in T-tubule integrity and assembly of cardiac dyads in adult ventricular myocytes. In the postnatal stage, one of the critical features of myocyte maturation is development of the T-tubule system, though the mechanisms remain poorly understood. In this study, we aim to determine whether JP2 is required for normal cardiac T-tubule maturation.
Methods and results
Using in situ confocal imaging of intact murine hearts, we found T-tubules were absent in both left- and right-ventricular myocytes at postnatal Day 8 and did not appear until Day 10. Quantification of T-tubule structural integrity using the T-tubule power (TTpower) index revealed a progressive increase in TTpower between postnatal Days 10 and 19. By postnatal Day 19, TTpower was similar to that in adult murine cardiomyocytes, indicative of a nearly matured T-tubule network. JP2 levels increased dramatically during development, reaching levels observed in adult hearts by postnatal Day 14. Deficiency of JP2, using a mouse model in which a JP2-specific shRNA is expressed during embryonic development, severely impaired T-tubule maturation, with equivalent decreases in the left- and right-ventricular TTpower. We also detected a gradual increase in the density of transverse but not longitudinal tubules during development, and JP2 deficiency abolished the increase in the density of transverse elements. Alterations in T-tubules caused significant reduction in Ca2+ transient amplitude and marked increase in Ca2+ release dyssynchrony, Ca2+ alternans, and spontaneous Ca2+ waves, leading to contractile failure.
Conclusion
Our data identify a critical role for JP2 in T-tubule and excitation–contraction coupling maturation during development.
Keywords: T-tubules, Junctophilin-2, Excitation–contraction coupling, Calcium, Heart development
1. Introduction
Cardiac excitation–contraction (E–C) coupling is the central mechanism governing heart muscle contraction.1 At the cellular level, E–C coupling requires precise communication, i.e. Ca2+-induced Ca2+ release, between voltage-gated Ca2+ channels located mainly on the T-tubule membrane and Ca2+ release channels/ryanodine receptor channels on the sarcoplasmic reticulum.2,3 The T-tubule and sarcoplasmic reticulum membranes are highly co-localized and form a tightly coupled ultrastructural unit, termed the cardiac dyad, within a 12–15 nm junctional space.4,5 At birth or a few days after birth, the cardiac T-tubule network is virtually absent in mammalian ventricular myocytes.6–8 T-tubule formation involves cell-membrane invaginations originating from the cell periphery and extending into the lumen of the cell,9 yet the mechanisms underlying T-tubule development remain incompletely defined.
Striking changes in the morphology of T-tubule system are associated with heart failure,10–24 including the loss of T-tubules and reappearance of prevalent longitudinal tubules, a foetal phenotype that normally disappears during development.9,11,25–27 At the molecular level, pathological T-tubule remodelling and E–C coupling dysfunction correlate with the loss of junctophilin-2 (JP2) expression in left-ventricular myocytes of failing heart.23,28–30 JP2 bridges the physical gap between the plasma membrane and the SR in excitable cells, maintaining the junctional membrane complex in normal ventricular myocytes.31–33 Conventional knockout of JP2 is embryonically lethal, and embryonic myocytes with JP2 deficiency have defective cardiac dyads, including more SR segments with no T-tubule couplings as well as reduced intracellular Ca2+ transients.31 These data collectively indicate a critical role for JP2 in developing hearts.
In this study, we used confocal imaging of intact hearts to examine the contribution of JP2 to normal T-tubule development and Ca2+ handling during the early stages of heart development. Studies in mice with cardiac-specific JP2 knockdown demonstrate that T-tubule maturation and Ca2+ handling are significantly impaired with JP2 deficiency, thereby defining a key role for JP2 in T-tubule and E–C coupling maturation during development.
2. Methods
2.1. Animals
Animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No.85 - 23, revised 1985) and were approved by the Institutional Animal Care and Use Committee at the University of Iowa. Cardiac-specific knockdown of JP2 protein was achieved with the use of transgenic mice expressing JP2-shRNA, which was reported recently.32 Specifically, JP2-shRNA mice were crossed with mice carrying the cardiac-specific α-myosin heavy chain promoter upstream of Cre (αMHC-Cre) to generate cardiac-specific JP2-shRNA expressing mice. JP2-shRNA mice were maintained on a C57BL/6 background for more than 10 generations. The αMHC-Cre mice were maintained on an FVB background. Only the first generation of JP2-shRNA×αMHC-Cre offspring were used for experiments in this study. Double transgenic mice of either sex (JP2-shRNA and αMHC-Cre) were used as the experimental group. Single transgenic (either JP2-shRNA or αMHC-Cre) mice were used as controls.
2.2. Transthoracic echocardiography
Transthoracic echocardiography was performed as previously described.16
2.3. In situ confocal imaging of T-tubules on intact hearts
Adult mice were heparinized (100 IU i.p.) and euthanized by pentobarbital (120 mg/kg, i.p.). Postnatal mice up to 21 days were sacrificed by cervical dislocation. Mouse hearts were excised and were performed with in situ confocal imaging of T-tubules as previously reported.16,29 T-tubule power analysis and quantitation of densities of longitudinal and transverse elements was performed as previously described, with Interactive Data Language (IDL).25,29
2.4. In situ confocal imaging of Ca2+ transients in intact hearts
Methods were adapted from published reports.34–36 Mice were heparinized (100 IU i.p.) and euthanized by pentobarbital (120 mg/kg, i.p.). Excised hearts were perfused with Rhod-2 AM (0.3 mM, AAT Bioquest, CA, USA) containing Kreb–Henseleit's solution (in mM: 120 NaCl, 24 NaHCO3, 11.1 glucose, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.42 KH2PO4, oxygenated with 95% O2 and 5% CO2) at room temperature for 30 min via retrograde Langendorff perfusion system. Hearts were later transferred to another Langendorff apparatus (37°C) attached to the confocal microscope system after Rhod-2 loading was completed. The hearts were placed onto a recording chamber for in situ confocal imaging (linescan) of Ca2+ signals from epicardial myocytes under sinus rhythm. To avoid motion artefacts in Ca2+ imaging, blebbistatin (10 μM, Sigma) and 2,3-butanedione monoxime (BDM, 5 mM, Sigma) were added to the perfusion solution. The confocal linescan images were acquired at a rate of 3.07 ms or 1.93 ms per line. Ca2+ transients were recorded either under autonomous beating (elicited by electrical signals from sinoatrial node) or under electrical pacing at 5 Hz (by placing a platinum electrode onto the surface of ventricle apex). Analysis of Ca2+ imaging data was performed offline using custom-compiled routines in IDL, as previously described.36
2.5. Western blotting assay of proteins
Frozen hearts were homogenized and then sonicated in lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 5 mM EGTA, 5 mM EDTA, 0.5% Triton X-100, 0.5% Na deoxycholate, 0.1% SDS), containing protease inhibitors (Sigma, P8340). Tissue lysates were then centrifuged at 12 000 g for 10 min to remove insoluble debris. Protein concentrations were determined by using the Pierce BCA assay (Pierce, Thermo Scientific). Samples (8 μg) were separated by SDS–PAGE (4–12% Bis–Tris gel, Invitrogen) and transferred to PVDF membranes. Primary antibodies that recognize JP2 (1:2000) (Santa Cruz, sc-51313), Caveolin-3 (Cav3, 1:10 000) (BD Transduction Laboratory, #610420), Bin1 (1:1000) (Santa Cruz, sc-23918), and GAPDH (1:10 000) (Cell Signaling, #2118) were used. HRP-linked anti-goat IgG (1:10 000), anti-mouse (1:10 000), and anti-rabbit IgG (1:10000) were used to visualize bound primary antibodies with the SuperSignal chemiluminescence substrate (Pierce, Thermo Scientific). The protein bands were quantified using Image J software (version 1.43d).
2.6. Statistics
Data were expressed as mean ± SE. Student's t-test or ANOVA test was applied when appropriate. P < 0.05 was considered statistically significant.
3. Results
3.1. Development of T-tubules in murine left and right ventricle
It is yet unknown whether T-tubules develop at the same rate in left- and right-ventricular myocytes. Our first objective was to characterize the progression of T-tubule maturation in the left and right ventricles using in situ confocal imaging of T-tubules in mice. At postnatal Day 8, we observed no apparent T-tubule network in either the left or right ventricles, whereas the beginnings of a T-tubule network appeared at postnatal Day 10 (Figure 1). A progressive increase in T-tubule organization was observed between Days 10 and 19 in both the left and right ventricles, with the degree of organization at Day 19 similar to that observed in adult mice. Analysis of heart weight and the heart weight to body weight ratio demonstrated the anticipated proportional increase in heart weight between postnatal Days 8 and 19 (Supplementary material online, Figure S1).
Figure 1.

Postnatal T-tubule development in the left and right ventricles of mice. Representative in situ confocal images of ventricular myocytes from WT mice at different ages. LV, left ventricle; RV, right ventricle. Scale bar = 50 µm.
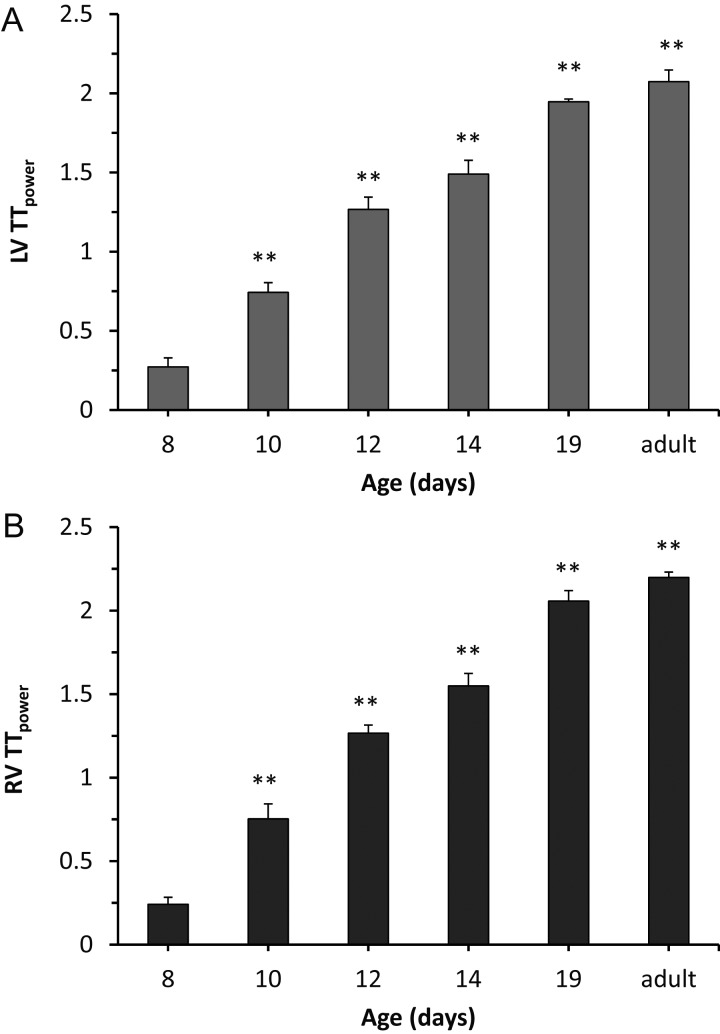
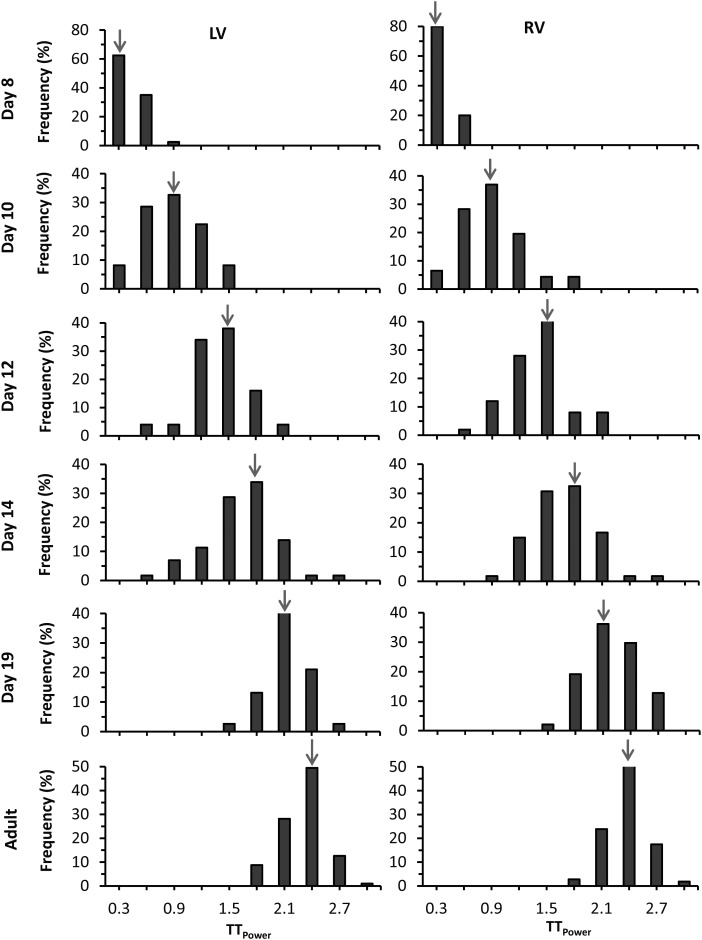
Quantitation of T-tubule organization using T-tubule power (TTpower) confirmed the developmental pattern of the T-tubule network, including the significant increase in TTpower between Days 8 and 10 (Figure 2). The time course of T-tubule maturation was similar between the left (Figure 2A) and right (Figure 2B) ventricles. We also observed a similar pattern in the shift of the TTpower histogram in the left and right ventricles (Figure 3), suggestive of a global mechanism that concomitantly regulates myocyte T-tubule maturation in each chamber.
Figure 2.
T-tubule development in murine left- and right-ventricular myocytes is initiated at Day 10. Average TTpower, an index of T-tubule regularity, in left (A) and right (B) ventricular myocytes in intact hearts of mice at different ages. n = 5–6 mice per age. ** P < 0.01 vs. 8 days of age.
Figure 3.
Histogram analysis of T-tubule power distribution in ventricles of developing mice. The count numbers at each bin size were normalized by the total images of each age and are shown as frequency. The histogram of TTpower in both the left and right ventricles and their modes shifted to the right with increasing age. Arrow indicates peak of mode. Each group is from 50 to 60 images of 5–6 mice.
To determine if this progressive increase in T-tubule network maturation is a general phenomenon in other species, we also examined T-tubule development in rats. Our data indicate that T-tubule development in rats is delayed as compared with mice, with minimal T-tubules at postnatal Day 13 in either the left or right ventricles (Supplementary material online, Figures S1–S4). Consistent with murine T-tubule development pattern, however, we observed a significant increase in TTpower at postnatal Day 15 and a gradual further increase between postnatal Days 17 and 23, though the TTpower at Day 23 remained less than that observed in adult rats (Supplementary material online, Figure S3).
3.2. Role of JP2 in T-tubule maturation
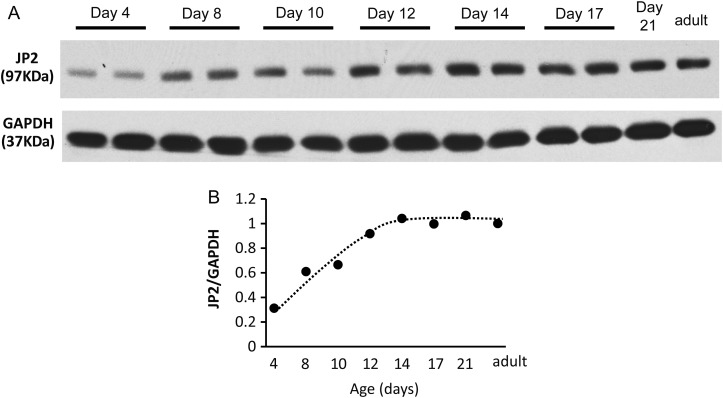
We recently reported a pivotal role for JP2 in maintaining T-tubule ultrastructure in development and progression of heart failure,29,32 and others have shown a correlation between JP2 expression and T-tubule maturation.37 However, it is unknown if JP2 is required for normal T-tubule maturation. Therefore, we first determined the expression pattern of JP2 in developing hearts. As early as postnatal Day 4, modest levels of JP2 were detectable in lysates from whole hearts (Figure 4A). JP2 levels increased greater than two-fold by postnatal Day 8 and continued to increase at Day 14, at which point levels reached those observed in adult mice (Figure 4A and B).
Figure 4.
JP2 expression is detectable in postnatal hearts as early as postnatal Day 4 and gradually increases with development. (A) Expression of JP2 in murine heart lysates by western blot. GAPDH, loading control. (B) Quantitation of JP2 protein levels normalized to GAPDH. n = 4 mice per age group.
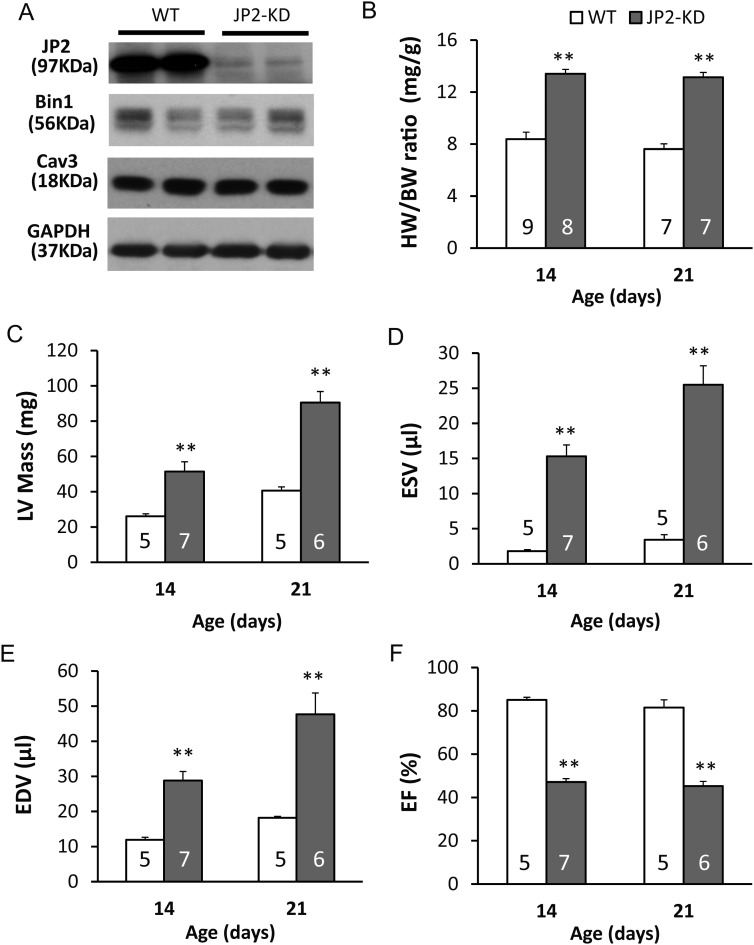
We next explored the hypothesis that JP2 is required for the maturation of the T-tubule network using a mouse model containing a cardiac-specific JP2 shRNA.32 In this transgenic model (see Methods), JP2-shRNA is expressed in the heart beginning in the embryonic stage. We first confirmed a 75% knockdown of JP2 at the protein level during the postnatal developmental stage (Figure 5A, see also Supplementary material online, Figure S5A). JP2 has been shown to interact with caveolin-3 (Cav3),38 and Cav3 knockout is associated with T-tubule disorganization in skeletal muscle.39 Furthermore, both Cav3 and amphiphysin 2 (Bin1) have been implicated in T-tubule biogenesis in skeletal muscle.40–42 However, JP2 deficiency had no effect on Cav3 or Bin1 abundance (Figure 5A, and Supplementary material online, Figure S5B). Morphometric and echocardiographic analysis showed that JP2-KD mice had larger hearts (Figure 5B and C) and reduced ejection fraction (Figure 5D–F).
Figure 5.
JP2 knockdown does not alter expression of other T-tubule associated proteins but produces global cardiac dysfunction. (A) Expression of JP2, Bin1, and Cav3 in murine heart lysates from WT or JP2-KD mice at postnatal Day 14. GAPDH, loading control. (B–F) Effect of JP2 deficiency on cardiac hypertrophy as determined by the ratio of heart weight to body weight (B) or LV mass (C), left-ventricular function as determined by end-systolic volume (ESV, D) end-diastolic volume (EDV, E), and ejection fraction (F). n values for each group are listed inside bars. **P < 0.01 vs. WT of the same age group.
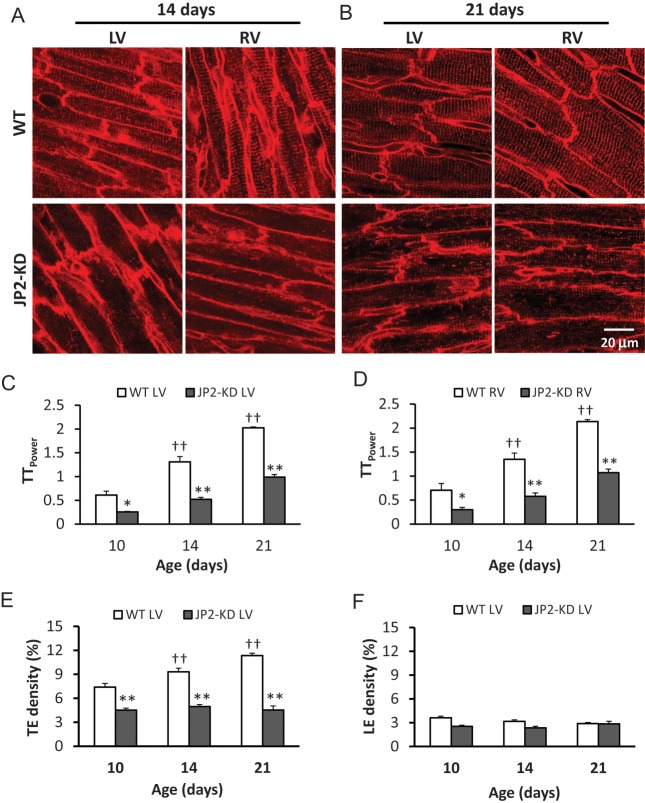
Next, using in situ confocal imaging of ventricular myocytes, we determined the impact of JP2 expression on T-tubule development. Deficiency of JP2 severely impaired T-tubule maturation when compared with wild-type (WT) mice (Figure 6A and B). This effect was observed in both the left- and right-ventricular myocytes as late as postnatal Day 21, a time point at which T-tubules were nearly mature in WT mice. Analysis of T-tubule power demonstrated a significant impairment in T-tubule organization with JP2 knockdown, with a >50% reduction in TTpower in the left- and right-ventricular myocytes at postnatal Days 10 and 14 (Figure 6C and D and Supplementary material online, Figure S6).
Figure 6.
JP2 deficiency severely impairs T-tubule maturation in postnatal hearts. (A and B) Representative in situ confocal images of LV and RV myocytes from WT and JP2-KD mice at 14 (A) and 21 (B) days of age. Scale bar = 20 µm. (C and D) Average TTpower in left (C) and right (D) ventricular myocytes of WT and JP2-KD mice at 10, 14, and 21 days of age. n = 4–5 hearts for each group, *P < 0.05, **P < 0.01 vs. WT at the same age. (E and F) Analyses of T-tubule density in transverse (E) and longitudinal directions (F). n = 30–40 cells per group.
Further analyses of tubule elements in transverse and longitudinal directions showed that during development, the density of transverse elements (TE density, Figure 6E) gradually increased with age in WT hearts, but the density of longitudinal elements (LE density, Figure 6F) remained unchanged. More interestingly, JP2 deficiency prohibited the maturation of transverse elements, resulting in a TE density that was essentially the same during the critical T-tubule developmental stage (Figure 6E). JP2 deficiency had no effect on the longitudinal elements (Figure 6F). The same analyses were performed in myocytes of right ventricle, and identical results were obtained (data not shown). These data identify a key role for JP2 in early T-tubule maturation by tethering T-tubules to the SR membrane, and also suggest that the longitudinal tubule components might be independent of JP2.
3.3. Role of JP2 in Ca2+ handling during early development
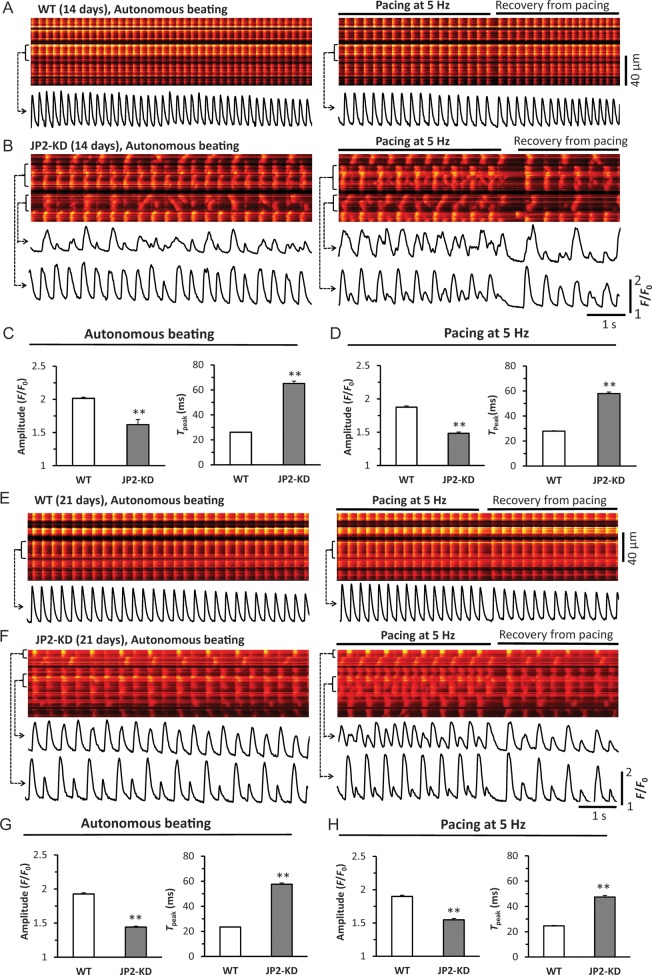
SR Ca2+ release was examined in epicardial myocytes of Langendorff-perfused hearts using an in situ confocal Ca2+ imaging technique as previously described.36 Figure 7 shows data from 14- and 21-day-old WT and JP2-KD mice. At 14 days, WT myocytes already developed uniform, synchronous and stable Ca2+ release among different myocytes and within individual myocytes (Figure 7A). JP2 deficient myocytes displayed high heterogeneity and instability of Ca2+ release under either autonomous beating or external pacing, characteristic of decreased amplitude and prolonged time to peak (dyssynchrony) in Ca2+ transients, Ca2+ alternans and frequently spontaneous Ca2+ waves (Figure 7B–D and Supplementary material online, Figure S7). Figure 7E–H demonstrates similar findings at the age of 21 days. This feature of heterogeneous and unstable Ca2+ release was consistently present in all examined hearts from JP2-deficient mice. The findings herein also underpin the important role of JP2 in (ultra)structural and functional maturation of cardiac E–C coupling.
Figure 7.
JP2 deficiency causes remarkable abnormalities in myocyte Ca2+ handling in situ, including dyssynchronous Ca2+ transients, Ca2+ alternans, frequent Ca2+ waves, and beat-to-beat variability. (A and B) Representative examples of confocal Ca2+ images from 14-day-old WT and JP2-KD hearts, respectively. Left panels are Ca2+ fluorescence under autonomous beating. Right panels are Ca2+ fluorescence under electrical pacing (5 Hz) and recovery after pacing. Bottom traces underneath each image are profiles of Ca2+ transients of the cell as indicated by the bracket. (C and D) Summary of the amplitude and time to peak of Ca2+ transients under autonomous beating or paced at 5 Hz. n = 311 and 350 cells from 4 to 5 hearts for WT and JP2-KD, respectively. **P < 0.01 vs. WT. (E and F) Representative examples of confocal Ca2+ images from 21-day-old WT and JP2-KD hearts. (G and H) Summary of the amplitude and time to peak of Ca2+ transients under autonomous beating or paced at 5 Hz. n = 223 and 192 cells from 4 to 5 hearts for WT and JP2-KD, respectively. **P < 0.01 vs. WT.
4. Discussion
In this study, we described a critical role for JP2 in the development of the T-tubule network in postnatal hearts using non-invasive in vivo confocal imaging. In murine hearts, T-tubules were mostly absent in both left- and right-ventricular myocytes at postnatal Day 8 and became apparent by postnatal Day 10. Levels of JP2 in heart lysates progressively increased starting at postnatal Day 8 and plateaued at Day 14, while T-tubules in the both left and right ventricles started to emerge at Day 10, and matured at Day 19 or slightly later. Furthermore, we provided compelling evidence that deficiency of JP2 significantly impairs T-tubule maturation using in vivo cardiac-specific JP2 knockdown. Lastly, we showed that JP2 deficiency caused marked abnormalities in Ca2+ handling. Taken together, our data demonstrate that JP2 is an important factor required for postnatal T-tubule and E–C coupling development. The findings of this study are in agreement with the pivotal role for JP2 in T-tubule integrity and homeostasis in the adult heart.29,32
The time course of postnatal T-tubule development varies among mammalian ventricular myocytes in rodents, with some studies suggesting that the T-tubule network is essentially absent at birth in mammalian hearts. For example, our data demonstrate that T-tubule development is initiated prior to postnatal Day 10 in the mouse ventricular myocytes but commences later in the rat, with the first evidence for T-tubules at Day 15. The observed time course for T-tubule development in the rat ventricle is similar to findings by Ziman and colleagues.8,37 Our use of TTpower to quantitate T-tubule regularity, combined with examination of T-tubule development in a much wider timespan with shorter intervals, allowed us to better define the progression of T-tubule initiation and maturation in rodent hearts. We also defined the density of tubule components in both transverse and longitudinal directions. In addition, in situ imaging of intact myocytes rather than imaging isolated myocytes, the dissociation of which is known to damage T-tubule structure, may be more reflective of the time course of T-tubule development in vivo.
Interestingly, with in situ confocal imaging, we detected a progressive increase in the density of ventricular transverse elements during normal postnatal mouse heart development, whereas the density of the longitudinal elements remained static. JP2 deficiency abolished this increase in transverse element density, which is an essential part of T-tubule maturation. Several previous studies in multiple heart failure models have demonstrated a reduction in transverse elements paralleled by a relative increase in longitudinal elements.20,25,27 Taken with our findings, these data suggest that the transverse elements are regulated by JP2, whereas the longitudinal elements are independent of changes in JP2 expression.
Our data demonstrate that JP2 deficiency impairs but does not completely preclude T-tubule maturation. As JP2 deficiency does not alter protein levels of Bin1 and Cav3 (Figure 5A and Supplementary material online, Figure S5), it remains possible that these and other unknown proteins participate in T-tubule formation during postnatal development. For example, Cav3 and Bin1 have also been implicated in T-tubule development or deformation of skeletal muscle cells.42–44 Bin1 or Cav3 may affect T-tubule formation independently via different mechanisms, for example, by providing the initial mechanical force for T-tubule invagination. By binding T-tubules in the dyads, JP-2 may serve to maintain T-tubules and prevent their degradation. Future studies are necessary to determine whether Cav3 or Bin1 plays a role in the mechanism by which JP2 mediates T-tubule maturation.
Reynolds et al.45 recently demonstrated that JP2-shRNA mice on a pure C57BL/6 background exhibit impaired T-tubule maturation associated with 100% mortality by postnatal Day 15. In this study, we used a first generation cross of FVB/C57BL/6 mice which results in a slightly delayed onset of the T-tubule maturation impairments, as well as an extended lifespan of the JP2-shRNA mice. It is known that FVB mice are less susceptible to sudden cardiac death phenotypes compared with C57BL/6 mice,46 which may explain the delayed onset of the phenotype in our mixed background mice. Regardless of the exact timing, we also observed that JP2-KD mice developed cardiac hypertrophy and heart failure as well as dysregulation of E–C coupling. Moreover, the degree of T-tubule development impairment at postnatal Day 10 was quite similar in the present study and that of Reynolds et al.45 The discrepancy in the sudden death phenotype between our study and that of Reynolds et al.45 suggests that other genes might modulate the severity and potential lethal consequences of JP2-mediated T-tubule disruption. Factors other than JP2-mediated T-tubule disruption are responsible for the differences in cardiac death phenotype between our study and that of Reynolds et al.45 warrants future investigation.
Consistent with the important role of the T-tubule system in controlling beat to beat SR Ca2+ release, we found that JP2 deficiency caused a severe reduction in the amplitude of Ca2+ transients and significantly slowed activation kinetics of SR Ca2+ release. In addition, dyssynchrous Ca2+ release, Ca2+ alternans, and spontaneous Ca2+ waves were often observed in JP2-deficient myocytes from intact hearts. These data support the notion that ‘orphaned RyRs’, i.e. without local control of L-type Ca2+ channels due to loss of T-tubules, are a source of Ca2+ release instability. JP2 may also have a direct impact on RyR activities as suggested by van Oort et al.32 and Beavers et al.47 In addition, a recent study from the Sejersted group demonstrated that spontaneous Ca2+ sparks are not associated with orphaned RyRs. Their mathematical modelling studies suggest that it may result from changes in altered density or distribution of Ca2+ release units.48
Our previous studies in a rat pressure overload cardiomyopathy model have shown that there is a differential remodelling in T-tubule structure between the left and the right ventricles: T-tubule remodelling starts from left-ventricular myocytes without affecting the right ventricle during the left-ventricular hypertrophy stages. With disease progression from compensated hypertrophy to early and late heart failure, T-tubule remodelling spreads from the left to the right ventricle.29 This is likely due to differential alterations in JP2 levels during different stages of heart disease in the pressure overload model. Supporting this notion, we also found that JP2 down-regulation following myocardial infarction is more severe in the border zone relative to the remote zone, consistent with the pattern of T-tubule damage in those regions.16 We therefore evaluated T-tubule development in both the right and left ventricles and found relatively little difference in the TTpower between the ventricles at each timepoint during physiological development, suggesting a pattern of parallel maturation of the T-tubule system between the left and right ventricles. Our data suggest that JP2 knockdown exerts the same impairment of T-tubule maturation in both ventricles.
In summary, these data provide in vivo evidence that JP2 critically contributes to the maturation of an organized T-tubule network by tethering T-tubules to the SR membrane in the developing heart, thereby expanding the emerging body of work demonstrating that JP2 functions to maintain T-tubule integrity in response to cardiac stress. By understanding the role of JP2 in normal cardiac development, we can potentially gain more valuable insight into how down-regulation of JP2 in response to cardiac stress promotes T-tubule remodelling and disruption of the cardiac dyad, E–C coupling dysfunction and heart failure development.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by NIH R01 HL090905 (L.S.S.) and an American Heart Association Scientific Development Grant 0635056N (L.S.S.), American Heart Association Midwest Postdoctoral Fellowship 13POST14630077 (A.G.), and a W.M. Keck Foundation Distinguished Young Scholar in Medical Research Award, an Established Investigator of the American Heart Association (AHA), NIH R01 HL089598 and HL091947 (X.H.T.W.), and NIH R01 HL079031, HL62494, HL70250, and HL113001 (M.E.A.) and a grant from the Foundation Leducq for the Alliance for CaMKII Signaling (X.H.T.W., M.E.A.)
Supplementary Material
Acknowledgments
Conflict of interest: none declared.
References
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92:1182–1192. doi: 10.1161/01.RES.0000074908.17214.FD. [DOI] [PubMed] [Google Scholar]
- 3.Bito V, Heinzel FR, Biesmans L, Antoons G, Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodelling. Cardiovasc Res. 2008;77:315–324. doi: 10.1093/cvr/cvm063. [DOI] [PubMed] [Google Scholar]
- 4.Sun XH, Protasi F, Takahashi M, Takeshima H, Ferguson DG, Franzini-Armstrong C. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J Cell Biol. 1995;129:659–671. doi: 10.1083/jcb.129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 6.Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, et al. Subcellular [Ca2+]i gradients during excitation-contraction coupling in newborn rabbit ventricular myocytes. Circ Res. 1999;85:415–427. doi: 10.1161/01.RES.85.5.415. [DOI] [PubMed] [Google Scholar]
- 7.Sedarat F, Xu L, Moore ED, Tibbits GF. Colocalization of dihydropyridine and ryanodine receptors in neonate rabbit heart using confocal microscopy. Am J Physiol Heart Circ Physiol. 2000;279:H202–H209. doi: 10.1152/ajpheart.2000.279.1.H202. [DOI] [PubMed] [Google Scholar]
- 8.Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, et al. Fetal and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res. 2003;58:535–548. doi: 10.1016/S0008-6363(03)00255-4. [DOI] [PubMed] [Google Scholar]
- 9.Takekura H, Flucher BE, Franzini-Armstrong C. Sequential docking, molecular differentiation, and positioning of T-Tubule/SR junctions in developing mouse skeletal muscle. Dev Biol. 2001;239:204–214. doi: 10.1006/dbio.2001.0437. [DOI] [PubMed] [Google Scholar]
- 10.Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, et al. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res. 2003;59:67–77. doi: 10.1016/S0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 11.Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, et al. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol. 2006;574:519–533. doi: 10.1113/jphysiol.2006.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swift F, Birkeland JA, Tovsrud N, Enger UH, Aronsen JM, Louch WE, et al. Altered Na+/Ca2+-exchanger activity due to downregulation of Na+/K+-ATPase alpha2-isoform in heart failure. Cardiovasc Res. 2008;78:71–78. doi: 10.1093/cvr/cvn013. [DOI] [PubMed] [Google Scholar]
- 13.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci USA. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, et al. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail. 2009;2:482–489. doi: 10.1161/CIRCHEARTFAILURE.109.852228. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim M, Navaratnarajah M, Siedlecka U, Rao C, Dias P, Moshkov AV, et al. Mechanical unloading reverses transverse tubule remodelling and normalizes local Ca(2+)-induced Ca(2+)release in a rodent model of heart failure. Eur J Heart Fail. 2012;14:571–580. doi: 10.1093/eurjhf/hfs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen B, Li Y, Jiang S, Xie YP, Guo A, Kutschke W, et al. beta-Adrenergic receptor antagonists ameliorate myocyte T-tubule remodeling following myocardial infarction. FASEB J. 2012;26:2531–2537. doi: 10.1096/fj.11-199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie YP, Chen B, Sanders P, Guo A, Li Y, Zimmerman K, et al. Sildenafil prevents and reverses transverse-tubule remodeling and Ca2+ handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension. 2012;59:355–362. doi: 10.1161/HYPERTENSIONAHA.111.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D'Hooge J, et al. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res. 2009;105:876–885. doi: 10.1161/CIRCRESAHA.109.206276. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, et al. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102:338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- 20.Wagner E, Lauterbach MA, Kohl T, Westphal V, Williams GS, Steinbrecher JH, et al. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res. 2012;111:402–414. doi: 10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemi OJ, Hoydal MA, Macquaide N, Haram PM, Koch LG, Britton SL, et al. The effect of exercise training on transverse tubules in normal, remodeled, and reverse remodeled hearts. J Cell Physiol. 2011;226:2235–2243. doi: 10.1002/jcp.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CY, Jia Z, Wang W, Ballou LM, Jiang YP, Chen B, et al. PI3Ks maintain the structural integrity of T-tubules in cardiac myocytes. PLoS One. 2011;6:e24404. doi: 10.1371/journal.pone.0024404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu HD, Xu M, Li RC, Guo L, Lai YS, Xu SM, et al. Ultrastructural remodelling of Ca2+ signalling apparatus in failing heart cells. Cardiovasc Res. 2012;95:430–438. doi: 10.1093/cvr/cvs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crossman DJ, Ruygrok PN, Soeller C, Cannell MB. Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS One. 2011;6:e17901. doi: 10.1371/journal.pone.0017901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swift F, Franzini-Armstrong C, Oyehaug L, Enger UH, Andersson KB, Christensen G, et al. Extreme sarcoplasmic reticulum volume loss and compensatory T-tubule remodeling after Serca2 knockout. Proc Natl Acad Sci USA. 2012;109:3997–4001. doi: 10.1073/pnas.1120172109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao W, Shi J, Dorn GW, II, Wei L, Rubart M. Spatial variability in T-tubule and electrical remodeling of left ventricular epicardium in mouse hearts with transgenic Galphaq overexpression-induced pathological hypertrophy. J Mol Cell Cardiol. 2012;53:409–419. doi: 10.1016/j.yjmcc.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu M, Zhou P, Xu SM, Liu Y, Feng X, Bai SH, et al. Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biol. 2007;5:e21. doi: 10.1371/journal.pbio.0050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, et al. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landstrom AP, Kellen CA, Dixit SS, van Oort RJ, Garbino A, Weisleder N, et al. Junctophilin-2 expression silencing causes cardiocyte hypertrophy and abnormal intracellular calcium-handling. Circ Heart Fail. 2011;4:214–223. doi: 10.1161/CIRCHEARTFAILURE.110.958694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 32.van Oort RJ, Garbino A, Wang W, Dixit SS, Landstrom AP, Gaur N, et al. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation. 2011;123:979–988. doi: 10.1161/CIRCULATIONAHA.110.006437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu M, Wu HD, Li RC, Zhang HB, Wang M, Tao J, et al. Mir-24 regulates junctophilin-2 expression in cardiomyocytes. Circ Res. 2012;111:837–841. doi: 10.1161/CIRCRESAHA.112.277418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, Kadish AH, et al. Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res. 2006;99:e65–e73. doi: 10.1161/01.RES.0000244087.36230.bf. [DOI] [PubMed] [Google Scholar]
- 35.Rubart M, Wang E, Dunn KW, Field LJ. Two-photon molecular excitation imaging of Ca2+ transients in Langendorff-perfused mouse hearts. Am J Physiol Cell Physiol. 2003;284:C1654–C1668. doi: 10.1152/ajpcell.00469.2002. [DOI] [PubMed] [Google Scholar]
- 36.Chen B, Guo A, Gao Z, Wei S, Xie YP, Chen SR, et al. In situ confocal imaging in intact heart reveals stress-induced Ca(2+) release variability in a murine catecholaminergic polymorphic ventricular tachycardia model of type 2 ryanodine receptor(R4496C+/-) mutation. Circ Arrhythm Electrophysiol. 2012;5:841–849. doi: 10.1161/CIRCEP.111.969733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziman AP, Gomez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol. 2010;48:379–386. doi: 10.1016/j.yjmcc.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minamisawa S, Oshikawa J, Takeshima H, Hoshijima M, Wang Y, Chien KR, et al. Junctophilin type 2 is associated with caveolin-3 and is down-regulated in the hypertrophic and dilated cardiomyopathies. Biochem Biophys Res Commun. 2004;325:852–856. doi: 10.1016/j.bbrc.2004.10.107. [DOI] [PubMed] [Google Scholar]
- 39.Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, et al. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- 40.Parton RG, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol. 1997;136:137–154. doi: 10.1083/jcb.136.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carozzi AJ, Ikonen E, Lindsay MR, Parton RG. Role of cholesterol in developing T-tubules: analogous mechanisms for T-tubule and caveolae biogenesis. Traffic. 2000;1:326–341. doi: 10.1034/j.1600-0854.2000.010406.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, et al. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 43.Al-Qusairi L, Laporte J. T-tubule biogenesis and triad formation in skeletal muscle and implication in human diseases. Skelet Muscle. 2011;1:26. doi: 10.1186/2044-5040-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tjondrokoesoemo A, Park KH, Ferrante C, Komazaki S, Lesniak S, Brotto M, et al. Disrupted membrane structure and intracellular Ca(2)(+) signaling in adult skeletal muscle with acute knockdown of Bin1. PLoS One. 2011;6:e25740. doi: 10.1371/journal.pone.0025740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds JO, Chiang DY, Wang W, Beavers DL, Dixit SS, Skapura DG, et al. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc Res. 2013;100:44–53. doi: 10.1093/cvr/cvt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnabei MS, Palpant NJ, Metzger JM. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol Genomics. 2010;42A:103–113. doi: 10.1152/physiolgenomics.00071.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beavers DL, Wang W, Ather S, Voigt N, Garbino AD, Dixit SS, et al. Mutation E169 K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.06.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louch WE, Hake J, Mork HK, Hougen K, Skrbic B, Ursu D, et al. Slow Ca(2)(+) sparks de-synchronize Ca(2)(+) release in failing cardiomyocytes: evidence for altered configuration of Ca(2)(+) release units? J Mol Cell Cardiol. 2013;58:41–52. doi: 10.1016/j.yjmcc.2013.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.