Abstract
Objective
The purpose of this pilot study was to compare the effects of 30 sessions of neurofeedback (NF) with stimulant medication on attention-deficit/hyperactivity disorder (ADHD) patients.
Methods
Thirty-two medication-naïve ADHD patients, ages 7–16, from a neuropsychiatric clinic, were randomized to NF (n=16) or drug treatment (n=16). Other actions, such as parent management training, information, or support in school were given as needed, with no differences between the groups. All participants were assessed before treatment on two rating scales, each with parent and teacher forms. In addition, quantitative electroencephalogram (QEEG) and event-related potentials (ERPs), which included behavioral data from a go/no go test were administered. NF training took place in the clinic over a period of 7–11 months, and was followed by a repeat of the same assessment tools. The mean time interval between pre- and postassesment was not significantly different in the two groups. The 18 symptoms of ADHD (American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV)) were used as the primary outcome measure.
Results
Analysis of covariance revealed a significant difference between the groups at evaluation in favor of medication, with a large effect size. This picture was confirmed by other outcome measures. The QEEG spectral power in the theta and beta bands did not change in either group. In ERP, the P3 no go component increased significantly in 8 of 12 patients who had a clinically relevant medication effect, but did not increase in the medication nonresponders or the NF group.
Conclusions
Our study supports effects for stimulants, but not for NF. Effects of NF may require thorough patient selection, frequent training sessions, a system for excluding nonresponders, and active transfer training. The P3 no go ERP component may be a marker for treatment response.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) (American Psychiatric Association 2000) is considered a neurodevelopmental disorder, with a prevalence of ∼3–5% in school-aged children (Polanczyk and Jensen 2008). The disorder is characterized by age-inappropriate levels of inattention and/or hyperactivity and impulsivity, creating significant impairment in social relations and in school and home environments. Comorbid conditions such as behavior-, anxiety-, and learning-related disorders are common (Gillberg et al. 2004).
Although pharmacological treatment with methylphenidate, amphetamine, and atomoxetine is the most effective treatment to date, its disadvantages and limitations include side effects, reservations against medication, and 10–25% nonresponse rate (Taylor et al. 2004; Banaschewski et al. 2006). Even among responders, there is room for improvement. Clinical guidelines recommend a multimodal treatment, encompassing both medication and cognitive-behavioral and family treatments (Taylor et al. 2004). Parent management training has been found effective for young children, but less effective for adolescents (Barkley 2002, 2006). Therefore, there remains a need for effective treatment strategies in improving attention and self-management capabilities in children and adolescents with ADHD.
In the search for additional or alternative treatment options, neurofeedback (NF) emerged as one of the most promising options (Heinrich et al. 2007). NF is an operant conditioning procedure in which participants (patients) learn to gain self-control over electroencephalogram (EEG) patterns. It can even be run as a computer game. Measures reflecting these patterns are converted into visual or acoustic signals that are continuously fed back in real time, so that changes in the desired direction can be reinforced. Foster and Drago (2007) claim that normalizing EEG patterns results in the normalization of behavior.
Neurofeedback protocols
Electrode placement and frequencies to be rewarded and inhibited can be considered as a definition of “protocol.” Finding the best patient protocol is a basic and complex question in the use of NF (Lubar and Lubar 1999; Kropotov 2009; Thompson and Thompson 2009). Standard ADHD protocols that inhibit theta (4–8 Hz) and reinforce beta (13–20 Hz) or sensorimotor rhythm (SMR) (13–15 Hz over the motor strip) have been used extensively and are based on experience and research showing that excess theta or a high theta/beta ratio is typical for ADHD (Barry et al. 2003a, 2005; Loo and Barkley 2005; Snyder and Hall 2006). Since the mid-1990s, an increasing number of clinicians have used quantitative electroencephalogram (QEEG)-based protocols, targeting sites and frequencies deviant from the norm to be trained (Johnstone et al. 2005). A newer form of NF is the training of slow cortical potentials (SCPs) related to phasic regulation of cortical excitability (Gevensleben et al. 2009).
Effects of NF in ADHD
There are a substantial number of publications related to clinical effects of NF in ADHD (Monastra et al. 2002; Monastra 2005a; Leins et al. 2006; Levesque et al. 2006; Heinrich et al. 2007; Hollup 2007; Arns et al. 2009; van As et al. 2010; Lofthouse et al. 2012). Only a few of them report negative results (Lansbergen et al. 2011; Skokauskas et al. 2011), a phenomenon that may, to some extent, reflect publication bias. Some researchers have noted that case studies in which patients or parents have actively sought and paid for NF are of limited scientific value. Evaluations before and after treatment in the absence of an adequate control group cannot demonstrate if clinical effects are related to NF or other factors such as elapsed time, contact with therapist, positive expectations, or general cognitive training. Small sample sizes, lack of randomization, inadequate control conditions, and the small number of studies of generalization and long-term outcomes make it difficult to conclude that NF has a lasting, clinically relevant effect on ADHD (Loo and Barkley 2005; Holtmann and Stadler 2006).
Arns et al. (2009) completed a meta-analysis comprising 15 studies. A total of 718 patients participated in pre-post studies (no control groups). In addition, 476 patients from controlled studies were included. In some of the controlled studies, participants received such semi-active control treatments as attention training. In three controlled studies, none of which were randomized, stimulant medication served as the control condition (Fuchs et al. 2003; Rossiter 2004). Based on test data for impulsivity, similar effects were found for NF and medication. Arns reports an effect size (ES; Cohen's d) of 0.81 for inattention, 0.69 for impulsivity, and 0.39 for hyperactivity, compared with control groups. There were no differences related to protocol (theta/beta, SMR, or SCP training; see section “Neurofeedback protocols”), which may reflect the influence of nonspecific factors or the possibility that the same relevant brain changes can be achieved by various training methods. Comparable effects of NF were found for medicated and unmedicated patients. In randomized studies, smaller effect sizes were found for hyperactivity, but not for inattention or impulsivity. The long-term effects of NF introduce a further issue. Strehl et al. (2006) found that the positive effects of SCP were maintained and even improved at a 6 month follow-up. Two years later the researchers found stable improvements and preservation of EEG self-regulation.
Reduced theta/beta ratio after treatment has been found (Monastra et al. 2002), but many studies do not report pre- and post-QEEG measures. Lubar et al. (1995) found that children who did not succeed in reducing theta activity were still rated as improved, and Kropotov et al. (2005) reported pre-post changes in ERPs (P3 no go) in good performers, but not in poor performers. Arns et al. (2009) argue that a high theta/beta ratio in eyes-open and eyes-closed conditions may represent a stable trait marker or phenotype, and should not be considered a valid end point of treatment.
In a recent double-blind, placebo-controlled pilot study (Lansbergen et al. 2011), eight children were randomized to 30 sessions of QEEG-based NF and six children received noncontingent or sham feedback. All 14 children completed the study, and no side effects were reported. It is noteworthy that six of eight members of the NF group thought that they had received sham feedback, as did half of the children in the sham group. The severity of inattention and hyperactivity/impulsivity symptoms declined over time, but to the same degree in both groups. The authors suggested that use of automatic thresholds for reinforcement (80% success), use of individualized rather than standard protocols, and lack of explicit transfer strategies (reinforcement for using new skills in home and school environment) may have contributed to the negative outcome.
One study, which employed 20 active and 20 placebo sessions (deBeus and Kaiser 2011), used a blinded, placebo-controlled, crossover design; 42 of the 56 children (75%) completed the study. An engagement index (EI), defined as a power ratio (12–20 Hz/4–12 Hz), was reinforced in a car race computer game. The placebo condition was identical, but controlled by a technician. Effects were assessed by the Conners' Teacher Rating Scale and results on a continuous performance test (CPT); 71% of participants were able to increase EI by ≥0.5 SD from the first to the last active sessions. For this group, defined as learners, ADHD symptoms were reduced by 0.5 SD compared with participants in the placebo group. The learners improved their test results by 0.6 SD.
Gevensleben et al. (2009, 2010) undertook a randomized, controlled, multisite study, including 6 month follow-up with ADHD-diagnosed children ages 8–12 years (n=102), none of whom were medicated. The treatment group received 18 sessions of theta/beta NF and 18 sessions of SCP training. The control group received computerized attention training. To avoid confounding variables, the researchers abstained from cognitive strategies and parent or teacher involvement. They found 52% responders in the NF group (i.e., 25% reduction of raw score on an ADHD rating scale) and 29% in the control group. NF was superior to attention training, with an effect size of 0.6. No difference was found between theta/beta training and SCP. At 6 month follow-up, the situation was similar.
A recent review article (Skokauskas et al. 2011), evaluating complementary medicine in children with ADHD concludes that most recent randomized controlled trials in NF have yielded negative results.
Another recent review article regarding improvements in NF by Lofthouse et al. (2012) reported an overall effect size of 0.79 (large) for inattention and 0.71(moderate) for hyperactivity/impulsivity. Fourteen randomized studies were critically reviewed. It is still unclear how much of the reported effects are related to feedback per se, and follow-up studies of high quality are lacking. They conclude that there is a need for large multisite triple-blind, randomized, sham-controlled trials. The authors suggest that NF for ADHD should be considered as “probably efficacious.” Arns et al. (2009) consider NF for ADHD to be “efficacious and specific.”
In the present pilot study, NF was compared with drug treatment in 32 ADHD patients, all drug-naïve before onset of the study, 16 of whom received 30 sessions of NF and 16 of whom were medicated with stimulants. Regarding QEEG and ERP, we hypothesized that a reduction in ADHD symptoms would be reflected in a reduced level of power in the theta band at Cz and an increase in the P3 no go ERP component, which is assumed to reflect inhibitory control (Kropotov et al. 2005).
Methods
Subjects
All 32 patients, 7–16 years of age, were referred to a neuropsychiatric team that is part of the child and adolescent psychiatry system in Østfold County, Norway. The school's psychology service and the patient's general practitioner had screened the cases and made a tentative diagnosis of ADHD based upon observation at school; a short medical history; a medical examination; Verbal, Performance, and Total Intelligence Quotient (IQ); achievement test results; and ADHD screening scales that had been completed by parents and teachers. We supplemented these data by taking a more detailed medical history and administering the clinical interview Development and Well Being Assessment (DAWBA) (Goodman et al. 2000), Conners' Rating Scale-revised (CRS-R), for parent (Pa) and teacher (Te) (Conners et al. 1998), the Behavior Rating Inventory of Executive Function (BRIEF) (Gioia and Isquith 2000), and attention testing (Quantitative Behavior test [QB-test], www.qbtech.se). The rating scales were also used as baseline information, because treatment started within a time frame of 1 month. When information from parents and teachers did not coincide, this issue was discussed with them before the team of two neuropsychologists and a pediatrician drew their diagnostic conclusions regarding ADHD and comorbidities. A summary is shown in Table 1.
Table 1.
Demographics of the 29 Patients Who Completed the Study
| NF group; n=14 | Med group; n=15 | p | |
|---|---|---|---|
| Age | 10.6 (SD: 2.0) | 11.2 (SD: 2.7) | n.s. |
| Gender | 8 male, 6 female | 10 male, 5 female | n.s. |
| Verbal IQ | M=90 (SD: 16) | M=88 (SD: 13) | n.s. |
| Performance IQ | M=95 (SD: 18) | M=92 (SD: 19) | n.s. |
| Total IQ | M=92 (SD: 17) | M=89 (SD: 16) | n.s. |
| ADHD-C | n=11 (79%) | n=11 (73%) | |
| ADHD-I | n=3 (21%) | n=4 (27%) | |
| No psychiatric comorbidity | n=4 (29%) | n=5 (33%) | |
| ODD-CD | n=7 (50%) | n=6 (40%) | |
| Anxiety/depression | n=3 (21%) | n=3 (20%) | |
| Other | n=0 (0%) | n=2 (13%) | |
| No learning disabilities (LD) | n=7 (50%) | n=5 (33%) | |
| General LD | n=5 (36%) | n=5 (33%) | |
| Specific LD | n=2 (14%) | n=5 (33%) | |
| CRS-R-Te: ADHD total | T score=72 | T score=73 | |
| (SD: 13) | (SD: 10) | ||
| CRS-R-Pa: ADHD total | T score=79 | T score=71 | |
| (SD: 12) | (SD: 9) |
Specific LD includes patients with dyslexia and dyscalculia and IQ>80. General LD includes IQ<80 and significant learning problems in several school subjects, requiring special education. Other comorbidity=Tourette's syndrome (1), Asperger's syndrome (1). ADHD total=T scores based on all 18 symptoms of ADHD in American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision DSM IV-TR
NF, neurofeedback; IQ, intelligence quotient; ADHD, attention-deficit/hyperactivity disorder; ADHD-C, ADHD-combined type; ADHC-I, ADHD-inattentive type; ODD, oppositional defiant disorder; CD, conduct disorder; CRS-R, Conners' Rating Scale revised; Te, Teacher edition; Pa, Parent edition.
All diagnostic conclusions, both ADHD and comorbidities, were based on American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) (American Psychiatric Association 1994) and accepted clinical guidelines (American Academy of Pediatrics 2000). As mentioned previously, information was obtained from DAWBA, medical history, rating scales, school reports and informal interviews. Patients with IQ and adaptive levels <70 were excluded. Subjects with learning disabilities and behavioral and emotional comorbidities were included, as was one patient with both ADHD and Asperger's syndrome. None of the patients were on or had been treated with stimulants when evaluated for diagnostic assessment, and none of them had received psychotherapy. Regarding sample size, one purpose of this pilot was to see if the results justified a larger study. If positive effects of NF (defined as >0.5 SD change on accepted outcome measures) were found in 12 of 16 patients (75%), the “true” effect was calculated to be >50%. If positive effects were found in 4 patients (25%), the “true” effect was considered <50%. The parents gave written consent for their children to participate in our project, which was approved by the Regional Committee for Medical Research Ethics (REK). Detailed written and oral information was given to parents and children. Patients randomized to NF were offered medication treatment after 30 sessions of NF or if they dropped out of the project.
Table 1 shows demographic information for the sample. The male/female ratio of this group and the pattern and frequency of psychiatric comorbidities were in accordance with the ADHD literature (Barkley 2006). As shown in Table 1, the total percentage of learning disabilities (59%) was higher than in most studies of ADHD, perhaps reflecting the fact that referrals were made from the school psychology service.
Assessment of behavior
Data from two rating scales, both of which encompassed parent and teacher forms; and from QEEG/ERP, including the Visual Continuous Performance Test (VCPT), were recorded before and after treatment in both groups. The time interval between pre- and post assessment was 8.5 months in the NF group and 10.5 months in the medication group. This difference was not significant.
CRS-R
The CRS-R (Conners et al. 1998) contains 80 items in the parent form and 59 in the teacher form, resulting in T scores on 14 subscales on the parent form and 13 on the teacher form. Diagnostic criteria for ADHD are included, in addition to screening of behavioral and emotional problems. Primary outcome measures were T scores from CRS-R parent and teacher forms (DSM-IV criteria for ADHD).
BRIEF
The BRIEF (Gioia and Isquith 2000) contains 86 items each for parent and teacher forms. The Global Executive Composite (GEC) is the average of the Behavior Regulation Index (BRI – three subscales) and the Metacognition Index (MI – five subscales).
VCPT
The VCPT is performed during registration of EEG, obtaining ERPs in addition to behavioral data. VCPT is a modification of the visual two-stimuli go/no go paradigm. Three categories of visual stimuli were selected: 20 pictures of animals, 20 pictures of plants, and 20 pictures of humans (presented together with a novel artificial sound). The trials consisted of the presentation of pairs of stimuli: animal–animal (go trials), animal–plant (no go trials), plant–plant (“ignore” trials), and plant–human (novel trials). The task required participants to press a button as fast as possible in response to all go trials. During the task, subjects were seated in a comfortable chair, 1.5 m in front of a computer screen. The stimuli were presented on a 38 cm monitor using Psytask (Mitsar Ltd.) software (Mueller et al. 2010). The VCPT, with 400 pairs of pictures, takes 20 minutes to complete.
Assessment of QEEG and ERP
EEG was recorded using a Mitsar 201 (www.mitsar-medical.com), a PC-controlled 19 channel EEG system. The input signals referenced to the linked ears were filtered between 0.5 and 50 Hz and digitized at a sampling rate of 250 Hz. Impedance was kept below 5 kΩ for all electrodes. Electrodes were placed according to the International 10–20 system, using an electrode cap with tin electrodes (Electro-cap International Inc.). Quantitative data were obtained using WinEEG software, common reference montage (see Kropotov [2009] for a definition) prior to data processing. Eye-blink artifacts were corrected by zeroing the activation curves of individual independent component analysis (ICA) components corresponding to eye blinks (Vigario 1997). In addition, epochs of the filtered electroencephalogram with excessive amplitude (>100 μV) and/or presented with excessively fast (>35 μV in 20–35 Hz band) and slow (>50 μV in 0–1 Hz band) frequency activities were automatically marked and excluded from further analysis. Finally, the EEG was manually inspected to verify artifact removal.
We registered theta (4–8 Hz), beta (13–21 Hz), and the theta/beta ratio at CZ as per Monastra et al. (1999, 2001) in eyes-open and VCPT conditions. (According to the international 10–20 system of electrode placement in EEG, Cz or vertex refers to a central, midline electrode placement). For each individual, we computed the mean power of the ERP component P3 no go at Cz in the time interval from 300 ms to 500 ms after stimulus 2 for each subject. We also computed the average P3 no go component for both groups before and after treatment. The WinEEG software allowed us to compare pre- and post-ERPs for each individual. All significant increases for each patient in four groups were registered: medication responders, medication nonresponders, NF responders, and NF nonresponders. (For a definition of responders, see “Statistical methods” and “Changes of clinical relevance”).
Statistical methods
To compare the treatment effects in the NF group and the medication group (Med group), we used one-way between-groups analysis of covariance (ANCOVA) with measurements at baseline (T1) as the covariate. This was done for the primary outcome measures (ADHD total T score in CRS-R, parent scale [Pa], and teacher scale [Te]) and for supplementary outcome measures. The effect of using the interval between T1 and evaluation (T2) as an additional covariate was checked, and did not change the results. T scores were used for inattention and hyperactivity/impulsivity scales from the CRS-R and BRI and the MI from BRIEF. Raw scores were used for measures of omission errors and reaction time variability (RTvar) in VCPT, with data from T1 as covariate.
We also addressed the question of clinical relevant change, defined as >1.0 SD change from pre- to postevaluation on two or more measures. (Negative changes of >1.0 SD were subtracted). This was done for the four CRS-R subscales; DSM-IV inattention; and hyperactivity/impulsivity, parent and teacher forms. An expanded version of “clinical relevant change” also included global scales (BRI, MI) from BRIEF, “omission errors,” and RTvar from the VCPT test. (Calculations of means and standard deviations for the VCPT measures were based on data from normal controls). Effect sizes were evaluated using Cohen's d and characterized as small 0.2–0.4), medium (0.5–0.8), or large (>0.8), according to guidelines (Cohen 1988).
Data were analyzed using the Statistical Package for the Social Sciences (SPSS), volume 18 (www.spss.com). Two-tailed tests were used. The significance level was set at 0.05.
Procedure of neurofeedback
The NF training was performed using Brain Tuner Version 1.4 from Mitsar. We used a bipolar montage and the ground electrode on the skull, which is optimal for artifact rejection. Training protocols were individually selected, combining behavioral information and data from the QEEG. Selection of protocols was discussed with Professor of Neurophysiology J. Kropotov. Excess theta, compared with norms, led to a theta/beta protocol, rewarding 13–20 Hz and inhibiting 4–8 Hz, with the active electrodes at parietal, central, or frontal midline (Pz, Cz, or Fz, respectively), depending upon the individual QEEG. In some cases, minor changes in band ranges were made; inhibiting 3–8 Hz, for example. We also found significant deviances in QEEG, such as excess occipital alpha, which were probably unrelated to ADHD. In accordance with principles of QEEG-based neurofeedback, we reasoned that training deviances toward normality might produce improvements in behavior. In these patients, a maximum of 30% of the training time addressed these deviances. No patients had more than two protocols. The following ADHD protocols were used: theta/beta (7), SMR (5), and beta inhibiting (2). Additional protocols were rewarding occipital alpha (3), inhibition of 4–13 Hz occipitally (1), and inhibition of 11–13 Hz at motor strip (1).
The training, which was conducted in the clinic, was completed by the first author, who has worked in the area for 30 years and with NF for 14 years; together with a licensed psychologist, receiving in-clinic training and working under close supervision. In clinic, modifications of protocols are often made based on feedback from the patients and lack of progress. A therapeutic relationship is also emphasized. We completed the predetermined protocols, however, and tried to be “friendly, but neutral” in order to reduce the influence of factors other than NF as much as possible. Each session lasted 45 minutes, with 30–35 minutes of training. A 2 minute baseline was recorded before the training procedure to determine the level of threshold for reinforcement. This baseline was sometimes adjusted during the session, however, to regulate the percentage of time above threshold between 70% and 85%. After 5 minutes of training, there was a 1 minute pause. During baseline and pauses, patients were asked to relax; to breathe deeply, not focusing on anything special; and to “lower their shoulders.” Every training session started with simple visual feedback: a green bar on a grey screen following the dynamics of the feedback parameters and a horizontal threshold line in the middle of the screen. It was explained to patients that this was a method of increasing their attention skills, and that the feedback was controlled by their brainwaves reflecting levels of attention. They were asked to raise the bar above the threshold without tightening their muscles and to notice their own mental state when they succeeded. The percentage of time above threshold was shown at the top of the screen. To increase motivation, most of the training time was in video mode. A DVD film was shown on the TV screen. When the bar sank below threshold level, the picture was blurred, because a unit called “jammer” was receiving the EEG signals. To see the film properly, patients were required to increase the feedback parameter above threshold.
After every session, the youngest children could choose a sticker. In addition, the children were offered a small toy every third session, and the adolescents received a lottery ticket every fifth session.
Our plan was to complete the 30 sessions within a period of 4 months, with two sessions a week. Illness, holidays, special events in school, and appointments forgotten by patients forced us to take 6–9 months (mean=8.5 months) to complete the training.
Medication
Six of the 15 patients randomized to medication underwent a double-blind procedure, which was part of another research project. For three periods of 2 weeks they received either low (10 mg×3) or high (15 mg×2 and 10 mg×1) doses of methylphenidate, low (5 mg×2) or high (10 mg×2) doses of dextroamphetamine, or a placebo. Each dose was reached after 2 days of titration. Based on daily parent and teacher ratings and attention testing, medication type and dose were individually adjusted; further adjustments were completed as needed. Our testing of these patients occurred ∼10 months after the onset of this procedure. The other patients underwent a 4 week tryout period, usually titrated up to a maximum of 15 mg of methylphenidate×3 or 10 mg of dextroamphetamine×2. Dextroamphetamine was tried if the methylphenidate produced too many side effects such as appetite loss, stomach pain, or insomnia, or if the effect of the medication (based on ratings, interview, and attention testing) was limited. At the time of evaluation, nine methylphenidate patients were receiving a daily dose of between 30 mg and 90 mg; six of these patients used long-acting agents (8 or 12 hours), including four patients who combined this agent with one or more short-acting tablets. Dextroamphetamine was the medication for six patients, whose daily doses were 10 mg (4), 15 mg (1), and 5 mg (1).
Results
Primary outcome measures
All statistical analyses were based on 29 of the 32 patients who were evaluated before and after treatment. Analyses based on all 32, using T1 measures at T2 for the 3 drop-outs (intention to treat model) gave similar results. There were two dropouts in the NF group. In one case, the single parent changed jobs, making further treatment for the child impossible. The second patient refused to participate after a few sessions. One patient in the Med group did not show a positive response to medication, and wanted to withdraw from the project. Table 2 shows data for the primary outcome measures: the American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM IV-TR)'s 18 symptoms for ADHD measured with CRS-R before and after treatment, parent and teacher forms (American Psychiatric Association 2000). As mentioned, the interval from T1 to T2 was 8.5 months in the NF group and 10.5 months in the Med group. A one-way between-groups ANCOVA was used, controlling for differences at baseline T1. There was a significant difference between the two groups after treatment, showing best results for the medication group. The effect sizes were large, according to Cohen (1988; see Table 2). Time between T1 and T2 as a second covariate did not change the results.
Table 2.
One-Way ANCOVA Comparing T Scores in NF and Med Groups on DSM IV ADHD Scales After Treatment (T2) Controlling for Scores Before Treatment (T1)
| |
NF T2 |
Med T2 |
|
|
|
|
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | df | F | p | Cohen's d | |
| ADHD- Conners' Rating Scale (CRS-R), parent scale | 78 (14) | 63 (13) | 1 | 5.14 | 0.033* | 1.11 Large |
| ADHD- Conners' Rating Scale (CRS-R), teacher scale | 74 (18) | 58 (9) | 1 | 6.87 | 0.015* | 1.12 Large |
Parent scale: NF group, n=13; Med group, n=14. Teacher scale: NF group, n=14; Med group, n=15. p: Significance level of the difference between NF and Med group at T2. Cohen's d=effect size (ES). Cohen's d>0.8 is considered a large effect. T scores at T1 for the NF group were 79 (12), parent scale, and 73 (10), teacher scale. Med group: 71(9), parent scale; 72(13), teacher scale.
Calculations based on all 32 participants; following guidelines of the “intention to treat” model (scores at T2=scores at T1) resulted in similar results.
ANCOVA, analysis of covariance; NF, neurofeedback; DSM IV, American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. ADHD, attention-deficit/hyperactivity disorder.
Other outcome measures
Scores on inattention and hyperactivity/impulsivity scales on CRS-R (DSM IV criteria) and global indexes on BRIEF rating scale, parent and teacher forms, were analyzed using one-way ANCOVA, with baseline T1 scores as the covariate. Data from VCPT related to omission errors and RTvar were also analyzed, because these measures are considered the best test indexes of attention. There were significant differences at evaluation (T2) between the two groups in 7 of the 10 measures, all favoring the Med group, with large effect sizes.
Changes of clinical relevance
Data regarding clinically relevant changes in individual patients were also assessed. We defined relevant change as improvement of ≥1 SD on two or more of the inattention and hyperactivity/impulsivity scales of CRS-R-P and CRS-R-T; DSM IV-TR criteria – four scales in all. (Any negative changes of ≥1 SD or more were subtracted). In the NF group, significant positive changes were seen in 2 of the 14 patients, and negative changes were seen in 3 patients. In the Med group, positive changes were seen in 9 of 15 patients; there were no negative changes. Similar results were found if we included results from BRIEF and VCPT: Med group=12 positive and 2 negative changes, NF group=4 positive and 5 negative changes. These data about individual patients supplement the group data reported in Tables 2 and 3, and are of interest from a clinical point of view.
Table 3.
One-Way ANCOVA Comparing T Scores in NF and Med Groups at Evaluation (T2) on 10 Measures Controlling for Baseline Scores (T1)
| |
|
NF T1 |
NF T2 |
Med T1 |
Med T2 |
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|
| n | M (SD) | M (SD) | M (SD) | M (SD) | df | F | p | Cohen's d | |
| CRS-R-Pa inattention | NF=13 Med=14 |
75 (10) | 75 (12) | 70 (12) | 64 (13) | 1 | 4.7 | 0.040 | 0.88 large |
| CRS-R-Pa hyper/imp | NF=13 Med=14 |
77 (13) | 76 (15) | 69 (12) | 62 (15) | 1 | 3.2 | 0.090 NS | 0.93 large |
| CRS-R-Te inattention | NF=14 Med=15 |
73 (12) | 72 (14) | 68 (14) | 56 (9) | 1 | 12.6 | 0.002 | 1.36 large |
| CRS-R-Te hyper/imp | NF=14 Med=15 |
68 (14) | 72 (23) | 76 (21) | 61 (21) | 1 | 4.2 | 0.052 | 0.50 medium |
| BRIEF-Pa BRI | NF=13 Med=14 |
71 (14) | 67 (12) | 63 (9) | 61 (14) | 1 | 0.3 | 0.591NS | 0.46 small |
| BRIEF-Pa MI | NF=13 Med=14 |
70 (10) | 68 (9) | 66 (7) | 62 (15) | 1 | 0.7 | 0.408 NS | 0.49 small |
| BRIEF-Te BRI | NF=14 Med=15 |
72 (19) | 76 (17) | 75 (14) | 65 (14) | 1 | 8.5 | 0.007 | 0.71 medium |
| BRIEF-Te MI | NF=14 Med=15 |
76 (12) | 78 (16) | 70 (13) | 62 (12) | 1 | 7.9 | 0.009 | 1.13 large |
| VCPT omissions | NF=11 Med=15 |
23.3 (20) | 20.6 (12) | 19.9 (13) | 8.9 (8) | 1 | 9.8 | 0.005 | 1.15 large |
| VCPT RT variation | NF=11 Med=15 |
15.3 (6) | 16.9 (5) | 17.3 (7) | 12.9 (4) | 1 | 6.9 | 0.015 | 0.88 large |
The table shows differences between the groups at T2 when controlling for scores at T1 (ANCOVA). For CRS-R and BRIEF, T scores are used. For VCPT measures, raw scores are used. NF 1 and Med 1=before treatment. NF 2 and Med 2=after treatment.
ANCOVA, analysis of covariance; NF, neurofeedback; DSM IV, American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. CRS-R, Conners' Rating Scale – revised, Pa (parent) and Te (teacher) forms; Inattention=DSM IV, 9 inattention symptoms; Hyper/imp, DSM IV, 9 symptoms of hyperactivity/impulsivity; BRIEF, Behavior Rating Inventory of Executive Function, Pa (parent) and Te (teacher) forms; BRI, Behavior Regulation Index; MI, Metacognition Index; VCPT, Visual Continuous Performance Test; RT variation, reaction time variation. Cohen's d, effect size (0.2–0.4: small, 0.5–0.8: medium, >0.8: large).
Changes in QEEG and ERP
We compared power (microvolt) in the theta and beta band at T1 and T2 for both groups, controlling for differences at baseline (ANCOVA). All changes were nonsignificant. In the NF group, theta T1=13.9 (10.0), T2=13.7 (9.3) and beta T1=1.9 (1.5), T2=1.8 (0.7). In the Med group, theta T1=11.9 (5.2), T2=11.5 (6.5) and beta T1=1.4 (0.4), T2=1.4 (0.5).
Splitting the sample into responders and nonresponders (see “Changes of clinical relevance”) gave no indications of reduced theta or increased beta for responders. The power of the P3 no go component (mean power in the time interval between 300 ms and 500 ms after stimulus 2) decreased in the NF group (T1=2.7 [4.5], T2=1.9 [7.3]) and increased in the Med group (T1=0.3 [4.9], T2=1.9 [3.9]). The difference was not statistically significant.
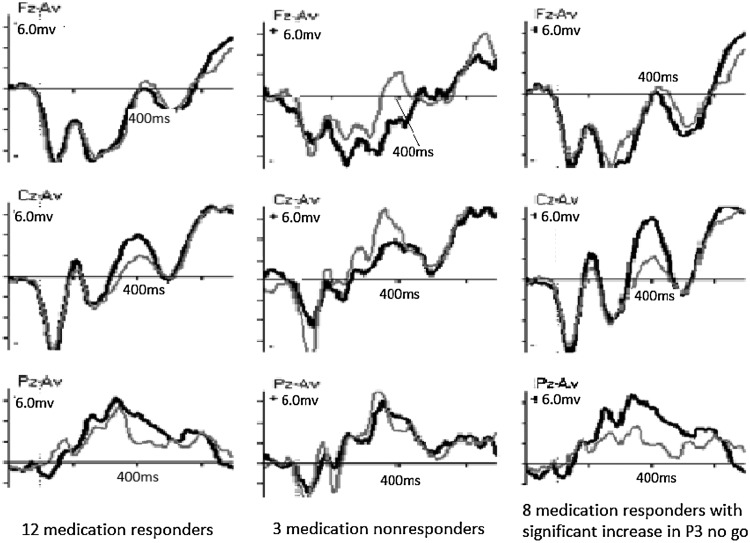
Using WinEEG software, changes in the P3 no go component from T1 to T2 were registered for all patients individually. We divided them into responders and nonresponders. (Nonresponders included patients with no clinically relevant positive changes.) For 3 NF patients, EEG data for T1 or T2 was missing, reducing the NF group to 11. For the four NF responders, a significant increase of the P3 no go component was found in two of them. For the seven NF nonresponders, a significant increase was also found in two patients. Among Med responders, 8 of 12 showed a significant increase of the P3 no go component. (Maximum increase was at Cz for four of them, at Pz for three, and at Fz for one). All three Med nonresponders showed a significant reduction of the P3 no go component, two of them at Cz and one at Pz. Changes in the P3 nogo component for the Med group (all 12 responders, 3 nonresponders, and the subgroup of 8 responders with individually significant increases in P3 no go) are shown in Figure 1. For this subgroup of eight responders, the increase was significant at site Pz.
FIG. 1.
Changes in ERP component P3 no go at sites Fz, Cz, and Pz for the Med group. (Left: All 12 with significant clinical improvement [responders]. Middle: Three nonresponders. Right: The subgroup of eight responders with significant increase of P3 no go). X-axis: Time in milliseconds. Y-axis: Power in microvolts. Fz, Cz, and Pz are sites of registration; frontal, central, and parietal midline. Left: P3 no go for all 12 medication responders at T1 (gray) and T2 (black), showing an increase at Cz and Pz. Middle: P3 no go for three medication nonresponders at T1 (gray) and T2 (black), showing a decrease in component at Cz. Right: P3 no go for 8 of 12 medication responders with significantly increased component, showing increased component at Cz and Pz. Cz: Increase from 2.14 mV to 5.96 mV at 400 ms. p=0.27. Pz: Increase from 1.80 mV to 5.78 mV at 396 ms. p=0.04.
Discussion
The key finding in this pilot study was that NF treatment did not produce significant positive changes on any of the primary outcome measures. Significant positive changes were found for the Med group. At the individual level, we found positive changes (defined in the Results section) for two patients in the NF group and negative changes for three. In the Med group, we found nine positive and no negative changes. The positive changes in the Med group measured in this study were related to ADHD symptoms and executive function. We did not make corrections for multiple comparisons, increasing the risk for positive and negative random changes. Our criteria for significant changes were relatively strict, however, and there is no reason to believe that random changes favored positive changes over negative changes in either group.
Excess power in the theta band (4–8 Hz) and/or reduced power of low beta (13–20 Hz) have been considered a hallmark of ADHD and a target for change in most NF protocols (Clarke et al. 2001; Chabot et al. 2005; Loo and Barkley 2005; Snyder and Hall 2006). Several studies (Monastra 2005b; Snyder et al. 2008) have used Cz as the site of registration. Reductions of power in the theta band after successful treatment with NF and medication have been reported (Monastra et al. 2002; Loo et al. 2004). Clinical improvement after NF treatment is not always accompanied by changes in QEEG, however (Lubar et al. 1995; Arns et al. 2009; Othmer, personal communication, 2010; Arns et al. 2012).
Because NF did not result in reduced symptoms of ADHD in our study, it comes as no surprise that the theta or beta level at Cz did not change from T1 to T2. We expected to see changes in the Med group, as they showed a significant reduction in ADHD symptoms (Tables 2 and 3). Theta and beta at Cz were remarkably stable in both groups, however. It may be that excess theta is a marker for ADHD at a more basic level, and not a good indicator of symptom change.
The P3 no go component in go/no go tasks such as VCPT has been reported to be smaller in ADHD. It has also been found to increase in a group of NF responders with ADHD (Kropotov et al. 2005; Kropotov 2009) and in medication responders (Barry et al. 2003b; Sawada et al. 2010). We hypothesized that the P3 no go component amplitude would increase in ADHD patients successfully treated, as this component is thought to reflect inhibitory control. There was a reduction in the NF group from T1 to T2 and, as predicted, an increase in the 12 medication responders using medication on a daily basis; however, neither of these findings reached statistical significance. A small n and large SDs may provide part of the explanation. When scored individually, 8 of these 12 responders showed a significant increase of the P3 no go component. The site of maximum increase was Cz (4), Pz (3), and Fz (1), which may explain the lack of significant changes when measuring at Cz only. Although we see this component increase as an indication that P3 no go may be a marker for medication response in ADHD, four of the responders did not show such a change in ERP pattern.
Studies examining the effects of NF in ADHD are inconclusive. Arns et al. (2009) conclude that “neurofeedback treatment for ADHD can be considered ‘Efficacious and Specific.’” Lofthouse et al. (2012) suggest “probably Efficacious,” whereas Lansbergen et al. (2011) found no effect of NF. The effect size reported in two other studies were modest (Gevensleben et al. 2010; deBeus and Kaiser 2011). In a recent review (Skokauskas et al. 2011), the conclusion regarding effects was negative.
It is paradoxical that protocol selection is the topic most discussed in the NF literature, and that researchers have found no differences related to protocol (Arns et al. 2009). Although we chose to use QEEG-based ADHD protocols in this study, the use of standard protocols for enhancing attention or reducing hyperactivity might have been a better solution. For some patients, we added a second protocol to address EEG deviances not covered by the ADHD protocol, and checked the effect by splitting the NF group into “improved” and “not improved.” This classification was not influenced by the inclusion of a second protocol. For each NF patient, we completed the predetermined protocols to make it clear what was actually done. Although most research studies follow the same principle, this is not always common practice in clinics, and may reduce the generalizability of our findings. On the other hand, we did not receive feedback of adverse effects or other information indicating a need of protocol change. In the Med group, individual adjustments were made.
NF is an expensive intervention, and it is critical to increase our knowledge about the patients who may benefit from the treatment. If (some of ) the training effects are related to active learning and everyday use of new skills, a certain level of maturity and motivation is an obvious advantage. In situations in which the level of conflict at home or school is high, a focus on training is rendered difficult, and NF may not be a good idea. Many clinicians argue that after 5–10 sessions, it should be possible to differentiate the responders from the nonresponders, so that NF can be stopped in time for the last group.
We have no systematic data that reveal whether or not the patients learned to change their EEG within each session. Improvements shown in performance curves were pointed out to them, but data were not saved. How useful QEEG and ERPs are in predicting good responders is still not clear, although a high level of power in the theta band is often considered a good indication.
Limitations
The results of this small pilot study with its obvious weaknesses cannot disprove studies showing that NF is effective. A number of factors may have contributed to the lack of effect in the present study.
The sample size was small, suited only for detection of large treatment effect sizes.
The patients were unselected clinical cases. We had few exclusion criteria; 59% of the patients in our study had learning disabilities; a much higher proportion than in most studies of the effect of NF on ADHD. Effects of NF regarding a beneficial outcome may be harder to achieve in this population.
We believe that the number and distribution of training sessions might have exerted a negative effect on our results. We were unable to reach our goal of two sessions per week. Frequent training sessions, especially in the beginning, and a combination of clinic training supplemented with home or school training might have been better, and more in accordance with general principles of learning.
A systematic collaboration with parents and teachers for motivating the children to use their new skills would be in line with the practice of many clinicians.
Conclusions
Our study supports positive effects for stimulants 7–11 months after onset of treatment, but not for NF. Clinical success reported in several NF studies may be related to nonspecific therapeutic factors, patient selection, frequency of sessions, supplementary training in school or home, and explicit use of new skills. Choosing the best protocol, standard or QEEG-based, is still a matter of controversy.
Power in the theta and beta band did not change for any of the groups. The P3 no go ERP component may be a marker for treatment response. We saw significant increases of this component in 8 of the 12 Med responders.
Clinical Significance
The way we completed NF in this highly comorbid sample of ADHD patients did not lead to significant clinical improvements. We suggest that NF may be best suited for carefully selected individuals.
Disclosures
No competing financial interests exist.
This study was financially supported by Østfold Hospital Trust, Norway. Professionally the project is connected to The Norwegian University of Science and Technology (NTNU), Trondheim, Norway, Institute of Psychology.
Acknowledgments
Psychologist Elisabeth Roinas was engaged as an assistant, conducting several training sessions and collecting data. Protocol selection was discussed with Professor Juri Kropotov. Professor Leiv Sandvik was our statistical adviser.
References
- American Academy of Pediatrics: Clinical practice guideline. Diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics. 2000;105:1158. doi: 10.1542/peds.105.5.1158. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Text Revision. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- American Psychiatric Association. Text Revision. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Arns M. de Ridder S. Strehl U. Breteler M. Coenen A. Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clin EEG Neurosci. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Arns M. Drinkenburg W. Kenemans J. The effects of QEEG-informed neurofeedback in ADHD: An open-label pilot study. Appl Psychophysiol Biofeedback. 2012;24:1–10. doi: 10.1007/s10484-012-9191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T. Coghill D. Santosh P. Zuddas A. Asherson P. Buitelaar J. Danckaerts M. Dopfner M. Faraone SV. Rothenberger A. Sergeant J. Steinhausen HC. Sonuga–Barke EJ. Taylor E. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry. 2006;15:476–495. doi: 10.1007/s00787-006-0549-0. [DOI] [PubMed] [Google Scholar]
- Barkley RA. 3rd. New York: The Guilford Press; 2006. Attention-Deficit Hyperactivity Disorder. A Handbook for Diagnosis and Treatment; pp. 184–219. [Google Scholar]
- Barkley RA. Psychosocial treatments for attention-deficit/hyperactivity disorder in children. J Clin Psychiatry. 2002;63(Suppl 12):36–43. [PubMed] [Google Scholar]
- Barry RJ. Clarke AR. Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003a;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Barry RJ. Clarke AR. Johnstone SJ. Oades RD. Electrophysiology in attention-deficit/hyperactivity disorder. Int J Psychophysiol. 2005;58:1–3. doi: 10.1016/j.ijpsycho.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Barry RJ. Johnstone SJ. Clarke AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol. 2003b;114:184–198. doi: 10.1016/s1388-2457(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Chabot RJ. di Michele F. Prichep L. The role of quantitative electroencephalography in child and adolescent psychiatric disorders. Child Adolesc Psychiatr Clin N Am. 2005;14:21–53. doi: 10.1016/j.chc.2004.07.005. v–vi. [DOI] [PubMed] [Google Scholar]
- Clarke AR. Barry RJ. McCarthy R. Selikowitz M. EEG-defined subtypes of children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2001;112:2098–2105. doi: 10.1016/s1388-2457(01)00668-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale: Laurence Erlbaum; 1988. [Google Scholar]
- Conners CK. Sitarenios G. Parker JD. Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- deBeus R. Kaiser DA. Neurofeedback with children with attention deficit hyperactivity disorder. a randomized double-blind placebo-controlled study. In: Coben R, editor; Evans JR., editor. Neurofeedback and Neuromodulation. Techniques and Applications. London: Academic Press; 2011. pp. 127–152. [Google Scholar]
- Foster PS. Drago V. Handbook of Neurofeedback: Dynamics, Clinical Applications. New York: Haworth Medical Press; 2007. [Google Scholar]
- Fuchs T. Birbaumer N. Lutzenberger W. Gruzelier JH. Kaiser J. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: A comparison with methylphenidate. Appl Psychophysiol Biofeedback. 2003;28:1–12. doi: 10.1023/a:1022353731579. [DOI] [PubMed] [Google Scholar]
- Gevensleben H. Holl B. Albrecht B. Vogel C. Schlamp D. Kratz O. Studer P. Rothenberger A. Moll GH. Heinrich H. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J Child Psychol Psychiatry. 2009;50:780–789. doi: 10.1111/j.1469-7610.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- Gevensleben H. Moll GH. Heinrich H. Neurofeedback training in children with ADHD: Behavioral and neurophysiological effects. Z Kinder Jugendpsychiatr Psychother. 2010;38:409–420. doi: 10.1024/1422-4917/a000070. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Gillberg IC. Rasmussen P. Kadesjo B. Soderstrom H. Rastam M. Johnson M. Rothenberger A. Niklasson L. Co-existing disorders in ADHD – implications for diagnosis and intervention. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):I80–192. doi: 10.1007/s00787-004-1008-4. [DOI] [PubMed] [Google Scholar]
- Gioia GA. Isquith PK. Lutz, FL: Psychological Assessment Resources (PAR); 2000. BRIEF Behavior Rating Inventory of Executive Function. [Google Scholar]
- Goodman R. Ford T. Richards H. Gatward R. Meltzer H. The Development and Well-Being Assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–655. [PubMed] [Google Scholar]
- Heinrich H. Gevensleben H. Strehl U. Annotation: Neurofeedback – train your brain to train behaviour. J Child Psychol Psychiatry. 2007;48:3–16. doi: 10.1111/j.1469-7610.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- Hollup ECA. Can EEG neurofeedback be an alternative treatment for ADHD?[in German] Dissertation Trondheim. 2007;76 [Google Scholar]
- Holtmann M. Stadler C. Electroencephalographic biofeedback for the treatment of attention-deficit hyperactivity disorder in childhood and adolescence. Expert Rev Neurother. 2006;6:533–540. doi: 10.1586/14737175.6.4.533. [DOI] [PubMed] [Google Scholar]
- Johnstone J. Gunkelman J. Lunt J. Clinical database development: Characterization of EEG phenotypes. Clin EEG Neurosci. 2005;36:99–107. doi: 10.1177/155005940503600209. [DOI] [PubMed] [Google Scholar]
- Kropotov JD. Amsterdam: Elsevier AP; 2009. Quantitative EEG Event-Related Potentials and Neurotherapy. [Google Scholar]
- Kropotov JD. Grin–Yatsenko VA. Ponomarev VA. Chutko LS. Yakovenko EA. Nikishena IS. ERPs correlates of EEG relative beta training in ADHD children. Int J Psychophysiol. 2005;55:23–34. doi: 10.1016/j.ijpsycho.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Lansbergen MM. van Dongen–Boomsma M. Buitelaar JK. Slaats–Willemse D. ADHD and EEG-neurofeedback: A double-blind randomized placebo-controlled feasibility study. J Neural Transm. 2011;118:275–284. doi: 10.1007/s00702-010-0524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leins U. Hinterberger T. Kaller S. Schober F. Weber C. Strehl U. Neurofeedback for children with ADHD: A comparison of SCP- and theta/beta-protocols. Prax Kinderpsychol Kinderpsychiatr. 2006;55:384–407. [PubMed] [Google Scholar]
- Levesque J. Beauregard M. Mensour B. Effect of neurofeedback training on the neural substrates of selective attention in children with attention-deficit/hyperactivity disorder: A functional magnetic resonance imaging study. Neurosci Lett. 2006;394:216–221. doi: 10.1016/j.neulet.2005.10.100. [DOI] [PubMed] [Google Scholar]
- Lofthouse N. Arnold LE. Hersch S. Hurt E. Debeus R. A review of neurofeedback treatment for pediatric ADHD. J Atten Disord. 2012;16:351–372. doi: 10.1177/1087054711427530. [DOI] [PubMed] [Google Scholar]
- Loo SK. Barkley RA. Clinical utility of EEG in attention deficit hyperactivity disorder. Appl Neuropsychol. 2005;12:64–76. doi: 10.1207/s15324826an1202_2. [DOI] [PubMed] [Google Scholar]
- Loo SK. Hopfer C. Teale PD. Reite ML. EEG correlates of methylphenidate response in ADHD: Association with cognitive and behavioral measures. J Clin Neurophysiol. 2004;21:457–464. doi: 10.1097/01.wnp.0000150890.14421.9a. [DOI] [PubMed] [Google Scholar]
- Lubar JF. Lubar JO. Neurofeedback assessment, treatment for attention deficit/hyperactivity disorders. In: Evans JR, editor; Abarbanel A., editor. Introduction to Quantitative EEG and Neurofeedback. London: Academic Press; 1999. pp. 103–143. [Google Scholar]
- Lubar JF. Swartwood MO. Swartwood JN. O'Donnell PH. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul. 1995;20:83–99. doi: 10.1007/BF01712768. [DOI] [PubMed] [Google Scholar]
- Monastra VJ. Electroencephalographic biofeedback (neurotherapy) as a treatment for attention deficit hyperactivity disorder: Rationale and empirical foundation. Child Adolesc Psychiatr Clin N Am. 2005a;14:55–82. doi: 10.1016/j.chc.2004.07.004. vi, [DOI] [PubMed] [Google Scholar]
- Monastra VJ. Overcoming the barriers to effective treatments for attention-deficit/hyperactivity disorder: A neuro-educational approach. Int J Psychophysiol. 2005b;58:71–80. doi: 10.1016/j.ijpsycho.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Monastra VJ. Lubar JF. Linden M. The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: reliability and validity studies. Neuropsychology. 2001;15:136–144. doi: 10.1037//0894-4105.15.1.136. [DOI] [PubMed] [Google Scholar]
- Monastra VJ. Lubar JF. Linden M. VanDeusen P. Green G. Wing W. Phillips A. Fenger TN. Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: An initial validation study. Neuropsychology. 1999;13:424–433. doi: 10.1037/0894-4105.13.3.424. [DOI] [PubMed] [Google Scholar]
- Monastra VJ. Monastra DM. George S. The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Appl Psychophysiol Biofeedback. 2002;27:231–249. doi: 10.1023/a:1021018700609. [DOI] [PubMed] [Google Scholar]
- Mueller A. Candrian G. Kropotov JD. Ponomarev VA. Baschera GM. Classification of ADHD patients on the basis of independent ERP components using a machine learning system. Nonlinear Biomed Phys. 2010;4(Suppl 1):S1. doi: 10.1186/1753-4631-4-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G. Jensen P. Epidemiologic considerations in attention deficit hyperactivity disorder: A review and update. Child Adolesc Psychiatr Clin N Am. 2008;17:245–260. doi: 10.1016/j.chc.2007.11.006. vii, [DOI] [PubMed] [Google Scholar]
- Rossiter T. The effectiveness of neurofeedback and stimulant drugs in treating AD/HD: Part II. Replication. Appl Psychophysiol Biofeedback. 2004;29:233–243. doi: 10.1007/s10484-004-0383-4. [DOI] [PubMed] [Google Scholar]
- Sawada M. Iida J. OtaT Negoro H. Tanaka S. Sadamatsu M. Kishimoto T. Effects of osmotic-release methylphenidate in attention-deficit/hyperactivity disorder as measured by event-related potentials. Japanese Society of Psychiatry and Neurology, Australia. Psychiatry Clin. Neurosci. 2010;64:491–498. doi: 10.1111/j.1440-1819.2010.02134.x. [DOI] [PubMed] [Google Scholar]
- Skokauskas N. McNicholas F. Masaud T. Frodl T. Complementary medicine for children and young people who have attention deficit hyperactivity disorder. Curr Opin Psychiatry. 2011;24:291–300. doi: 10.1097/YCO.0b013e32834776bd. [DOI] [PubMed] [Google Scholar]
- Snyder SM. Quintana H. Sexson SB. Knott P. Haque AF. Reynolds DA. Blinded, multi-center validation of EEG and rating scales in identifying ADHD within a clinical sample. Psychiatry Res. 2008;159:346–358. doi: 10.1016/j.psychres.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Snyder SM. Hall JR. A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. J Clin Neurophysiol. 2006;23:440–455. doi: 10.1097/01.wnp.0000221363.12503.78. [DOI] [PubMed] [Google Scholar]
- Strehl U. Leins U. Goth G. Klinger C. Hinterberger T. Birbaumer N. Self-regulation of slow cortical potentials: A new treatment for children with attention-deficit/hyperactivity disorder. Pediatrics. 2006;118:e1530–1540. doi: 10.1542/peds.2005-2478. [DOI] [PubMed] [Google Scholar]
- Taylor E. Dopfner M. Sergeant J. Asherson P. Banaschewski T. Buitelaar J. Coghill D. Danckaerts M. Rothenberger A. Sonuga–Barke E. Steinhausen HC. Zuddas A. European clinical guidelines for hyperkinetic disorder – first upgrade. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):I7–30. doi: 10.1007/s00787-004-1002-x. [DOI] [PubMed] [Google Scholar]
- Thompson L. Thompson M. QEEG, neurofeedback for assessment, effective intervention with attention deficit hyperactivity disorder (ADHD) In: Budzynski TH., editor. Introduction to Quantitative EEG and Neurofeedback: Advanced Theory and Applications. 2nd. Vol. 502. New York: AP; 2009. pp. 337–364. [Google Scholar]
- van As J. Hummelen JW. Buitelaar JK. Neurofeedback and attention deficit hyperactivity disorder: What is it and is it working? Tijdschr Psychiatr. 2010;52:41–50. [PubMed] [Google Scholar]
- Vigario RN. Extraction of ocular artefacts from EEG using independent component analysis. Electroencephalogr Clin Neurophysiol. 1997;103:395–404. doi: 10.1016/s0013-4694(97)00042-8. [DOI] [PubMed] [Google Scholar]