Abstract
Background. CD4+/CD8+ T-cell activation levels often remain elevated in chronic human immunodeficiency virus (HIV) infection despite initiation of antiretroviral therapy (ART). T-cell activation predicts early death and blunted CD4+ T-cell recovery during ART and may affect persistent HIV reservoir size. We investigated whether very early ART initiation is associated with lower on-therapy immune activation and HIV persistence.
Methods. From a cohort of patients with early HIV infection (<6 months duration since infection) we identified persons who started ART early (<6 months after infection) or later (≥2 years after infection) and maintained ≥2 years of virologic suppression; at-risk HIV-negative persons were controls. We measured CD4+/CD8+ T-cell activation (percent CD38+/HLA-DR+) and HIV reservoir size (based on HIV DNA and cell-associated RNA levels).
Results. In unadjusted analyses, early ART predicted lower on-therapy CD8+ T-cell activation (n = 34; mean, 22.1%) than achieved with later ART (n = 32; mean, 28.8%; P = .009), although levels in early ART remained elevated relative to HIV-negative controls (P = .02). Early ART also predicted lower CD4+ T-cell activation than with later ART (5.3% vs 7.5%; P = .06). Early ART predicted 4.8-fold lower DNA levels than achieved with later ART (P = .005), and lower cell-associated RNA levels (difference in signal-to-cutoff ratio (S/Co), 3.2; P = .035).
Conclusions. ART initiation <6 months after infection is associated with lower levels of T-cell activation and smaller HIV DNA and RNA reservoir size during long-term therapy.
Keywords: HIV antiretroviral therapy, early ART, T-cell activation, inflammation, HIVreservoir, HIV eradication, HIV cure
(See the editorial commentary by Henrich and Gandhi on pages 1189–93 and the major article by Yukl et al 1212–20.)
T-cell immune activation, defined by coexpression of CD38 and HLA-DR on CD4+ and CD8+ T cells, has been linked to morbidity and mortality in untreated human immunodeficiency virus (HIV) infection, both AIDS-related and non-AIDS-related [1, 2]. In patients receiving HIV antiretroviral therapy (ART), higher levels of T-cell activation has been linked to diminished CD4+ T-cell count recovery [3–5], surrogate markers of cardiovascular disease [6, 7], and increased mortality [8].
ART-mediated virologic suppression reduces both CD4+ and CD8+ T-cell activation levels [3, 9]. However, patients frequently continue to exhibit T-cell activation levels higher than those seen in HIV-negative controls, indicating that ART reduces but does not fully reverse HIV-related immune activation [3, 9, 10]. A key unanswered question, therefore, is whether ART initiated early (within the first months after HIV infection) can reduce on-therapy immune activation more than ART initiated during chronic HIV infection.
A related question is whether early initiation of ART durably limits the size of persistent HIV reservoirs. Cellular reservoirs of latent genomically integrated HIV are established quickly after infection [11–14], but studies investigating whether ART initiated in the first few months after infection can limit the size of the established reservoir have reached inconsistent conclusions. One study found that ART initiated <6 months after HIV infection was associated with substantially decreased cell-associated HIV infectivity [15], but other studies have reached different conclusions [11, 16–18]. Controversy has persisted in part because of the small size of these studies and the lack of direct comparison between early- and later-treated individuals. The growing research effort aimed at eradicating HIV infection will depend on more precisely elucidating the role early ART may play in limiting the growth of the viral reservoir.
We hypothesized that ART initiated in the first 6 months after HIV infection, compared with ART initiated during chronic disease, would lead to lower levels of on-therapy T-cell activation and a smaller HIV reservoir size. To test this hypothesis, we studied individuals with HIV infection diagnosed during acute infection and who either started ART ≤6 months after infection or deferred therapy for >2 years. We assessed T-cell activation levels and measured the HIV reservoir (based on both HIV DNA and cell-associated RNA levels) to study the impact of early ART on these outcomes.
METHODS
Study Setting and Participants
This study included individuals diagnosed with early HIV infection (<6 months after infection) and enrolled in the Options Project at San Francisco General Hospital, University of California, San Francisco (UCSF). The UCSF Human Research Protection Program approved this study, and participants gave informed, written consent before enrollment.
The Options Project, formed in 1996, is a prospective cohort enrolled <12 months after HIV antibody seroconversion (since 2003, this was restricted to <6 months after seroconversion). Early HIV infection is diagnosed if participants meet any of the following criteria [19]: (1) 2 plasma HIV-1 RNA levels ≥3000 copies/mL with a negative or indeterminate HIV-1 antibody test result; (2) positive HIV-1 antibody result with history of negative HIV-1 antibody result ≤12 months earlier (in 2003, this was changed to ≤6 months); or (3) clinical history suggesting recent HIV acquisition along with reactive standard HIV-1 antibody test result but nonreactive result with less sensitive (“detuned”) HIV-1 antibody test [20–22]. Standardized estimated infection dates are calculated from these data.
We selected Options Project participants enrolled between 1996 and 2009 who fit in 1 of 4 categories: (1) early ART group: participants who started ART <6 months after estimated HIV infection date and had undetectable plasma RNA values for ≥2 years (continuous ART-mediated virologic suppression); (2) later ART group: participants who started ART ≥2 years after estimated HIV infection date and had ≥2 years of virologic suppression with ART; (3) untreated group: participants who remained ART naive for ≥1 year after HIV diagnosis; and (4) HIV-negative controls: at-risk persons who sought acute HIV testing, tested HIV negative, and attended ≥2 study visits (baseline visit plus ≥1 visit ≥12 weeks later).
We analyzed T-cell activation and HIV persistence at key visits. In the early ART group, outcomes were determined at baseline (acute HIV infection), 1 year after ART initiation (1Y ART), and at the participant's final observed time point in the cohort (max ART) if this was ≥2 years after ART initiation. In the later ART group, outcomes were analyzed at baseline (acute HIV), at the participant's final pretherapy time point (pre-ART), 1 year after ART initiation (1Y ART), and at the participant's final time point (max ART) if ≥2 years after ART initiation. In the untreated group, outcomes were assessed at baseline (acute HIV infection), after 1 year of untreated HIV infection (1Y No-TX), and at the participant's final time point. Among HIV-negative controls, outcomes were assessed at baseline and at 1 follow-up time point (12–48 weeks after baseline).
T-cell activation was measured in all treatment groups at all time points described above. In the early and later ART groups, cell-associated HIV DNA/RNA levels were measured only at the on-therapy time points (1 year after ART initiation and at the participant's final on-ART time point).
Clinical and Laboratory Evaluations
Demographic and behavioral data were collected in standardized interviews. CD4+ T-cell counts and HIV-1 plasma RNA levels were measured at baseline and approximately every 3–4 months. The total pre-ART cumulative viremia experienced by patients in the early or late ART groups (akin to total pretreatment area under the viral load curve) was estimated by summing the trapezoidal areas bounded by each pair of viral load measurements, ranging from infection to ART initiation (described in detail in Supplementary Figure 1) [23, 24].
Analysis of T-Cell Activation
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood, cryopreserved, and stored at the UCSF AIDS Specimen Bank. T-cell activation markers were measured at the UCSF Core Immunology Laboratory; multiparameter flow cytometry was used to detect CD3, CD4, CD38, HLA-DR, CCR5, and PD-1, according to methods described elsewhere [25]. Data were analyzed with FlowJo software (Tree Star).
Analysis of HIV DNA and Cell-Associated RNA Levels
PBMC DNA was extracted and HIV DNA was measured using real-time polymerase chain reaction (PCR) targeting Gag DNA sequences; total amplifiable DNA (indicated cell input) was measured using real-time PCR of a single-copy gene (conserved region of HLA-DQ alpha locus), as described elsewhere [26–28], with values normalized per million PBMCs. The HIV DNA assay has a detection limit of 1 HIV DNA copy/3 μg input DNA, equivalent to approximately 450 000 PBMCs [29, 30].
Cell-associated RNA levels were measured with the transcription-mediated amplification assay (Aptima; Gen-Probe), using modified PBMC extraction and transcription-mediated amplification of cell-associated hepatitis C virus RNA [27, 28], recently reported by our group [31]. This yields HIV RNA values expressed as the signal-to-cutoff ratio (S/Co; range 0–30; undetectable, <1.0; detectable, >1.0), with direct correlation of S/Co and viral RNA copy number from 3 to 100 copies. S/Co values were normalized per million PBMCs, as with proviral DNA.
Data Analysis
The 4 participant groups (early ART, later ART, untreated, and HIV-negative controls) were compared with respect to CD4+/CD8+ T-cell activation, as follows. First, median (interquartile range [IQR]) T-cell activation levels were visualized with box plots. All baseline values from the early ART, later ART, and untreated groups (representing HIV-positive subjects) were grouped and analyzed (because these represented immune activation values during early HIV disease, before ART). Median (IQR) values were calculated in the untreated, early ART, and later ART groups using participants' final observed time points. For HIV-negative controls, median (IQR) values were calculated using participants' second (nonbaseline) visit, to decrease the possibility that an intercurrent illness had led them to seek HIV screening and that baseline samples might not represent steady-state immune activation levels. Wilcoxon rank-sum tests were used to compare immune activation levels between early and later ART groups (during ART), and HIV-negative controls.
In the early and later ART groups, longitudinal mixed-effects regression modeling was done to assess the impact of ART timing (early vs later) on immune activation levels during ART. After exploratory analyses, we fit models that assumed a flat slope between the 2 on-ART time points, and used these to predict mean on-therapy CD4+/CD8+ T-cell activation levels.
To assess HIV reservoir size, we visualized median (IQR) log10-transformed HIV DNA levels and cell-associated RNA S/Co ratios with box plots. We then used longitudinal modeling (as described above) to assess the impact of early vs later ART on geometric mean on-ART HIV DNA and RNA levels.
For all outcomes (CD4+/CD8+ T-cell activation, and HIV DNA/RNA levels), both univariate and multivariate models were used to elucidate relative associations of early vs later ART, pre-ART CD4+ T-cell count, and pre-ART cumulative viremia. All analyses were performed using Stata/SE 10.0 software (StataCorp.).
RESULTS
Patient Characteristics
Participant groups were composed almost exclusively of young men with median ages ranging from 33 to 38 years whose main risk factor for HIV acquisition was sexual activity with other men, reflecting local epidemiology (Table 1). Intravenous drug use was infrequent in the cohort.
Table 1.
Baseline Characteristics of Participants
| Variable | Early ART (n = 34) | Later ART (n = 32) | Untreated (n = 37) | HIV-Negative Controls (n = 19) |
|---|---|---|---|---|
| Age, median (IQR), y | 37.3 (33.8–42.2) | 36.6 (34.7–43) | 33.4 (27.3–36.5) | 37.6 (30.2–42) |
| Male, No. (%) | 33 (97) | 32 (100) | 36 (97) | 16 (84) |
| MSM, No. (%) | 33 (97) | 32 (100) | 36 (97) | 15 (79) |
| Recent IVDU, No. (%) | 0 | 0 | 2 (5) | 4 (21) |
| Baseline CD4+ T-cell count, median (IQR), cells/µL | 533 (434–742) | 562 (499–704) | 580 (514–676) | … |
| Baseline plasma HIV RNA level (IQR), log10 copies/mL | 5.2 (4.5–5.7) | 4.2 (3.5–4.7) | 4.2 (3.3–5.0) | … |
| Duration of HIV infection at diagnosis, median (IQR), mo | 2.4 (0.8–2.4) | 2.4 (2.4–2.4) | 2.4 (2.4–2.4) | … |
| Immediate pre-ART CD4+ T-cell count (IQR), cells/µL | 533 (434–742) | 325 (246–435) | … | … |
| Immediate pre-ART plasma HIV RNA level (IQR), log10 copies/mL | 4.8 (4.3–5.5) | 4.7 (4.0–5.0) | … | … |
| Duration of observation during ART (IQR), y | 2.8 (1.8–4.6) | 2.3 (1.8–3.3) | … | … |
| Total duration of observation (IQR), y | 3.0 (2.0–4.7) | 6.1 (4.8–7.7) | 3.5 (2.4–4.4) | … |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; IVDU, intravenous drug use; MSM, men who have sex with men.
Patients in the early and later ART groups were estimated to have acquired HIV a median of 2.4 months before cohort enrollment. The median CD4+ T-cell count at diagnosis of HIV infection was 533 cells/µL in the early ART group, 562 cells/µL in the later ART group, and 580 cells/µL in the untreated group. The median plasma HIV RNA level at this baseline visit was higher in the early ART group than in the later ART and untreated groups (5.2 log copies/mL vs 4.2 and 4.2, respectively). Participants in the later ART group showed a CD4+ T-cell decline to a median of 325 cells/µL before ART initiation. The median HIV RNA level at this time was 4.59 log copies/mL. Early ART participants were observed for a median of 2.8 years on therapy, compared with 2.3 years for later ART participants.
CD4+ T-Cell Activation
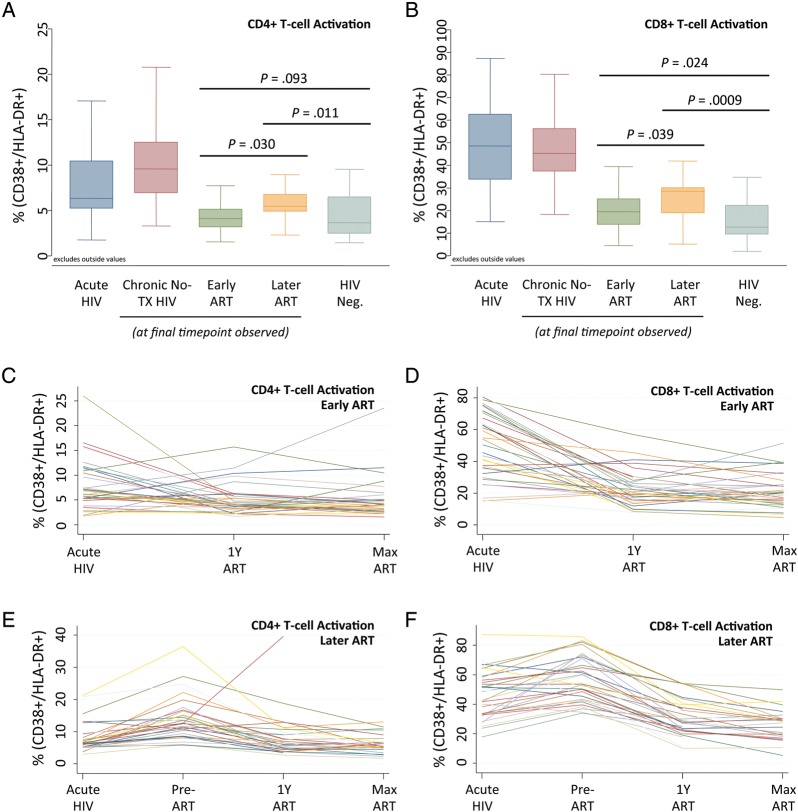
As shown in the cross-sectional data in Figure 1A, during early HIV infection the median CD4+ T-cell activation was 6.3% (IQR, 5.3%–10.4%); this level was similar among the 3 HIV-positive groups (early ART, later ART, and untreated). In untreated participants, levels rose to 9.6% at participants' final observed time point (IQR, 7.0%–12.5%). In the early ART group, the median on-therapy immune activation level decreased from baseline to 4.2% (IQR, 3.6%–6.0%) at 1 year of ART and to 4.1% (IQR, 3.2%–5.1%) at the final observed time point. In the later ART group, on-therapy immune activation decreased less substantially, to 5.9% (IQR 4.2%–9.4%) at 1 year of ART, and 5.5% (IQR, 4.9%–6.8%) at the final time point (P = .01 for early vs later difference at 1 year after ART initiation; P = .03 for difference at final time point). Although the early ART group had lower levels of CD4+ T-cell activation during therapy, median levels remained higher than in HIV-negative controls (4.1% vs 3.1%; P = .09). Similarly, although CD4+ T-cell activation declined in the later ART group, median levels remained significantly higher than in negative controls (5.5% vs 3.1%; P = .01). Individual patients' CD4+ T-cell activation trajectories are displayed for the early and later ART groups (Figure 1C and 1E).
Figure 1.
CD4+ and CD8+ T-cell activation in participants initiating early versus later antiretroviral therapy (ART). A, Box plots showing median proportions (line), interquartile range (IQR; box), and adjacent values (1.5 × IQR; whiskers) of patients with CD4+ T-cells positive for both CD38 and HLA-DR (CD38+/HLA-DR+) within each of 5 participant groups: (1) acute human immunodeficiency virus (HIV) infection (composite of all participants' baseline time points during acute/early HIV infection before therapy; dark blue box), (2) ART-naive (participants remaining off therapy throughout observation, assessed at final available time point; red box), (3) early ART group (assessed at final available time point; green box), (4) later ART group (assessed at final available time point; orange box), and (5) HIV-negative controls (assessed at nonbaseline time point; light blue box). Values beyond adjacent values are not shown. B, Analogous values of CD8+ CD38+/HLA-DR+ T-cells in the same 5 subject groups. C, CD38+/HLA-DR+ CD4+ T-cell levels in 34 patients with early ART initiation. Values from baseline (Acute HIV; n = 34), at 1 year after ART initiation (1 y ART; n = 34), and at the participant's final available time point (Max ART; n = 33) are shown. D, CD38+/HLA-DR+ CD8+ T-cell levels in patients with early ART initiation. E, CD4+ T-cell activation levels in 32 patients with later ART initiation during acute HIV infection (n = 32), at the final pretherapy time point (Pre-ART; n = 32), at 1 y after ART initiation (n = 32), and at the final time point (n = 25). F, CD8+ T-cell activation levels in patients with later ART initiation.
With mixed-effects modeling, the mean T-cell CD4+ activation level during ART was 5.2% in the early ART group, compared with 7.5% in the later ART group (P = .06) (Table 2). We next examined how residual T-cell activation during subsequent ART was influenced by the timing of therapy (early vs later), pre-ART CD4+ T-cell count, and cumulative exposure to HIV (cumulative viremia) during the untreated period (see Table 2). Activation levels seemed to be predicted primarily by the pre-ART CD4+ T-cell count. The impact of later ART was 79% attenuated when modeling controlled for the pre-ART CD4+ T-cell count. Furthermore, the pre-ART CD4+ T-cell count remained substantially associated with on-ART CD4+ T-cell activation (P = .04) even when cumulative viremia was considered (P = .06).
Table 2.
Predictors of On-ART Levels of CD4+ T-Cell Activation
| Predictor | CD38+/HLA-DR+ T Cells, % |
P Value | Effect Attenuation, % | |
|---|---|---|---|---|
| Estimate | 95% CI | |||
| Univariate Models | ||||
| Later ART group | +2.23a | −.11 to +4.58 | .061 | … |
| Pre-ART CD4+ T-cell count | −0.77b | −1.31 to −.23 | .006 | … |
| Pre-ART cumulative viremia | +1.20c | +.14 to +2.30 | .03 | … |
| Pre-ART time | +0.66d | +.05 to +1.27 | .035 | … |
| Model With Later ART and Pre-ART CD4+ T-cell Count | ||||
| Later ART group | +0.47 | −2.36 to +3.30 | .74 | 79e |
| Pre-ART CD4+ T-cell count | −0.70 | −1.37 to −.03 | .041 | 9f |
| Model With Later ART and Cumulative Viremia | ||||
| Later ART group | +1.00 | −1.87 to +3.87 | .49 | 55e |
| Pre-ART cumulative viremia | +0.98 | −.32 to +2.30 | .14 | 19g |
| Model With Later ART, Pre-ART CD4+ T-cell Count, and Cumulative Viremia | ||||
| Later ART group | −0.47 | −3.65 to +2.72 | .77 | 121e |
| Pre-ART CD4+ T-cell count | −0.67 | −1.37 to +.03 | .062 | 13f |
| Pre-ART cumulative viremia | +0.74 | −.55 to +2.00 | .25 | 39g |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval.
a Increase in T-cell activation in later ART group relative to early ART group.
b Increase in T-cell activation per increase in immediate pre-ART CD4+ T-cell count of 100 cells/µL.
c Increase in T-cell activation per increase in total pre-ART cumulative viremia of 100 000 viral load copy years (viral load × years).
d Increase in T-cell activation per increase in pre-ART time (from infection to ART initiation) of 1 year.
e Attenuation relative to unadjusted estimate for later ART group.
f Attenuation relative to unadjusted estimate for pre-ART CD4+ T-cell count.
g Attenuation relative to unadjusted estimate for pre-ART cumulative viremia.
CD8+ T-Cell Activation
The baseline median CD8+ T-cell activation level during early HIV infection was 48.6% (IQR, 33.8%–62.6%; Figure 1B); this remained similar in untreated participants (median at final time point; 45.3%; IQR, 37.4%–56.2%). In the early ART group, CD8+ T-cell activation was reduced to a median of 20.8% (IQR 15.3%–27.9%) after 1 year of ART, and 19.4% (IQR, 13.9%–25.1%) at participants' final observed time point. In the later ART group, activation decreased to a median of 28.6% (IQR 22.7%–32.7%) after 1 year of ART and 28.6% (IQR, 18.9%–30.1%) at the final time point. In HIV-negative controls, the median CD8+ T-cell activation level was 12.5% (IQR, 9.5%–22.2%). As with CD4+ T-cell activation, the early ART group had lower levels of on-ART CD8+ T-cell activation than the later ART group at both 1 year (P = .037) and the final time point (P = .039). Moreover, although ART substantially reduced CD8+ T-cell activation, levels in both the early and later ART groups remained higher than in HIV-negative controls (P = .02 and P < .001, respectively). Individual patients' CD8+ T-cell activation trajectories are displayed within the early and later ART groups (Figure 1D and 1F).
Mixed-effects modeling showed a mean on-ART CD8+ T-cell activation level of 22.1% in the early ART group, compared with 28.8% in the later ART group (P = .009) (Table 3). The impact of delayed ART in raising on-ART activation seemed to be driven jointly by the pre-ART CD4+ T-cell count, cumulative viremia, and effects not captured by either predictor. This assessment is supported by balanced attenuation of each predictor's effects in models controlling for all factors (Table 3).
Table 3.
Predictors of On-ART Levels of CD8+ T-Cell Activation
| Predictor | CD38+/HLA-DR+ T Cells, % |
P Value | Effect Attenuation, % | |
|---|---|---|---|---|
| Estimate | 95% CI | |||
| Univariate Models | ||||
| Later ART group | +6.73a | +1.74 to +11.73 | .009 | … |
| Pre-A T-cell RT CD4+ T-cell count | −1.82a | −2.99 to −.66 | .003 | … |
| Pre-ART cumulative viremia | +3.2a | +.90 to +5.50 | .007 | … |
| Pre-ART time | +1.75a | +.43 to +3.07 | .01 | … |
| Model With Later ART and Pre-ART CD4+ T-cell Count | ||||
| Later ART group | +3.35 | −2.70 to +9.39 | .27 | 50a |
| Pre-ART CD4+ T-cell count | −1.35 | −2.78 to +.08 | .064 | 26a |
| Model With Later ART and Cumulative Viremia | ||||
| Later ART group | +4.38 | −1.63 to +1.39 | .15 | 35a |
| Pre-ART cumulative viremia | +2.1 | −.60 to +4.80 | .13 | 34a |
| Model With Later ART, Pre-ART CD4+ T-cell Count, and Cumulative Viremia | ||||
| Later ART group | +1.91 | −4.82 to +8.63 | .57 | 72a |
| Pre-ART CD4+ T-cell count | −1.13 | −2.61 to +.35 | .13 | 38a |
| Pre-ART cumulative viremia | +1.7 | −1.00 to +4.40 | .22 | 47a |
Abbreviation: ART, antiretroviral therapy; CI, confidence interval.
a Defined in Table 2.
HIV DNA Levels
In the early ART group, log-transformed HIV DNA levels were similar 1 year after ART initiation and at participants' final observed time points (P = .73; Figure 2A). This was also true within the later ART group (P = .69). Individual patients' HIV DNA trajectories are displayed for the early and later ART groups (Supplementary Figure 2A and 2C).
Figure 2.
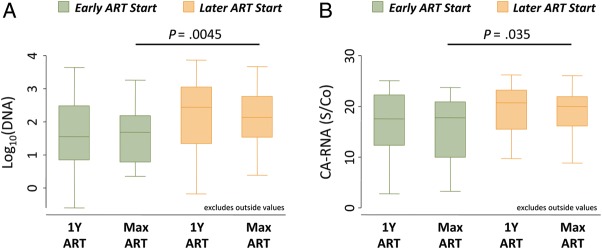
Human immunodeficiency virus (HIV) DNA and cell-associated RNA levels in participants with early versus later ART initiation. A, Box plots showing median log10 HIV DNA level, interquartile range (IQR; box), and adjacent values (1.5× IQR; whiskers) in patients receiving early ART (green boxes) or later ART (orange boxes) assessed 1 year after ART initiation (1 y ART) and at the participant's final available time point (Max ART). B, Analogous box plots depicting cell-associated HIV RNA levels for the same subject groups and time points. Abbreviation: S/Co, signal-to-cutoff ratio.
Mixed-effects modeling (Table 4) showed that patients in the early ART group had 4.8-fold lower on-ART HIV DNA levels than those in the later ART group (P = .005). The effect of ART timing on the HIV DNA reservoir size was not substantially altered when the pre-ART CD4+ cell count or pretherapy cumulative viremia were controlled for (Table 4).
Table 4.
Predictors of On-ART HIV DNA Levels
| Predictor | HIV DNA Levels (Log10 Copies/106 PBMCs) |
P Value | Effect Attenuation, % | |
|---|---|---|---|---|
| Estimate | 95% CI | |||
| Univariate Models | ||||
| Later ART group | +0.67a | +.22 to +1.14 | .005 | … |
| Pre-ART CD4+ T-cell count | −0.104b | −.22 to +.008 | .069 | … |
| Pre-ART cumulative viremia | +0.21c | −.10 to +.43 | .061 | … |
| Pre-ART time | +0.15d | +.03 to +.28 | .015 | … |
| Model With Later ART and Pre-ART CD4+ T-cell Count | ||||
| Later ART group | +0.64 | +.07 to +1.22 | .03 | 5e |
| Pre-ART CD4+ T-cell count | −0.012 | −.15 to +.12 | .86 | 88e |
| Model With Later ART and Cumulative Viremia | ||||
| Later ART group | +0.64 | +.08 to +1.20 | .03 | 5e |
| Pre-ART cumulative viremia | +0.05 | −.21 to +.30 | .71 | 77e |
| Model With Later ART, Pre-ART CD4+ T-cell Count, and Cumulative Viremia | ||||
| Later ART group | +0.65 | +.01 to +1.29 | .047 | 4e |
| Pre-ART CD4+ T-cell count | +0.003 | −.14 to +.15 | .96 | 103e |
| Pre-ART cumulative viremia | +0.05 | −.21 to +.31 | .70 | 77e |
Abbreviation: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; PBMCs, peripheral blood mononuclear cells.
a Increase in HIV DNA in later ART group relative to early ART group.
b Increase in HIV DNA per increase in immediate pre-ART CD4+ T-cell count of 100 cells/µL.
c Increase in HIV DNA per increase in total pre-ART cumulative viremia of 100 000 viral load copy years (viral load × years).
d Increase in HIV DNA per increase in pre-ART time (from infection to ART initiation) of 1 year.
e Defined in Table 2.
HIV Cell-Associated RNA Levels
As with HIV DNA levels, HIV cell-associated RNA levels were similar 1 year after ART initiation and at participants' final observed time points for early and later ART initiators (Figure 2B and Supplementary Figure 2B and 2D). Mixed-effects modeling (Table 5) showed that the mean on-ART cell-associated RNA level was lower in the early ART than in the later ART group (S/Co difference, 3.2; P = .035). These models also suggest that the RNA reservoir size was primarily driven by the total amount of pre-ART cumulative viremia (Table 5). Overall, on-therapy HIV DNA and cell-associated RNA values were not substantially correlated (data not shown), consistent with data recently published by our group [32].
Table 5.
Predictors of On-ART of Cell-Associated HIV RNA Levels
| Predictor | Cell-Associated HIV RNA Level, S/Co |
P Value | Effect Attenuation, % | |
|---|---|---|---|---|
| Estimate | 95% CI | |||
| Univariate Models | ||||
| Later ART group | +3.24a | +.24 to +6.25 | .035 | … |
| Pre-ART CD4+ T-cell count | −0.49b | −1.22 to +.24 | .18 | … |
| Pre-ART cumulative viremia | +2.0c | +.70 to +3.40 | .004 | … |
| Pre-ART time | +0.81d | +.01 to +1.61 | .048 | … |
| Model With Later ART and Pre-ART CD4+ T-cell Count | ||||
| Later ART group | +3.13 | −.62 to +6.88 | .01 | 3e |
| Pre-ART CD4+ T-cell count | −0.044 | −.93 to +.84 | .92 | 91e |
| Model With Later ART and Cumulative Viremia | ||||
| Later ART group | +1.06 | −2.53 to +4.65 | .56 | 67e |
| Pre-ART cumulative viremia | +1.8 | +.16 to +3.40 | .03 | 10e |
| Model With Later ART, Pre-ART CD4+ T-cell Count, and Cumulative Viremia | ||||
| Later ART group | +1.41 | −2.70 to +5.51 | .49 | 57e |
| Pre-ART CD4+ T-cell count | +0.16 | −.75 to +1.06 | .73 | 132e |
| Pre-ART cumulative viremia | +1.8 | +.19 to +3.50 | .03 | 10e |
Abbreviation: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; S/Co, signal-to-cutoff ratio.
a Increase in cell-associated HIV RNA in later ART group relative to early ART group.
b Increase in cell-associated HIV RNA per increase in immediate pre-ART CD4+ T-cell count of 100 cells/µL.
c Increase in cell-associated HIV RNA per increase in total pre-ART cumulative viremia of 100 000 viral load copy years (viral load × years).
d Increase in cell-associated HIV RNA per increase in pre-ART time (from infection to ART initiation) of 1 year.
e Defined in Table 2.
DISCUSSION
Debate continues about the optimal timing of ART in patients with early-stage disease. During long-term effective therapy, residual T-cell activation or dysfunction and inflammation have consistently been correlated with disease progression, but the ability of early ART to prevent these potentially irreversible outcomes remains unclear. Our analysis of the impact of early ART on post-ART T-cell activation in a unique cohort of individuals presenting with early infection reveals that early ART results in durable benefits, measured by CD8+ T-cell activation and, to a lesser degree, CD4+ T-cell activation. We also found on-ART levels of activation relatively stable, consistent with the generation of an immune activation “set point” during effective therapy, similar to previous observations from untreated individuals [33], and found that early ART is associated with smaller HIV DNA and RNA reservoir sizes.
In this study, we assessed the relative roles of ART timing (early vs later), pre-ART CD4+ T-cell loss over time, and the cumulative pre-ART effects of ongoing HIV replication (defined by cumulative viremia). Our prospective early infection cohort, with data from both early and later ART periods, made this type of analysis feasible.
The impact of ART timing on CD4+ T-cell activation was predicted largely by the pre-ART CD4+ T-cell count, suggesting that the potentially detrimental effect of delayed therapy on CD4+ T-cell activation was caused by the effect of untreated HIV on immune function. This supports other findings suggesting that the CD4+ T-cell count rather than viral load is more strongly associated with CD4+ T-cell activation [34, 35]. The robust impact of pretherapy CD4+ T-cell counts on subsequent CD4+ T-cell activation has been observed among chronically infected individuals in an AIDS Clinical Trials Group clinical trial [9]. Our early infection cohort extends and strengthens these findings by isolating the impact of ART timing and CD4+ T-cell loss as distinct predictors of on-ART T-cell activation.
In contrast to CD4+ T-cell activation, the benefit of early therapy on long-term CD8+ T-cell activation seemed to be mediated primarily by cumulative exposure to HIV replication and changes in CD4+ T-cell count. This again supports prior findings indicating that viral replication and CD8+ T-cell activation during untreated disease are strongly linked [34] and extends these findings to the effectively treated state. The independent harm of sustained viremia seen in our study has been explored in a study of seroconverters (Multicenter AIDS Cohort Study [MACS]) in which “viremia copy-years” (similar to our cumulative viremia metric) independently predicted AIDS and death [23]. Similar findings were noted in a large multicenter cohort of treated individuals (CFAR Network of Integrated Clinical Systems [CNICS]) [24]. Our finding that on-therapy CD8+ T-cell activation is influenced by pre-ART cumulative viremia is further evidence that pre-ART viral replication may establish an irreversible proinflammatory environment, perhaps through damage from HIV replication, (eg, at gut mucosal surfaces, causing bacterial translocation and chronic inflammation) [36, 37] or by establishing more active cycles of viral replication, producing chronic low-level viremia and triggering inflammation [38].
A recent study showed that ART initiation within 1 year after infection can normalize levels of soluble CD163 (sCD163)—a marker of monocyte and macrophage activation—to levels seen among HIV-negative individuals [39]. Monocyte activation may be driven by bacterial translocation causing exposure to lipopolysaccharide, and sCD163 expression is linked to on-ART levels of CD8+ T-cell activation [39]. These observations collectively suggest that residual T-cell activation among persons treated during acute infection may be related to pathways distinct from those captured by sCD163.
With interest in developing curative interventions for HIV disease burgeoning [40, 41], we investigated whether early ART is associated with a smaller chronic persistent HIV reservoir. We found that it was associated with substantially lower on-therapy cell-associated HIV DNA and RNA levels, similar to results from other studies using different assays [14, 42]. We also found that HIV persistence remained relatively stable during ART. This stability strongly suggests that delaying ART in patients diagnosed during early infection may result in a permanently enlarged reservoir, potentially reducing a person's ability to clear the reservoir, assuming that curative interventions become available.
As to why early therapy so durably affects reservoir size, our modeling suggests that ART timing (early vs later), rather than pre-ART cumulative viremia or CD4+ T cell count, was the primary determinant of cellular HIV DNA levels, suggesting that time is a critical determinant of the degree to which HIV penetrates the reservoir. In contrast, for reasons that are not clear, on-ART levels of cell-associated RNA were partially predicted by pre-ART cumulative viremia. It is possible that exposure to HIV replication results in a more inducible reservoir or that the cumulative effect of HIV replication on the host results in an environment that irreversibly enhances HIV transcription.
Our study has some limitations. First, because patients were not randomized to receive early vs later ART, bias is possible, for example, if early ART initiation was influenced by factors also linked to chronic inflammation, such as intravenous drug use. However, most of these biases are conservative; for example, factors such as baseline HIV RNA levels were higher in those who started therapy early. Furthermore, T-cell activation levels during early HIV infection were similar between those starting ART early and those starting it later. Both the demographic and behavioral homogeneity within our cohort and the rarity of drug use also reduce the probability of significant bias.
Second, our study design only included 2 on-therapy observations of T-cell activation and HIV reservoir size per patient measured after an average of 2.5–4 years of therapy, where other smaller studies have assessed patients after even longer-term ART [32, 42]. Although our modeling helps maximize the predictive power of our data, uncertainties in our estimates of the impact of early ART remain. A larger sample size, studied over more time points, would permit more precise estimates. Even more optimal would be a trial randomizing patients with early HIV to immediate ART versus deferral of ART until CD4+ T-cell counts were <500 cells/µL. The Strategic Timing of AntiRetroviral Treatment (START) study [43] is enrolling individuals with early HIV infection, which we hope will allow for increased precision on this question.
Our study argues for more pathophysiologic research to confirm the biologic pathways mediating the positive impact of early-initiated ART. Our group and others are assessing plasma biomarkers of inflammation, hypercoagulability, and microbial translocation to assess which of these are affected by the timing of ART. Likewise, detailed characterizations are needed of T-cell functional parameters, such as markers of immunosenescence, T-cell proliferative capacity, and the specific distribution of effector and memory T-cell subsets. These will help elucidate more specific immunologic pathways affected by early ART. In summary, findings from our observational study strongly support the idea that early ART initiation may be valuable in 2 significant areas of HIV pathophysiology and clinical outcomes: minimizing chronic CD4+ and CD8+ T-cell activation and limiting the size of the persistent HIV reservoir.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was primarily supported by the National Institutes of Health (NIH; grant R01 AI087145). The UCSF Options Project was supported by NIH/National Institute of Allergy and Infectious Diseases (grants P01 AI071713 and U01 AI41531). This work was also supported by NIH grant K24 AI069994, the Delaney AIDS Research Enterprise (grant U19AI096109), the UCSF/Gladstone Institute of Virology and Immunology Center for AIDS Research (grant P30 AI027763), and NIH UCSF-CTSI (grant KL2TR000143 to V. J.). J. M. M. is a recipient of the NIH Director's Pioneer Award Program, part of the NIH Roadmap for Medical Research (grant DPI OD003290). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bofill M, Mocroft A, Lipman M, et al. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS. 1996;10:827–34. doi: 10.1097/00002030-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–70. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 3.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42:426–34. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 5.Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–13. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25:2123–31. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins GK, Spritzler JG, Chan ES, et al. Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis. 2009;48:350–61. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–66. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 11.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–73. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schacker T, Little S, Connick E, et al. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J Infect Dis. 2000;181:354–7. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]
- 13.Schacker T, Little S, Connick E, et al. Productive infection of T cells in lymphoid tissues during primary and early human immunodeficiency virus infection. J Infect Dis. 2001;183:555–62. doi: 10.1086/318524. [DOI] [PubMed] [Google Scholar]
- 14.Archin NM, Vaidya NK, Kuruc JD, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci USA. 2012;109:9523–8. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191:1410–8. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 16.Lori F, Jessen H, Lieberman J, et al. Treatment of human immunodeficiency virus infection with hydroxyurea, didanosine, and a protease inhibitor before seroconversion is associated with normalized immune parameters and limited viral reservoir. J Infect Dis. 1999;180:1827–32. doi: 10.1086/315113. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Ramratnam B, Tenner-Racz K, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. New Engl J Med. 1999;340:1605–13. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 18.Blankson JN, Finzi D, Pierson TC, et al. Biphasic decay of latently infected CD4+ T cells in acute human immunodeficiency virus type 1 infection. J Infect Dis. 2000;182:1636–42. doi: 10.1086/317615. [DOI] [PubMed] [Google Scholar]
- 19.Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16:1119–29. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 20.Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–8. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 21.Kothe D, Byers RH, Caudill SP, et al. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J Acquir Immune Defic Syndr. 2003;33:625–34. doi: 10.1097/00126334-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Hecht FM, Wellman R, Busch MP, et al. Identifying the early post-HIV antibody seroconversion period. J Infect Dis. 2011;204:526–33. doi: 10.1093/infdis/jir304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole SR, Napravnik S, Mugavero MJ, Lau B, Eron JJ, Jr, Saag MS. Copy-years viremia as a measure of cumulative human immunodeficiency virus viral burden. Am J Epidemiol. 2010;171:198–205. doi: 10.1093/aje/kwp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mugavero MJ, Napravnik S, Cole SR, et al. Viremia copy-years predicts mortality among treatment-naive HIV-infected patients initiating antiretroviral therapy. Clin Infect Dis. 2011;53:927–35. doi: 10.1093/cid/cir526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinclair E, Tan QX, Sharp M, et al. Protective immunity to cytomegalovirus (CMV) retinitis in AIDS is associated with CMV-specific T cells that express interferon- gamma and interleukin-2 and have a CD8+ cell early maturational phenotype. J Infect Dis. 2006;194:1537–46. doi: 10.1086/508997. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, el-Amad Z, Reis M, et al. Absence of HIV-1 DNA in high-risk seronegative individuals using high-input polymerase chain reaction. AIDS. 1991;5:1201–7. doi: 10.1097/00002030-199110000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Bernardin F, Tobler L, Walsh I, Williams JD, Busch M, Delwart E. Clearance of hepatitis C virus RNA from the peripheral blood mononuclear cells of blood donors who spontaneously or therapeutically control their plasma viremia. Hepatology. 2008;47:1446–52. doi: 10.1002/hep.22184. [DOI] [PubMed] [Google Scholar]
- 28.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol. 2009;83:329–35. doi: 10.1128/JVI.01763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee TH, Chafets DM, Reed W, et al. Enhanced ascertainment of microchimerism with real-time quantitative polymerase chain reaction amplification of insertion-deletion polymorphisms. Transfusion. 2006;46:1870–8. doi: 10.1111/j.1537-2995.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee TH, Paglieroni T, Utter GH, et al. High-level long-term white blood cell microchimerism after transfusion of leukoreduced blood components to patients resuscitated after severe traumatic injury. Transfusion. 2005;45:1280–90. doi: 10.1111/j.1537-2995.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 31.Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208(1):50–6. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksson S, Graf EH, Dahl V, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 34.Catalfamo M, Di Mascio M, Hu Z, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci USA. 2008;105:19851–6. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasula S, Lempicki RA, Adelsberger JW, et al. Differential effects of HIV viral load and CD4 count on proliferation of naive and memory CD4 and CD8T lymphocytes. Blood. 2011;118:262–70. doi: 10.1182/blood-2011-02-335174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–61. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 38.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Ann Rev Med. 2011;62:141–55. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–63. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deeks SG, Barre-Sinoussi F. Public health: Towards a cure for HIV. Nature. 2012;487:293–4. doi: 10.1038/487293a. [DOI] [PubMed] [Google Scholar]
- 41.Deeks SG, Autran B, Berkhout B, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12:607–14. doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chun TW, Justement JS, Moir S, et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis. 2007;195:1762–4. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 43.Babiker AG, Emery S, Fatkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2013;10(1):S5–S36. doi: 10.1177/1740774512440342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.