Abstract
Objective
The purpose of this study was to evaluate the extended efficacy, safety, and tolerability of escitalopram relative to placebo in adolescents with major depressive disorder (MDD).
Methods
Adolescents (12–17 years) who completed an 8-week randomized, double-blind, flexible-dose, placebo-controlled, lead-in study of escitalopram 10–20 mg versus placebo could enroll in a 16–24-week, multisite extension trial; patients maintained the same lead-in randomization (escitalopram or placebo) and dosage (escitalopram 10 or 20 mg/day, or placebo) during the extension. The primary efficacy was Children's Depression Rating Scale-Revised (CDRS-R) change from the lead-in study baseline to treatment week 24 (8-week lead-in study plus 16-week extension); the secondary efficacy was Clinical Global Impressions-Improvement (CGI-I) score at week 24. All efficacy analyses used the last observation carried forward (LOCF) approach; sensitivity analyses used observed cases (OC) and mixed-effects model for repeated measures (MMRM). Safety was evaluated via adverse event (AE) reports and the clinician-rated Columbia-Suicide Severity Rating Scale (C-SSRS).
Results
Following lead-in, 165 patients enrolled in the double-blind extension (82 placebo; 83 escitalopram); 40 (48.8%) placebo and 37 (44.6%) escitalopram patients completed treatment. CDRS-R total score improvement was significantly greater for escitalopram than for placebo (p=0.005, LOCF; p=0.014; MMRM). Response rates (CDRS-R ≥40% reduction from baseline [adjusted and unadjusted] and CGI-I ≤2) were significantly higher for escitalopram than for placebo (LOCF); remission rates (CDRS-R ≤28) were 50.6% for escitalopram and 35.7% for placebo (p=0.002). OC analyses were not significantly different between groups. The most frequent escitalopram AEs (≥5% and more frequent than placebo) were headache, nausea, insomnia, vomiting, influenza-like symptoms, diarrhea, and urinary tract infection. Most AEs were mild/moderate and not related to the study drug. AEs suggestive of self-harm occurred in 5.7% and 7.1% of placebo and escitalopram patients. Occurrence of suicidal behavior and/or suicidal ideation assessed by C-SSRS was 10.9% (14/128) for placebo and 14.5% (19/131) for escitalopram.
Conclusions
Extended use of escitalopram was generally safe and resulted in modest improvement in efficacy in adolescents with MDD.
Introduction
The concept of depressive disorders in children and adolescents is a relatively new phenomenon that dates back only to empirical studies conducted in the late 1980s (Birmaher et al. 1996). However, major depressive disorder (MDD) is a serious and common disorder that affects all age groups, and is accompanied by significant social and functional impairment. Approximately 8.5% of adolescents 12–17 years of age, an estimated 2,100,000 people, experienced at least one major depressive episode in the past year according to recently reported data (2004–2006). Almost half (48.3%) reported severe impairment in home life, school/work, family relationships, and/or social life, and 21% reported very severe impairment in at least one of these areas (National Survey on Drug Use and Health 2008). Suicide attempts and completion are among the most significant and catastrophic sequelae of MDD; over 7% of youths aged 12–17 years had thoughts about killing themselves, 3.6% made a plan to do so, and 2.9% made a suicide attempt during their worst or most recent major depressive episode (National Survey on Drug Use and Health 2005). In fact, the Treatment for Adolescents with Depression Study (TADS), a major study designed to evaluate the effectiveness of treatments for adolescents with MDD, reported that clinically significant suicidal thinking was present in 29% of participants at baseline (March et al. 2004, 2007).
Although most children and adolescents recover from a first depressive episode, longitudinal studies show that the probability of recurrence is 20–60% by 1–2 years after remission and 70% after 5 years (Birmaher et al. 2007). Recurrences can persist, and a substantial proportion of early-onset MDD continues into adulthood (Cheung et al. 2005). Longer-term consequences of MDD for adolescents include high risk of substance abuse; legal problems; physical illness; and poor work, academic, and psychosocial functioning (Birmaher et al. 2007). For these reasons, safe and effective long-term treatments for juvenile-onset MDD are needed.
Only limited prospective data on the efficacy of antidepressant treatment in adolescents exist, and results across trials are difficult to interpret because of variability in trial methodology, inclusion/exclusion criteria, study design, outcome measures, and high placebo response rates (Cheung et al. 2005). Fluoxetine (Masi et al. 2010), the most extensively studied selective serotonin reuptake inhibitor (SSRI), has been shown to be superior to treatment with placebo in both acute (Emslie et al. 1997, 2002) and maintenance therapy for pediatric MDD (Emslie et al. 2004, 2008).
In spite of the high prevalence and significant impairment associated with MDD in adolescents, pharmacologic treatment options are limited; only two agents have been approved by the United States Food and Drug Administration (FDA) for the treatment of MDD in patients under 18 years of age. In 2003, fluoxetine was the first SSRI approved for acute and maintenance treatment of MDD in patients 8–18 years of age. In 2009, escitalopram was approved for acute and maintenance treatment of MDD in adolescent patients 12–17 years of age; escitalopram is not approved for use in younger children.
The rationale to investigate escitalopram for the treatment of MDD in adolescents was based on results of evaluations of citalopram, the racemic parent compound. In an 8-week, flexible-dose, placebo-controlled study of MDD in children and adolescents (7–17 years of age), racemic citalopram 20–40 mg/day demonstrated significant improvement in depressive symptoms versus placebo within 1 week (Wagner et al. 2004). In subsequent clinical trials of citalopram (age range, 13–18 years) (von Knorring et al. 2006) and escitalopram (age range, 6–17 years) (Wagner et al. 2006), active treatment failed to separate from placebo. However, age-group adjusted post-hoc analyses of the escitalopram trial (Wagner et al. 2006) revealed significant differences in favor of escitalopram compared with placebo in the adolescent subgroup (age range, 12–17 years).
To further investigate the efficacy and tolerability of escitalopram in patients 12–17 years of age, an 8-week randomized, double-blind, placebo-controlled study was conducted (ClinicalTrials.gov Identifier NCT00107120). In this study, treatment with escitalopram was found to be associated with significantly greater improvement on the primary outcome measure than was placebo (Emslie et al. 2009). These positive results, as well as extrapolation from the positive citalopram study in children and adolescents, were used to establish the indication for acute and maintenance treatment of MDD for escitalopram (Lexapro®) in adolescents 12–17 years of age.
The frequently chronic and recurrent nature of adolescent MDD requires that antidepressant treatments ameliorate acute symptomatology, maintain efficacy over time, and provide good safety and tolerability. To investigate the extended effects of escitalopram in an adolescent population, a 16-week, randomized, controlled, continuation study was conducted to further the tolerability and efficacy findings of the positive, 8-week acute clinical trial previously reported (Emslie et al. 2009). Of particular interest was that methodology to prospectively evaluate suicidality in this highly vulnerable patient population was employed for the first time in an industry-sponsored, multisite trial. Results from this combined 24-week efficacy and tolerability data set helped to elucidate the effects of extended escitalopram treatment in adolescents with moderately severe MDD.
Methods
This study (ClinicalTrials.gov Identifier: NCT00107120) was designed to evaluate the extended efficacy and tolerability of escitalopram relative to placebo in adolescent patients (12–17 years of age) with MDD. The study was conducted as a 16–24-week, double-blind extension of an 8-week randomized, double-blind, placebo-controlled trial that evaluated the acute efficacy and tolerability of escitalopram 10–20 mg/day in adolescent patients with MDD. Detailed methods, inclusion and exclusion criteria, and results of the 8-week study have been previously reported (Emslie et al. 2009).
Prospectively defined study design
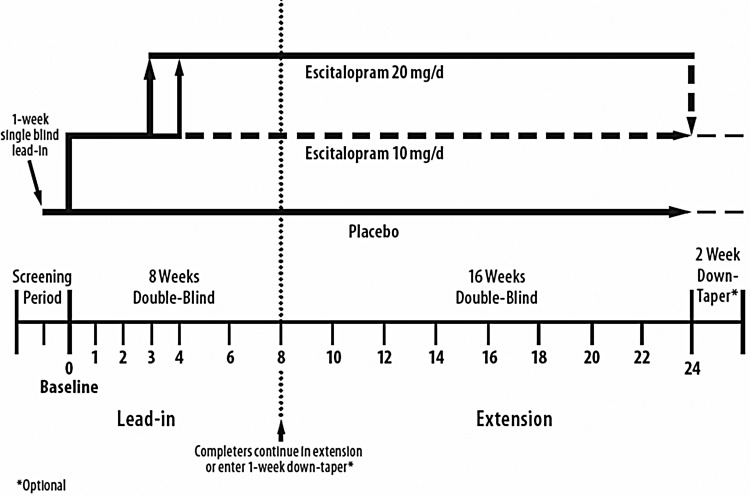
Adolescent patients who completed the 8-week, double blind, flexible-dose, placebo-controlled study (lead-in study) were eligible to enroll in this extension study regardless of response in the lead-in study. This extension study was initially designed as a 24-week open-label continuation of escitalopram treatment. It was subsequently modified to 24-weeks of additional double-blind treatment, to allow for extended evaluation of efficacy and tolerability using a placebo-control group. However, given concerns regarding placebo for longer-term use in adolescents with depression, the duration of the extension study was ultimately reduced by 8-weeks and patients received 16-weeks of double-blind treatment in the extension study. The design of the study following adoption of the final protocol amendment is shown in Figure 1.
FIG. 1.
Final study design with 16-week extension period.
During double-blind extension treatment, patients maintained the same lead-in randomization assignment (escitalopram or placebo) and continued at the same dose (escitalopram 10 or 20 mg/day, or placebo) as they had been receiving at the end of the lead-in study. No dose adjustments were allowed during the course of double-blind continuation treatment. Upon study completion or premature discontinuation from the extension study, patients were eligible to enter a 2-week double-blind taper-down period. Patients who discontinued for insufficient therapeutic response were eligible to receive up to 6 months of aftercare, provided at the discretion of the investigator. Treatment codes for both the lead-in and the extension studies were unblinded only after the last patient had completed the extension study, and both databases were locked.
Eligibility criteria (required at lead-in study entry)
To be included in the study, male or female outpatients 12–17 years of age were required to meet the Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text Revision (DSM-IV-TR) (American Psychiatric Association, 2000) criteria for MDD, with an ongoing MDD episode of at least 12 weeks' duration. Additionally, participants were required to have a Children's Depression Rating Scale, Revised (CDRS-R) (Poznanski and Mokros 1996) score ≥45 at initial screening and baseline, and a Clinical Global Impressions-Severity (CGI-S) (Guy 1976) score of at least 4 (i.e., moderately ill) at baseline. The diagnosis of MDD was established by two independent clinicians using the Kiddie Schedule for Affective Disorders and Schizophrenia–Present and Lifetime (K-SADS-PL) (Kaufman et al. 1997).
Patients meeting DSM-IV criteria for an Axis I disorder, other than MDD, were not eligible for inclusion in the trial. Patients who were considered to be a suicide risk or who had made a suicide attempt were excluded. Concurrent medical conditions that might interfere with the conduct of the study, concomitant treatment with a prohibited medication, being a female of childbearing potential who was not practicing a reliable method of birth control, or a positive test for alcohol were reasons for exclusion. Patients who planned to initiate or change ongoing psychotherapy or behavioral therapy during the course of the study were also excluded.
Patients completing the lead-in study provided assent to continue participation, and a parent or legal guardian provided written informed consent before any study-specific procedures were conducted. Patients were required to have normal physical examination, clinical laboratory test, and electrocardiography (ECG) results at the last visit of the lead-in study. Female patients of childbearing potential were required to have a negative serum β-human chorionic gonadotropin pregnancy test at study entry. All patients were required to have a parent or primary caregiver who was capable of providing information about the patient's condition, and who agreed to accompany the patient to all study visits. Additional requirement included family support that was sufficiently organized and stable to guarantee adequate safety monitoring.
Efficacy assessments
Efficacy measures included the CDRS-R, Clinical Global Impressions-Improvement (CGI-I) Scale (Guy 1976) CGI-S, and the Children's Global Assessment Scale (CGAS) (Shaffer et al. 1983). The clinician-rated CDRS-R was administered separately to the patient and the identified parent or primary caregiver at lead-in study screening, baseline, and weeks 2, 4, 6, 8 (end of lead-in study/beginning of extension study), 12, 16, and 24 (end of extension study). The CGI-I was administered at weeks 1 through 8, 12, 16, 20, and 24. The CGI-S was administered at baseline and weeks 1 through 8, 12, 16, 20, and 24. CGAS was administered at baseline and weeks 4, 8, 12, 16, and 24. All efficacy assessments were administered upon early termination from the study.
Safety assessments
Safety assessments were conducted at various visits throughout the lead-in and extension trials, and included vital sign measurement, adverse event (AE) reports, clinical laboratory determinations, and monitoring of concomitant medications. Body weight was measured at screening, baseline, and weeks 1 through 8, 10, 12, 16, 20, 24, and end of taper-down. Blood pressure and pulse rate were measured during the same visits and were recorded after the patients had been sitting for 5 minutes, and 1 minute after the patients had been standing. Height was recorded at screening and at weeks 8 and 24. Laboratory and ECG assessments were made at screening, week 1 (ECG only), and weeks 6, 20, and 24. All safety assessments were also performed at early termination.
Ongoing AEs that were present at the final visit of the lead-in study (week 8) were carried forward and assessed at week 10 (extension phase). At the week 10 visit and each subsequent visit, patients were asked to respond to nonleading/open-ended questions to elicit information about AEs; answers were recorded on the patient's case report form. The relationship of an AE to study medication and an assessment of severity (mild, moderate, or severe) were provided by the investigator. AEs were assessed by the investigator to determine the suggestion of self-harm; all AEs considered to be suggestive of self-harm were further categorized as suicide attempt, suicidal ideation, self-injurious behavior (nonsuicidal), accidental overdose, or other.
The clinician-rated Columbia-Suicide Severity Rating Scale (C-SSRS) (Posner et al. 2011) was administered at screening and baseline and during all study visits, weeks 1–24. The C-SSRS rates the severity of suicidal behavior and ideation from the least to the most serious (0–5 points). The classification of suicidal behavior ranges from nonsuicidal to multiple attempts; suicidal ideation ranges from nonsuicidal to active suicidal ideation with plan and intent. For each patient individually, the modal (most common) and the most severe type of suicidal ideation since the last assessment were recorded.
The Suicide Ideation Questionnaire-Junior High School version (SIQ-JR) (Reynolds 1987), a patient-rated scale that identifies thoughts and cognitions about suicide, was administered at screening, baseline, and weeks 1, 4, 8, 12, 16, and 24. The C-SSRS and the SIQ-JR were also administered to participants at the end of the down-titration period or at early termination.
Potentially clinically significant (PCS) changes in postbaseline vital sign values were defined, and assessed for the combined double-blind treatment period relative to baseline. Assessments and PCS criteria included standing or sitting systolic blood pressure, standing or sitting diastolic blood pressure, standing or sitting pulse, ECG parameters, and weight. The effect of escitalopram on growth was evaluated using Z-scores (the number of standard deviations a patient is from the age/sex standardized mean); a negative change in the Z-score indicates that the observed growth is less than the normally expected growth.
Data analyses
All efficacy and tolerability analyses are based on the combined double-blind periods from the lead-in study and double-blind extension study. The combined double-blind period for efficacy analyses was limited to 24-weeks (8-week lead-in plus 16-week double-blind extension); patients receiving open-label escitalopram were not included in the analyses. For tolerability analyses, the combined double-blind period was 24–32 weeks (8-week lead-in plus 16–24-week double-blind extension). Tolerability data for patients with open-label escitalopram were also included.
The safety population comprised all subjects who received at least one dose of randomized double-blind study medication in the lead-in study. The intent-to-treat (ITT) population included all patients in the safety population who had at least one postbaseline CDRS-R assessment in the lead-in study. Baseline of the lead-in study was used as the baseline for all analyses for this extension study.
Patients who received open-label escitalopram during the extension study are summarized separately.
The primary outcome measure was CDRS-R change from baseline (week 0) of the lead-in study to treatment week 24 (8-weeks in lead-in plus 16-weeks in extension). An analysis of covariance (ANCOVA) model, with treatment group and study center as factors and baseline CDRS-R total score as covariate, was used for between-treatment group comparison.
The secondary efficacy measure was the CGI-I score at week 24. Between-treatment group comparison was performed using an analysis of covariance (ANCOVA) model, with treatment group and study center as factors, and CGI-S score at baseline as covariate. Additional per protocol efficacy parameters included CDRS-R response rate (≥40% reduction from baseline), CGI-I response rate (CGI-I ≤2, “very much improved/much improved”), and CDRS-R remission rate (CDRS-R ≤28, indicating minimal symptoms). A post-hoc analysis of response rates was performed using CDRS-R total score adjusted for the 17 point minimum CDRS-R score; adjusted CDRS-R response was defined as at least 50% reduction from CDRS-R baseline minus 17.
All efficacy analyses were conducted using the last observation carried forward (LOCF) approach, in which the last observed value before a missing postbaseline value is carried forward to impute the missing postbaseline value. Sensitivity analyses were conducted using the observed cases (OC) approach, in which only reported values are used with no imputation of missing values, and the mixed-effects model for repeated measures (MMRM), which estimates missing values based on observed postbaseline longitudinal data. Between-group comparisons for response and remission rates were performed by using the logistic regression model, with treatment group and CDRS-R baseline score as explanatory variables. Statistical tests were two sided, with a significance level of α=0.05.
Tolerability analysis was based on the safety population; baseline of the lead-in study was used as the baseline for all safety analyses. Data were summarized for the entire 24-week double-blind treatment period. For all safety parameters, only descriptive statistics or frequency distributions were used; no formal statistical analyses were conducted.
The number and percentage of patients who had treatment-emergent AEs (TEAEs) were tabulated by treatment group for the combined double-blind treatment periods. Descriptive statistics for clinical laboratory values, ECG parameters, and vital signs during the combined double-blind treatment period were summarized; only patients with a screening assessment and at least one postbaseline assessment were included. The number and percentage of patients who had a worsening from baseline in the suicidal behavior or suicidal ideation scores on the C-SSRS at any time during the study were tabulated.
Study conduct
The lead-in study was conducted from April 1, 2005 to May 31, 2007. The extension study was conducted from June 16, 2005 to September 24, 2007. Approximately 40 sites participated in the lead-in and/or the extension study. The studies were conducted in compliance with International Conference on Harmonisation (ICH)-E6 Good Clinical Practice. Institutional review board approval was obtained for each study site and each protocol amendment.
Results
Patient disposition
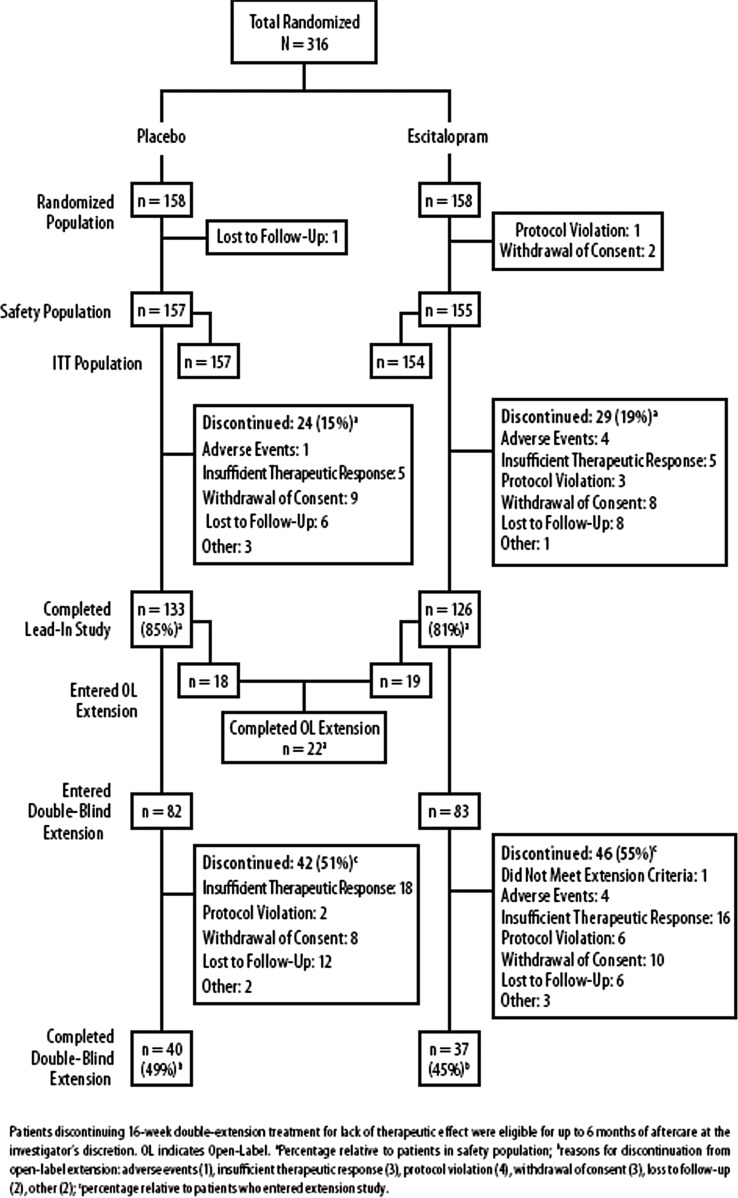
A total of 584 patients were screened for the lead-in study; 316 were randomized to receive double-blind treatment. Of the randomized patients, 157 placebo- and 155 escitalopram-treated patients received at least one dose of double-blind study medication. The lead-in study was completed by 133 (84.7%) placebo patients and 126 (81.3%) escitalopram patients (Emslie et al. 2009) (Fig. 2).
FIG. 2.
Patient disposition.
A total of 202 patients enrolled in the extension study. Thirty-seven patients enrolled prior to the protocol amendment that changed the study design to double-blind treatment, and they received open-label escitalopram. Open-label escitalopram treatment was completed by 22 (59.5%) patients; the overall mean dosage was 13.7 mg/day. A total of 165 patients enrolled in the double-blind extension study; 82 patients received placebo and 83 patients received escitalopram. Of these patients, 40 (48.8%) placebo- and 37 (44.6%) escitalopram-treated patients completed treatment (Fig. 2).
Demographic and clinical characteristics
As previously reported (Emslie et al. 2009), the treatment groups in the lead-in study were comparable in regard to demographic and clinical characteristics; baseline CDRS-R and CGI-S scores were statistically higher in the escitalopram group than in the placebo group, but the difference was not clinically meaningful; imbalances were adjusted in analyses in which the respective baseline CDRS-R total score and CGI-S score were included in the ANCOVA model as covariates. Baseline patient characteristics are presented in Table 1.
Table 1.
Baseline Characteristics (Safety Population)
| Placebo (n=157) | Escitalopram (n=155) | |
|---|---|---|
| Age, years, mean±SD | 14.52±1.48 | 14.74±1.64 |
| Female, n (%) | 92 (58.6) | 92 (59.4) |
| Race, n (%) | ||
| White | 123 (78.3) | 113 (72.9) |
| Black | 24 (15.3) | 30 (19.4) |
| Asian | 0 | 3 (1.9) |
| Other | 10 (6.4) | 9 (5.8) |
| Mean age of onset, years±SD | 12.29±2.5 | 12.36±2.6 |
| Mean duration of current MDD episode, months±SD | 16.46±15.4 | 15.68±17.4 |
| Patients receiving psychosocial treatment, n (%)a | 25 (15.9) | 24 (15.4) |
Includes individual psychotherapy, group therapy, or behavioral therapy.
Double-blind escitalopram treatment
The extent of exposure to placebo and escitalopram during double-blind treatment is presented in Table 2. A total of 71 patients (13 open-label patients and 58 double-blind patients [placebo=30; escitalopram=28]) received treatment for >168 days (24-weeks). The combined double-blind period for efficacy analyses was limited to 24-weeks, which comprised 8-weeks of double-blind treatment in the lead-in study plus 16-weeks of double-blind treatment in the extension study.
Table 2.
Extent of Double-Blind Exposure (Safety Population)
| Placebo (n=157) | Escitalopram (n=155) | |
|---|---|---|
| Treatment duration, days, mean±SDa | 100.23±66.70 | 96.62±64.13 |
| Overall mean dosage in mg/day, mean±SD | — | 14.04±3.5 |
| Overall mean dosage in tablets/day, mean±SD | 1.44±0.32 | 1.40±0.35 |
| Patient-yearsb | 43.08 | 41.00 |
Treatment duration=last date of double-blind study drug before taper- down–first date of double-blind study drug+1.
Patient-years=total treatment duration in days/365.25.
Efficacy
Primary (CDRS-R change from lead-in study baseline to week 24), secondary (CGI-I score at week 24), and additional efficacy analyses are presented in Table 3.
Table 3.
Efficacy Analyses at Week 24 (ITT Population)
| |
Placebo |
Escitalopram |
||
|---|---|---|---|---|
| n | Value | n | Value | |
| Primary efficacy: CDRS-R | ||||
| Baseline score mean±SEM | 157 | 56.0±0.7 | 154 | 57.6±0.7 |
| Change from baseline at week 24, LS mean±SE (LOCF) | 157 | −18.7±1.4 | 154 | −23.1±1.3 |
| Change from baseline at week 24, LS mean±SE (MMRM) | 153 | −24.1±1.4 | 154 | −28.9±1.4 |
| Change from baseline at week 24, LS mean±SE (OC) | 40 | −28.9±1.6 | 39 | −30.4±1.5 |
| Secondary efficacy: CGI-I score | ||||
| Score at week 24, mean±SEM (LOCF) | 157 | 2.5±0.1 | 154 | 2.2±0.1* |
| Score at week 24, mean±SEM (OC) | 40 | 1.5±0.2 | 39 | 1.7±0.1 |
| Additional efficacy measures | ||||
| CGI-S | ||||
| Baseline score mean±SEM | 157 | 4.4±0.0 | 154 | 4.6±0.1 |
| Change from baseline at week 24, LS mean±SE (LOCF) | 157 | −1.4±0.1 | 154 | −1.8±0.1** |
| Change from baseline at week 24, LS mean±SE (OC) | 40 | −2.5±0.2 | 39 | −2.5±0.2 |
| CGAS | ||||
| Baseline score mean±SEM | 157 | 51.9±0.4 | 154 | 51.9±0.5 |
| Change from baseline at week 24, LS mean±SE (LOCF) | 152 | 11.7±1.3 | 149 | 15.3±1.2* |
| Change from baseline at Week 24, LS mean±SE (OC) | 40 | 18.7±2.12 | 39 | 23.7±2.0 |
p<0.05; **p<0.01 (based on analysis of covariance [ANCOVA] model).
ITT, intent-to-treat; CDRS-R, Children's Depression Rating Scale-Revised; CGI-I, Clinical Global Impressions-Improvement; CGI-S, CGI-Severity; CGAS, Children's Global Assessment Scale; SE, standard error of least squares [LS] mean; SEM, standard error of the mean; LOCF, last observation carried forward; MMRM, mixed-effects model for repeated measures; OC, observed cases.
Primary efficacy
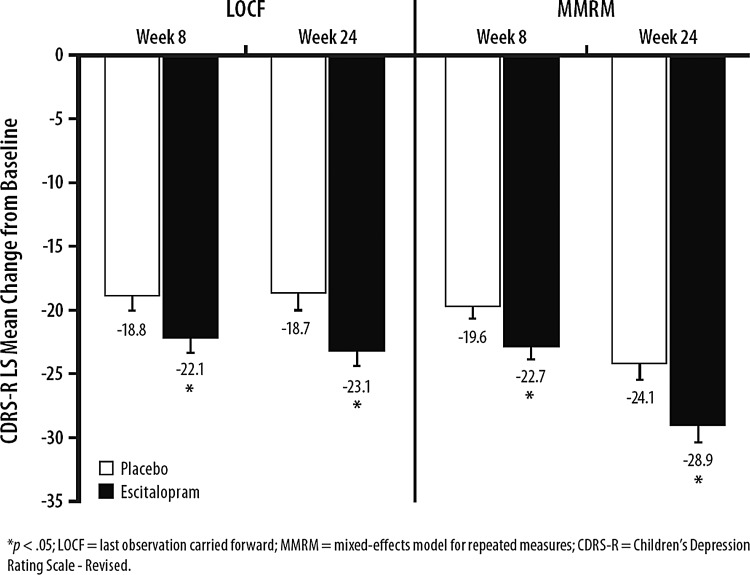
The primary efficacy parameter was change from lead-in study baseline (prior to administration of double-blind medication) to week 24 in CDRS-R total score. At the end of the 24-week double-blind treatment period, the mean (SD) CDRS-R total scores for patients in the escitalopram- and placebo-treatment groups were 34.4 (15.2) and 37.8 (14.9); respectively. Escitalopram-treated patients had significantly greater CDRS-R total score improvement than did placebo-treated patients (least squares mean difference [LSMD]=−4.5; p=0.005; LOCF); the difference in CDRS-R total score was also significant for escitalopram using the MMRM (LSMD=−4.8; p=0.014) (Fig. 3, Table 3), but not the OC (LSMD=−1.5; p=0.502, Table 3), approach. For the patients who received open-label escitalopram and had at least one post baseline CDRS-R assessment, the mean change in CDRS-R total score from baseline to 24-week end-point was −26.0.
FIG. 3.
Children's Depression Rating Scale-Revised (CDRS-R) total score change from baseline to week 24 (intent-to-treat [ITT] population).
To investigate if the decision to enter the extension study introduced a potential bias against detecting a between-group difference, CDRS-R total score change at week 8 was analyzed in relation to whether patients did or did not enter the double-blind extension. For escitalopram-treated patients, change from baseline to week 8 in CDRS-R total score was similar regardless of whether they entered or did not enter the double-blind extension (Table 4). CDRS-R change for patients in the placebo group was greater for patients who entered the double-blind extension than for those who did not.
Table 4.
Change from Baseline to Week 8 in CDRS-R Total Score for Patients Who Entered or Did Not Enter Double-Blind Extension
| |
|
Placebo |
Escitalopram |
||
|---|---|---|---|---|---|
| n | Mean±SEM | n | Mean±SEM | ||
| Entered double-blind extension (n=164) | Baseline | 82 | 55.6±0.8 | 82 | 57.2±0.9 |
| Change at week 8 | 82 | −22.7±1.2 | 82 | −22.8±1.6 | |
| Did not enter double-blind extension (n=57) | Baseline | 33 | 55.0±1.4 | 24 | 59.1±1.7 |
| Change at week 8 | 33 | −15.9±2.5 | 24 | −24.9±2.6 | |
CDRS-R, Children's Depression Rating Scale-Revised.
Outcomes for patients who responded to lead-in treatment were further explored in post-hoc analyses. In the subset of patients who were responders (≥40% reduction in the CDRS-R) at end of week 8, continued improvement was seen in patients in the escitalopram group, but not in the placebo group (LOCF and OC) (Table 5).
Table 5.
Change in CDRS-R Scores for Responders Entering Extensiona
| |
Placebo n=52 |
Escitalopram n=49 |
||
|---|---|---|---|---|
| LOCF | Baseline | Change | Baseline | Change |
| Week 8 | 55.3 | −29.2 | 58.3 | −31.9 |
| Week 24 | −26.0 | −33.1 | ||
| Week 8 to week 24 | 3.2 | −1.1 | ||
| |
Placebo n=28 |
Escitalopram n=29 |
||
|---|---|---|---|---|
| OC | Baseline | Change | Baseline | Change |
| Week 8 | 55.6 | −29.8 | 56.7 | −30.9 |
| Week 24 | −29.3 | −33.6 | ||
| Week 8 to week 24 | 0.5 | −2.7 | ||
Restricted to patients who were responders at week 8, and who had at least one CDRS-R assessment during the double-blind extension phase.
CDRS-R, Children's Depression Rating Scale-Revised; LOCF, last observation carried forward; OC, observed cases.
Secondary and additional efficacy
At the end of 24-weeks of double-blind treatment, patients receiving escitalopram compared with placebo-treated patients showed statistically greater CGI-I score improvement when the LOCF approach was used for analysis (LSMD=−0.4; p=0.003); no significant difference between treatment groups was observed using the OC approach (LSMD=−0.1; p=0.555). Significant improvement was also seen for the escitalopram group compared with the placebo group on CGI-S and CGAS scores (LOCF).
Response rates
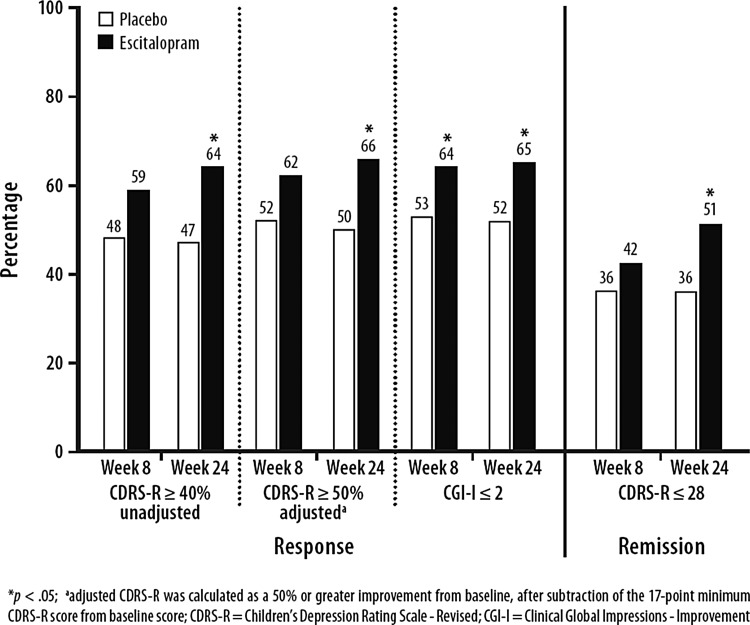
Response rates for unadjusted CDRS-R (≥40% reduction from baseline in total score) and CGI-I (CGI-I ≤2) at week 24 were significantly higher for the escitalopram group than for the placebo group (LOCF), and post-hoc analysis of CDRS-R response adjusted for the 17 point minimum CDRS-R score (≥50% reduction from baseline) was also significant. Response based on unadjusted CDRS-R total score (at least 40% reduction from baseline) was 63.6% for escitalopram patients and 47.1% for placebo patients (p=0.003); response using the adjusted CDRS-R total score (at least 50% reduction in CDRS-R total score adjusted for baseline [baseline −17]) was 65.6% for escitalopram patients and 50.3% for placebo patients (p=0.006). Response based on a CGI-I score ≤2 was 64.9% for escitalopram patients and 51.6% for placebo patients (p=0.006) (Fig. 4). Remission rates (CDRS-R ≤28) were also significantly higher for escitalopram than for placebo (LOCF). OC analyses of response and remission rates were not significantly different between treatment groups at week 24.
FIG. 4.
Response and remission rates at week 24 (last observation carried forward [LOCF], intent-to-treat [ITT] population).
Safety and tolerability
AEs
An overall summary of AEs during the 8-week lead-in trial, the open-label and double-blind 16–24-week extension periods, and combined lead-in and extension double-blind treatment periods (24–32 weeks) is presented in Table 6. No deaths occurred during the study. TEAEs were common in both groups of patients during both the acute and extension trials. During the combined double-blind treatment period, nine patients discontinued treatment prematurely because of an AE (one placebo; eight escitalopram; p<0.05); four escitalopram patients discontinued during the extension study. Failure to thrive (i.e., patient with severe weight loss), also reported as a serious AE (SAE), inflicted injury (superficial cutting on the arm that was categorized as suggestive of self-harm), fatigue, and insomnia were the AEs that led to discontinuation of escitalopram treatment during the extension trial (Table 7). Additional AE information for the 37 patients who continued the study under the open-label escitalopram protocol is included in Tables 6 and 7.
Table 6.
Summary of Patients with Adverse Events (Safety Population)
| |
Lead-in study (8-weeks) |
Extension study (16–24-weeks) |
Combined double-blind treatment (24–32 weeks)a |
||||
|---|---|---|---|---|---|---|---|
| |
|
|
Double-blind |
|
|
|
|
| Placebo n (%) n=157 | Escitalopram n (%) n=155 | Placebo n=82 | Escitalopram n=83 | Open-label escitalopram n=37 | Placebo n (%) n=157 | Escitalopram n (%) n=155 | |
| Patients with SAE | 2 (1.3) | 4 (2.6) | 2 (2.4) | 2 (2.4) | 2 (5.4) | 4 (2.5) | 6 (3.9) |
| Patients who discontinued because of AE | 1 (0.6) | 4 (2.6) | 0 | 4 (4.8) | 2 (5.4) | 1 (0.6) | 8 (5.2)* |
| Patients with TEAEs | 118 (75.2) | 121 (78.1) | 7 (8.5) | 7 (8.4) | 32 (86.4) | 125 (79.6) | 128 (82.6) |
| Patients with TEAEs suggestive of self harmb | 6 (3.8) | 6 (3.9) | 3 (3.7) | 5 (6.0) | 3 (8.1) | 9 (5.7) | 11 (7.1) |
p<0.05.
For safety analyses, the combined double-blind treatment period consisted of the 8-week lead-in period and 16–24-week double-blind extension.
For each AE, the Investigator indicated whether it was considered suggestive of self-harm and, if so, categorized it as nonsuicidal self-injurious behavior, suicidal ideation, suicide attempt, accidental overdose, or other.
AE, adverse event; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Table 7.
Patients with Serious AEs (SAE) or Discontinuation Because of an AE During Extension Study (Weeks 16–24) (Safety Population)
| Age/Sex | Days on drug | AE preferred term | AE investigator term | AE start day | Type of event |
|---|---|---|---|---|---|
| Double-blind placebo | |||||
| 17 years/female | 228 | Suicidal tendencya | Threatening to cut herself | 149 | SAE |
| 16 years/male | 174 | Suicide attempta,b | Suicide attempt | 197 | SAE |
| Double-blind escitalopram | |||||
| 17 years/ female | 128 | Fatigue | Increased fatigue | 30 | ADO |
| 12 years/ male | 104 | Failure to thrivec | Failure to thrive | 99 | SAE, ADO |
| 14 years/ male | 75 | Suicidal tendencya | Self-harm gesture | 95 | SAE |
| 16 years/female | 73 | Inflicted injury | Superficial cutting on arm | 66 | ADO |
| 13 years/ female | 98 | Insomnia | Insomnia | 84 | ADO |
| Open-label escitalopram | |||||
| 15 years/ female | 80 | Nonaccidental overdosea | Intentional overdose of study drug | 80 | SAE, ADO, |
| 12 years/ male | 153 | Pleuritis | Viral pleurodynia | 130 | SAE |
| 13 years/ male | 18 | Abdominal pain | Intermittent stomachaches | 10 | ADO |
Event was considered suggestive of self-harm by the investigator.
Event reported during treatment with commercial escitalopram ∼3 weeks after completing study.
Failure to thrive is defined as severe weight loss.
SAE, serious adverse event; ADO; dropout because of adverse event.
AEs that the investigator considered suggestive of self-harm were reported in 11 patients (18 events) during the extension phase (3 double-blind placebo, 5 double-blind escitalopram, 3 open-label escitalopram; Table 6). The majority of the events included self-inflicted cuttings to arm/thigh/leg and lacerations; these were categorized as “nonsuicidal self-injurious behavior” by the investigator. For four patients (two placebo, one double-blind escitalopram, one open-label escitalopram), the event was also considered serious and/or led to discontinuation from study (Table 7).
The most frequent double-blind treatment AEs in the escitalopram group (incidence at least 5% and more frequent than with placebo) were headache, nausea, insomnia, vomiting, influenza-like symptoms, diarrhea, and urinary tract infection. The great majority of the most commonly reported AEs in both treatment groups occurred during lead-in treatment (Table 8). Most AEs were considered to be either mild or moderate (placebo, 97.9%; escitalopram, 97.5%) and not related to the double-blind study drug (placebo, 70.1%; escitalopram, 62.4%).
Table 8.
Summary of Common Adverse Events (≥5% in Any Group) During Double-Blind Treatment (Safety Population)
| |
Lead-in study double-blind period (8-weeks) |
Combined double-blind treatment (24–32 weeks)a |
||
|---|---|---|---|---|
| Most frequent (≥5%) adverse events | Placebo % n=157 | Escitalopram % n=155 | Placebo % n=157 | Escitalopram % n=155 |
| Headache | 25.5 | 25.2 | 28.0 | 28.4 |
| Menstrual crampsb | 15.2 | 10.9 | 15.2 | 13.0 |
| Insomnia | 6.4 | 10.3 | 7.0 | 11.0 |
| Nausea | 8.3 | 10.3 | 9.6 | 11.6 |
| Abdominal pain | 7.0 | 9.0 | 9.6 | 9.0 |
| Inflicted injury | 13.4 | 9.0 | 20.4 | 15.5 |
| Pharyngitis | 9.6 | 8.4 | 12.7 | 11.0 |
| Fatigue | 8.3 | 7.7 | 9.6 | 8.4 |
| Influenza-like symptoms | 3.2 | 7.1 | 5.7 | 8.4 |
| Rhinitis | 8.9 | 7.1 | 14.6 | 9.7 |
| Vomiting | 5.7 | 6.5 | 5.7 | 9.0 |
| Diarrhea | 3.2 | 5.2 | 3.8 | 5.2 |
| Upper respiratory tract infection | 7.6 | 5.2 | 14.0 | 9.0 |
| Appetite decreased | 3.8 | 2.6 | 5.1 | 2.6 |
| Urinary tract infection | 0.6 | 2.6 | 1.3 | 5.2 |
| Coughing | 4.5 | 1.3 | 8.3 | 3.9 |
For safety analyses, the combined double-blind treatment period consisted of the 8-week lead-in period and 16–24-week double-blind extension.
Percentages are relative to the number of females: 92 placebo; 92 escitalopram.
Change in suicide rating scale scores
The number and percentage of patients with suicidal behavior and suicidal ideation based on C-SSRS assessment is provided in Table 9. The majority of the events were noted during the 8-week lead-in period. During the double-blind extension period, suicidal behavior or ideation was reported in eight new patients (one placebo, seven escitalopram); an associated AE (insomnia, anxiety, abrasion, injury) was reported in three of the eight patients (one placebo, two escitalopram).
Table 9.
Summary of Patients with Increase from Baseline in C-SSRS Scores (Safety Population)
| |
Lead-in study (8-weeks) |
Combined double-blind treatment (24–32 weeks)a |
||
|---|---|---|---|---|
| Placebo n (%) n=128 | Escitalopram n (%) n=131 | Placebo n (%) n=128 | Escitalopram n (%) n=131 | |
| Any suicidal behavior and/or ideation | 13 (10.2) | 12 (9.2) | 14 (10.9) | 19 (14.5) |
| Suicidal behavior | 3 (2.3) | 2 (1.5) | 3 (2.3) | 4 (3.1) |
| Suicidal Ideation | 12 (9.4) | 12 (9.2) | 13 (10.2) | 19 (14.5) |
For safety analyses, the combined double-blind treatment period consisted of the 8-week lead-in period and the 16–24-week double-blind extension.
The mean (±SD) SIQ-JR score at baseline was 15.2±15.5 for the placebo group and 14.3±14.4 for the escitalopram group. At end-point, the mean (±SD) change from baseline was −5.8±12.8 for the placebo group and −3.0±11.7 for the escitalopram group.
Vital signs, laboratory and ECG results
Mean changes in vital signs from baseline to double-blind end-point were small in magnitude.
More patients in the escitalopram-treatment group than in the placebo-treatment group had PCS weight gain (≥7% increase) (5.8% placebo; 12.4% escitalopram) or weight loss (≥7% decrease) (0.6% placebo; 4.6% escitalopram) during the combined double-blind treatment period. None of the PCS weight values were reported as an SAE or as an AE resulting in discontinuation. Changes from baseline to end-point in sex- and age-corrected Z-score values for height and weight were similar between treatment groups (Table 10).
Table 10.
Change in Z-Scores from Baseline to End-Point
| |
Mean±SD |
Mean±SD |
|
|---|---|---|---|
| Height Z-score, cm | Placebo n=44 | Escitalopram n=52 | p Valuea |
| Baseline of lead-in study | 0.59±1.07 | 0.22±1.19 | |
| End-point | 0.74±0.98 | 0.32±1.22 | |
| Change | 0.16±0.36 | 0.10±0.24 | 0.210 |
| Weight Z-score, kg | Placebo n=77 | Escitalopram n=80 | |
|---|---|---|---|
| Baseline of lead-in study | 1.38±1.16 | 1.06±1.20 | |
| End-point | 1.45±1.13 | 1.15±1.19 | |
| Change | 0.08±0.15 | 0.09±0.21 | 0.944 |
| Body mass index Z-score, kg/m2 | Placebo n=44 | Escitalopram n=52 | |
|---|---|---|---|
| Baseline of lead-in study | 1.30±1.26 | 0.96±1.19 | |
| End-point | 1.32±1.12 | 1.01±1.20 | |
| Change | 0.03±0.37 | 0.05±0.22 | 0.999 |
Analysis of covariance (ANCOVA) analysis with baseline values as a covariate.
Mean changes in laboratory values or ECG parameters (heart rate, PR interval, and QTc interval) were small and not clinically significant; no PCS ECG values were reported.
Discussion
Results from this study of escitalopram treatment in adolescents add important prospective clinical data to the literature pertaining to long-term antidepressant use in this population. Improvement from baseline of the lead-in study to the end of treatment in the extension study (week 24), albeit modest, was significantly superior for escitalopram than for placebo on the CDRS-R total score using the primary (LOCF) and MMRM analytic approaches. CDRS-R total scores at week 24 for escitalopram and placebo were not significantly different using OC analysis. Secondary outcome measures, as well as significantly better response and remission rates, support statistically superior efficacy for escitalopram-treated patients than for placebo-treated patients across a time frame of several months, although the magnitude of the differences in clinical outcomes reflect a modest advantage for the active treatment over placebo. Improvement in escitalopram-treated patients largely occurred in the first 8-weeks of double-blind treatment and was maintained, with some additional improvement, during the 16-week double-blind extension.
Safety and tolerability are elements of primary importance when evaluating and utilizing pharmaceutical agents in adolescent patients. In this extension study, escitalopram was generally well tolerated. The incidence of AEs contributing to premature discontinuation was relatively small, and a similar percentage of patients in both treatment groups reported TEAEs during the 24-week double-blind treatment period. However, significantly more patients in the escitalopram-treatment group than in the placebo-treatment group discontinued because of AEs during the study. The majority of AEs were reported during the first 8-weeks of treatment (75.2% placebo, 78.1% escitalopram); most AEs were considered by the investigator to be either mild or moderate (97.9% placebo, 97.6% escitalopram) and not related to the study drug (70.1% placebo, 62.4% escitalopram). Overall, the incidence of PCS clinical laboratory values and vital signs was low for both treatment groups.
At week 24, remission (CDRS-R ≤28) rates were 50.6% (78/154) for escitalopram-treated adolescents and 35.7% (56/157) for placebo-treated adolescents (p=0.002). Acute remission data in adolescents are limited and long-term data are sparse, but high risk of relapse in youth with residual symptoms, as well as slower recovery and subsequent episodes of MDD in relation to subsyndromal symptoms, have been reported (Lewinsohn et al. 2000; Fergusson et al. 2005; Emslie et al. 2008). Remission rates in antidepressant treatment trials in youth are reported as ranging from 23% to 63% (Cheung et al. 2005; Kennard et al. 2009). Data from the TADS trial demonstrated remission rates of 24% in the fluoxetine arm at week 12, 37% at week 18, and 55% at week 36. These rates appear to be comparable to the remission rates for escitalopram within a similar time frame, and the fluoxetine results suggest increasing remission with longer duration of treatment (Kennard et al. 2009).
Although antidepressant pharmacotherapy is generally effective and well tolerated in adolescents, safety concerns regarding antidepressant use and young patients have been raised by regulatory agencies in the United States and Europe. Results from a meta-analysis of 24 controlled clinical trials of nine antidepressants used across indications identified a modestly increased risk of suicidality in pediatric patients using SSRIs and serotonin norepinephrine reuptake inhibitors (SNRIs) (Hammad et al. 2006). The overall risk ratio for SSRIs in depression trials was 1.66 (95% CI, 1.02–2.68). Subsequently, a black box warning to describe an increased risk of suicidality associated with antidepressant use in adolescents and children was issued by the FDA to change the labeling of all antidepressants (United States Food and Drug Administration 2004). However, methodological limitations of retrospective data analysis and interpretation, as well as subsequent reanalyses of published and unpublished data, epidemiologic studies, and safety outcomes from TADS have resulted in continued controversy regarding the risk/benefit relationship of antidepressant use in the adolescent population (Kratochvil et al. 2006). Adolescents with MDD who are being treated with antidepressants may have continued risk for suicidality, and may require continued monitoring during the treatment phase and thereafter.
This study is the first multicenter, double-blind, placebo-controlled, industry-sponsored trial to use prospective methodology to evaluate suicidality in adolescents using an SSRI. AEs that were considered to be suggestive of self-harm occurred in 5.7% of placebo-treated patients and 7.1% of escitalopram-treated patients. On the clinician-rated C-SSRS, the overall incidence of increase in any suicidal behavior and/or ideation was 10.9% (14/128) in the placebo group and 14.5% (19/131) in the escitalopram group. More patients in the escitalopram group than in the placebo group reported emerging suicidal behavior and/or ideation in the extension phase. One suicide attempt occurred in a participant who had finished double-blind treatment in the placebo group and was subsequently taking commercially available Lexapro. No deaths occurred in this study.
It is of additional interest that placebo-treated patients who entered the extension study had greater improvement in CDRS-R than did those who did not continue (−22.7 vs. −15.9); escitalopram-treated patients had similar scores regardless of whether or not they entered the double-blind extension. As such, the placebo group in the extension trial was enriched with highly placebo-responsive patients, which is not unusual in an extension trial. This may have created a potential bias against escitalopram when using OC analysis in which only observed values are analyzed, with no missing data imputed.
To explore the effect of a responder-enriched placebo group, a post-hoc analysis that included lead-in study responders only was conducted. In the extension study, escitalopram responders continued to modestly improve over 24-weeks of treatment (LOCF or OC approach) whereas continued improvement for placebo responders was not observed (LOCF approach). The extension study provided a unique opportunity to observe a large number of placebo responders over time.
Limitations
Several limitations of this study should be recognized. Restrictive inclusion and exclusion criteria, a relatively long period during which placebo-treated patients did not receive pharmacologic treatment for MDD, and the relatively small number of subjects who entered the extension trial might have limited the ability to generalize these results to other adolescents. High placebo response rates, which accounted for more placebo-treated patients completing both the lead-in and extension trials, were a further limitation of this study. Additionally, although statistically significant differences on the primary and secondary efficacy parameters were observed in favor of escitalopram over placebo, it must be noted that differences between treatments were modest.
Conclusions
This 24-week trial of escitalopram in adolescents with MDD (combined 8-week lead-in and 16-week extension data) adds valuable information to the literature concerning the effects of extended antidepressant treatment, and provides a prospective look at treatment-related suicidality in this vulnerable population. Based, in part, on these data, escitalopram is an FDA-approved treatment for adolescents with MDD, because of its efficacy and tolerability in extended use.
Clinical Significance
The chronic and recurrent nature of MDD and its serious acute and long-term complications make identifying appropriate treatments for the disorder a healthcare imperative for adolescents. Results from this randomized, placebo-controlled extension study of escitalopram in the treatment of adolescents with MDD add important prospective clinical data to the literature. Statistically significant efficacy for escitalopram in adolescents with MDD was demonstrated with several analytic approaches and across a time frame of several months, although clinically, the improvement was modest in magnitude. It is of note that this study is the first multicenter, double-blind, placebo-controlled, industry-sponsored trial to use prospective methodology to evaluate suicidality in adolescents using an SSRI. Under the auspices of this trial, and using this prospective methodology to evaluate suicidality, escitalopram was found to be generally well tolerated.
Acknowledgments
Dr. Graham Emslie of the University of Texas Southwestern Medical Center at Dallas, Texas provided review and critical commentary. Writing assistance and editorial support for the preparation of this manuscript were provided by Adam Ruth and Carol Dyer, of Prescott Medical Communications Group, Chicago, Illinois, a contractor of Forest Research Institute. Additional editorial support was provided by Dr. John B. Edwards, an employee of Forest Research Institute, Jersey City, New Jersey.
Disclosures
Dr. Findling receives or has received research support, acted as a consultant, received royalties from, and/or served on a speaker's bureau for Abbott, Addrenex, Alexza, American Psychiatric Press, AstraZeneca, Biovail, Bracket, Bristol-Myers Squibb, Clinsys, Dainippon Sumitomo Pharma, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson & Johnson, KemPharm Lilly, Lundbeck, Merck, National Institutes of Health, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Physicians' Post-Graduate Press, Rhodes Pharmaceuticals, Roche, Sage, Sanofi-Aventis, Schering-Plough, Seaside Therapeutics, Sepracore, Shionogi, Shire, Solvay, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, WebMD and Wyeth. Dr. Robb receives or has received research support from, acted as a consultant for, received royalties from, and/or served on a speaker's bureau for: American Academy of Child and Adolescent Psychiatry (AACAP), Abbott, Bracket, Bristol Myers Squibb, Eli Lilly, Epocrates, Forest, Glaxo Smith Kline, Johnson and Johnson, Lundbeck, McNeil Pediatrics, Merck/Scherring Plough, National Institute of Child Health and Human Development (NICHD), National Institute of Mental Health (NIMH), Otsuka Pharmaceuticals, Pfizer, Sepracor, and Supernus. Dr. Bose was an employee of Forest Research during study conduct and manuscript preparation.
References
- American Psychiatric Association. Text Revision. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Birmaher B. Brent D. Bernet W. Bukstein O. Walter H. Benson RS. Chrisman A. Farchione T. Greenhill L. Hamilton J. Keable H. Kinlan J. Schoettle U. Stock S. Ptakowski KK. Medicus J. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Ryan ND. Williamson DE. Brent DA. Kaufman J. Childhood and adolescent depression: A review of the past 10 years. Part II. J Am Acad Child Adolesc Psychiatry. 1996;35:1575–1583. doi: 10.1097/00004583-199612000-00008. [DOI] [PubMed] [Google Scholar]
- Cheung AH. Emslie GJ. Mayes TL. Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry. 2005;46:735–754. doi: 10.1111/j.1469-7610.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- Emslie GJ. Heiligenstein JH. Hoog SL. Wagner KD. Findling RL. McCracken JT. Nilsson ME. Jacobson JG. Fluoxetine treatment for prevention of relapse of depression in children and adolescents: A double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 2004;43:1397–1405. doi: 10.1097/01.chi.0000140453.89323.57. [DOI] [PubMed] [Google Scholar]
- Emslie GJ. Heiligenstein JH. Wagner KD. Hoog SL. Ernest DE. Brown E. Nilsson M. Jacobson JG. Fluoxetine for acute treatment of depression in children and adolescents: A placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Emslie GJ. Kennard BD. Mayes TL. Nightingale–Teresi J. Carmody T. Hughes CW. Rush AJ. Tao R. Rintelmann JW. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165:459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ. Rush AJ. Weinberg WA. Kowatch RA. Hughes CW. Carmody T. Rintelmann J. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry. 1997;54:1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- Emslie GJ. Ventura D. Korotzer A. Tourkodimitris S. Escitalopram in the treatment of adolescent depression: A randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry. 2009;48:721–729. doi: 10.1097/CHI.0b013e3181a2b304. [DOI] [PubMed] [Google Scholar]
- Fergusson DM. Horwood LJ. Ridder EM. Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62:66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976. The Clinician Global Severity and Impression Scales; pp. 218–222. DHEW Publication No. 276–338. [Google Scholar]
- Hammad TA. Laughren T. Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kennard BD. Silva SG. Tonev S. Rohde P. Hughes JL. Vitiello B. Kratochvil CJ. Curry JF. Emslie GJ. Reinecke M. March J. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): Acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry. 2009;48:186–195. doi: 10.1097/CHI.0b013e31819176f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochvil CJ. Vitiello B. Walkup J. Emslie G. Waslick BD. Weller EB. Burke WJ. March JS. Selective serotonin reuptake inhibitors in pediatric depression: Is the balance between benefits and risks favorable? J Child Adolesc Psychopharmacol. 2006;16:11–24. doi: 10.1089/cap.2006.16.11. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM. Solomon A. Seeley JR. Zeiss A. Clinical implications of “subthreshold” depressive symptoms. J Abnorm Psychol. 2000;109:345–351. [PubMed] [Google Scholar]
- Lexapro [package insert] St Louis, MO: Forest Pharmaceuticals, Inc; 2011. [Google Scholar]
- March JS. Silva S. Petrycki S. Curry J. Wells K. Fairbank J. Burns B. Domino M. McNulty S. Vitiello B. Severe J. The Treatment for Adolescents With Depression Study (TADS): Long–term effectiveness and safety outcomes. Arch Gen Psychiatry. 2007;64:1132–1143. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- March J. Silva S. Petrycki S. Curry J. Wells K. Fairbank J. Burns B. Domino M. McNulty S. Vitiello B. Severe J. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- Masi G. Liboni F. Brovedani P. Pharmacotherapy of major depressive disorder in adolescents. Expert Opin Pharmacother. 2010;11:375–386. doi: 10.1517/14656560903527226. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health, Office of Applied Studies. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005. Results from the 2004 National Survey on Drug Use and Health: National findings (DHHS Publication No. SMA 05-4062, NSDUH Series H-28). Suicidal Thoughts among Youths Aged 12 to 17 with Major Depressive Episode. [Google Scholar]
- National Survey on Drug Use and Health, Substance Abuse and Mental Health Services Administration, Office of Applied Studies: The NSDUH Report. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2008. Major Depressive Episode among Youths Aged 12 to 17 in the United States: 2004 to 2006. [Google Scholar]
- Posner K. Brown GK. Stanley B. Brent DA. Yershova KV. Oquendo MA. Currier GW. Melvin GA. Greenhill L. Shen S. Mann JJ. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski EO. Mokros HB. Los Angeles: Western Psychological Services; 1996. Children's Depression Rating Scale–Revised (Manual) [Google Scholar]
- Reynolds WM. Odessa, FL: Psychological Assessment Resources, Inc; 1987. Professional Manual for the Suicide Ideation Questionnaire. [Google Scholar]
- Shaffer D. Gould MS. Brasic J. Ambrosini P. Fisher P. Bird H. Aluwahlia S. A Children's Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration. Washington, DC: US Food and Drug Administration; 2004. [Nov 14;2011 ]. FDA Launches a Multi-Pronged Strategy to Strengthen Safeguards for Children Treated with Antidepressant Medications [press release] [Google Scholar]
- von Knorring AL. Olsson GI. Thomsen PH. Lemming OM. Hulten A. A randomized, double-blind, placebo-controlled study of citalopram in adolescents with major depressive disorder. J Clin Psychopharmacol. 2006;26:311–315. doi: 10.1097/01.jcp.0000219051.40632.d5. [DOI] [PubMed] [Google Scholar]
- Wagner KD. Jonas J. Findling RL. Ventura D. Saikali K. A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry. 2006;45:280–288. doi: 10.1097/01.chi.0000192250.38400.9e. [DOI] [PubMed] [Google Scholar]
- Wagner KD. Robb AS. Findling RL. Jin J. Gutierrez MM. Heydorn WE. A randomized, placebo–controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psychiatry. 2004;161:1079–1083. doi: 10.1176/appi.ajp.161.6.1079. [DOI] [PubMed] [Google Scholar]