Abstract
Background
MicroRNAs (miRNAs) are increasingly being recognized as being involved in cancer development and progression in gliomas.
Methods
Using a model cell system developed in our lab to study glioma progression comprising human neuroglial culture (HNGC)–1 and HNGC-2 cells, we report here that miR-145 is one of the miRNAs significantly downregulated during malignant transformation in glioblastoma multiforme (GBM). In a study using tumor samples derived from various glioma grades, we show that expression of miR-145 is decreased in a graded manner, with GBM patients showing lowest expression relative to lower-grade gliomas (P < .05) and normal brain tissues (P < .0001). Functional studies involving ectopic expression of miR-145 in glioma cells had a negative impact on cell proliferation and tumor development, as well as invasion and induced apoptosis, providing further support to the concept that inactivation of miR-145 is important for glioma disease pathogenesis. More notably, these growth-suppressive effects of miR-145 are mediated through its target proteins Sox9 and the cell adhesion-associated molecule adducin 3 (ADD3).
Results
Inhibiting Sox9 and ADD3 rescued effects of miR-145 loss. Interestingly, miR-145 loss in glioma cells led to overexpression of molecules involved in cell proliferation, like cyclin D1, c-myc, and N-myc, as well as enhanced expression of cell adhesion- and invasion-related molecules N-cadherin and E-cadherin, an effect which was again restored upon miR-145 overexpression in glioma cells. The miR-145 promoter was methylated at its cytosine–phosphate–guanine (CpG) islands in the glioma cell lines studied.
Conclusion
Our study demonstrates that miR-145 has a tumor-suppressive function in glioblastoma in that it reduces proliferation, adhesion, and invasion of glioblastoma cells, apparently by suppressing the activity of oncogenic proteins Sox9 and ADD3. Reduced levels of miR-145 may lead to neoplastic transformation and malignant progression in glioma due to unregulated activity of these proteins.
Keywords: adducin 3, glioma stem cells, invasion, neoplastic transformation, promoter methylation, Sox9
Malignant gliomas are the most common tumors of the CNS, with dismal prognosis. Among gliomas, glioblastoma multiforme (GBM; World Health Organization [WHO] grade IV) is one of the most aggressive CNS tumors and is unresponsive to most modalities of treatment. The chemo- and radioresistance of GBM tumors stems from high levels of heterogeneity displayed by the tumor, often attributed to a small subpopulation of tumor-initiating cells driving tumor growth.1 These tumor-initiating cells or glioma stem cells display the ability to self-renew, yielding progeny comprising additional stem cells as well as cells differentiating into various nontumor cells within the tumor.2 It is not evident whether glioma arises from neural stem cells, from differentiated progenitors, or from fully differentiated glial cells themselves.
The high level of molecular diversity and heterogeneity in GBM have led to its classification into 4 subtypes based on the gene-expression data derived from The Cancer Genome Atlas. The heterogeneity possibly arises from complex interactions of the tumor-initiating cells with the host microenvironment. Hence, it is critical to identify specific molecular signaling pathways activated in glioma stem cells or nontumor stem cell population regulating processes like cell survival, proliferation, and angiogenesis. This will help outline novel approaches to target resistance mechanisms as well as define strategies for drug delivery across the blood–brain barrier. Most of the GBM tumors exhibit abnormalities in pathways regulating the cell cycle, leading to failure of growth arrest and senescence due to defects in core signaling pathways like p53, retinoblastoma, and receptor tyrosine kinase.3
Importantly, aberrations in core signaling pathways identified through genome-wide screening studies are frequently driven by abnormal expression of microRNAs (miRNAs). Several miRNAs are implicated in almost all biological processes by virtue of their role in regulating gene expression. Using phenomena of RNA interference, these miRNAs induce gene silencing of their cognate target genes, through either translational repression or mRNA decay. The distinct patterns of aberrant DNA methylation, histone modifications, and varied miRNA expression profiles reiterate the heterogeneity evident in glial tumors. Interestingly, either overexpression of miRNAs with pro-oncogenic functions or downregulation of miRNAs with growth-suppressive effects contributes to tumorigenicity and invasiveness in glioma. Recent studies have demonstrated that miR-483-5p,4 miR-137,5 miR-128,6 and miR-1247 are significantly downregulated in glioma tumor tissues and cell lines, while miR-34a is specifically repressed in the proneural subtype of gliomas.8 In contrast, miRNAs like miR-21 and the miR-221/222 cluster are overexpressed in glioma cells and exert their effects by affecting multiple genes associated with glioma cell proliferation. The role of microRNAs like miR-143 and miR-145 as suppressors of tumor metastasis and invasion is reported in several colon and ovarian cancer tissues and cell lines,9,10 prostate cancers,11 and several cervical cancer tissues.12 The role of miR-145 in glioma is not clearly understood. While there are studies indicating its role as a tumor suppressor,13–16 few studies suggest it to be associated with an invasive phenotype in glioblastoma cell lines.17 We here provide evidence for the role of miR-145 as one of the tumor-suppressive miRNAs that is frequently downregulated in a graded manner during malignant transformation in glioblastomas. Next, we demonstrate that miR-145 affects GBM tumorigenesis through its targets Sox9 and adducin 3 (ADD3). Sox9, a member of the Sry-related high-mobility group box family, functions as a transcription factor that plays a significant role in the development and differentiation of multiple cell lineages and has recently been reported to be overexpressed in gliomas.18 Interestingly, ADD3, a membrane protein involved in stabilization of epithelial junctions, is presumed to have a role in epithelial cancers19 but is not reported in brain tumors. In this study, we demonstrate that downregulation of miR-145 leads to activation of its targets Sox9 and ADD3 in GBM, causing pro-invasive and malignant characteristics in GBM.
Materials and Methods
Clinical Samples
The use of human tumor tissues in the present study was approved by the Institutional Ethics Committee of the National Centre for Cell Science (NCCS), Pune, and KEM Hospital, Mumbai, India. Tumor tissue samples were collected from KEM Hospital and assigned specific tumor stages and pathological grades by a neuropathologist according to WHO criteria for gliomas.20 Signed consent to use tissues for research purposes was obtained from patients prior to surgery. Human brain tumor biopsies were taken during standard neurosurgical resection of brain tumors. A total of 29 glioma tumor tissue samples and 5 normal brain tissues were collected and processed for extraction of RNA, and a part of the remaining tissue was used for generation of long-term glioma cultures.
Cell Culture
The development of the human neuroglial culture (HNGC) stem cell lines HNGC-1 and HNGC-2 from glioma tissue has been described.21,22 The cell lines HNGC-1 and HNGC-2 and other long-term glioma cultures generated from tumor tissue specimens (Supplementary Table S1) were cultured in Dulbecco's modified Eagle's medium (DMEM)/Ham's F12 medium (1 : 1; Invitrogen) with 1× B27 supplement (Invitrogen), epidermal growth factor (10 ng/mL; Invitrogen) and basic fibroblast growth factor (20 ng/mL; Invitrogen), 1× nonessential amino acids (Invitrogen), and 1× Glutamax (Invitrogen) at 37°C with 5% CO2 in a humidified incubator. The stem cell line NSG-K16, derived from GBM tumor tissue, was established as a neurosphere culture and maintained under serum-free conditions.23 Human glioblastoma cell lines LN-18 and LN-229 were obtained from the American Type Culture Collection and maintained in DMEM (Sigma-Aldrich) supplemented with 5% (volume/volume) fetal bovine serum (FBS; Gibco), 50 U/mL penicillin (Sigma-Aldrich), and 50 µg/mL streptomycin (Sigma-Aldrich) and incubated in 5% CO2. The glioma cell line U373MG (American Type Culture Collection) was grown in minimum essential medium with sodium pyruvate in 5% FBS.
Real-time PCR
Total RNA was extracted from tissues and cells with Trizol Reagent (Invitrogen) in accordance with the protocol specified by the manufacturer, and its quality was assessed with a BioPhotometer (Eppendorf). 100 ng of total RNA was reverse transcribed into cDNA using the MirVana MiRNA Detection Kit (Ambion). Expression of mature miR-145 was quantified using SYBR green master mix (Applied Biosystems) with the 7500 Fast Real Time PCR System (Applied Biosystems). The amplification reaction (10 μL) included 2× SYBR green master mix, gene-specific primers, and cDNA. Fold changes in gene expression were calculated using the 2−ΔΔCt method with 18S rRNA serving as an internal control.
Construction of Plasmids
The pre–miR-145 lentiviral vector was a kind gift from Dr Yin-Yuan Mo (Southern Illinois University). It was constructed by ligating the miR-145 precursor (1.5 kb) into the EcoR1 and NotI sites of the pCDH-CMV-MCS-EF1-copGFP lentivector.24 The lentivector was packaged using PACKH1 Packaging Plasmid mix (System Biosciences) and transiently cotransfected into HEK-293 cells to generate recombinant virus particles. After 48 h of infection, lentivirus in the supernatant was transduced into glioma cells—HNGC-2, NSG-K16, and LN-229—using 8 µg/mL of polybrene (Sigma-Aldrich). Stable clones were maintained on 10 µg/mL of puromycin (Sigma-Aldrich). The control lentiviral vector pCDH-CMV-MCS-EF1-copGFP stably expressed green fluorescent protein (GFP), while the miR-145 lentiviral vector, Lenti-GFP-miR-145, stably expressed GFP and miR-145. In our experiments, cells were divided into 2 groups, designated the EV group (empty vector; transduced with vector only) and the miR-145 group (transduced with miR-145). The luciferase-3′ untranslated region (UTR) reporter plasmid was constructed by introducing Sox9 and ADD3 3′UTR regions carrying a putative miR-145 binding site into luciferase plasmid pMIR-REPORT miRNA Expression Reporter Vector (Life Technologies). We amplified Sox9 and ADD3 3′UTR sequences from U87MG DNA using PCR primers (Supplementary Table S2). The PCR products were gel eluted and ligated into pMIR-REPORT Luciferase plasmid. The specificity of PCR products was confirmed by DNA sequencing.
MTT Assay
Proliferation potential of cells was determined by assay by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] .25 Briefly, cells were seeded into 96-well plates (BD Biosciences) at a cell density of 1 × 103 cells/well in growth medium. Cell growth was assayed by addition of 20 μL of MTT (5 mg/mL; Sigma-Aldrich) to each well, and the plate was incubated at 37°C for 4 h. The proliferation assay was performed for 3 days and cell growth was assayed at every 24 h interval. Later, the reaction was stopped by addition of 200 μL dimethyl sulfoxide (Sigma-Aldrich). Optical density was measured at 570 nm with a microplate reader (Bio-Rad).
Cell Cycle Analysis
Glioma cell lines HNGC-2, NSG-K16, and LN-229 transfected with EV and miR-145 were used for determination of cell cycle kinetics using flow cytometry. EV cell lines were also transfected with small interfering (si)RNAs to Sox9 or ADD3 (50 ng/mL; Santa Cruz Biotechnology) with Lipofectamine 2000 reagent (Invitrogen). After overnight transfection, cells were incubated for an additional 36 h. Later, the siRNA-transfected cells as well as the EV and miR-145 stable glioma cell lines were harvested, washed twice in phosphate buffered saline (PBS), and fixed with 75% ethanol for 30 min on ice. After washing in cold PBS 3 times, cells were resuspended in 500 µL PBS with 50 µg/mL of propidium iodide (PI) solution and 10 µg/mL of DNase-free RNase A for 30 min at 37°C. Samples were analyzed for DNA content by FACSCalibur (Becton Dickinson) using a blue 488 nm laser for excitation and an FL2 detector 585 ± 21 nm for emission. DNA histograms were analyzed with CellQuest Pro analysis software 5.2.1 (BD Bioscience).
Apoptosis Assay
Apoptosis occurring via active caspases was detected in the EV and miR-145 glioma cells using the SR-FLICA [Fluorescent Labeled Inhibitor of Caspases] Poly Caspases Assay kit (ImmunoChemistry Technologies). In brief, cells were grown on coverslips and 1× FLICA solution was added to the cells for 1 h at 37°C. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 10 min and mounted in medium containing fixative. Confocal images were acquired with a Leica TCS SP5II microscope.
Analyses of caspase 3, caspase 9, and poly (ADP-ribose) polymerase (PARP) activities were performed using an apoptosis sampler kit (Cell Signaling Technology) per manufacturer's protocol. Briefly, cells were fixed, permeabilized, and blocked in blocking buffer (2% bovine serum albumin in 1× PBS) for 60 min followed by 1 h incubation with unconjugated caspase 3, caspase 9, or PARP antibodies separately (1 : 100 dilutions). Later, the cells were washed in blocking buffer followed by 30 min incubation with Alexa Fluor 594 anti-rabbit antibody (1 : 200; Invitrogen). The cells were resuspended in 0.5 mL 1× PBS and data were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analyzed with CellQuest Pro analysis software 5.2.1.
Confocal Microscopy
Glioma cells (EV and miR-145 transfected) were grown on coverslips, fixed, permeabilized, and blocked with 5% bovine serum albumin in 1× PBS for 1 h at room temperature. Cells were incubated with Ki67 antibody (1 : 100; Chemicon International) for 2 h at room temperature and washed thrice with PBS, followed by incubation for 1 h with Alexa Fluor 594 anti-rabbit antibody (1 : 200; Invitrogen). Nuclei were stained with DAPI (Sigma-Aldrich) for 10 min. Confocal images were acquired with a Leica TCS SP5II microscope.
Western Blot Analysis
Cells were lysed in 1× radioimmunoprecipitation assay lysis buffer (Thermo Scientific) with protease inhibitor cocktail (1×; Sigma-Aldrich). Homogenates were clarified by centrifugation at 12 000 rpm for 15 min at 4°C. Cell lysates (40 µg) were loaded on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene difluoride membranes (Millipore). The blots were incubated with primary antibodies to ADD3 (Santa Cruz), Sox9 (Santa Cruz), N-myc (Santa Cruz), cyclin D1 (Abcam), c-Myc (Santa Cruz), E-cadherin (Cell Signaling Technology), or N-cadherin (1 : 1000 dilution; Cell Signaling Technology), followed by incubation with horseradish peroxidase–conjugated, species-specific secondary antibodies (1:2000 dilution; Thermo Scientific). The specific proteins were detected using a Super Signal protein detection kit (Thermo Scientific). Protein levels were normalized by reprobing blots with β-actin antibody (MP Biomedicals).
Luciferase Assay
EV and miR-145 stably expressing glioma cells were seeded into 24-well plates (1 × 104 cells/well). After 24 h, the cells were cotransfected with 200 ng per well of pMIR-REPORT Luciferase reporter vector (Applied Biosystems) or pMIR-REPORT vector containing either Sox9 or ADD3 3′UTR variants. Luciferase activity was measured 48 h posttransfection using the Dual-Glo Luciferase Assay System according to the manufacturer's instructions (Promega). For each sample, Renilla luciferase activity was normalized to firefly luciferase expression.
Matrigel Invasion Assay
Invasion assay in vitro was performed in 24-well cell culture plates using Matrigel Invasion Chambers of 8.0 μm pore size membrane using manufacturer's protocol (BD Biosciences) and the method described by Albini et al.26 Briefly, 5 × 103 cells of either EV or miR-145 glioma cells in 0.5 mL serum-free DMEM were seeded in the upper compartment of each well of the test and control inserts. DMEM with 10% FBS served as a chemoattractant in lower compartments of both inserts. The plate was incubated for 22 h. After incubation, the noninvading cells on the upper surface were mechanically scrubbed and removed, and the filters were fixed and stained with 0.1% (weight/volume) crystal violet (Sigma-Aldrich) for 15 min and washed with 1× PBS to remove excess stain. Migrated cells on the bottom side of the membrane were counted in 10 different fields to acquire the average number of migrating cells per cell line with an Olympus IX51 fluorescent microscope and quantified with cellSens 1.3 software (Olympus).
In vivo Tumor Growth Assay
The animal studies were approved by the Institutional Animal Ethics Committee of NCCS and experiments were performed in accordance with the Animal Ethics guidelines of NCCS. For in vivo tumorigenicity assays, 6-week-old nonobese diabetic severe combined immunodeficient (NOD-SCID) mice were used. Briefly, 1 × 104 EV cells and miR-145 transfectant glioma cells were injected subcutaneously in NOD-SCID mice (n = 18), who were periodically observed for tumor development. Tumor volumes were determined using the formula: 4/3π (√major axis/2 × minor axis/2). Tumors were fixed with formalin and analyzed by hematoxylin and eosin (H&E) staining.
Orthotopic Grafts With GFP Transfected Cells
Into the striatum of NOD-SCID mice (n = 0.5) were intracranially implanted unilaterally 1 × 104 EV cells and miR-145 transfected glioma cells in 1× PBS. The mice were euthanized after 4 weeks. The brain tumor tissue was analyzed for tumor foci under the Olympus IX51 fluorescent microscope followed by H&E staining of the brain tumor sections.
Methylation Assays
Genomic DNA was isolated using the Gen Elute mammalian DNA isolation kit (Sigma-Aldrich). About 2 μg of genomic DNA was subjected to bisulphite modification and purified using the Epitect Bisulphite kit (Qiagen) according to the manufacturer's instructions. A primer pair targeting a 0.3 kb fragment relative to the start site of the primary transcript of miR-145 (Supplementary Table S2) was designed using Methprimer software.27 Using bisulphite modified genomic DNA as a template, PCR was performed with the designed primer pair, and the products were thymine-adenine cloned in pGEM-T vector (Promega). About 6 positive clones per template were sequenced using the Applied Biosystems PRISM DNA sequencer, and the data were analyzed using Bismark software.28 For inhibition of DNA methylation, cells were treated with 5 pmol/mL 5-azacytidine (Sigma-Aldrich) for 4 days at 37°C. Cells were fed with fresh medium containing 5-azacytidine every 24 h. Control cells were treated with 1× PBS without the demethylating agent 5-azacytidine and underwent fresh media change in parallel with the treated group. After treatment, genomic DNA and total RNA were isolated for further analyses.
Bioinformatics
Candidate miR-145 targets were generated using the publicly available algorithms TargetScan (http://www.targetscan.org/), miRanda (http://www.microrna.org/), PicTar (pictar.mdc-berlin.de), and miRDB (http://www.mirdb.org).
Statistical Analysis
Data are presented as mean ± SEM from at least 3 independent experiments. Statistical analysis was performed using a 1-way ANOVA followed by either Student's t-test or Fisher's exact test. Differences between groups were considered to be statistically significant at P ≤ .05. Graphs were made in GraphPad Prism 5.01.
Results
MiR-145 Is Downregulated in Glioblastomas and Glioma Stem Cells
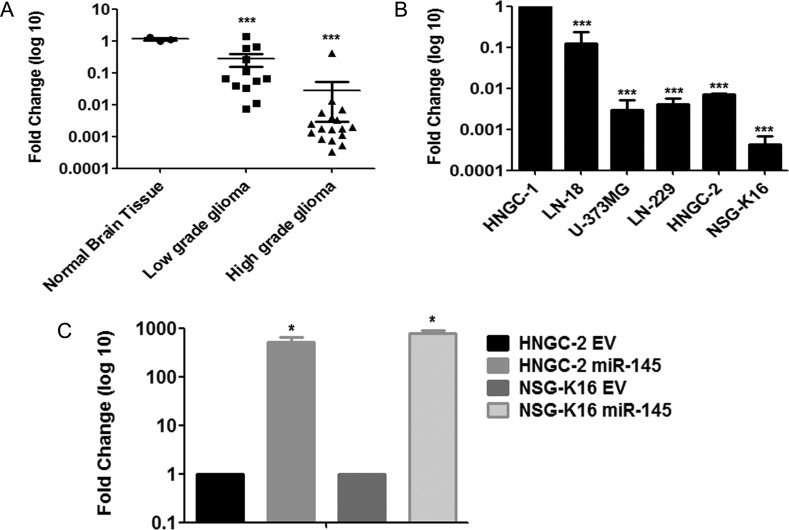
Our gene expression microarray data for global miRNA analyses of glioma cells HNGC-2 compared with the nontumorigenic neural stemlike cells HNGC-1 indicated that miR-145 was one of the miRNAs predominantly downregulated in HNGC-2 cells (Supplementary Fig. S1). To determine whether miR-145 levels were similarly low in glioma tumor tissues and GBM cell lines, we performed expression analyses using stem-loop quantitative real time (qRT)–PCR. For this, we quantified miR-145 expression in 29 glioma tumor tissue samples derived from all 4 grades, 5 established glioma cell lines, and 5 normal brain tissues. As shown in Fig. 1A, miR-145 levels were significantly downregulated in higher-grade gliomas and established GBM cell lines, compared with normal brain tissues. The expression levels negatively correlated to glioma grades, with lower grades (n = 2) exhibiting downregulation >5-fold and higher grades (n = 17) demonstrating a >50-fold decrease in miR-145 expression. These data provided us with experimental evidence that miR-145 expression was downregulated in a graded manner in gliomas, with higher grades showing lowest expression. Similarly, the various GBM cell lines analyzed (LN-18, U373MG, LN-229, HNGC-2, and NSG-K16) showed a >100-fold decrease in miR-145 levels (Fig. 1B) compared with expression of miR-145 normalized to one with respect to expression in the nontransformed neural stemlike cell line HNGC-1.
Fig. 1.
MiR-145 levels are downregulated in higher-grade gliomas and upregulated after transduction of cells with Lenti-GFP-miR-145 (A) Real-time quantitative RT-PCR assay for expression of miR-145 in low-grade and high-grade glioma tissues, compared with expression in normal brain tissues. The qRT-PCR assay revealed downregulation of miR-145 in glial tumors in a graded manner compared with normal brain tissue. ***P < .0001 vs control; (B) qRT-PCR analyses of miR-145 in glioma cell lines. The data were normalized with respect to expression of miR-145 in nontumorigenic neural stem cell line HNGC-1. Expression of miR-145 was downregulated in GBM cell lines. ***P < .0001 vs control. (C) The miR-145 level was significantly upregulated after transduction of HNGC-2 and NSG-K16 glioma cells with Lenti-GFP-miR-145, compared with EV-GFP groups. *P < .05 vs control. Transcript levels were normalized with respect to 18S rRNA expression. Each bar represents the means ± SEM of 3 independent experiments, with P-values vs controls generated using Student's t-test.
Because qRT-PCR data demonstrated that miR-145 levels were downregulated in higher-grade gliomas and GBM cell lines, we sought to understand the biological role of miR-145 by performing miR-145 overexpression studies. For this, we chose to use HNGC-2, NSG-K16, and LN-229 GBM cell lines, as they showed low expression of miR-145. We generated miR-145 overexpressing stable GBM cell lines that displayed increased expression of miR-145 by >500-fold in HNGC-2 and NSG-K16 cells (Fig. 1C) and by ∼5000-fold in LN-229 cells (Supplementary Fig. S3A) compared with corresponding EV cells. Here, EV cells were glioma cell lines transfected with vector alone.
MiR-145 Overexpression in Glioma Cells Induces Growth Arrest
Effects of miR-145 on cell growth, tumorigenicity, and invasion in glioma cell lines overexpressing miR-145 using a plethora of in vitro and in vivo assays. The MTT assay displayed a remarkable inhibition in cell growth due to miR-145 were studied overexpression in HNGC-2, NSG-K16 (Fig. 2A), and LN-229 (Supplementary Fig. S3B) cells compared with EV cells. Further, staining for Ki67, a proliferation marker, confirmed inhibitory effects of miR-145 on glioma cell growth. The EV groups comprising HNGC-2 and NSG-K16 cells (transfected with EV) showed most of the cells positive for Ki67, whereas glioma cells expressing miR-145 were almost devoid of Ki67 positivity (Fig. 2B), confirming growth-suppressive effects of miR-145 on cell proliferation. A marked inhibition in cell growth was also apparent at the morphological level, wherein HNGC-2 and NSG-K16 cells expressing miR-145 showed sparse growth in culture compared with EV cells seeded equally over a 3-day period (Supplementary Fig. S2A). Further, we investigated whether overexpression of miR-145 in GBM cells affected cell cycle progression by flow cytometry. As shown in Fig. 2C, compared with EV cells, introduction of miR-145 into both HNGC-2 and NSG-K16 cells significantly arrested cells at the G0/G1 transition, evidenced by a marked accumulation of cells in the G0/G1 peak. There was an increase in the number of cells arrested in G0/G1 phase by about 34.6% in HNGC-2 and by 30% in NSG-K16 cells compared with EV cells (Fig. 2C). A similar effect occurred in LN-229 cells, wherein there was significant increase in the number of cells arrested in the G0/G1 phase (Supplementary Fig. S3C). These results indicated that miR-145 overexpression produced a consistent reduction in cell proliferation together with defects in cell cycle progression leading to accumulation of cells in G0/G1 and extensive decrease in percentage of cells in S phase.
Fig. 2.
Overexpression of miR-145 inhibits cell proliferation and induces G1/S transition arrest in vitro. (A) Effects of miR-145 overexpression on proliferation of HNGC-2 and NSG-K16 cells compared with EV–control group cells as measured by MTT assay. MTT data are representative of 3 independent experiments, presented as mean ± SEM of triplicates. OD, optical density. (B) Immunofluroscence staining of proliferation marker Ki67 in EV and miR-145–overexpressing glioma cells and detected using anti-rabbit Alexa Flour 595 (red). Nuclei are stained with DAPI (blue). (C) Representative cell cycle distribution determined by flow cytometric analysis after staining with PI and showing distribution of cells in G0/G1, S, and G2/M phases in HNGC-2 and NSG-K16–EV and miR-145 cells (upper panel). MiR-145 overexpression promoted accumulation of cells in G0/G1 phase and resulted in G1/S transition arrest compared with EV-control cells. The lower panel represents data showing percentage distribution of cells in various phases of cell cycle generated from 3 independent experiments of cell numbers in various phases and represented as means ± SEM (n = 3).
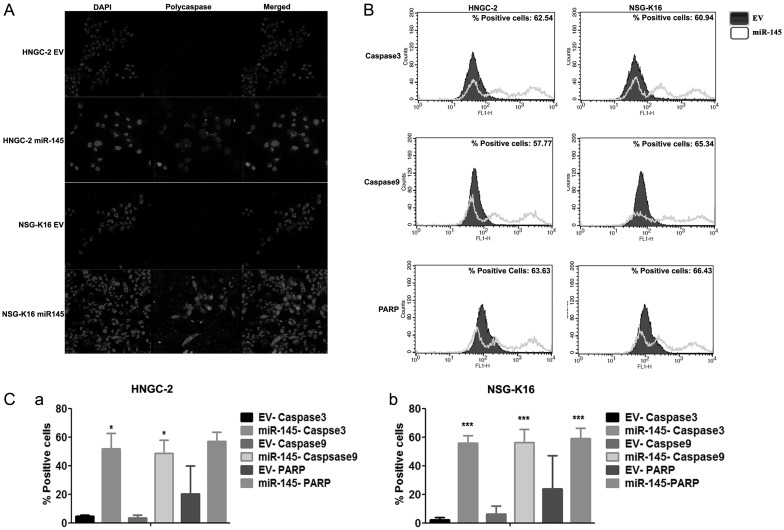
The growth-suppressive effects on glioma cells led us to analyze whether these effects were an outcome of the potential of miR-145 to induce apoptosis. To determine the number of cells that were driven to apoptosis by miR-145, we performed annexin V and PI staining in EV cells and the miR-145 group. For this, HNGC-2 cells transfected independently with the miR-145 construct or EV were costained with annexin V/PI. Interestingly, the HNGC-2 and NSG-K16 miR-145 cells possessed an increased number of cells in apoptotic phase compared with EV cells (data not shown). To investigate whether apoptosis induced by miR-145 in glioma cells was mediated through caspase-dependent mechanisms, we stained cells for polycaspases. We found a significantly higher number of miR-145-expressing HNGC-2, NSG-K16 (Fig. 3A), and LN-229 (Supplementary Fig. S3D) cells stained for polycaspases, whereas there were no apoptotic cells in EV cells. Next, by flow cytometry we analyzed expression of proteases, caspase 3, caspase 9, and PARP, which are involved in initiation and execution of apoptosis. As shown in Fig. 3B, 3 distinct peaks were obtained in miR-145–overexpressing HNGC-2 and NSG-K16 cells. The first cluster was overlaid with an EV cell population, while the second and third peaks represent cells in early and late apoptotic phases, respectively (Fig. 3B). The data showing number of apoptotic cells are represented as a histogram and show percentage positivity for caspase 3, caspase 9, and PARP in HNGC-2 (Fig. 3Ca) and NSG-K16 cells (Fig. 3Cb). The same data represented quantitatively as a bar diagram in Fig. 3C shows a large percentage of glioma cells undergoing apoptosis due to miR-145 in both HNGC-2 (Fig. 3Ca) and NSG-K16 (Fig. 3Cb) cells. These studies confirmed that one of the mechanisms through which miR-145 functions as a growth suppressor is induction of apoptosis in a caspase-dependent manner.
Fig. 3.
MiR-145 sensitizes glioma cells to caspase-dependent apoptosis. HNGC-2 and NSG-K16 cells stably expressing miR-145 or cells transfected with EV alone were analyzed for (A) fluorescence detection of polycaspases using FAM (carboxyfluorescein)-FLICA reagent and (B) measuring positivity for cleaved caspase 3, caspase 9, and PARP by flow cytometry. Iso-type control is represented as a filled plot. An unfilled histogram indicates staining for all 3 caspases in miR-145–overexpressing cells. (C) Quantitative representation of fluorescence activated cell sorting data showing relative expression of cleaved caspase 3, caspase 9, and PARP in HNGC-2 (a) and NSG-K16 (b) miR-145 transfected and control cells. Each bar represents the means ± SEM of 3 independent experiments. *P < .05, ***P < .0001 vs EV cells generated using t-test.
MiR-145 Inhibits Tumor Formation and Invasion in Glioma Cells
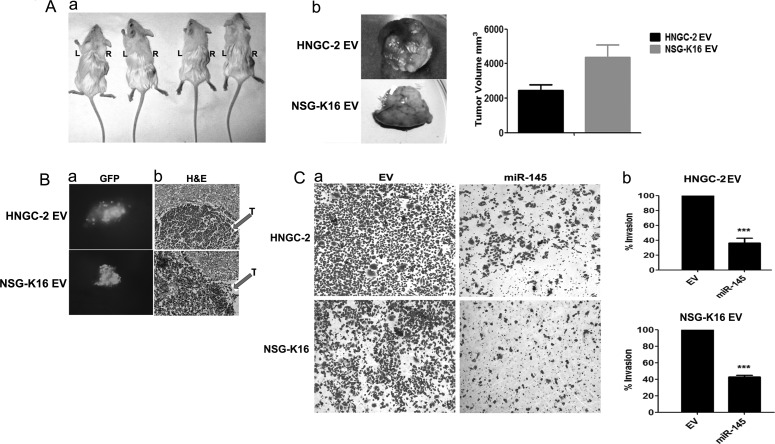
The dramatic effects of miR-145 overexpression leading to effects on cell cycle progression and apoptosis analyzed at in vitro levels prompted us to investigate effects of miR-145 on tumor development and xenograft formation in NOD-SCID mice. The EV cells introduced subcutaneously generated rapidly growing tumors within a month (Fig. 4Aa), while the miR-145–expressing cells introduced similarly were unable to form tumors even upon periodic inspection for up to 2 months. The mice were euthanized after this period. The tumors formed by EV cells on excision displayed an average tumor volume of 4356 mm3 for HNGC-2 and 2459 mm3 for NSG-K16 cells within 30 days (Fig. 4Ab). More notably, mice on intracranial implantation of GFP-labeled HNGC-2 EV and NSG-K16 EV cells demonstrated GFP-positive tumor foci within the brain tissue (Fig. 4Ba). The brain tumor tissues on histopathological analyses and H&E staining were confirmed to be glioma mainly on the basis of their highly infiltrative and necrotic nature (Fig. 4Bb). In contrast, the miR-145 glioma cells failed to get implanted within the brain parenchyma and colonize the brain tissue. Since invasion plays a critical role in glioma pathogenesis, we analyzed the role of miR-145 overexpression on cell migration and invasion using transwell invasion chambers. A profound decrease in invasion potential was induced due to miR-145 in HNGC-2 and NSG-K16 cells as shown in Fig. 4Ca and quantified in Fig. 4Cb, signifying the role of miR-145 in invasion. These experimental findings demonstrate the role of miR-145 as a tumor suppressor, the loss of which in glioma cells contributes to enhanced cell growth, proliferation, tumorigenicity, and invasion.
Fig. 4.
Overexpression of miR-145 inhibits xenograft growth in vivo and suppresses cell invasion in vitro. (A) HNGC-2 cells (1 × 104) stably transduced with EV (right) or stably expressing miR-145 (left) were injected subcutaneously in NOD-SCID mice (n = 18 mice). Representative picture of mice bearing tumors with HNGC-2-EV cells, while the HNGC-2 expressing miR-145 cells failed to form tumors (a). The HNGC-2 and NSG-K16 EV mice on bearing tumors (∼4 wk) were sacrificed and the tumors were dissected (b) and quantitated for tumor volumes (c). (B) NOD-SCID mice were injected intracranially with each of 1 × 103 EV and miR-145 HNGC-2 and NSG-K16 GFP cells (n = 5 mice/group). GFP-positive tumor focus seen within brain tissue (a). The H&E image shows tumor tissue (T) within the whole brain tissue section (b). (C) Invasion assay showing migrating HNGC-2 and NSG-K16–EV and miR-145 cells stained with crystal violet and observed at 10× magnification (a) and quantified (b). The percentages of invading cells were determined by considering migration of HNGC-2 and NSG-K16–EV cells as 100%. Data are shown as mean number of cells in triplicate wells. Data represent means ± SEM (n = 3), ***P < .0001.
Sox9 and ADD3 Are Direct Targets of MiR-145
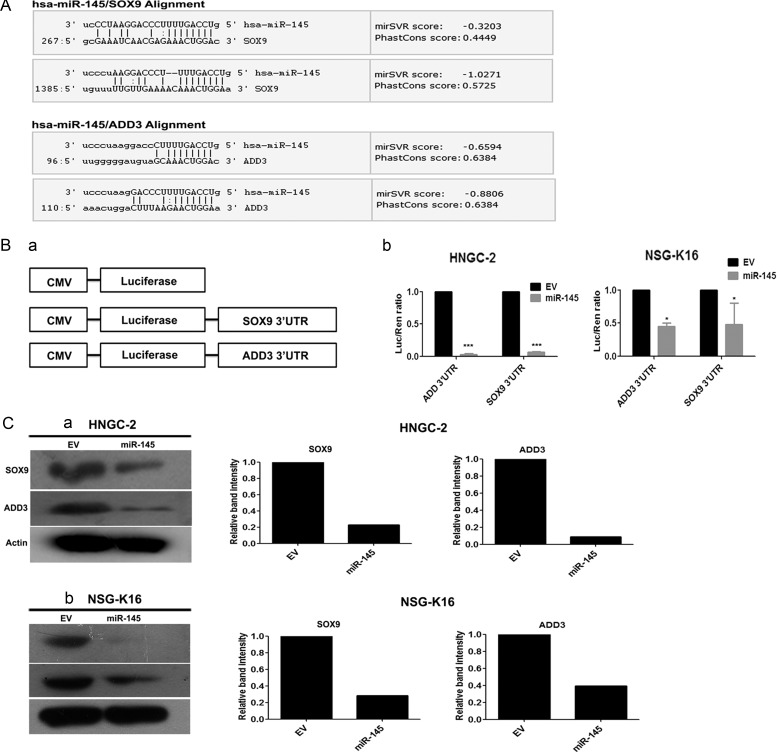
To identify novel targets for miR-145, we searched miRNA target prediction databases that included miRanda, PicTar, miRDB, and TargetScan. Using a multiplicity of these prediction algorithms, we were able to predict 51 common targets. On the basis of them, we determined that the seed sequence of miR-145 matched the 3′UTR of Sox9 and ADD3 mRNA (Fig. 5A). Further, we performed luciferase reporter assays to determine whether Sox9 and ADD3 were direct targets of miR-145 in glioma cells. For this, we cloned the 3′UTR of these genes downstream of the luciferase promoter and performed luciferase activity assays (Fig. 5Ba). MiR-145 significantly suppressed expression of Renilla luciferase reporter carrying the wild-type putative targets of Sox9 and ADD3 in HNGC-2 and NSG-K16 (Fig. 5Bb) and LN-229 cells (Supplementary Fig. S3F) compared with control cells transfected with their respective wild-type 3′UTR constructs. Further, we confirmed that expression of Sox9 and ADD3 was indeed regulated by miR-145 by analyzing whether miR-145 overexpression into GBM cells reduced their endogenous protein expression by Western blotting. Consistent downregulation in Sox9 and ADD3 protein levels were found in miR-145–overexpressing HNGC-2 (Fig. 5Ca), NSG-K16 (Fig. 5Cb), and LN-229 (Supplementary Fig. S3Ea) cells compared with their respective EV cells. The same data were quantified by densitometry (Fig. 5Ca, b and Supplementary Fig. S3Eb). These experiments confirmed that Sox9 and ADD3 genes were functional targets of miR-145, as their binding to miR-145 specifically inhibited their expression in glioma cells.
Fig. 5.
(A) Sox9 and ADD3 are direct primary targets of miR-145. SOX9 and ADD3 possess binding sites for miR-145 in their 3′UTRs. (B) Schematic depiction of Renilla luciferase reporter vectors containing wild-type Sox9 and ADD3 3′UTRs (a); CMV, cytomegalovirus. MiR-145 directly downregulates expression of Sox9 and ADD3 in luciferase assay. Relative luciferase values were determined with dual-luciferase assay of HNGC-2 and NSG-K16 cells cotransfected with reporter constructs and miR-145 vector (b). Renilla luciferase activity is normalized to firefly luciferase activity as control for transfection efficiency. Columns: mean of triplicate experiments; bars: SEM. *P < .05, ***P < .0001, the significant difference from EV. (C) Total cell lysates were used to determine levels of Sox9 and ADD3 by Western blotting in HNGC-2 (a), NSG-K16 (b)–EV, and miR-145 cells. Beta-actin served as loading control. The band intensities of Sox9 and ADD3 were quantified by densitometry using ImageJ software.
Inhibition of Sox9 and ADD3 Mimics MiR-145 Effects
Next, we determined whether glioma tumor tissue samples that showed downregulation of miR-145 in a graded manner expressed correspondingly higher levels of its targets Sox9 and ADD3. For this we performed qRT-PCR, wherein we individually analyzed low-grade (n = 12) and high-grade gliomas (n = 17) along with normal brain tissues (n = 5) for expression of Sox9 and ADD3. These same glioma tumor tissues were quantified earlier for expression of miR-145 (Fig. 1A; Supplementary Table SI). Expectedly, Sox9 was overexpressed to a ∼10-fold greater extent in low-grade gliomas and by ∼500-fold in GBM tumor samples compared with normal brain tissues (n = 5; Fig. 6Aa). Similarly, ADD3 levels increased with grades of glioma malignancy, wherein ADD3 levels were >5-fold in low-grade gliomas and >500-fold in higher-grade tumors compared with normal brain tissues (n = 5; Fig. 6Ab). The luciferase reporter assays and Western blotting experiments provided experimental evidence that Sox9 and ADD3 were indeed targets for miR-145. Further, we analyzed whether Sox9 and ADD3 were responsible for miR-145–mediated effects on proliferation and tumorigenicity of glioma cells. For this, we used siRNAs to knock down endogenous ADD3 and Sox9 expression from EV cells and determined whether that led to effects on cell proliferation and cell cycle progression that were similar to those seen with miR-145 restoration. For rescue experiments, siRNAs were transiently transfected into 3 glioma cell lines—HNGC-2, NSG-K16, and LN-229—and cells were analyzed for proliferation by MTT assay and for cell cycle kinetics by flow cytometry. The MTT assay demonstrated that while the HNGC-2 and NSG-K16 EV cells displayed exponential growth, the Sox9 and ADD3 siRNA transfected cells were retarded in their growth potential and displayed similar growth as did miR-145–overexpressing HNGC-2, NSG-K16 (Fig. 6B), and LN-229 glioma cells (Supplementary Fig. S3G). The effects of these siRNA's on glioma cells were also evident at levels of cell cycle kinetics assayed by flow cytometry. A marked increase in percentage of cells in G0/G1 phase and decrease in S-phase cells occurred on treatment of HNGC-2 cells with miR-145. The siRNA's to Sox9 and ADD3 mirrored the effects of miR-145 on glioma cells. The Sox9 and ADD3 siRNA treated cells showed similar distribution of cells in each of the 3 phases as was displayed by miR-145–overexpressing HNGC-2 cells and LN-229 cells (Fig. 6C, Supplementary Fig. S3H). These studies indicate that downregulation of the miR-145 targets Sox9 and ADD3 in glioma cells generates cells with growth characteristics similar to those manifested by miR-145–expressing cells.
Fig. 6.
Sox9 and ADD3 are functional targets of miR-145. Total RNA was extracted from tissues by Trizol Reagent and converted into cDNA using a cDNA synthesis kit. (A) Quantitative RT-PCR analysis of Sox9 and ADD3 expression in glial tumors. The low grade (n = 12) represents samples derived from grades I and II glial tumors, whereas high grade (n = 17) represents grades III and IV tumors. RNA expression levels were normalized with respect to levels in normal brain tissue samples (n = 5) collected from frontal lobectomy surgeries (Supplementary Table S1). Data represent means ± SEM from 3 independent experiments. *P < .05, ***P < .001. (B) MTT assay of HNGC-2 and NSG-K16 glioma cells stably expressing EV, miR-145, or cells transfected with siRNAs to ADD3 or Sox9. Inhibition of Sox9 and ADD3 using specific siRNAs mimics the growth-suppressive function of miR-45. Data represent means ± SEM (n = 3); OD, optical density. (C) Representative plots showing cell cycle analyses of HNGC-2 cells stably expressing EV, miR-145, or cells transfected with siRNA's to ADD3 or Sox9 and stained with PI after 72 h transfection (a). Cell cycle distribution is represented as a table showing distribution of cells in 3 different phases: G0/G1, S, and G2/M (b). Data are shown as mean ± SEM (n = 3), P < .0001.
MiR-145 Is Regulated by Promoter Hypermethylation
To determine the DNA methylation status of miR-145 promoter in glioma cells, we performed bisulphite genome sequencing. The distribution of cytosine–phosphate–guanine (CpG) dinucleotides is shown for a 0.3 kb region upstream of the miR-145 promoter (Fig. 7A). Hence, we designed primers that spanned a 1.5 kb region upstream of the start site to the miR-145 promoter and cloned this region in a pcDNA vector followed by bisulphite DNA sequencing in glioma cells. Among the multiple positive clones obtained, we screened 6 clones of each cell line for miR-145 promoter analysis at the CpG islands. The data generated from bisulphite genome sequencing for each of these clones were analyzed using Bismark tools and are represented in Fig. 7, wherein DNA hypermethylation is represented by red and blue color and depicts hypomethylation of the CpG islands. In all 6 clones analyzed for each of these cell lines, the majority of CpG islands showed hypermethylation of miR-145 promoter, indicating that it is epigenetically silenced in HNGC-2 and NSG-K16 (Fig. 7A) glioma cells. To further corroborate that miR-145 is epigenetically silenced in these glioma cell lines, we treated HNGC-2 and NSG-K16 cell lines with 5-azacytidine (5 μM), a potent inhibitor of DNA methyltransferases, for 24 h. Later, cells were harvested and analyzed for expression of miR-145 by RT-PCR. We found that 5-azacytidine treatment reversed the repression of miR-145 in the HNGC-2 and NSG-K16 (Fig. 7B) cell lines. Together these results indicated that miR-145 promoter was epigenetically regulated in the glioma cell lines HNGC-2 and NSG-K16 studied by us.
Fig. 7.
CpG island hypermethylation causes silencing of miR-145 in glioma cell lines. (A) Schematic representation of hsa-miR-145 genomic locus on chromosome 17. Bisulphite genome sequence analyses for miR-145 CpG islands in glioma cell lines. The distribution of CpG dinucleotides is shown for a 0.3 kb region upstream of miR-145 promoter. Presence of methylated (red squares) or unmethylated CpG islands (blue squares) in each of the 6 clones for HNGC-2 and NSG-K16 cells is shown. (B) Expression of mature miR-145 by qRT-PCR in cells treated with DNA demethylation agent 5-aza-2-deoxycytidine (AZA). cDNA synthesized from glioma cells was used for expression of miR-145 using PCR conditions as specified in Fig. 1A and primer sequences as specified in Supplementary Table S2. U6 was used as an internal control, and values were normalized to one with respect to cells not treated with AZA. The use of the DNA methylation inhibitor restores miRNA expression in the methylated glioma cell lines. Data represent means ± SEM, P < .05.
Interaction of MiR-145 With Its Targets
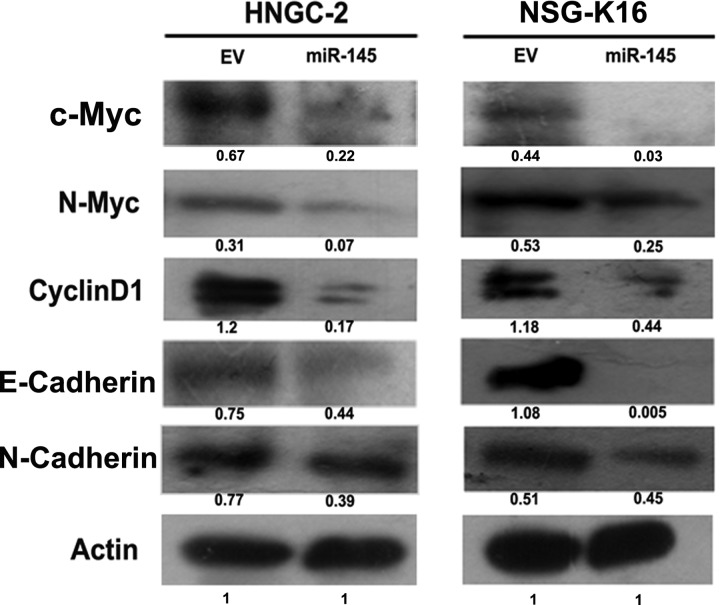
The in vitro and in vivo data exemplify the role of miR-145 as a tumor suppressor in gliomas. The effects of miR-145 overexpression on inhibiting cell cycle progression from G0/G1 to S phase and adhesion suggest interaction of this miRNA with molecules involved in cell cycle progression, proliferation, and invasion. An analysis of cell cycle regulatory molecules like c-myc, N-myc, and cyclin D1 by Western blotting demonstrated their substantial downregulation in miR-145–overexpressing glioma cells HNGC-2 and NSG-K16 compared with EV glioma cells (Fig. 8). Importantly, molecules important for invasion and metastasis like E-cadherin and N-cadherin were also downregulated in HNGC-2 and NSG-K16 miR-145 glioma cells compared with the control group (Fig. 8). These findings indicate that the cellular signaling effects mediated by miR-145 are manifested through direct and indirect effects of miR-145 on genes involved in cell cycle progression, adhesion, and invasion.
Fig. 8.
Western blot analysis. Decreased expression of molecules involved in cell growth (c-myc, N-myc, cyclin D1) and adhesion and invasion (E-cadherin, N-cadherin) in miR-145–HNGC-2 and NSG-K16 cells compared with EV cells. Beta-actin served as loading control. Values represent relative band intensity normalized to actin using ImageJ software 1.44p.
Discussion
MiRNAs are small (∼21 nucleotides) noncoding RNAs that are evolutionarily conserved and encoded within the genomes of almost all eukaryotes. MiRNAs posttranscriptionally regulate protein synthesis by base pairing to partially complementary sequences in the 3′UTRs of target mRNAs, leading to gene repression.29 Essentially, miRNAs execute their functions in a dynamic and context-dependent manner by affecting diverse downstream target genes from transcriptional factors to epigenetic regulators.30 With a large number of miRNAs known to be involved in regulating cell growth and survival, they play a pivotal role as modulators of carcinogenesis.31 While many oncogenic miRNAs are involved in pathogenesis of solid tumors, a general downregulation in their expression compared with normal tissues is reported in several cancers, suggesting that the majority of miRNAs are tumor suppressors.32,33
The current hypothesis regarding cell of origin for gliomas proposes that these tumors arise from a population of cells that share with normal neural stem cells a requirement for self-renewal and multilineage differentiation.34 Thus, identification of miRNAs regulating multiple steps of neurogenesis, from neural stem cell proliferation to neuronal differentiation and maturation, is vital for understanding mechanisms of cellular signaling and gene regulation. In glioblastomas, miRNAs like miR-21 are overexpressed, which causes tumor growth and survival in vivo, an effect mediated by downregulating several tumor suppressors like phosphatase and tensin (PTEN) homolog and promoting migration by targeting pro-invasion genes.35
Glioma stem cells or brain tumor–initiating cells are small populations of cells within a tumor that drive tumor growth, propel progression, enhance invasion, and induce therapeutic resistance.36 Recent studies suggest that miRNAs are involved in regulating properties of these cells through their targets, like genes essential for pluripotency and stem cell function.37 The present study was aimed at identifying miRNAs critically involved in transformation of nontumorigenic neural stem cells to brain tumor stem cells (BTSCs). For this we used a cell culture model developed by us to study glioma progression comprising the cell lines HNGC-1, representing a nontumorigenic neural stem cell line, and HNGC-2, demonstrating features of cancer stem cells.21 The extent and kind of differential expression of miRNAs in this model were achieved through a microarray service wherein we compared miRNA profiles of nontumorigenic HNGC-1 cells with BTSCs like HNGC-2. Similar studies were performed with another newly developed BTSC culture, NSG-K16. Among the several differentially expressed miRNAs, miR-145 was most discretely represented in this cell system as a tumor-suppressive RNA.38 Studies indicate that miR-145 causes growth inhibition by targeting oncogenes like c-Myc and represses proliferation and promotes differentiation by regulating pluripotency factors Oct4, Sox2, and Krüppel-like factor 4 (KLF4).39 MiR-145, while not significantly affecting tumor growth, slowed potential to inhibit both migration and invasion in breast cancer cell lines, possibly related to its ability to inhibit tumor cell invasion and metastasis by targeting Mucin1 and Fascin1.40 MiR-145 blocks migration of vascular smooth muscle cells by inhibiting Fli1, while miR-143 targets versican protein, which is involved in migration and is induced by platelet-derived growth factor (PDGF).41 The specific expression of miR-145 in pericytes, wherein it targets the hematopoietic transcription factor Fli1, causes inhibition of migration in response to growth factor gradients and supports its role in vascularization and angiogenesis.42 MiR-145, a regulator of the mammalian target of rapamycin (mTOR) pathway, directly targets p70S6K1 and downregulates the expression of hypoxia-inducible factor–1α (HIF-1α) and vascular endothelial growth factor (VEGF), thus inhibiting invasion and angiogenesis. Similar to embryonic stem cells, 2 specific miRNAs expressed from the same genetic locus, miR-143 and miR-145, downregulate Sox2, and Sox2 downregulates miR-143 and miR-145, suggesting that Sox2 and these miRNA's form a double-negative feedback loop in GBM cells; however, their relationship to GBM has not been studied.43 There have been recent reports about involvement of miR-145 as a tumor suppressor in gliomas with a more prominent role in glioma invasion mediated through its targets, like ADAM17 (a disintegrin and metalloproteinase metallopeptidase domain 17), a member of the Zn-dependent metalloproteinase family,14 or through NEDD9 (neural precursor cell expressed, developmentally downregulated 9), a scaffold protein involved in invasion in high-grade gliomas.16 In contrast, miR-145 is regarded as a positive regulator of glioblastoma invasion, a variation from its tumor-suppressor role, attributed mainly to the slower growth rate of glioma cells during invasion.13,17 Our study demonstrates the potential of miR-145 as a miRNA with tumor-suppressive, antimigratory, and anti-invasive potential in glioma cells as well as in glioma stem cells. By qRT-PCR analyses, we obtained >50-fold downregulation of miR-145 in most of the GBM tumors as well as in glioma stem cells. However, the tumor-suppressive effects were not specific to a stem cell population, as they were also evident in other established glioma cell lines, like LN-229 (Supplementary Fig. S3).
We further established that miR-145 directly targets Sox9 and ADD3. These genes are related to cell proliferation and invasion and were identified by a bioinformatics approach using various algorithms and validated by 3′UTR luciferase assays. Higher-grade gliomas with minimal expression of miR-145 showed overexpression of Sox9 and ADD3. The levels of Sox9 were found to be overexpressed in several brain tumors that included sonic hedgehog–dependent medulloblastomas and gliomas, as well as GBM samples, indicating that high Sox9 mRNA expression was a prognostic factor for glioma patients.18,42 Human malignant glioma and primitive neuroectodermal tumor samples, as well as glioma cell lines, showed strong nuclear positivity for Sox9.44 Interestingly, there is a role for Sox9 in PDGF signaling, as Sox9 together with Sox10 regulate PDGF receptor-α expression in oligodendrocyte progenitors of the spinal cord.45 Sox9 is also present in the neural crest, neural stem cells, and neurospheres46 and is coexpressed with Sox1 and Sox2 in brain regions that harbor neural stem cells.47 When Sox9 in U87MG cells was reduced by siRNA treatment, there was retardation in cell proliferation. Our studies demonstrating Sox9 as a target for miR-145 specify its role as an oncogene in glioma and a potential drug target. There are few studies proposing miR-145 as an important regulator of human chondrocyte function, wherein levels of miR-145 inversely correlate with Sox9 expression during human chondrocyte dedifferentiation, thereby inducing characteristic changes, as occur in osteoarthritis.48 Importantly, overexpression or suppression of miR-145 resulted in inhibiting or promoting, respectively, chondrogenic differentiation, suggesting that miR-145 acts as a key mediator to antagonize early chondrogenic differentiation via attenuating the effect of transcription factor Sox9.48 There are presently no reports specifying ADD3 as a target to miR-145, though there are reports linking it to miR-143.49 Ours is the first study to validate ADD3 as a genuine target to miR-145 using both Western blotting and luciferase assay. Adducins are heteromeric proteins composed of different subunits referred to as adducin alpha, beta, and gamma encoded by distinct genes and belong to a family of membrane skeletal proteins involved in the assembly of a spectrin-actin network in erythrocytes and at sites of cell–cell contact in epithelial tissues.50,51 Alternatively spliced adducin gamma transcripts encoding different isoforms are recognized, though with unknown functions. The role of ADD3 in gliomas is not known, although there may be some associations derived from the infiltrative growth behavior of GBM. Since the neural cell membrane lacks typical extracellular matrix proteins like collagen, fibronectin, and type II laminin, migrating glioma cells may invade neighboring brain tissue by coordinated cross-bridging between nonmuscle myosin II and actin. The involvement of miR-143 in cardiac myogenesis is known and occurs through direct repression of ADD3, which encodes an F-actin capping protein. MiR-143 and ADD3 act cell autonomously in zebrafish to control F-actin dynamics and cell morphology.49 Our data suggest that upregulation of ADD3 is one of the mechanisms contributing to the infiltrative behavior of gliomas. Additional studies are required to establish the role of this molecule in glioma invasion.
The growth-suppressive effects of miR-145 possibly stem from its potential to inhibit cell cycle progression, evident in terms of decreased levels of proteins like c-Myc, N-myc, and cyclin D1, as well as genes important to invasion and epithelial-mesenchymal transition like E-cadherin and N-cadherin in miR-145–overexpressing glioma cells. These findings point to a regulatory framework centered on miR-145 in glioma cells. MiR-145 coordinately downregulates expression of oncogenes, such as Sox9 and ADD3. The role of Sox9 in proliferation, survival, and invasion in gliomas is well documented and is reported in phenomena like epithelial-mesenchymal transition. Interestingly, molecules like N-cadherin, E-cadherin, N- myc, C-myc, and cyclin D1, downregulated in response to miR-145 overexpression, are either downstream targets or interacting partners of Sox9. Although our study does not signify a direct correlation between Sox9 and these genes, we hypothesize that miR-145 executes its tumor-suppressor function by inhibiting Sox9, causing downregulation of these prosurvival genes. The direct function of ADD3 has not yet been addressed in glioma biology, but microarray-based profiling has shown it's overexpression in various malignancies, like small cell lung cancer.52 As ADD3 is an important regulator of both the spectrin-based membrane skeleton and the actin cytoskeleton, we envisage that it functions along with other cytoskeletal components, like N-cadherin and E-cadherin. Another miRNA, miR-195, downregulated during GBM progression, is shown to function through its target genes E2F3 and CCND1, which are involved in cell cycle progression.53 Based on our data, we propose a model highlighting the possible mediators and signaling molecules through which miR-145 may exert its growth- and tumor-suppressive effects (Fig. 9). Hence, our study demonstrates that interaction of miR-145 with its targets Sox9 and ADD3 has crucial ramifications on tumor progression and invasion in glioma cells. Our studies showing the role of miR-145 as a tumor suppressor propose it to be an important therapeutic target for glioblastomas.
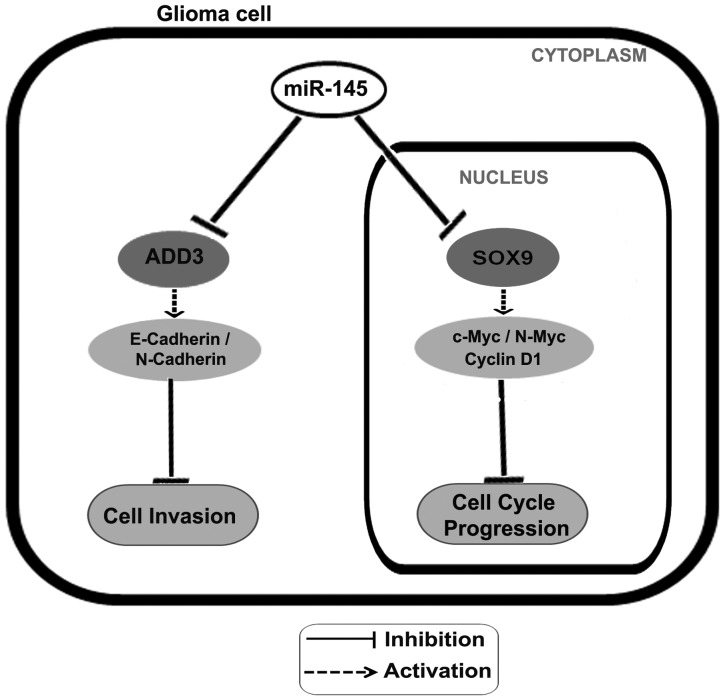
Fig. 9.
Model depicting role of miR-145 on cell cycle progression and invasion in glioma cells. The levels of miR-145 are downregulated in glioma cells. Sox9 and ADD3 levels inversely correlate with miR-145 expression in glioma cell lines, as they are the direct targets of miR-145. The downregulation of Sox9 can suppress the transcription of cell cycle progression–related genes (c-Myc, N-myc, cyclin D1) and cause accumulation of cells in G1 phase leading to inhibition of cell cycle progression. The attenuation of ADD3 levels can downregulate expression of genes involved in cell migration (E-cadherin, N-cadherin), resulting in inhibiting GBM cell invasion.
Supplementary Material
Funding
This work was supported by grants from Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, India and National Centre for Cell Science (NCCS), Pune, India.
Grant support for carrying out research was provided by Stem Cell Task Force, Department of Biotechnology (DBT) (grant no. BT/PR10557/MED/31/29/2008), Government of India, New Delhi, India, and National Centre for Cell Science (NCCS), Pune, India. The fellowship of S.R. was supported by ICMR, New Delhi. Fellowship funding for S.R and K.S. was provided by DBT, and the fellowship for N.K. was provided by Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Supplementary Material
Acknowledgments
We are grateful to Dr Avinash Pradhan, KEM Hospital, Pune, for his expert help in histopathogical analyses of glioma tissue sections. We are thankful to M. L. Shaikh for his technical help in mice tumorigenicity experiments.
Conflict of interest statement. None declared.
References
- 1.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 2.Chen R, Nishimura MC, Bumbaca SM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 3.Chow LM, Endersby R, Zhu X, et al. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19:305–316. doi: 10.1016/j.ccr.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, Shi M, Hou S, et al. MiR-483–5p suppresses the proliferation of glioma cells via directly targeting ERK1. FEBS Lett. 2012;586:1312–1317. doi: 10.1016/j.febslet.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Wang X, Wang H, et al. MiR-137 is frequently down-regulated in glioblastoma and is a negative regulator of Cox-2. Eur J Cancer. 2012;48:3104–3111. doi: 10.1016/j.ejca.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Shi ZM, Wang J, Yan Z, et al. MiR-128 inhibits tumor growth and angiogenesis by targeting p70S6K1. PLoS One. 2012;7:e32709. doi: 10.1371/journal.pone.0032709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia H, Cheung WK, Ng SS, et al. Loss of brain-enriched miR-124 microRNA enhances stem-like traits and invasiveness of glioma cells. J Biol Chem. 2012;287:9962–9971. doi: 10.1074/jbc.M111.332627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fineberg SK, Datta P, Stein CS, et al. MiR-34a represses Numbl in murine neural progenitor cells and antagonizes neuronal differentiation. PLoS One. 2012;7:e38562. doi: 10.1371/journal.pone.0038562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Q, Liu LZ, Qian X, et al. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–774. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Y, Yan ZP, Lu NN, et al. Downregulation of miR-145 associated with cancer progression and VEGF transcriptional activation by targeting N-RAS and IRS1. Biochim Biophys Acta. 2013;1829:239–247. doi: 10.1016/j.bbagrm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Zaman MS, Chen Y, Deng G, et al. The functional significance of microRNA-145 in prostate cancer. Br J Cancer. 2010;103:256–264. doi: 10.1038/sj.bjc.6605742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi M, Du L, Liu D, et al. Glucocorticoid regulation of a novel HPV-E6-p53-miR-145 pathway modulates invasion and therapy resistance of cervical cancer cells. J Pathol. 2012;228:148–157. doi: 10.1002/path.3997. [DOI] [PubMed] [Google Scholar]
- 13.Lee HK, Bier A, Cazacu S, et al. MicroRNA-145 is downregulated in glial tumors and regulates glioma cell migration by targeting connective tissue growth factor. PLoS One. 2013;8:e54652. doi: 10.1371/journal.pone.0054652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Chopp M, Zheng X, et al. MiR-145 reduces ADAM17 expression and inhibits in vitro migration and invasion of glioma cells. Oncol Rep. 2013;29:67–72. doi: 10.3892/or.2012.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou C, Xu Q, Mao F, et al. MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle. 2012;11:2137–2145. doi: 10.4161/cc.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speranza MC, Frattini V, Pisati F, et al. NEDD9, a novel target of miR-145, increases the invasiveness of glioblastoma. Oncotarget. 2012;3:723–734. doi: 10.18632/oncotarget.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo S, Martin GS, Schulz KJ, et al. Serial selection for invasiveness increases expression of miR-143/miR-145 in glioblastoma cell lines. BMC Cancer. 2012;12:143. doi: 10.1186/1471-2407-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, He S, Yuan J, et al. Oncogenic role of SOX9 expression in human malignant glioma. Med Oncol. 2012;29:3484–3490. doi: 10.1007/s12032-012-0267-z. [DOI] [PubMed] [Google Scholar]
- 19.Syed V, Zhang X, Lau KM, et al. Profiling estrogen-regulated gene expression changes in normal and malignant human ovarian surface epithelial cells. Oncogene. 2005;24:8128–8143. doi: 10.1038/sj.onc.1208959. [DOI] [PubMed] [Google Scholar]
- 20.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiras A, Chettiar ST, Shepal V, et al. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- 22.Shiras A, Bhosale A, Shepal V, et al. A unique model system for tumor progression in GBM comprising two developed human neuro-epithelial cell lines with differential transforming potential and coexpressing neuronal and glial markers. Neoplasia. 2003;5:520–532. doi: 10.1016/s1476-5586(03)80036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur N, Chettiar S, Rathod S, et al. Wnt3a mediated activation of Wnt/beta-catenin signaling promotes tumor progression in glioblastoma. Mol Cell Neurosci. 2013;54C:44–57. doi: 10.1016/j.mcn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Sachdeva M, Zhu S, Wu F, et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Albini A, Benelli R, Noonan DM, et al. The “chemoinvasion assay”: a tool to study tumor and endothelial cell invasion of basement membranes. Int J Dev Biol. 2004;48:563–571. doi: 10.1387/ijdb.041822aa. [DOI] [PubMed] [Google Scholar]
- 27.Li LC, Dahiya MR. Primer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 28.Krueger F, Andrews SR. Bismark: a flexible aligner and methylation caller for bisulfite-seq applications. Bioinformatics. 2011;27:1571–1572. doi: 10.1093/bioinformatics/btr167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djuranovic S, Nahvi A, Green R. MiRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang MF, Shi Y. Dynamic roles of microRNAs in neurogenesis. Front Neurosci. 2012;6:71. doi: 10.3389/fnins.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avraham R, Yarden Y. Regulation of signalling by microRNAs. Biochem Soc Trans. 2012;40:26–30. doi: 10.1042/BST20110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melo SA, Kalluri R. Molecular pathways: microRNAs as cancer therapeutics. Clin Cancer Res. 2012;8:4234–4239. doi: 10.1158/1078-0432.CCR-11-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho WC. MicroRNAs as therapeutic targets and their potential applications in cancer therapy. Expert Opin Ther Targets. 2012;16:747–759. doi: 10.1517/14728222.2012.696102. [DOI] [PubMed] [Google Scholar]
- 34.Swartling FJ, Savov V, Persson AI, et al. Distinct neural stem cell populations give rise to disparate brain tumors in response to n-Myc. Cancer Cell. 2012;21:601–613. doi: 10.1016/j.ccr.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong YW, Ferland-McCollough D, Jackson TJ, et al. MicroRNAs in cancer management. Lancet Oncol. 2012;13:e249–e258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 36.Venere M, Fine HA, Dirks PB, et al. Cancer stem cells in gliomas: identifying and understanding the apex cell in cancer’s hierarchy. Glia. 2011;59:1148–1154. doi: 10.1002/glia.21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li MA, He L. MicroRNAs as novel regulators of stem cell pluripotency and somatic cell reprogramming. Bioessays. 2012;34:670–680. doi: 10.1002/bies.201200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachdeva M, Mo YY. MiR-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res. 2010;2:170–180. [PMC free article] [PubMed] [Google Scholar]
- 39.Chivukula RR, Mendell JT. Abate and switch: miR-145 in stem cell differentiation. Cell. 2009;137:606–608. doi: 10.1016/j.cell.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 40.Lee TK, Poon RT, Man K, et al. Fascin over-expression is associated with aggressiveness of oral squamous cell carcinoma. Cancer Lett. 2007;254:308–315. doi: 10.1016/j.canlet.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Hu G, Zhou J. Repression of versican expression by microRNA-143. J Biol Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larsson E, Fredlund FP, Heldin J, et al. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009;1:108. doi: 10.1186/gm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang X, Yoon JG, Li L, et al. The SOX2 response program in glioblastoma multiforme: an integrated ChIP-seq, expression microarray, and microRNA analysis. BMC Genomics. 2011;12:11. doi: 10.1186/1471-2164-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swartling FJ, Ferletta M, Kastemar M, et al. Cyclic GMP-dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines. Oncogene. 2009;28:3121–3131. doi: 10.1038/onc.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finzsch M, Stolt CC, Lommes P, et al. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development. 2008;135:637–646. doi: 10.1242/dev.010454. [DOI] [PubMed] [Google Scholar]
- 46.Stolt CC, Lommes P, Sock E, et al. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alcock J, Sottile V. Dynamic distribution and stem cell characteristics of Sox1-expressing cells in the cerebellar cortex. Cell Res. 2009;19:1324–1333. doi: 10.1038/cr.2009.119. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Sanchez A, Dudek KA, Murphy C. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145) J Biol Chem. 2012;287:916–924. doi: 10.1074/jbc.M111.302430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deacon DC, Nevis KR, Cashman TJ, et al. The miR-143-adducin3 pathway is essential for cardiac chamber morphogenesis. Development. 2010;137:1887–1896. doi: 10.1242/dev.050526. [DOI] [PubMed] [Google Scholar]
- 50.Naydenov NG, Ivanov A. Adducins regulate remodeling of apical junctions in human epithelial cells. Mol Biol Cell. 2010;21:3506–3517. doi: 10.1091/mbc.E10-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavaur J, Mineur YS, Picciotto MR. The membrane cytoskeletal protein adducin is phosphorylated by protein kinase C in D1 neurons of the nucleus accumbens and dorsal striatum following cocaine administration. J Neurochem. 2009;111:1129–1137. doi: 10.1111/j.1471-4159.2009.06405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma PC, Tretiakova MS, Nallasura V, et al. Downstream signalling and specific inhibition of c-MET/HGF pathway in small cell lung cancer: implications for tumour invasion. Br J Cancer. 2007;97:368–377. doi: 10.1038/sj.bjc.6603884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang QQ, Xu H, Huang MB, et al. MicroRNA-195 plays a tumor-suppressor role in human glioblastoma cells by targeting signaling pathways involved in cellular proliferation and invasion. Neuro Oncol. 2012;14:278–287. doi: 10.1093/neuonc/nor216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.