Abstract
Multiple myeloma (MM) cells rely on protein homeostatic mechanisms for survival. These mechanisms could be therapeutically targeted via modulation of the heat shock response. We studied the roles of Hsp72 and Hsc70, and show that the two major cytoplasmic Hsp70s play a key role in regulating protein homeostasis and controlling multiple oncogenic pathways in MM, and their inhibition can lead to myeloma cell death. Our study provides further evidence that targeting Hsp70 represents a novel therapeutic approach which may be effective in the treatment of MM.
Keywords: Haematology, Multiple myeloma, Protein folding, ER stress, Hsp70
1. Introduction
Heat shock proteins are involved in a wide range of biochemical processes including folding of nascent polypeptides, degradation of misfolded proteins, intracellular trafficking, modulating signalling pathway activity and regulating immune responses [1–4]. The multi-functional nature of heat shock proteins and their critical roles in the regulation of protein homeostasis and cell survival make them good therapeutic targets in cancer [5,6].
Heat shock proteins are found to be overexpressed in a wide range of cancers, with Hsp90 and Hsp70 being the most studied of the family. Accumulating evidence implicate them in cancer cell survival, apoptosis, invasion, metastasis and escape of immune surveillance. In recent years, more than ten Hsp90 inhibitors have entered clinical trials against a range of solid and haematological malignancies, and promising activity has been observed in many cancers but not others [7]. In addition, efforts are also being made to develop Hsp70 inhibiting compounds, although these projects are still in the drug discovery phase [6,8].
Increasing evidences suggest that the functions of heat shock proteins are complex in cells, with isoforms of the same heat shock protein at different subcellular locations playing distinctive roles [7,9]. New co-chaperones are also being identified linking the heat shock proteins to previously unknown functions. All of these factors can influence the effectiveness of heat shock protein inhibitors in specific cell types. A better understanding of the mechanisms that heat shock proteins exert their cellular effects will enable the development of targeted therapeutic strategies with increased activity and reduced toxicity. One approach to achieving this is via the development of isoform specific inhibitors and co-chaperone inhibitors.
To investigate the clinical utility of such an approach in cancer we investigated multiple myeloma as a model system. Multiple myeloma (MM) results from the malignant proliferation of plasma cells in the bone marrow, which is characterised by the secretion of monoclonal paraprotein [10]. The mechanisms underlying the production and secretion of the paraprotein are a potential therapeutic target as myeloma cells may be sensitive to the generation of proteotoxic stress mediated via the heat shock protein pathway [11]. Previous studies have shown that MM cells are sensitive to Hsp90 inhibitors; however, the inhibition of Hsp90 is usually accompanied by the induction of the pro-survival cytosolic Hsp70 family proteins, which may attenuate the pro-apoptotic effect [12,13]. Hsp70 chaperones act upstream of Hsp90 and present client proteins for further processing [14], as well as modulating apoptosis [15–17]. Thus targeting the Hsp70s alone or in combination with Hsp90 offers an appealing target for the treatment of myeloma as well as other secretory tumours [6]. The roles of Hsp72 and Hsc70, the two major cytosolic isoforms of Hsp70 in MM have not been well defined. The aim of this study was to increase our understanding of the roles of cytosolic Hsp70 in MM, as well as to investigate in vitro a MM-specific therapy based on targeting the heat shock response.
2. Materials and methods
2.1. Cell lines
All MM cell lines were grown in RPMI1640 containing GlutaMax™(Gibco, Invitrogen), supplemented with 10% heat-inactivated fetal calf serum (PAA Laboratories). H293T cells were grown in DMEM (Gibco, Invitrogen) supplemented with 10% heat-inactivated fetal calf serum. Cells were cultured at 37 °C in a humidified gas chamber with 95% air and 5% carbon dioxide. All cell lines used were mycoplasma-free as confirmed by PCR. Primary CD138+ myeloma cells and bone marrow stromal cells were obtained and cultured as previously described [18]. Blood samples were obtained from patients during routine practice following informed consent, and peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque Premium (GE Healthcare).
2.2. Lentiviral transduction of shRNAs
Hsp72 shRNA sequence #1 (GGACGAGTTTGAGCACAAG), a scramble control corresponding to sequence #1 (GGACGAGTTGTAGCACAAG), and shRNA #2(GCCATGACGAAAGACAACAAT) were cloned into the pLVTHM vector as described previously [19]. Hsc70 shRNAs #1 (CCAAGACTTCTTCAATGGAAA) and #2(GCAACTGTTGAAGATGAGAAA) cloned into pLKO.1 vectors were obtained from The RNAi Consortium (TRC) via Open Biosystems. A scramble control corresponding to sequence #1 GAAGACTACCACGACTTATAT was cloned into the pLKO.1 vector according to supplier’s protocol (Addgene).
Virus was produced according to protocols published by TRC (http://www.broadinstitute.org/rnai/public/resources/protocols). Cells transduced with pLVTHM vectors were sorted for the GFP positive population using a FACSAria (BD Biosciences) and stably transduced cells were grown in normal culture media. For double transductions, cells were then transduced with pLKO.1 vectors. All experiments were performed within 10 days after transduction with pLKO.1 vectors.
2.3. Cell proliferation assay
Cell proliferation was measured by the colorimetric WST-1 assay (Roche) according to the manufacturer’s instructions. The WST-1 tetrazolium compound is bioreduced into a coloured formazan by NADPH or NADH in metabolically active cells, therefore the amount of absorbance is directly proportional to the number of living cells in culture. Cells were plated in 100 μl media at a density of 1 × 104/well in 96-well plates. To perform WST-1 assay, 10 μl of WST-1 solution was added to each well and the plates were incubated in a humidified incubator in 5% CO2 at 37 °C. Two hours after incubation, plates were read on a MRX plate reader (Dynatech Laboratories). For lentiviral knockdown studies, cells were harvested 5 days after pLKO.1 transduction, plated in 96-well plates and the proliferation followed every 24 h for four days. For small-molecule inhibition studies, cells were treated with the Hsp70 inhibitor Ver-155008 (Tocris) and WST-1 assays were performed at indicated time points. For drug combination study, KMS11 and RPMI8226 cells were treated with an 11-point concentration range of 17-AAG, Ver-155008 or in combination for 24 h before cell viability was measured by WST-1 assay. The combination index (CI) methods of Chou and Talalay were used to calculate the effect of drug combination, with values <1 indicating synergism, 1–1.2 additive and values >1.2 antagonism.
2.4. Apoptosis assays
For the Annexin V/PI double staining assay, cells were collected and resuspended in 1X binding buffer (10 mM Hepes/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). 1 × 105 cells in 200 μl binding buffer were double stained with 2.5 μl Annexin V–APC (BD Biosciences) and 2.5 μl 50 μg/ml PI (BD Biosciences). 10,000 cells were analysed per sample using a BD LSRII flow cytometer.
For PI cell cycle analysis, cells were resuspended in 1ml PBS, then fixed by adding 2.5 ml ice cold absolute ethanol for 15 min. Fixed cells were pelleted by spinning at 1500 rpm for 5 min, then the pellets were resuspended in 500 μl PI solution (50 μg/ml propidium iodide (BD), 0.1 mg/ml RNase A (Life Technologies) and 0.05% Triton X-100 (BDH) in PBS) and incubated at 37 °C for 40 min. After incubation, cells were pelleted and resuspended in 500 μl PBS, then analysed on a BD LSRII flow cytometer.
2.5. Western blotting and immunoprecipitation
After transduction or small-molecule inhibitor treatment, cells were harvested and lysed in RIPA buffer (1% w/v sodium deoxycholate, 1% v/v TritonX-100, 1% v/v Nonident P-40, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 50 mM Tris, 30 mM NaF) supplemented with 1X protease inhibitor cocktail (Roche), 200 mM phenylmethylsulfonyl fluoride (Fluka), and 1 mM Na3VO4 (Sigma–Aldrich). Proteins were resolved on 10% SDS–PAGE gels, transferred to PVDF membranes (Millipore), blocked with 5% milk and incubated with primary antibodies for two hours at room temperature. Primary antibodies to Hsp90 (#SPS-771, Stressgen), Hsp72 (#SPA-810, Stressgen); Hsc70, GRP78 (#sc-376768, Santa Cruz Biotechnology); CDK4, C-RAF, Caspase 3, ubiquitin (#2906, #9422, #9662 and #3936, Cell Signaling); LAMP-2, Hsf-1, IgK, Igλ (#ab25631, #ab2923, #ab134083 and #ab124719, Abcam) and anti-β actin antibody (AC-15, Sigma–Aldrich) were used. Membranes were then incubated with corresponding secondary antibodies conjugated to horseradish peroxidase (Amersham Biosciences) for 1 h at room temperature. ECL-Plus (Amersham Biosciences) was used for protein band detection on Kodak XAR films.
For immunoprecipitation studies, cells were lysed in RIPA buffer supplemented with 1X protease inhibitor cocktail, 200 mM phenylmethylsulfonyl fluoride, and 1 mM Na3VO4. Equal amounts of protein were incubated with primary anti-IgK or anti-Igλ antibody for 2 h at room temperature followed by incubation with protein A/G magnetic beads (Thermo Scientific) for one hour at room temperature. Protein samples were eluted in 1X sample buffer (50 mM Tris pH6.8, 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue and 10% glycerol) and analysed on SDS–PAGE gels as described above.
For transduction studies, all protein samples were harvested 5 days after transduction with pLKO.1 vectors.
2.6. Enzyme-linked immunosorbent assay (ELISA)
ELISA analysis on myeloma cells was performed as described previously [20]. Secreted and intracellular light chain was detected using the human kappa or lambda ELISA quantification kit (Bethyl Laboratories) according to the manufacturer’s instructions. Cells were cultured for 24 h before the supernatant from 1 × 106 cells was diluted 1:100 and used for the quantification of Ig secretion. For intracellular Ig, whole-cell lysates were prepared as described for western blotting and 500 ng of total protein was used per assay.
2.7. Reactive oxygen species detection
Global levels of reactive oxygen species was detected using Total ROS detection kit (Enzo) according to the manufacturer’s instructions. Cells were treated with 5 μM Ver-155008 for 24 h before analysis on a BD LSRII flow cytometer. As a negative control, 5 μM ROS inhibitor N-acetyl-L-cysteine was added to the treated cells 30 min before analysis. Histograms were generated using the free software Flowing (Version 2.5).
2.8. Statistics
All experiments were performed in triplicate. Statistical analysis was performed using two-tailed student t-test. P-value < 0.05 was considered statistically significant.
3. Results
3.1. Silencing of Hsc70 increases immunoglobulin retention and reduces the ubiquitin tagging required for proteasome degradation
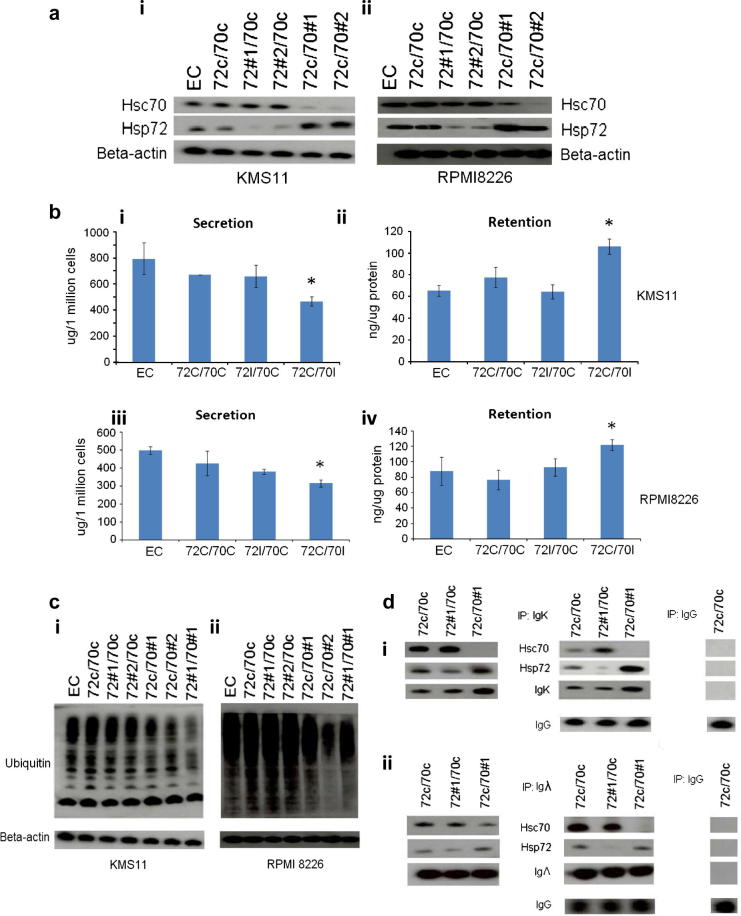
One of the main characteristics of MM cells is the synthesis of a large amounts of immunoglobulin (Ig), creating a protein folding load [21]. To understand if Hsp70 isoforms have a role in Ig homeostasis, we studied the level of Ig light chains (IgK or Igλ) in myeloma cells after knockdown of Hsp72 or Hsc70. The expression levels of Hsp72 and Hsc70 were silenced using two specific shRNAs against each protein and the experiments were controlled using a scrambled shRNA for each isoform. In western blot analysis of KMS11 and RPMI8226 human MM cells good knockdown of the individual isoforms was demonstrated, with the empty vector and scramble controls having no effect. Hsc70 and Hsp72 expression levels were reduced by more than 90% five days following lentiviral transduction in both cell lines using two shRNAs, apart from Hsc70 shRNA #1 in RPMI8226 cells, which achieved 50% knockdown of Hsc70 (Fig. 1a i&ii). The suppression of Hsp72 expression had no effect on the level of Hsc70 protein; whereas silencing of Hsc70 resulted in a significant compensatory induction of Hsp72 in both cell lines (Fig. 1a i&ii).
Fig. 1.
Silencing of Hsc70 increased paraprotein load and reduced total ubiquitination. (a) KMS11 and RPMI8226 human MM cells were transduced with shRNAs, empty vector (EC) or scramble controls (C). Western blot analysis was performed to determine the level of Hsp72 or Hsc70 protein. (b) (i) ELISA analysis of the level of IgK secretion after lentiviral transduction in KMS11 cells. (ii) ELISA assay of the level of IgK retention in KMS11 cells. (iii) ELISA analysis of Igλ secretion in RPMI8226 cells. (iv) ELISA assay of Igλ retention in RPMI8226 cells. Results represent the average readings taken from two shRNAs. Results are representative of three independent experiments, ∗p < 0.05. (c) Western blot analysis of the level of ubiquitination in protein samples collected from (i) KMS11 cells and (ii) RPMI8226 cells after lentiviral transduction. (d) Immunoprecipitation study on protein samples collected after transduction. Cell lysate was immunoprecipitated with (i) IgK in KMS11 and (ii) Igλ in RPMI8226 followed by western blot detection of Hsc70 and Hsp72.
Further, we performed ELISA assay on cells knocked down for Hsp72 or Hsc70, and measured the level of Ig light chain secretion and retention. The data were normalised to an equal cell number or protein level respectively. Interestingly, silencing of Hsc70 but not Hsp72 significantly reduced the level of Ig light chain secretion (Fig. 1b i&iii), with a consequent increase in the intracellular retention level (Fig. 1b ii&iv). The net result of this inhibition would be to increase the intracellular protein load, leading to increased cell stress and cell death.
Since only correctly folded Ig can be secreted from the cell, incompletely folded or misfolded Ig is either retained in the cell for further folding, or targeted for proteasomal degradation [22–24]. In this respect, Hsp70 isoforms are known to mediate the ubiquitination of their substrate proteins via interaction with their co-chaperone CHIP, an E3 ubiquitin ligase, thereby targeting them for proteasomal degradation [20,25]. To study the mechanism of Ig light chain retention in cells following Hsp70 silencing, we investigated whether Hsp70 isoforms were involved in proteasomal degradation in MM cells. Following Hsc70 but not Hsp72 silencing, total ubiquitination levels were decreased in both cell lines and in KMS11 dual silencing reduced ubiquitination levels further (Fig. 1c i&ii). This result suggests that Hsp70 isoforms are involved in proteasomal targeting and is consistent with the observed increase in Ig retention following Hsp70 silencing described above.
To determine if the Ig light chains physically interacted with Hsc70 or Hsp72, we immunoprecipitated IgK or Igλ, secreted by KMS11 and RPMI8226 cells respectively, followed by western blotting and staining for Hsp72 and Hsc70. We show that although intracellular Ig retention was only observed following Hsc70 silencing, Ig light chains interacted with both Hsp72 and Hsc70 (Fig. 1f), a result which suggests that both Hsp70 isoforms are involved in Ig homeostasis in MM, with Hsc70 playing the key role.
3.2. Dual silencing of Hsp72/Hsc70 enhances MM cell death compared to Hsc70 silencing alone
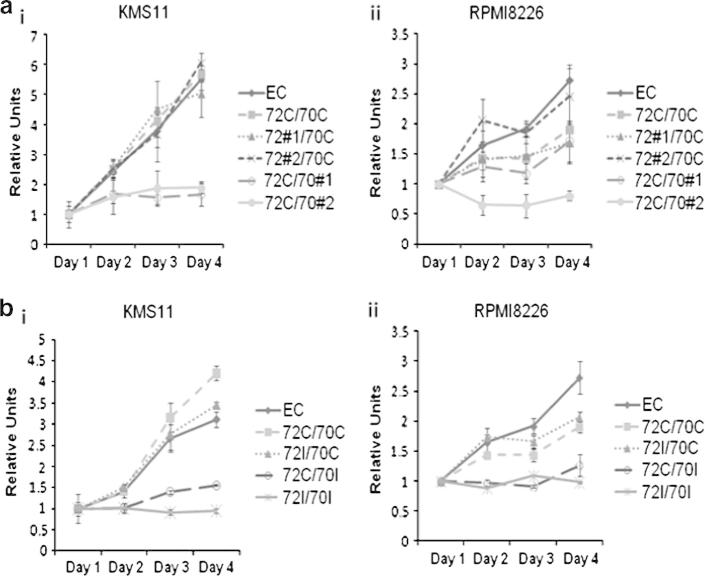
In order to determine if the intracellular accumulation of immunoglobulin and proteotoxic stress resulted in an alteration in myeloma cell growth and survival, we performed WST-1 assays on cells knocked down for Hsp72 or Hsc70 to measure the number of metabolically active cells. The results of these experiments show that the silencing of Hsc70 significantly reduced the number of cells over time compared to the controls, whilst silencing Hsp72 had no significant effect (Fig. 2a i&ii). Following Hsc70 silencing using two shRNAs (Fig. 2a ii), cell number was reduced by 80% in both cell lines despite a significant upregulation of Hsp72 (Fig. 1a). ShRNA #1 in RPMI8226 cells did not induce a significant cell number change in RPMI8226 cells and this is in line with the much lower knockdown level achieved with this particular shRNA (Fig. 1a ii). This decrease in metabolically active cells correlates with the increased level of Ig retention following Hsc70 silencing (Fig. 1b). Thus overall, our data suggests that Hsc70 plays an important role in Ig homeostasis that is critical to MM cell survival and that its function cannot be compensated for by Hsp72.
Fig. 2.
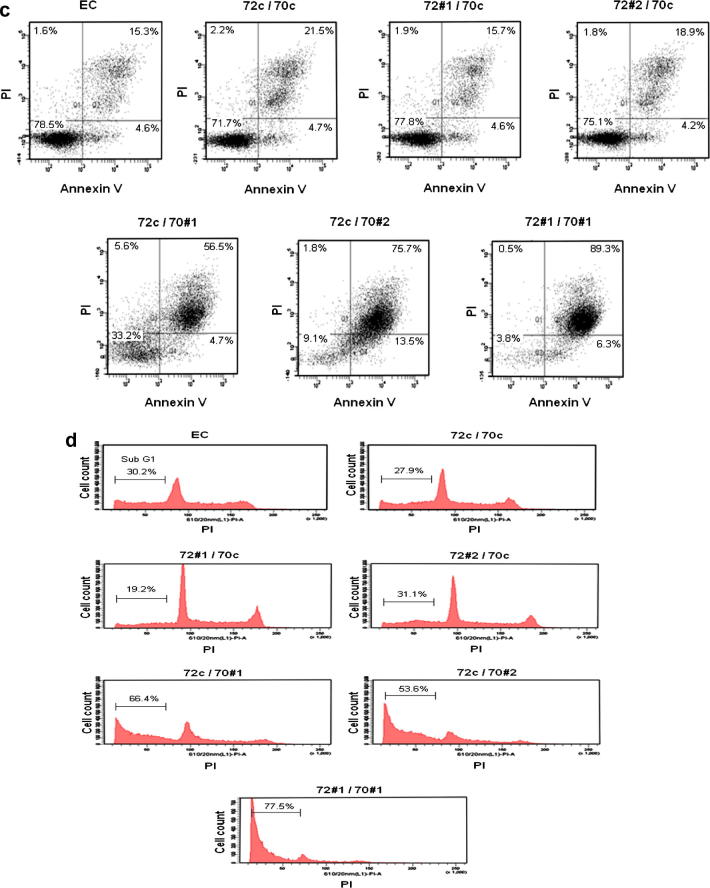
Lentiviral shRNA knockdown of Hsp72 and Hsc70 significantly reduced cell growth and induced apoptosis. (a) WST-1 assay to assess cell proliferation. (i) KMS11 cells and (ii) RPMI8226 cells. (b) WST-1 assay to assess cell proliferation following dual knockdown. Results represent the average readings taken from two shRNAs. Graph represents mean values of triplicate determinations. Error bars represent standard deviation. (c) Annexin V/PI double staining assay. The corresponding % live (AV−/PI−), apoptotic (AV+/PI−), dead (AV+/PI+) and necrotic (AV−/PI+) cells are shown in quadrants. (d) PI exclusion assay. The % cell population with sub G1 staining is shown on graph. All results shown are representative of three independent experiments.
We next studied the effect of dual silencing of Hsp72 and Hsc70 on proliferation and apoptosis using WST-1, Annexin V/PI staining and PI exclusion assays. The results confirm that silencing of Hsc70 but not Hsp72 significantly reduced cell numbers over time in KMS11 and RPMI8226 cells (Fig. 2b i&ii). In addition, combined knockdown of both Hsp72 and Hsc70 consistently reduced the survival of MM cells to a greater extent than single isoform silencing. Annexin V/PI double staining and PI exclusion assays show that the change in cell numbers is at least in part via apoptosis in KMS11 cells (Fig. 2c and d) and similar results were obtained in RPMI8226 cells (data not shown). Ten days after knockdown, most cells had died with dual silencing (Fig. 2c and d). These results show that while targeting Hsc70 alone is effective in killing MM cells, combined inhibition of both Hsp72 and Hsc70 is better.
3.3. Silencing Hsp72 and Hsc70 triggers multiple cellular responses that contribute to MM cell death
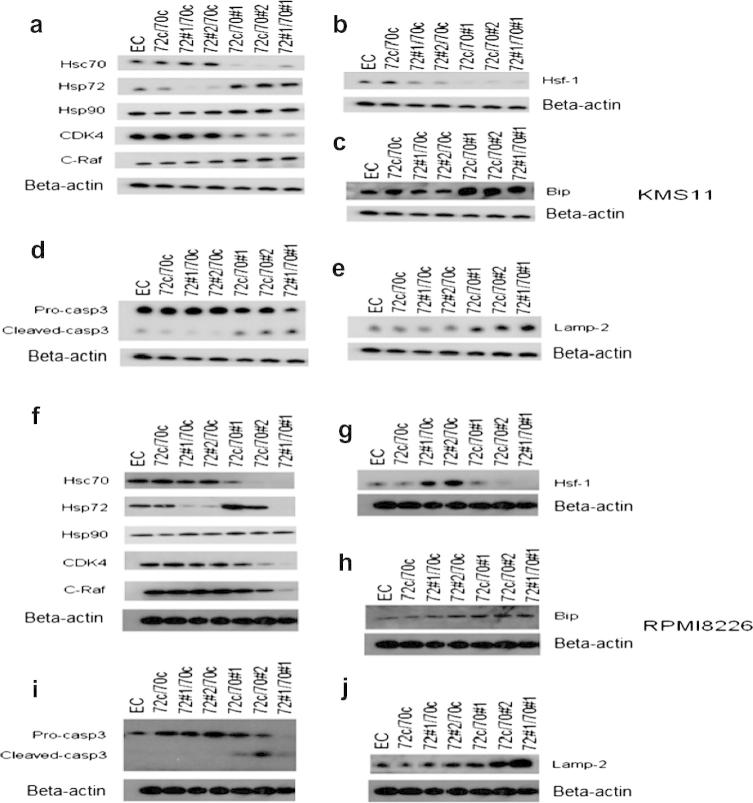
In addition to increasing intracellular Ig, based on their functional roles, we postulated that other mechanisms of Hsp70 may also be important in MM. To investigate the other potential roles of Hsp70, we studied the effect of Hsp72 and Hsc70 silencing on pathways known to be important for MM survival. As previously noted, knockdown of Hsp72 had no effect on the level of Hsc70, whereas silencing Hsc70 resulted in a significant upregulation of Hsp72 (Fig. 3a and f). The level of Hsp90 protein did not change following silencing of either Hsp72 or Hsc70 alone, or in combination (Fig. 3a and f). To understand the complex interactions of Hsp70 isoforms with Hsp90 chaperone activity, we examined the Hsp90 client proteins CDK4 and C-RAF after Hsp70 knockdown. The level of client proteins remained constant after single Hsp72 knockdown, whereas following single Hsc70 knockdown, CDK4 was depleted in both KMS11 and RPMI8226 cells and C-RAF was depleted in RPMI8226 cells. Dual knockdown further depleted CDK4 in both cell lines and C-RAF in RPMI8226 cells. These results confirm that both Hsp70 isoforms are actively involved in Hsp90-dependent chaperoning system, but in MM Hsc70 seems to play a more important role.
Fig. 3.
Silencing of Hsp72 and Hsc70 depleted Hsp90 client proteins, enhanced Caspase 3 cleavage and induced protein stress pathways. Western blot analysis was performed on lentivirally transduced KMS11 and RPMI8226 cells with Hsp72 and/or Hsc70. Beta-actin was used as a loading control.
Hsf-1 is the major heat shock transcription factor responsible for the transcriptional induction of a range of heat shock proteins during the stress response [26]. Therefore, we studied the effect of Hsp72 and Hsc70 knockdown on Hsf-1 expression. Interestingly, Hsf-1 expression was induced following Hsp72 knockdown in RPMI8226 cells (Fig. 3g) and reduced in both cell lines following Hsc70 knockdown and dual knockdown (Fig. 3b and g), whereas in KMS11 cells, following single Hsp72 knockdown, Hsf-1 expression is also reduced (Fig. 3b). To further control this experiment we also tested the cells with heat shock at 42 °C for 30 min, which resulted in induced level of Hsf-1 protein (data not shown). The biological differences in the two cell lines may account for the discrepancies observed, but based on these observations we conclude that 72/70 knockdown does not induce Hsf-1 at protein level.
In addition to Hsp72 and Hsc70, BIP/GRP78 is a critical ER isoform of Hsp70 which plays a role in ER protein folding and targeting misfolded proteins for proteasomal degradation as well as regulating the unfolded protein response (UPR) via IRE1, ATF6 and PERK [27]. We show that the level of BIP expression does not change following Hsp72 knockdown, whereas following Hsc70 knockdown it is upregulated (Fig. 3c and h). This upregulation of BIP may act as a compensatory mechanism increasing protein folding capacity and elevating pro-survival UPR signalling pathways following Hsp70 knockdown, and could in part explain the differential effects of Hsc70 and Hsp72 outlined above.
As the Hsp70 isoforms are known to play important anti-apoptotic roles, we next studied the effect of Hsp72 and Hsc70 knockdown on Caspase-3 cleavage, the main effector caspase involved in both the intrinsic and extrinsic apoptotic pathways. We show that the level of pro-Caspase 3 was reduced and that the level of cleaved-Caspase 3 increased following Hsc70 but not Hsp72 knockdown, suggesting that Hsc70 knockdown induced cell death, at least in part, was mediated via caspase dependent apoptosis. Simultaneous silencing of Hsc70 and Hsp72 further reduced pro-Caspase 3, consistent with the results of cell apoptosis assays (Fig. 3d and i).
Hsp70 is also known to assist in chaperone-mediated autophagy in mammalian cells [28,29], and it is known that autophagy plays a role in MM cell survival [30–32]. We, therefore, studied the impact of Hsp72 and Hsc70 knockdown on the level of the chaperone-mediated autophagy marker LAMP-2A. Following Hsp72 silencing, LAMP-2A levels were unchanged but following Hsc70 silencing it was significantly increased. Dual silencing of Hsp72 and Hsc70 further increased the LAMP-2A level (Fig. 3e and j). We suggest that the induction of LAMP-2A following Hsp70 knockdown may be a compensatory response to maintain chaperone-mediated autophagy activity, and thus promote cell survival.
Taken together in these experiments we show that Hsp70s, in particular Hsc70, are involved in multiple oncogenic pathways contributing to MM survival and targeting the Hsp70s for therapy could be useful clinically.
3.4. A small-molecule inhibitor of Hsp70 reduces the viability of MM cell lines and caused molecular level changes similar to the shRNA silencing of Hsp70 isoforms
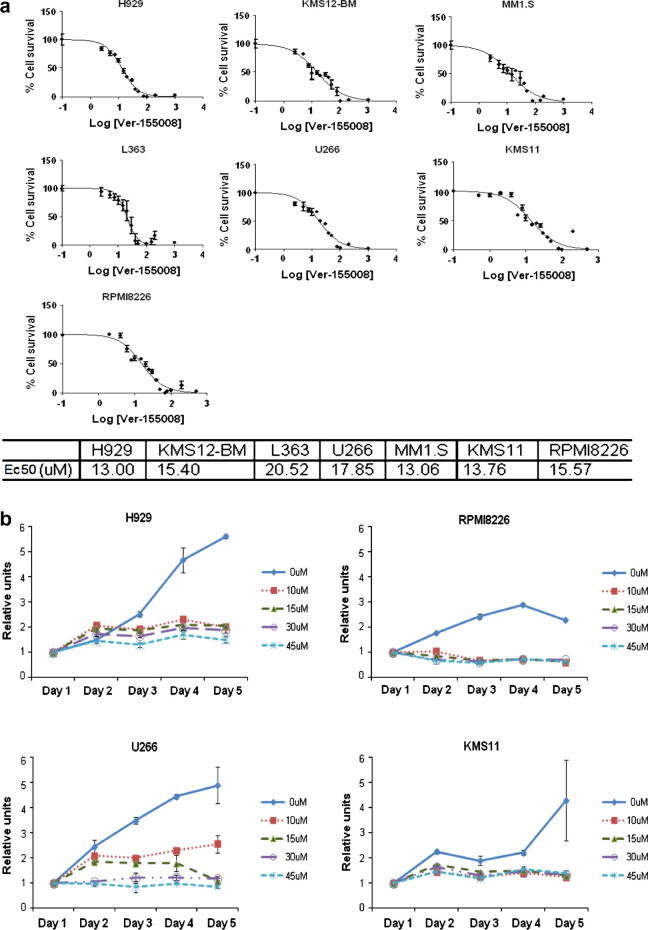
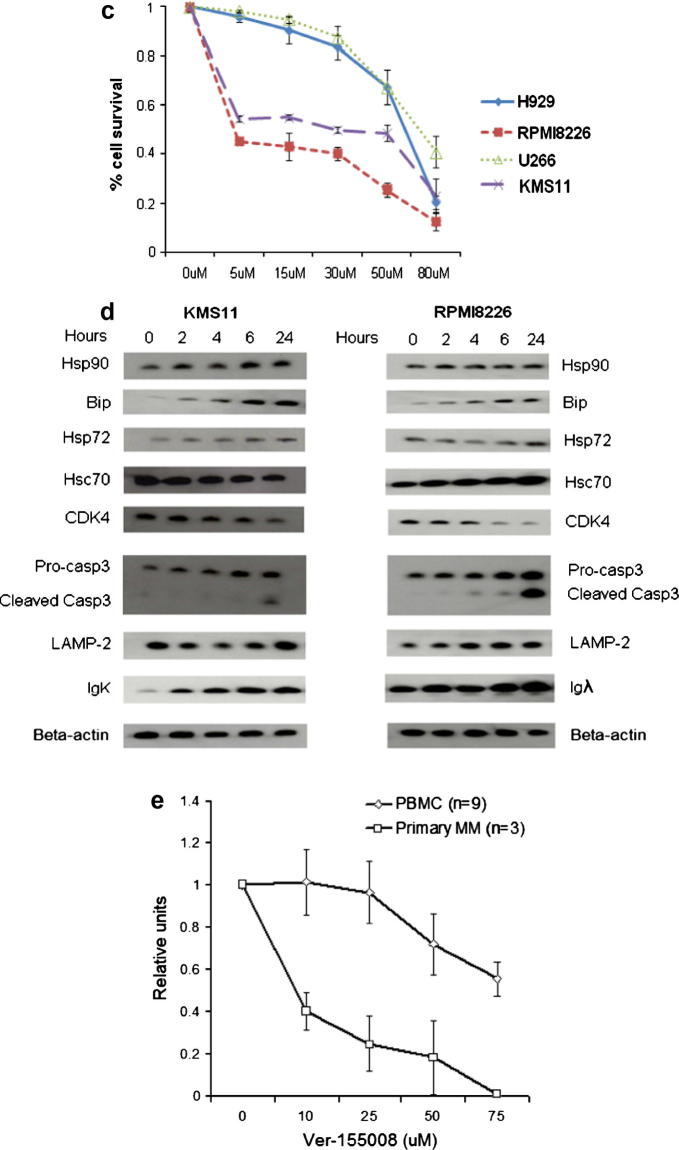
To validate our shRNA findings, we investigated the impact of the cell-permeable chemical inhibitor Ver-155008, which is known to inhibit both Hsp72 and Hsc70 [33], in a panel of MM cell lines. We showed in a WST-1 assay that all MM cell lines tested were sensitive to the inhibitor (Fig. 4a), with significant inhibition of cell number changes over time, even at the lowest concentration tested (10 μM) (Fig. 4b). There was however differential sensitivity, with U266 cells being more resistant in comparison to KMS11 and RPMI8226 cells (Fig. 4c). In addition, exposure to Ver-155008 for 24 h resulted in increased reactive oxygen species (ROS) production in KMS11 and RPMI8226 cells (Supplementary Fig. 1), consistent with the induction of apoptosis observed by Annexin V/PI double staining assay.
Fig. 4.
Small-molecule Hsp70 inhibitor strongly induced apoptosis and inhibited cell growth of MM cell lines. (a) EC50 curves of a panel of MM cell lines treated with Ver-155008 for 24 h as determined by WST-1 assay. (b) Dose- and time-dependent response of four human MM cell lines treated with Ver-155008. (c) Annexin V/PI staining assay for cell survival following 24-h treatment of four MM cell lines using Ver-155008. (a–c) Graphs represent mean values taken from triplicate determinations, error bars represents standard deviation. (d) Western blot analysis on KMS11 and RPMI8226 cells treated with 15 Ver-155008 for 24 h. (e) Dose dependent response of primary MM and normal peripheral blood mononuclear cells treated with Ver-155008 for 24 h as determined by WST-1 assay. Graph represents mean values taken from all cell samples with duplicate readings. Error bars represents standard deviation.
Treatment with Ver-155008 also resulted in Hsp90 client protein depletion, Caspase-3 cleavage, LAMP-2 and Ig protein retention (Fig. 4d). All of these molecular changes are consistent with those observed following shRNA knockdown. Thus small molecule inhibition of Hsc70 and Hsp72 is able to recapitulate the effects of specific shRNA knockdown especially reducing Hsp90 client oncogenic protein levels, altering chaperone-mediated autophagy, and deregulating protein homeostasis resulting in MM cell death.
We then went onto study the effect of chemical Hsp70 inhibition in primary MM and peripheral blood mononuclear cell (PBMC) cells which were used as a non-myeloma control. The primary CD138 + MM cells were significantly more sensitive to Hsp70 inhibition compared to the PBMC cells (Fig. 4e), providing further evidence that targeting Hsp70 is a good treatment strategy for MM. Moreover, we observed that the lethal effect of Hsp70 chemical inhibition on MM cells could not be rescued by co-culturing with bone marrow stromal cells (BMSC) (data not shown), suggesting that the bone marrow microenvironment cannot protect MM cells from hsp70 inhibition induced cell death. In addition, We also show that the combination treatment with Ver-155008 and 17-AAG for 24 h induced either additive or synergistic antiproliferative effects in KMS11 and RPMI8226 cells (Supplementary Fig. 2), supporting the use of hsp70/hsp90 double inhibition as an effective therapeutic strategy in MM.
4. Discussion
Myeloma remains an incurable malignancy and more effective biologically-based therapeutic strategies are required. In this study, we investigated the role of therapeutically targeting the main cytoplasmic Hsp70 isoforms. We hypothesized that myeloma cells are differentially sensitive compared to other cells within the body to proteotoxic stress because of their role in immunoglobulin synthesis. Further, their survival is supported by both the heat shock pathway and UPR because these molecular processes are essential for Ig folding and efficient protein degradation via the proteosome and autophagy, which both regulate intracellular proteotoxic stress maintaining cellular survival.
Targeting protein homeostasis via the inhibition of Hsp90 is effective in in vitro and in vivo models, but so far has been disappointing in clinical myeloma studies [11,20], possibly because of Hsp70 induction [34]. The Hsp70 isoforms have a range of functions including protein folding, protection from apoptosis and the control of protein degradation pathways. Hsp70s act as co-chaperones of the Hsp90 chaperone complex by presenting client proteins to Hsp90; recruiting E3 ubiquitin ligases such as CHIP to tag target proteins for proteasomal degradation [35]; participating in chaperone-mediated autophagy and inhibiting both the caspase-dependent and -independent apoptosis pathways at multiple levels [36]. All of these observations suggest that Hsp70 targeting would be an effective therapeutic strategy for myeloma.
In this study, we confirm and extend previous works showing the importance of Hsp70 family members in myeloma and other cancers [34,37]. Here, we show that silencing of Hsc70 alone significantly reduces the proliferation and increases apoptosis of MM cells. In contrast, silencing of Hsp72 alone did not have an impact on cell survival, whereas simultaneous silencing of both Hsp70 isoforms produced a more lethal effect. These results suggest that both Hsp70 isoforms have a role in MM cell survival. The inducible Hsp72 and constitutively active Hsc70 isoforms share 85% sequence homology, and are thought to have some compensatory roles [34]. However, the significant induction of Hsp72 protein following Hsc70 knockdown could not rescue the cell death phenotype, suggesting that Hsc70 may have specific functions in MM that cannot be recapitulated by Hsp72. The pro-survival importance of Hsp72 and Hsc70 has been shown to vary between different cancer types. For example, only simultaneous knockdown of both Hsp72 and Hsc70 was able to induce cell death in solid tumour cell lines [34], yet in the case of MM cell lines we were able to induce cell death by a single knockdown of Hsc70, consistent with Hsc70 playing a potentially more important role in MM survival.
One of the most interesting findings of this study is the accumulation of immunoglobulin in MM cells following Hsc70 silencing, which increased proteotoxic stress and cell death. The reduction in Ig secretion that mirrors intracellular retention suggests that the immunoglobulin increase following shRNA knockdown is caused by increased cellular accumulation instead of increased protein production. Since unfolded Ig is degraded via the ubiquitin proteasome pathway [38,39], we investigated the effect of Hsp70 knockdown on ubiquitination level. We show that Hsc70 knockdown reduced total ubiquitinated protein levels that were further reduced by dual Hsc70/Hsp72 knockdown. Since only correctly folded Ig can be secreted from cells, our observations suggest that the accumulation of Ig which follows Hsp70 knockdown is also accompanied by reduced clearance via proteasomal degradation. The net result of reduced secretion and increased cellular retention is to significantly increase the proteotoxic stress in MM cells, which triggers apoptosis. We also show that both Hsc70 and Hsp72 interact directly with Ig, suggesting that the Hsp70s have a role in regulating Ig stability and the two isoforms may play compensatory roles. However, since only Hsc70 silencing resulted in Ig accumulation, our results provide further evidence that the Hsc70 has an irreplaceable role in MM.
In addition to the effects on immunoglobulin, silencing of Hsp70 isoform functions also resulted in a number of other effects that may specifically promote MM cell death. Chaperone-mediated autophagy is a type of lysosomal degradation responsible for the clearance of a range of soluble cytosolic proteins bearing the KFERQ-related motif, which can be recognised by the Hsp70 chaperone complex [28,40]. Hsp70 delivers its target protein to the lysosome surface and presents it to the chaperone-mediated autophagy receptor LAMP-2A [41]. At this site, Hsp70 assists the unfolding of the substrate protein, enabling its translocation into the lysosomal lumen [29]. Autophagocytosis plays an important role in protein homeostasis and recent evidence suggests that macroautophagy regulates MM cell survival [30–32]. How chaperone-mediated autophagy contributes to MM survival is still unclear. The increase in LAMP-2A expression following Hsp70 knockdown suggests a compensatory mechanism to maintain chaperone-mediated autophagy for survival, since substrate loading to LAMP-2A is the rate-limiting step [42,43].
We also studied the roles of Hsp70 as a co-chaperone of Hsp90 in MM. Hsp90 is required in the activation and maturation of a range of client proteins, many of which are key transcription factors and kinases involved in cancer related signalling pathways [44]. It has been previously shown in solid tumour cells that Hsc70 acts as the primary co-chaperone for Hsp90; Hsp72, on the other hand, can compensate for the loss of Hsc70 and replace it in the Hsp90 chaperone complex [34]. We studied two client proteins of Hsp90, known to be involved in cell cycle control (CDK4) and proliferative signal transduction (C-RAF). Interestingly, Hsc70 silencing alone resulted in CDK4 depletion in MM cells and dual Hsc70/Hsp72 silencing induced further depletion, whereas C-RAF was only depleted in RPMI8226 cells following Hsc70 silencing. These results confirm the involvement of both Hsp70 isoforms in Hsp90 client protein maturation/degradation, contributing to MM cell survival. The varying responses of the different client proteins to Hsp70 isoform silencing suggests that Hsp90 client proteins may be differentially dependent on Hsp70 isoforms as co-chaperones. Hsc70 may act as the main co-chaperone for CDK4 in both cell lines. C-RAF, on the other hand, appears not to be dependent on these Hsp72 isoforms in KMS11 cells.
Both Hsp72 and Hsc70 have been known to directly interact with Hsf-1 [45,46], the master regulator of the heat shock response, responsible for the upregulation of a subset of inducible heat shock proteins [47]. Hsp72 negatively regulates Hsf-1 transcriptional activity, whereas Hsc70 is required for the activation of Hsf-1 [45,46]. We observed an upregulation of Hsf-1 following Hsp72 knockdown in RPMI8226 cells, which may be the heat shock response of RPMI8226 cells trying to re-balance heat shock protein levels following the loss of Hsp72. The downregulation of Hsf-1 we observed following Hsp70 knockdown and dual knockdown in both KMS11 and RPMI8226 cells suggests a negative feedback loop in the heat shock response, which may be a late-stage event after the induction of Hsp72. The downregulation of Hsf-1 following Hsp72 knockdown in KMS11 cells by an unknown mechanism is in contrast to RPMI8226 cells, and may reflect the biological difference between the myeloma cell lines. Overall, the downregulation of Hsf-1 may contribute to MM cell death following Hsp70 silencing, by weakening the ability of MM cells to cope with proteotoxic stress.
The observation of consistent biological outcomes following small-molecule inhibition or shRNA knockdown provides confidence in the biological effect of Hsp70 inhibition, and points to the involvement of particular functional mechanisms rather than scaffolding effects [48]. By using a chemical inhibitor acting on both Hsp72 and Hsc70 to block ATP binding and hydrolysis [33], we were able to validate and extend our shRNA data. We show that in a panel of MM cell lines the Hsp70 inhibitor was able to induce MM cell apoptosis with or without the protection of the bone marrow microenvironment, with associated changes in Hsp90 client proteins and chaperone-mediated autophagy. Moreover, we showed that primary MM cells were also killed in response to the Hsp70 inhibitor and that they are significantly more sensitive compared to normal peripheral blood mononuclear cells. These results suggest that there is a potential therapeutic window that could be exploited in the clinic [34].
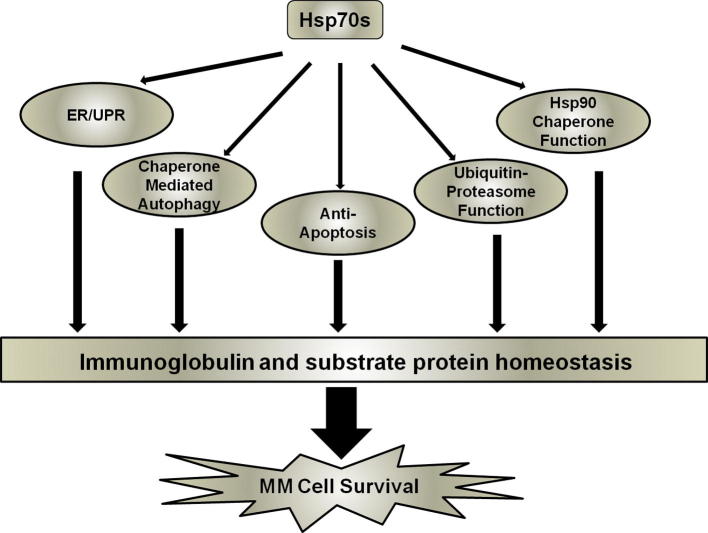
We have previously shown that the inhibition of Hsp72 enhances Hsp90 inhibitor-induced apoptosis in myeloma cells, confirming the potential of targeting Hsp70s in myeloma in combination with Hsp90 [49]. In this study, we were able to provide additional data supporting the use of Hsp70 inhibition in myeloma treatment, either alone or in combination with Hsp90 inhibition. We have also extended our work to show the different roles that Hsp72 and Hsc70 play in myeloma cells, and their involvement in the various molecular pathways known to be important to myeloma survival. We propose that the mechanism of effective cell killing observed following Hsp70 inhibition is the collective result of targeting multiple pathways, leading to the alteration in protein homeostasis - the key to myeloma cell survival (summarised in Fig. 5). The development of an effective Hsp70 inhibitor suitable for clinical use is, therefore, eagerly awaited.
Fig. 5.
Proposed mechanisms of Hsp70s contributing to MM cell survival. Our results demonstrate that the Hsp70 isoforms Hsc70 and Hsp72 play a variety of roles within the MM cell particularly with respect to protein homeostasis and the regulation of proteotoxic stress.
Funding
This work was supported by Cancer Research UK and Myeloma UK. F.E.D is a Cancer Research UK Senior Cancer Research Fellow (C20826/A12103). P.W. is a Cancer Research UK Life Fellow. We acknowledge NHS funding to the NIHR Biomedical Research Centre at the Royal Marsden Hospital.
Conflict of Interest
The authors are employees of The Institute of Cancer Research which has a commercial interest in the development of chaperone and stress pathway inhibitors. Work on molecular chaperones and stress pathway signaling in the Cancer Research UK Centre for Cancer Therapeutics has been funded by Vernalis and AstraZeneca and HSP90 inhibitors have been licensed to Vernalis and Novartis.
Contributions
L.Z., G.J.M. and F.E.D. designed the research, analysed data. L.Z., J.J.L.F., F.M., L.I.A., R.A.F. performed research and analysed data. L.Z., G.J.M., P.W., and F.E.D. wrote the paper.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.canlet.2013.07.023.
Appendix A. Supplementary material
Supplementary material contains Figs. S1 and S2.
References
- 1.Hartl F.U., Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295(5561):1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 2.Craig E.A. Chaperones: helpers along the pathways to protein folding. Science. 1993;260(5116):1902–1903. doi: 10.1126/science.8100364. [DOI] [PubMed] [Google Scholar]
- 3.Hartl F.U. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 4.Robert J. Evolution of heat shock protein and immunity. Dev. Comp. Immunol. 2003;27(6–7):449–464. doi: 10.1016/s0145-305x(02)00160-x. [DOI] [PubMed] [Google Scholar]
- 5.Travers J., Sharp S., Workman P. HSP90 inhibition: two-pronged exploitation of cancer dependencies. Drug Discov. Today. 2012;17(5–6):242–252. doi: 10.1016/j.drudis.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Powers M.V. Targeting HSP70: the second potentially druggable heat shock protein and molecular chaperone? Cell Cycle. 2010;9(8):1542–1550. doi: 10.4161/cc.9.8.11204. [DOI] [PubMed] [Google Scholar]
- 7.Trepel J. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer. 2010;10(8):537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A.R. Goloudina, O.N. Demidov, C. Garrido, Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett., vol. 325, no. 2, (2012) pp. 117–24. [DOI] [PubMed]
- 9.Voss A.K., Thomas T., Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127(1):1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Palumbo A., Anderson K. Multiple myeloma. N. Engl. J. Med. 2011;364(11):1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 11.Davenport E.L. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110(7):2641–2649. doi: 10.1182/blood-2006-11-053728. [DOI] [PubMed] [Google Scholar]
- 12.Clarke P.A. Gene expression profiling of human colon cancer cells following inhibition of signal transduction by 17-allylamino-17-demethoxygeldanamycin, an inhibitor of the hsp90 molecular chaperone. Oncogene. 2000;19(36):4125–4133. doi: 10.1038/sj.onc.1203753. [DOI] [PubMed] [Google Scholar]
- 13.Maloney A. Gene and protein expression profiling of human ovarian cancer cells treated with the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res. 2007;67(7):3239–3253. doi: 10.1158/0008-5472.CAN-06-2968. [DOI] [PubMed] [Google Scholar]
- 14.Dittmar K.D., Pratt W.B. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation. J. Biol. Chem. 1997;272(20):13047–13054. doi: 10.1074/jbc.272.20.13047. [DOI] [PubMed] [Google Scholar]
- 15.Arispe N., De Maio A. ATP and ADP modulate a cation channel formed by Hsc70 in acidic phospholipid membranes. J. Biol. Chem. 2000;275(40):30839–30843. doi: 10.1074/jbc.M005226200. [DOI] [PubMed] [Google Scholar]
- 16.Jiang R. Hsc70 chaperones clathrin and primes it to interact with vesicle membranes. J. Biol. Chem. 2000;275(12):8439–8447. doi: 10.1074/jbc.275.12.8439. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y. Heat shock protein 70 together with its co-chaperone CHIP inhibits TNF-alpha induced apoptosis by promoting proteasomal degradation of apoptosis signal-regulating kinase1. Apoptosis. 2010;15(7):822–833. doi: 10.1007/s10495-010-0495-7. [DOI] [PubMed] [Google Scholar]
- 18.Moore H.E. Aminopeptidase inhibition as a targeted treatment strategy in myeloma. Mol. Cancer Ther. 2009;8(4):762–770. doi: 10.1158/1535-7163.MCT-08-0735. [DOI] [PubMed] [Google Scholar]
- 19.Szulc J., Aebischer P. Conditional gene expression and knockdown using lentivirus vectors encoding shRNA. Meth. Mol. Biol. 2008;434:291–309. doi: 10.1007/978-1-60327-248-3_18. (Clifton, NJ) [DOI] [PubMed] [Google Scholar]
- 20.Obeng E.A. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meister S. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67(4):1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 22.Klausner R.D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- 23.Mancini R. Degradation of unassembled soluble Ig subunits by cytosolic proteasomes: evidence that retrotranslocation and degradation are coupled events. FASEB J. 2000;14(5):769–778. doi: 10.1096/fasebj.14.5.769. [DOI] [PubMed] [Google Scholar]
- 24.Fagioli C., Mezghrani A., Sitia R. Reduction of interchain disulfide bonds precedes the dislocation of Ig-mu chains from the endoplasmic reticulum to the cytosol for proteasomal degradation. J. Biol. Chem. 2001;276(44):40962–40967. doi: 10.1074/jbc.M107456200. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J. Biol. Chem. 2001;276(46):42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- 26.Pirkkala L., Nykanen P., Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15(7):1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 27.Davenport E.L., Morgan G.J., Davies F.E. Untangling the unfolded protein response. Cell Cycle. 2008;7(7):865–869. doi: 10.4161/cc.7.7.5615. [DOI] [PubMed] [Google Scholar]
- 28.Chiang H.L. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246(4928):382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 29.Agarraberes F.A., Terlecky S.R., Dice J.F. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J. Cell. Biol. 1997;137(4):825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoang B. Effect of autophagy on multiple myeloma cell viability. Mol. Cancer Ther. 2009;8(7):1974–1984. doi: 10.1158/1535-7163.MCT-08-1177. [DOI] [PubMed] [Google Scholar]
- 31.Aronson L.I., Davies F.E. DangER: protein ovERload. Targeting protein degradation to treat myeloma. Haematologica. 2012;97(8):1119–1130. doi: 10.3324/haematol.2012.064923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aronson L.I. Understanding the interplay between the proteasome pathway and autophagy in response to dual PI3K/mTOR inhibition in myeloma cells is essential for their effective clinical application. Leukemia. 2013 doi: 10.1038/leu.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massey A.J. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells. Cancer Chemother. Pharmacol. 2010;66(3):535–545. doi: 10.1007/s00280-009-1194-3. [DOI] [PubMed] [Google Scholar]
- 34.Powers M.V., Clarke P.A., Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14(3):250–262. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Park S.H. The cytoplasmic Hsp70 chaperone machinery subjects misfolded and endoplasmic reticulum import-incompetent proteins to degradation via the ubiquitin-proteasome system. Mol. Biol. Cell. 2007;18(1):153–165. doi: 10.1091/mbc.E06-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans C.G., Chang L., Gestwicki J.E. Heat shock protein 70 (hsp70) as an emerging drug target. J. Med. Chem. 2010;53(12):4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatterjee M. The PI3K/Akt signalling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica. 2012 doi: 10.3324/haematol.2012.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Hare T. Cutting edge: proteasome involvement in the degradation of unassembled Ig light chains. J. Immunol. 1999;163(1):11–14. [PubMed] [Google Scholar]
- 39.Ho S.C. Accelerated proteasomal degradation of membrane Ig heavy chains. J. Immunol. 2000;164(9):4713–4719. doi: 10.4049/jimmunol.164.9.4713. [DOI] [PubMed] [Google Scholar]
- 40.Dice J.F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem. Sci. 1990;15(8):305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 41.Cuervo A.M., Dice J.F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273(5274):501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 42.Majeski A.E., Dice J.F. Mechanisms of chaperone-mediated autophagy. Int. J. Biochem. Cell. Biol. 2004;36(12):2435–2444. doi: 10.1016/j.biocel.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Massey A., Kiffin R., Cuervo A.M. Pathophysiology of chaperone-mediated autophagy. Int. J. Biochem. Cell. Biol. 2004;36(12):2420–2434. doi: 10.1016/j.biocel.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Neckers L., Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin. Cancer Res. 2012;18(1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn S.G. Heat-shock cognate 70 is required for the activation of heat-shock factor 1 in mammalian cells. Biochem. J. 2005;392(Pt 1):145–152. doi: 10.1042/BJ20050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Y., Mosser D.D., Morimoto R.I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12(5):654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciocca D.R., Arrigo A.P., Calderwood S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch. Toxicol. 2013;87(1):19–48. doi: 10.1007/s00204-012-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Workman P., Collins I. Probing the probes: fitness factors for small molecule tools. Chem. Biol. 2010;17(6):561–577. doi: 10.1016/j.chembiol.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davenport E.L. Targeting heat shock protein 72 enhances Hsp90 inhibitor-induced apoptosis in myeloma. Leukemia. 2010;24(10):1804–1807. doi: 10.1038/leu.2010.168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material contains Figs. S1 and S2.