Abstract
Human immunodeficiency virus type 1 (HIV) infection and antiretroviral therapy (ART) have long been associated with abnormalities in adipose tissue distribution and metabolism. More-recent evidence demonstrates that adipocytes and adipose-resident immune cells have a role in the response to HIV. Clinical and laboratory studies indicate that viral proteins and antiretroviral medications alter adipocyte biology to enhance the persistent, systemic inflammatory state characteristic of untreated and treated HIV infection. Relationships between body composition and lymphocyte populations, cellular immune activation, and immune reconstitution in HIV-infected individuals receiving ART suggest that adipose tissue may also affect cellular immune function. This is further supported by in vitro studies demonstrating the effect of adipocytes and adipokines on lymphocyte proliferation, differentiation, and activation. Synthesis of the literature on adipose tissue biology and immune function in uninfected individuals may shed light on major outstanding research questions in the HIV field.
Keywords: HIV, antiretroviral therapy, adipose tissue, obesity, inflammation, immune activation
Since the introduction of effective antiretroviral therapy (ART), the prevalence of human immunodeficiency virus (HIV)–associated wasting has declined, while the proportion of overweight and obese HIV-infected individuals has increased [1]. Obese HIV-infected individuals have a greater prevalence of cardiovascular and metabolic diseases, compared with lean patients [2, 3]. Beyond these classic comorbidities, the observations that obese HIV-patients often have greater increases in systemic inflammation and that they possibly have a different pattern of immune reconstitution have increased interest in the interaction of adiposity and HIV immunology.
Adipose tissue represents one of the largest organs in the body and comprises a range of cell types with diverse energy storage, metabolic regulation, neuroendocrine, and immunologic functions. HIV infection and ART cause alterations to adipose tissue distribution and biology, with broad effects on cytokine and hormone expression, lipid storage, and the composition of adipose-resident immune cell populations. The resultant changes appear to have important consequences for basal systemic inflammation and the adaptive immune response—2 aspects of immunology with direct relevance to HIV infection. In this review, we synthesize current literature on the role of adipose tissue in innate and cellular immune function (Table 1), which reveals the multifaceted contribution of adiposity to the body's response to HIV infection and highlights key outstanding research questions in the field.
Table 1.
Summary of Current Findings and Important Future Research Areas
| Summary Points |
| Part 1: Adipose Tissue and Inflammation |
| • Increased adiposity in HIV-infected individuals is associated with proportionally higher serum inflammation biomarkers implicated in the development of cardiometabolic diseases [41] • HIV proteins (eg, Vpr and Tat) disrupt adipogenesis and promote adipocytes expression of inflammatory mediators in vitro [51, 52, 54] • Both nucleoside reverse-transcriptase inhibitors and protease inhibitors promote lipodystrophy and adipose tissue inflammation; intraclass variability exists, and some effects are reversible [56–58, 64–66] • Adipose tissue from obese subjects has a higher density of inflammatory (M1) macrophages, CD4+ T cells and CD8+ T cells and fewer T-regulatory cells in both animal and human studies [28–35] • Subcutaneous adipose tissue exhibits greater fibrosis and disrupted adipogenesis as compared to visceral adipose tissue in treated HIV infection but similar expression of inflammatory cytokines [10, 71, 72] |
| Part 2: Adipose Tissue and Cellular Immune Function • Higher body mass index was associated with slower CD4+ T-cell decline and progression to AIDS in the pre-ART era [73–75] • Obesity is associated with diminished early CD4+ T-cell recovery during ART in preliminary studies [76–78] • Congenital lipodystrophy and malnutrition in HIV-uninfected individuals is associated with lower peripheral lymphocyte counts and decreased T-cell responses [79–81, 84–86] • Obesity in HIV-uninfected individuals is associated with higher peripheral T-cell counts, T-cell activation, and Th1-type CD4+ T-cell polarization in preliminary studies [28, 87, 88] • Leptin is constitutively produced in proportion to adipose tissue mass and promotes T-cell proliferation, T-cell activation, and T-helper cell polarization in vitro [101–106] • Pilot studies of recombinant methionyl human leptin have shown beneficial effects in HIV lipodystrophy but no effect on CD4+ T-cell recovery [107–109] |
| Key Outstanding Research Questions |
| • What are the long-term health outcomes among overweight and obese HIV-infected patients? • Does the relationship between serum inflammation biomarkers and cardiometabolic disease risk differ between obese and nonobese HIV-infected patients? • What is the proportional contribution of in situ adipose tissue inflammation to circulating biomarker levels in HIV-infected patients? • Does dysregulated lipid storage in peripheral fat promote visceral and dorsocervical fat hypertrophy? • How do immune cell populations in the ectopic accumulations of pericardial, muscle, and hepatic fat observed in treated HIV infection contribute to metabolic disruptions and disease states? • Why does visceral adipose tissue demonstrate less disrupted adipogenesis than subcutaneous adipose tissue? • Does adipose tissue inflammation attract T cells, or do activated T cells preferentially infiltrate adipose tissue? • What mechanism promotes T-cell infiltration and retention in adipose tissue? Does it differ between subcutaneous and visceral deposits? • Do adipose-resident immune cell populations in HIV-infected individuals of equivalent adiposity differ by sex, age, or race/ethnicity? • What are genetic determinants of adipose tissue inflammation and immune cell infiltration? • Does adipose tissue promote increased peripheral cellular immune activation in treated HIV infection? • Does circulating leptin affect peripheral T-cell numbers, T-cell activation status, or T-helper cell polarization in vivo? |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; Th1, T-helper cell type 1.
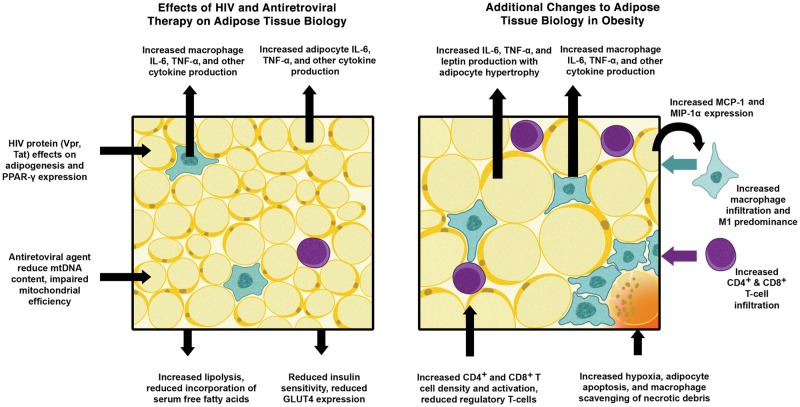
Figure 1.
Effects of human immunodeficiency virus (HIV) infection, antiretroviral therapy, and obesity on adipose tissue biology. Abbreviations: IL-6, interleukin 6; MCP-1, macrophage chemotactic protein 1; MIP-1α, macrophage inflammatory protein 1α; mtDNA, mitochondrial DNA; PPAR-γ, peroxisome proliferator-activated receptor γ; TNF-α, tumor necrosis factor α [9–13, 21–26, 28–33, 36–38, Supplementary data 51–55, 64-66, 71, 72].
PART I: ADIPOSE TISSUE PROMOTES A PERSISTENT INFLAMMATORY RESPONSE DURING TREATMENT OF HIV INFECTION
HIV Infection Alters Adipose Tissue Distribution and Metabolic Characteristics
Approximately 50% of HIV-infected individuals receiving long-term ART demonstrate altered adipose tissue distribution, characterized by peripheral lipoatrophy of the limbs, face, and buttocks; lipohypertrophy of the visceral, cervical, and dorsocervical area (ie, the “buffalo hump”); or a combination of these changes [4, 5]. The cause of HIV-associated lipodystrophy is hypothesized to be multifactorial and due to antiretroviral toxicity, direct effects of HIV infection, and dysregulation of lipid metabolism, among other factors [6]. Irrespective of etiology, the resultant differences in adipose tissue metabolic activity are informative for understanding the pathogenesis of this condition. Whole-body positron emission tomography (PET) of 18F-fluorodeoxyglucose (FDG) uptake by regional fat deposits demonstrates higher metabolic activity in subcutaneous extremity and abdominal adipose tissue deposits of ART treated adults relative to uninfected controls, while uptake in visceral adipose tissue remains normal despite the frequent enlargement of this compartment on ART [7, 8]. Furthermore, FDG uptake in subcutaneous extremity fat is positively correlated with the degree of lipoatrophy, independent of body mass index (BMI; calculated as the weight in kilograms divided by the height in meters squared), indicating an accelerated cellular metabolism in lipoatrophic deposits [8]. Subcutaneous fat biopsy specimens from individuals with HIV-associated lipoatrophy demonstrate reduced mitochondrial DNA (mtDNA) and structural changes characterized by increased fibrosis and apoptosis, heterogeneous mitochondrial mass, and formulation of lipogranulomas, while the adipocytes demonstrate reduced expression of several transcription factors necessary for cellular differentiation and fatty acid uptake but higher tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6) expression [9–13]. Taken together, these findings indicate a shift to a proinflammatory, profibrotic, and dysregulated metabolic state within the subcutaneous fat tissue of these patients.
The enlargement of the visceral adipose compartment and the hypertrophy of dorsocervical adipose deposits (ie, the “buffalo hump”) in ART-treated individuals has been attributed to an “energy excess” state resulting from increased circulating lipids in combination with deficient energy storage in atrophied and fibrotic subcutaneous fat [14, 15]. Studies of dorsocervical fat from HIV-infected individuals have shown less dysregulated adipogenesis and in situ inflammation, compared with other subcutaneous fat deposits, and a unique pattern of glucose uptake and gene expression different from the highly thermogenic, mitochondria-rich brown fat depot characteristically found in this region in uninfected persons [16]. HIV-infected adults with clinically apparent neck fat accumulation demonstrate higher expression of DIO2 (which encodes type II iodothyronine deiodinase, a deiodinase that converts T4 to active T3 and contributes to thermogenesis), characteristic of brown fat, but uncharacteristically low PET-FDG uptake and expression of UCP-1 (the gene that encodes uncoupling protein 1 and is expressed in typical brown adipose tissue, which separates oxidative phosphorylation from adenosine triphosphate synthesis to dissipate energy as heat), suggesting a “whitening” of this ordinarily brown depot [17].
In addition to visceral and dorsocervical fat enlargement, the accumulation of ectopic adipose tissue in a variety of organs in treated HIV infection may contribute to local inflammation and end-organ disease. HIV-infected men have increased epicardial fat, associated with a higher risk of CVD events, and increased hepatic and muscle fat infiltration, associated with greater insulin resistance, compared with uninfected men with similar cardiovascular and metabolic disease risk profiles [18–20].
Adipose Tissue Constitutively Expresses a Range of Proinflammatory Factors
Obesity is a proinflammatory condition in which hypertrophied adipocytes and adipose-resident immune cells (primarily macrophages and, to lesser extent, lymphocytes and neutrophils) contribute to increase the levels of circulating proinflammatory cytokines [21–23]. Higher adipose tissue mass is primarily due to adipocyte hypertrophy, rather than to hyperplasia, and interval increases in adipocyte size result in disproportionate increases in IL-6 and TNF-α expression [24–26]; it is estimated that adipose tissue–derived IL-6 constitutes up to 35% of circulating levels in obese individuals and serves as a major signaling pathway for C-reactive protein (CRP) production [27].
Immune cell infiltration of adipose tissue accompanies progressive weight gain and contributes to in situ inflammation. Biopsy specimens from obese individuals and animal models contain higher absolute numbers of resident macrophages and proportionally greater polarization toward an M1 proinflammatory phenotype (characterized by high IL-6, TNF-α, and inducible nitric oxide synthase production), higher numbers of CD4+ and CD8+ T cells, but fewer T-regulatory cells [28–33]. Additionally, there is increased neutrophil infiltration, which is accompanied by greater in situ inflammation and reduced insulin sensitivity in a murine model and by increased vascular inflammation in humans [34, 35]. Adipocyte hypertrophy is associated with increased production of macrophage chemotactic protein 1 and macrophage inflammatory protein 1α, which promote macrophage infiltration, and with increased production of interleukin 8, which promotes neutrophil chemotaxis [36–38]. In mice, diet-induced obesity also results in increased macrophage CCR5 expression, accompanied by greater insulin resistance and hepatic lipid content, and a shift toward M1 predominance [39]. Future studies are needed to evaluate whether CCR5 inhibitor treatment (eg, maraviroc) affects macrophage activation or other aspects of adipose tissue biology in obese HIV-infected patients.
Greater Adiposity Is Associated With Increased Systemic Inflammation in Treated HIV Infection
Treated HIV infection may be equivalent to obesity in terms of the level of basal inflammation and the presence of other risk factors for chronic cardiovascular and metabolic diseases. HIV-infected men who are receiving ART and have a normal BMI (ie, <25) have statistically equivalent serum CRP levels, as measured by the high-sensitivity assay (hsCRP; a predictor of cardiovascular events in several studies), IL-6 levels, and TNF-α levels and similar lipid profiles and insulin levels, compared with HIV-uninfected men who are resistant to insulin and obese (defined as a BMI of >30) [40].
As observed in the general population, serum levels of hsCRP are also higher among HIV-infected adults with greater adiposity [41–44]. In the Fat Redistribution and Metabolic Change in HIV Infection (FRAM) cohort, each 2-fold increase in visceral adipose tissue mass was associated with a 17% higher hsCRP level, while a similar increase in subcutaneous adipose tissue mass was associated with a 21% higher level [41]. However, the proportional increase in hsCRP level with each doubling of visceral or subcutaneous fat mass in HIV-infected participants was smaller than the change observed in uninfected controls (34% and 61%, respectively). CRP is a terminal product of the inflammation cascade and may be an imprecise measure of circulating cytokine levels earlier in the signaling pathway. For example, there is some evidence that the serum hsCRP level increases after starting treatment with nonnucleoside reverse-transcriptase inhibitors but decreases during treatment with protease inhibitors, and hsCRP levels in HIV-infected patients may also be affected by hepatitis C virus coinfection, statin use, hepatic steatosis, and other factors [41, 45–48]. Given these and other potential confounders, translational studies of viral protein and antiretroviral agent effects on adipocyte function are useful to understand potential mechanisms underlying the association between adiposity and circulating inflammatory biomarkers in HIV-infected individuals.
HIV Proteins and Some Antiretroviral Agents Disrupt Adipocyte Biology
There are conflicting reports on whether adipocytes express HIV entry receptors, but if entry does occur it appears that adipocytes may produce early, but not late, viral transcripts unless further stimulated via exogenous TNF-α [49, 50]. Irrespective of direct infection, many adipose-resident immune cells (eg, lymphocytes and macrophages) are susceptible to infection, and adipocytes may be persistently exposed to high local levels of viral proteins. The viral protein Vpr, in particular, can enter adipocytes from the extracellular fluid in the absence of direct viral infection and acts as a potent repressor of peroxisome proliferator-activated receptor γ (PPAR-γ). PPAR-γ is a transcriptional regulator of adipogenesis (ie, the differentiation of preadipocytes into adipocytes) and the maintenance of normal lipogenesis, insulin sensitivity, and normal cytokine/adipokine expression [51, 52]. The interaction of viral proteins and adipocyte biology is complex and an area of continuing research; for instance, HIV Tat protein has been shown to impair adipogenesis and increase inflammatory cytokine expression in preadipocytes in vitro, while HIV Tat-interacting protein 60 has been shown to act as an adipogenic, positive regulator of PPAR-γ transcriptional activity [53, 54]. Interestingly, the effects of HIV proteins on adipose tissue appear to be independent of plasma viremia; a recent study found significantly lower PPAR-γ and higher TNF-α, interleukin 18, and β2-microglobulin messenger RNA (mRNA) expression in adipose tissue from long-term nonprogressors (ie, HIV-infected individuals with minimal CD4+ T-lymphocyte loss and low plasma viremia for many years), compared with uninfected controls [55].
Early studies reported that nucleoside reverse-transcriptase inhibitors (NRTIs) promoted lipoatrophy, whereas protease inhibitors predisposed to visceral lipohypertrophy, but evolving data reveals intraclass variability in these effects and complex synergistic relationships [56–58]. Loss of limb fat, attributed to mtDNA polymerase γ inhibition and impaired respiratory chain efficiency in adipocytes, is more prevalent with older thymidine analogues (stavudine and zidovudine) than with newer agents (lamivudine, abacavir, and tenofovir), and there is evidence that mtDNA haplogroup may influence susceptibility [11, 57, 59–63]. Adipose tissue samples from lipoatrophic individuals treated with zidovudine or stavudine demonstrate higher macrophage infiltration and proinflammatory cytokine production, both of which increase with treatment duration, compared with samples from lipoatrophic individuals receiving an abacavir- or tenofovir-based regimen [64]. Some adverse NRTI effects on adipose tissue appear reversible; patients receiving a stavudine- or zidovudine-containing regimen with reduced mtDNA cellular content and activity in subcutaneous adipose tissue demonstrated increased mtDNA and mitochondrial gene expression, improved respiratory chain function, and reduced adipocyte apoptosis after switching to a newer NRTI [65, 66].
Protease inhibitors are implicated in visceral fat accumulation and a combined phenotype of insulin resistance and dyslipidemia, in addition to higher adipocyte oxidative stress, proinflammatory cytokine expression, and apoptosis and reduced adipokine expression and insulin signaling [58, 67–69]. A recent study investigating the in vitro effect of nelfinavir on subcutaneous versus intraabdominal murine preadipocyte cell lines found increased lipolysis and a greater reduction in insulin signaling and leptin secretion in subcutaneous cells, compared with visceral cells, illustrating a possible mechanism for the preferential gain in central adiposity observed with protease inhibitor treatment [70].
Given the invasive nature of intraabdominal fat biopsies, there are fewer data on visceral adipocytes than on subcutaneous adipocytes from human subjects. In a study of paired subcutaneous and visceral adipose tissue samples from lean HIV-uninfected individuals, in vitro exposure to nelfinavir, lopinavir, or ritonavir, but not atazanavir, resulted in increased lipolysis and release of free fatty acids, decreased glyceroneogenesis (ie, the recycling of free fatty acids into adipocytes), and increased IL-6 and TNF-α secretion in subcutaneous but not visceral tissue [71]. A small study of HIV-infected individuals with lipodystrophy found similar mtDNA content and mRNA expression of TNF-α and markers of macrophage infiltration in both subcutaneous and visceral adipose biopsy specimens but lower mRNA expression of markers of adipogenesis (PPAR-γ, adiponectin, GLUT4, and lipoprotein lipase) in subcutaneous tissue specimens, compared with visceral tissue specimens [72]. These results suggest less derangement of cellular metabolism and storage capacity in visceral adipocytes. Thus, multiple lines of evidence across ART classes reveal differential and unique effects in visceral versus subcutaneous adipose tissue, potentially explaining the range of lipophenotypes during HIV infection.
PART II: ADIPOSE TISSUE AFFECTS LYMPHOCYTE FUNCTION AND MAY ALTER IMMUNE RECONSTITUTION
A Higher BMI Was Associated With Slower HIV Disease Progression in the Pre-ART Era
Studies from the pre-ART era found that a higher BMI was associated with slower disease progression in untreated HIV infection. In a cohort of HIV-infected drug users, 19% of the obese participants exhibited a >25% decline in CD4+ T cell counts after 18 months, compared with 61% of nonobese participants, despite similar starting CD4+ T-cell counts [73]. In a longitudinal study of HIV-infected women, a BMI of ≥30 was associated with a slower progression to a CD4+ T-cell count of <200 cells/µL and a lower risk of an AIDS-defining event or HIV-related death, while in a similar study each 1-unit decrement in initial BMI was independently associated with a 24% higher risk of progression to AIDS [74, 75]. These results suggest that a high BMI has a protective effect, but it is unclear whether the slower disease progression and lower mortality observed in the pre-ART era were due to adiposity or to related factors, such as fewer secondary infections, fewer micronutrient deficiencies, reduced gut permeability, or the absence of HIV-associated wasting.
Recent Evidence Suggests Obesity Is Associated With Decreased Early Immune Reconstitution
In contrast to data from the pre-ART era that showed a slower decline in CD4+ T-cell counts in heavier patients, recent studies have shown that obesity does not increase CD4+ T-cell count recovery during ART and may actually impair it. An analysis from the US Military HIV Natural History Study found that obesity was associated with a significantly lower adjusted gain in CD4+ T-cell counts after ART, compared with normal-weight patients [76]. A similar analysis of the HIV Outpatient Study cohort, however, found that patients who were overweight or obese had a statistically equivalent increase in CD4+ T-cell count 3–9 months after ART initiation, relative to patients with a normal weight [77].
A more recent cohort study of HIV-infected adults with a high prevalence of obesity found that 12-month increases in CD4+ T-lymphocyte counts after treatment initiation were greatest among those with a pretreatment BMI of 25–30, compared with those with BMIs above or below this range [78]. Among patients with a pretreatment CD4+ T-lymphocyte count of <200 cells/µL, those with a BMI of 25 had the highest odds of reaching a CD4+ T-lymphocyte count of >350 cells/µL at 12 months. These findings indicate that body composition affects peripheral CD4+ T-lymphocyte recovery, but the mechanisms underlying this observation and the reproducibility in other populations are important areas for further study.
Excess Adiposity Is Associated With Changes in Peripheral Immune Cell Populations and Increased Markers of Cellular Immune Activation in HIV-Uninfected Persons
A minimum quantity of adipose tissue appears necessary to maintain normal-range lymphocyte subset counts, but assessing the relationship between adiposity and peripheral T-cell populations in the setting of HIV infection is confounded by CD4+ T-cell depletion, variations in immune recovery during ART, and the effects of cellular immune activation. Thus, studies of HIV-uninfected individuals may be revealing in this area, and a synthesis of this literature suggests a positive relationship between adipose tissue and lymphocyte proliferation, CD4+ T-cell counts, and lymphocyte activation.
In small studies of persons with congenital and acquired lipodystrophy, lymphocyte subsets, including CD4+ T cells, were in the low-normal range, but peripheral blood mononuclear cell TNF-α expression in response to antigen stimulation was significantly reduced, compared with that for normal controls [79, 80]. Similarly, studies of individuals with advanced clinical malnutrition have found reduced peripheral lymphocyte and eosinophil counts [81], a reversal of the T-helper/suppressor ratio [82, 83], decreased T-cell primary antibody response and memory response [84], and atrophy of the lymph tissues, compared with nonmalnourished persons [85, 86]. Last, a longitudinal survey of HIV-uninfected women found that being overweight, obese, or morbidly obese was independently associated with higher CD4+ and total lymphocyte counts, compared with being normal weight, while morbid obesity was associated with a higher CD8+ T-cell count [87].
A recent cross-sectional study of obese, overweight, and normal-weight HIV-uninfected adults found a 3-fold higher expression of the surface activation marker CD25 on CD3+ T cells from obese subjects, compared with nonobese subjects, and the ratio of T-helper 1 (Th1; proinflammatory) to T-helper 2 (Th2; inflammation-repressing) CD4+ T-lymphocytes was also significantly higher in this group [88]. Additionally, BMI was closely associated with the total number of Th1-type CD4+ T cells. Similarly, an analysis of the European CODAM cohort of HIV-uninfected individuals found that greater waist circumference was associated with higher circulating markers of cellular immune activation (neopterin and soluble CD25) [28]. Last, the recent finding that the ratio of mtDNA to nuclear DNA in subcutaneous adipocytes from HIV-infected individuals positively correlates with CD38 and HLA-DR expression on peripheral CD8+ and CD4+ T cells suggests a linkage between cellular immune activation and adipose tissue biology [89]. Taken together, these data suggest that insufficient fat stores may be associated with lower immune cell populations and reduced response to antigen, whereas excess adiposity and the preservation of adipocyte mtDNA content may be associated with increased cellular immune activation.
Obesity Is Characterized by Increased Adipose Tissue T-Cell infiltration
Progressive adipose tissue accumulation is accompanied by increased CD8+ T-cell infiltration and a shift toward a higher ratio of CD8+ T cells to CD4+ T cells. CD8+ T-cell infiltration appears to be a key event preceding the depletion of visceral adipose tissue T-regulatory cells and the increased CD4+ T-cell activation observed in murine models of diet-induced obesity [32, 33]. Increased adipose-resident CD8+ T-cell activation also potentiates adipocyte expression of IL-6 and TNF-α in mice, whereas CD8+ T-cell depletion reverses this effect [33]. Last, T-cell infiltration appears to be an early and necessary step preceding proinflammatory macrophage recruitment into adipose tissue; in murine models, antibody-induced CD8+ T-cell depletion resulted in reduced M1 macrophage infiltration but no change in M2 macrophages [33].
While these findings are intriguing, the relative ease of obtaining surgical biopsy specimens from mice and the higher density of T cells per unit of adipose tissue in rodents contributes to a preponderance of animal data, which may or may not be relevant to human health. Additional studies are needed to determine whether the density and phenotype of adipose-resident T cells differs between HIV-infected and HIV-uninfected individuals and to further characterize the relationship between peripheral CD4+ and CD8+ T-cell activation and adipose tissue biology in the setting of HIV infection.
Adipose Tissue Hormones Alter Lymphocyte Function and May Alter the Immune Response to HIV
Adipokines are hormones produced by adipocytes, which demonstrate a range of metabolic, neuroendocrine, and immunomodulatory properties (Table 2). Adiponectin has important insulin-sensitizing effects, and circulating levels decline with increasing adiposity and during HIV infection [90]. Adiponectin also has a potent antiinflammatory effect in vitro by inhibiting macrophage differentiation and production of TNF-α, reducing adipose tissue endothelial adhesion molecule expression, stimulating the production of interleukin 10 and interleukin 1 receptor antagonist, and reducing T-cell proliferation [91–93], all of which would be predicted to be salutary effects for reducing inflammation and immune activation.
Table 2.
Summary of Adipokine Effects on Immune Cells
| Leptin [97–99, 101–106, 110–112] |
| • Produced in proportion to fat mass • Structural similarity to IL-6 • Increases TNF-α, IL-6, and IL-12 production from monocytes and macrophages • Stimulates neutrophil proliferation and reactive oxygen species production • Increases naive CD4+ T-cell proliferation and Th1 polarization • Increases antigen-stimulated CD4+ and CD8+ T-cell activation marker expression • Promotes increased antigen-stimulated Th1-type cytokine release (principally INF-γ) • Suppresses Th2 production of IL-4 • Stimulates hepatocytes to express C-reactive protein |
| Adiponectin [90–93] • Markedly decreased in visceral obesity and HIV infection • Serum levels inversely correlate with insulin resistance • Inhibits macrophage differentiation and decreases IL-6 and TNF-α production • Increases IL-10 production • Decreases endothelial adhesion molecules • Reduces T-cell proliferation |
Abbreviations: IL-4, interleukin 4; IL-6, interleukin 6; IL-10, interleukin 10; IL-12, interleukin 12; HIV, human immunodeficiency virus; IFN-γ, interferon γ; Th1, T-helper cell type 1; Th2, T-helper cell type 2; TNF-α, tumor necrosis factor α.
Leptin, an adipokine encoded by the ob gene and produced roughly in proportion to fat cell mass, was initially characterized as a regulator of appetite via receptors in the hypothalamus but also appears to have a range of local and potentially systemic immune effects [94–96]. Leptin independently induces expression of proinflammatory cytokines by macrophages and monocytes [97, 98] and acts directly on hepatocytes to promote CRP expression [99]. Mature CD4+ T cells express the long isoform of the leptin receptor [100, 101], and in vitro leptin stimulates T-cell proliferative responses, polarizes naive CD4+ T-cell proliferation toward the Th1 phenotype, and promotes a marked increase in Th1-type cytokine production, principally interferon γ [101–105]. Leptin also enhances in vitro expression of activation markers (CD69, CD25, and CD71) on both CD4+ and CD8+ T cells after antigen stimulation in a dose-dependent manner [104, 106].
A single-arm study administering physiologic quantities of recombinant methionyl human leptin (r-metHuLeptin) to HIV-uninfected adults with congenital or acquired lipodystrophy found an increase in total CD4+ and CD8+ T cells, but trials in HIV patients to date have not shown a clear benefit for immune recovery during ART [79]. In a placebo-controlled crossover study of 7 HIV-infected men with ART-associated lipodystrophy, 2 months of r-metHuLeptin did not significantly alter CD4+ T-cell counts (although the mean baseline level was 454 cells/µL) but did result in significantly lower trunk fat and fasting insulin levels, and there was a trend toward lower serum IL-6 levels [107]. A single-arm, dose-escalation trial involving HIV-infected adults receiving ART found no change in CD4+ T-cell counts but decreased visceral adipose tissue volume, insulin resistance, and dyslipidemia, whereas another trial found no effect on lipolysis or fasting lipid kinetics [108, 109]. Metreleptin, a commercial version of r-metHuLeptin, is under evaluation by the Food and Drug Administration and will be important for further in vivo studies of leptin therapy in patients with advanced CD4+ T-cell deficiency or marked visceral adipose hypertrophy.
CONCLUSIONS
The complex alterations in adipose tissue biology and energy storage observed in HIV-infected individuals have been an active research area since early in the epidemic, but adipose tissue may also have important roles in modulating the immune response to HIV infection. As the proportion of overweight and obese HIV-infected individuals continues to rise, understanding the role of adipose tissue in the pathogenesis of immune activation will be critical to optimizing long-term health outcomes.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Dr Steven Grinspoon for his assistance in reviewing a draft manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant K23 100700 to J. K.) and the Tennessee Valley Healthcare System (to K. N.).
Potential conflicts of interest. T. H. has served as principal investigator on research grants from Merck to Vanderbilt University. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5:e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26:303–14. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 3.Kim DJ, Westfall AO, Chamot E, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr. 2012;61:600–5. doi: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–31. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunfeld C, Saag M, Cofrancesco J, Jr, et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. AIDS. 2010;24:1717–26. doi: 10.1097/QAD.0b013e32833ac7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caron-Debarle M, Lagathu C, Boccara F, Vigouroux C, Capeau J. HIV-associated lipodystrophy: from fat injury to premature aging. Trends Mol Med. 2010;16:218–29. doi: 10.1016/j.molmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Hadigan C, Kamin D, Liebau J, et al. Depot-specific regulation of glucose uptake and insulin sensitivity in HIV-lipodystrophy. Am J Physiol Endocrinol Metab. 2006;290:E289–98. doi: 10.1152/ajpendo.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torriani M, Zanni MV, Fitch K, et al. Increased FDG uptake in association with reduced extremity fat in HIV patients. Antivir Ther. 2013;18:243–48. doi: 10.3851/IMP2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastard JP, Caron M, Vidal H, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359:1026–31. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 10.Jan V, Cervera P, Maachi M, et al. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir Ther. 2004;9:555–64. [PubMed] [Google Scholar]
- 11.Nolan D, Hammond E, Martin A, et al. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS. 2003;17:1329–38. doi: 10.1097/00002030-200306130-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kannisto K, Sutinen J, Korsheninnikova E, et al. Expression of adipogenic transcription factors, peroxisome proliferator-activated receptor gamma co-activator 1, IL-6 and CD45 in subcutaneous adipose tissue in lipodystrophy associated with highly active antiretroviral therapy. AIDS. 2003;17:1753–62. doi: 10.1097/00002030-200308150-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lihn AS, Richelsen B, Pedersen SB, et al. Increased expression of TNF-alpha, IL-6, and IL-8 in HALS: implications for reduced adiponectin expression and plasma levels. Am J Physiol Endocrinol Metab. 2003;285:E1072–80. doi: 10.1152/ajpendo.00206.2003. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Albu JB, Engelson ES, et al. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2004;286:E261–71. doi: 10.1152/ajpendo.00056.2003. [DOI] [PubMed] [Google Scholar]
- 15.Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012;205(Suppl 3):S383–90. doi: 10.1093/infdis/jis205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guallar JP, Gallego-Escuredo JM, Domingo JC, et al. Differential gene expression indicates that ‘buffalo hump’ is a distinct adipose tissue disturbance in HIV-1-associated lipodystrophy. AIDS. 2008;22:575–84. doi: 10.1097/QAD.0b013e3282f56b40. [DOI] [PubMed] [Google Scholar]
- 17.Torriani M, Fitch K, Stavrou E, et al. Deiodinase 2 expression is increased in dorsocervical fat of patients with HIV-associated lipohypertrophy syndrome. J Clin Endocrinol Metab. 2010;97:E602–7. doi: 10.1210/jc.2011-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo J, Abbara S, Rocha-Filho JA, Shturman L, Wei J, Grinspoon SK. Increased epicardial adipose tissue volume in HIV-infected men and relationships to body composition and metabolic parameters. AIDS. 2010;24:2127–30. doi: 10.1097/QAD.0b013e32833c055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlando G, Guaraldi G, Zona S, et al. Ectopic fat is linked to prior cardiovascular events in men with HIV. J Acquir Immune Defic Syndr. 2012;59:494–7. doi: 10.1097/QAI.0b013e31824c8397. [DOI] [PubMed] [Google Scholar]
- 20.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46:312–7. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 22.Bourlier V, Bouloumie A. Role of macrophage tissue infiltration in obesity and insulin resistance. Diabetes Metab. 2009;35:251–60. doi: 10.1016/j.diabet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 24.Bastard JP, Lagathu C, Caron M, Capeau J. Point-counterpoint: Interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J Appl Physiol. 2007;102:821–2. doi: 10.1152/japplphysiol.01353.2006. [DOI] [PubMed] [Google Scholar]
- 25.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 26.Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T. Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–10. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 28.Thewissen MM, Damoiseaux JG, Duijvestijn AM, et al. Abdominal fat mass is associated with adaptive immune activation: the CODAM Study. Obesity (Silver Spring) 2011;19:1690–8. doi: 10.1038/oby.2010.337. [DOI] [PubMed] [Google Scholar]
- 29.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–10. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 31.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–38. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 32.Deiuliis J, Shah Z, Shah N, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 2011;6:e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 34.Shah TJ, Leik CE, Walsh SW. Neutrophil infiltration and systemic vascular inflammation in obese women. Reprod Sci. 2010;17:116–24. doi: 10.1177/1933719109348252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talukdar S, Oh da Y, Bandyopadhyay G, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–12. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–9. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 37.Lee YH, Nair S, Rousseau E, et al. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia. 2005;48:1776–83. doi: 10.1007/s00125-005-1867-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jernas M, Palming J, Sjoholm K, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–2. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 39.Kitade H, Sawamoto K, Nagashimada M, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–90. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring) 2009;17:53–9. doi: 10.1038/oby.2008.500. [DOI] [PubMed] [Google Scholar]
- 41.Reingold J, Wanke C, Kotler D, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–8. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boger MS, Shintani A, Redhage LA, et al. Highly sensitive C-reactive protein, body mass index, and serum lipids in HIV-infected persons receiving antiretroviral therapy: a longitudinal study. J Acquir Immune Defic Syndr. 2009;52:480–7. doi: 10.1097/qai.0b013e3181b939e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 44.Koethe JR, Dee K, Bian A, et al. Circulating interleukin-6, soluble CD14, and other inflammation biomarker levels differ between obese and non-obese HIV-infected adults on antiretroviral therapy. AIDS Res Hum Retroviruses. 2013;29:1019–25. doi: 10.1089/aid.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shikuma CM, Ribaudo HJ, Zheng Y, et al. Change in high-sensitivity C-reactive protein levels following initiation of efavirenz-based antiretroviral regimens in HIV-infected individuals. AIDS Res Hum Retroviruses. 2011;27:461–468. doi: 10.1089/aid.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henry K, Kitch D, Dube M, et al. C-reactive protein levels over time and cardiovascular risk in HIV-infected individuals suppressed on an indinavir-based regimen: AIDS Clinical Trials Group 5056s. AIDS. 2004;18:2434–7. [PubMed] [Google Scholar]
- 47.McComsey GA, Kitch D, Daar ES, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS. 2012;26:1371–85. doi: 10.1097/QAD.0b013e328354f4fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aslangul E, Fellahi S, Assoumou LK, Bastard JP, Capeau J, Costagliola D. High-sensitivity C-reactive protein levels fall during statin therapy in HIV-infected patients receiving ritonavir-boosted protease inhibitors. AIDS. 2011;25:1128–31. doi: 10.1097/QAD.0b013e328346be29. [DOI] [PubMed] [Google Scholar]
- 49.Munier S, Borjabad A, Lemaire M, Mariot V, Hazan U. In vitro infection of human primary adipose cells with HIV-1: a reassessment. AIDS. 2003;17:2537–9. doi: 10.1097/00002030-200311210-00019. [DOI] [PubMed] [Google Scholar]
- 50.Maurin T, Saillan-Barreau C, Cousin B, Casteilla L, Doglio A, Penicaud L. Tumor necrosis factor-alpha stimulates HIV-1 production in primary culture of human adipocytes. Exp Cell Res. 2005;304:544–51. doi: 10.1016/j.yexcr.2004.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.