Abstract
Background
Spine stereotactic body radiotherapy (SBRT) is increasingly being applied to the postoperative spine metastases patient. Our aim was to identify clinical and dosimetric predictors of local control (LC) and survival.
Methods
Eighty patients treated between October 2008 and February 2012 with postoperative SBRT were identified from our prospective database and retrospectively reviewed.
Results
The median follow-up was 8.3 months. Thirty-five patients (44%) were treated with 18–26 Gy in 1 or 2 fractions, and 45 patients (56%) with 18–40 Gy in 3–5 fractions. Twenty-one local failures (26%) were observed, and the 1-year LC and overall survival (OS) rates were 84% and 64%, respectively. The most common site of failure was within the epidural space (15/21, 71%). Multivariate proportional hazards analysis identified systemic therapy post-SBRT as the only significant predictor of OS (P = .02) and treatment with 18–26 Gy/1 or 2 fractions (P = .02) and a postoperative epidural disease grade of 0 or 1 (0, no epidural disease; 1, epidural disease that compresses dura only, P = .003) as significant predictors of LC. Subset analysis for only those patients (n = 48/80) with high-grade preoperative epidural disease (cord deformed) indicated significantly greater LC rates when surgically downgraded to 0/1 vs 2 (P = .0009).
Conclusions
Postoperative SBRT with high total doses ranging from 18 to 26 Gy delivered in 1–2 fractions predicted superior LC, as did postoperative epidural grade.
Keywords: spinal cord compression, spine metastases, postoperative radiation, spine radiosurgery, spine stereotactic body radiotherapy
Although surgery plays a major role in the management of patients with symptomatic single level malignant epidural spinal cord compression,1 one of the major questions that remains is the optimal degree of epidural disease resection and whether there is an association with local control (LC). With respect to adjuvant therapy, radiation has been the standard in reducing the risk for local recurrence,2,3 although the rates of LC following the current standard of conventional low-dose external beam radiotherapy (CRT) ranges widely from 40% to 80% at 1 year.2–4
With modern radiation technology, the technique of stereotactic body radiotherapy (SBRT) has emerged and is being applied to various disease sites, including the spine.5–7 The Canadian Association of Radiation Oncology recently defined SBRT as “the precise delivery of highly conformal and image-guided hypo-fractionated external beam radiotherapy, delivered in a single or few fraction(s), to an extra-cranial body target with doses at least biologically equivalent to a radical course when given over a conventionally fractionated (1.8–3.0 Gy/fraction) schedule.”7 This definition translates to treating metastatic patients with locally “curative” intent, as opposed to locally “palliative” intent. In the postoperative spine metastases patient, spine SBRT makes even more philosophical sense because after exposing patients to the risks of a major spinal surgery,8 it is only logical to offer an aggressive local treatment to consolidate the therapeutic intent. The aim of our study was to report the University of Toronto postoperative spine SBRT experience and specifically analyze the impact of epidural disease extension on LC.
Materials and Methods
A total of 80 patients with spine metastases who were operated upon and treated with postoperative SBRT between October 2008 and February 2012 were identified from a prospective database and retrospectively reviewed. The primary outcome of this study was to evaluate LC and overall survival (OS) and to identify clinical and dosimetric predictors. Local control was defined as imaging-based disease progression compared with the initial postoperative MRI. We categorized surgery according to whether stabilization alone was performed with no epidural disease resection and decompression (eg, instrumentation alone, a cement augmentation procedure), as opposed to epidural disease resection and decompression (eg, an en bloc vertebral spinal segment resection, vertebrectomy, partial vertebrectomy, minimal access spine surgical procedures aimed to resect epidural disease) with or without instrumentation for stabilization. Given the heterogeneity of the surgical procedures, we focused our analysis on preoperative and postoperative epidural grade according to the validated Bilsky criteria,9 which is summarized in Table 1. In addition, we graded the postoperative neurologic status according to the American Spinal Injury Association (ASIA) scale.10 An ASIA E rating is normal motor and sensory function, D is incomplete motor impairment with more than half of the key muscles below the affected level having a power of at least 3 out of 5, C is incomplete motor impairment with key muscles below the affected level having a power under 3 out of 5, B is incomplete motor impairment with sensory but no motor function preserved, and A is complete impairment with neither sensory nor motor function preserved. All patients were treated with SBRT by a single radiation oncologist (A.S.). Follow-up imaging was based on our institutional practice, consisting of a full spine MRI study at 2- to 3-month intervals.
Table 1.
Summary of the Bilsky epidural disease grading classification
| Bilsky Grade | Description |
|---|---|
| 0 | No epidural disease |
| 1a | Epidural impinging the thecal sac but without deformation |
| 1b | Epidural disease deforming the thecal sac but not the spinal cord |
| 1c | Epidural disease deforming the thecal sac and spinal cord contact |
| 2 | Epidural spinal cord compression with CSF visible |
| 3 | Epidural spinal cord compression and no CSF visible |
Stereotactic Body Radiotherapy Technique
Our spine SBRT technique has been previously described.11 All patients were treated on the Elekta Synergy unit equipped with a 4-mm multileaf collimator, a kilovoltage cone-beam CT image guidance system, and a HexaPOD robotic couch (Elekta). The treatment planning process involved CT simulation with a slice thickness of 1 mm. Thin-slice (1.5-mm) axial T1 and T2 volumetric MRI sequences (noncontrast) scanning at least 1 vertebral body above and below the target were fused to the treatment planning CT scan. If the surgical hardware distorted the MRI such that the spinal canal and spinal cord could not be visualized, then a treatment planning CT myelogram was performed (Fig. 1). With respect to the delineation of the clinical target volume (CTV), the principles as outlined by Sahgal et al.5 were followed. Essentially, if the preoperative MRI described near circumferential epidural disease, then a circumferential “donut” type CTV was contoured, as opposed to excluding epidural space. Figure 1 describes a typical clinical scenario requiring a donut volume, and 90% of the patients in this study were treated similarly. We followed the spinal cord guidelines as previously described for both radiation-naive and re-irradiation indication regardless of the epidural disease grade to maintain an acceptable risk for radiation myelopathy.12,13
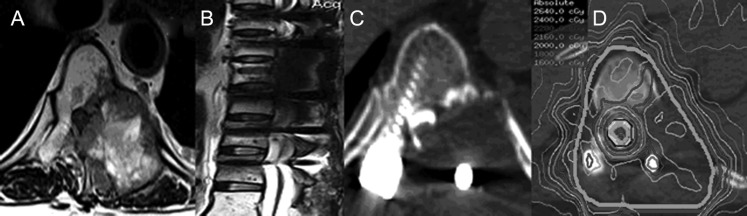
Fig. 1.
This patient with metastatic breast cancer involving T8 (vertebral body, posterior elements, and paraspinal tissue), with near circumferential epidural disease (Bilsky grade 3), presented with pain and no neurologic deficit. (A) The preoperative axial T2-weighted MRI. (B) The postoperative sagittal T2-weighted MRI following circumferential decompression, epidural resection, and instrumentation. This image clearly illustrates the artifact induced by the surgical hardware that prevents accurate delineation of the spinal cord. (C) The axial CT myelogram that allowed the spinal cord to be delineated despite the surgical hardware. (D) The “donut” type of dose distribution where radiation conforms around the circumference of the spinal cord/thecal sac and encompasses the entire spinal segment and postoperative bed. The patient was treated with 24 Gy in 2 fractions with the thecal sac limited to a maximum dose of 17 Gy.
Statistical Analysis
Descriptive statistics were used to assess patient demographics, disease characteristics, and related covariates of interest (Table 2). A description of the dosimetric variables analyzed is provided in Table 3. Categorical variables investigated—such as sex, primary diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status, baseline neurologic function according to ASIA, number of metastases in the target volume, location of the tumor, type of surgery, use of systemic therapy post-SBRT, and prior radiation exposure—were expressed as count and proportions, whereas continuous variables such as age, total dose, dose per fraction, and follow-up were expressed as mean ± SD or median + range. The outcome variable of interest was the time to local failure and OS. The time-to-event data were calculated in months from the date of SBRT to the date of the event (date of local failure and death data for OS) or last follow-up if the event had not yet occurred. LC probabilities and survival probabilities were calculated using the Kaplan–Meier product-limit method. The log-rank test was used as a univariate analysis to compare local probability with a potential predictor of interest. A multivariate Cox proportional hazards regression model was used to determine the joint effect of potential factors that were found significant on univariate analysis. All P-values were 2-sided. Results were considered significant at P < .05. Statistical analyses were performed using SAS version 9.2 and its user's guide.
Table 2.
Baseline patient and tumor characteristics
| Patient Characteristics | n= 80 Patients | Treatment Characteristics | n= 80 Patients |
|---|---|---|---|
| Median age, y (range) | 58.5 (18–81) | Median total dose (Gy)/frx | 24 Gy/2 |
| Gender | Number of frx | ||
| Male | 44 (55%) | (24 Gy) 1 | 3 (3.7%) |
| Female | 36 (45%) | (18–26 Gy) 2 | 32 (40%) |
| (18–40 Gy) >/=3 | 45 (56.2%) | ||
| Primary cancer | Prior CRT | ||
| Breast | 13 (16%) | (median 20 Gy/5 frx) | |
| NSCLC | 11 (13.7%) | Yes | 60 (75%) |
| Thyroid | 10 (12.5%) | No | 20 (25%) |
| RCC | 7 (8.7%) | ||
| HCC | 4 (5%) | ||
| Other | 35 (43.7%) | ||
| Location | CTV description | ||
| Cervical | 11 (13.7%) | Circumferential donut type | 72 (90%) |
| Thoracic | 45 (56.2%) | Nondonut | 8 (10%) |
| Lumbar | 22 (27.5) | ||
| Sacral | 2 (2.5%) | ||
| ECOG | Type of surgery | ||
| −0 | 4 (5%) | Instrumented Stabilization alone | 11 (14%) |
| −1 | 71 (88.7%) | Decompression, no stabilization | 29 (36%) |
| −2 | 3 (3.7%) | Decompression with instrumented stabilization | 40 (50%) |
| −3 | 2 (2.5%) | ||
| Baseline VCF | 44 (55%) | Number of spinal segments in CTV | |
| 1 | 44 (55%) | ||
| 2 | 18 (22.5%) | ||
| 3 | 10 (12.5%) | ||
| 4 | 5 (6.25%) | ||
| 5 | 2 (2.5%) | ||
| Preop Bilsky epidural grade | Postop Bilsky epidural grade | ||
| 0 | 4 (5%) | 0 | 6 (7.5%) |
| 1a | 3 (3.7%) | 1a | 25 (31.2%) |
| 1b | 12 (15%) | 1b | 26 (32.5%) |
| 1c | 13 (16.2%) | 1c | 15 (18.7%) |
| 2 | 28 (35%) | 2 | 8 (10%) |
| 3 | 20 (25%) | 3 | 0 (0%) |
| Paraspinal extension | Postop ASIA | ||
| Present | 62 (77.5%) | A | 2 (3%) |
| Absent | 18 (22.5%) | B | 3 (4%) |
| C | 1 (1%) | ||
| D | 16 (20%) | ||
| E | 57 (72%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NSCLC, non–small cell lung cancer; RCC, renal cell carcinoma; HCC, hepatocellular carcinoma; frx, fractions.
Table 3.
SBRT dosimetric factors
| SBRT Dosimetric Factors | n = 80 patients |
|---|---|
| Median CTV V80 (range) | 90% (60–99) |
| Median PTV V80 (range) | 88% (57–93) |
| Median spinal cord Dmax/BED-Dmax (range) | 11.8 Gy (2–18.4)/37.8 Gy2 (2.7–74.7) |
| Median spinal cord PRV Dmax/BED-Dmax (range) | 14.3 Gy (2–20)/50.9 Gy2 (2.7–80) |
| Median thecal sac Dmax/BED-Dmax (range) | 17 Gy (2.9–30)/70.13 Gy2 (4.9–125.9) |
Abbreviations: PTV, planning target volume; V80, volume (%) receiving 80% of the prescribed dose; BED, biologically effective dose; Gy10, BED calculated with a/b = 10; Gy2, BED calculated with a/b = 2; Dmax, dose to the maximum point volume; thecal sac dose refers to levels below the spinal cord and typically below the 1st lumbar vertebrae.
Results
Patient and Treatment Characteristics
Patient, tumor and treatment characteristics are summarized in Table 2, and treatment plan dosimetric data in Table 3. High-grade epidural disease (grades 2 and 3) was observed preoperatively in 55% of patients. Thirty-five patients (44%) were treated with 18–26 Gy in 1 or 2 fractions, and 45 patients (56%) with 18–40 Gy in 3 to 5 fractions (the median total dose was 24 Gy delivered in 2 fractions).
Clinical Outcomes
The median follow-up time was 8.3 months (range, 0.13–39.1). The median time from surgery to SBRT was 4.7 weeks. The OS rate at 1 year was 64%. Thirty-five patients (44%) died at a median of 8.3 months (range, 0.1–37.4) from the time of spine SBRT. The majority of patients (71%) died of systemic disease progression. A total of 21 local failures (26%) were observed. The LC rate at 1 year was 84%, the crude median time to local failure was 6.9 months (range, 0.1–37.4), the actuarial median time to local failure was 19.91 months, and the median follow-up time in those with local failure was 4.32 months (range, 0.23–33.41). With respect to patterns of failure, the most common site of progression was isolated to the epidural space in 15/21 patients (71%), posterior elements alone when not included in the CTV in 1/21 patients (5%), and mixed patterns of in-field bony anatomic involvement in addition to epidural disease progression in the remaining 5/21 patients (24%).
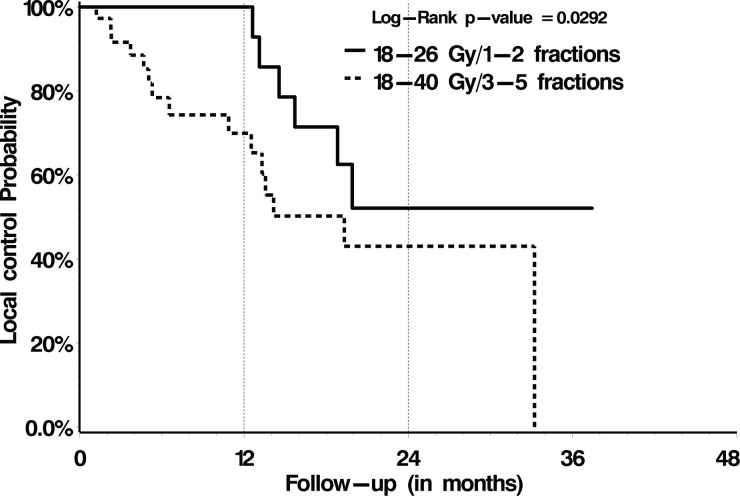
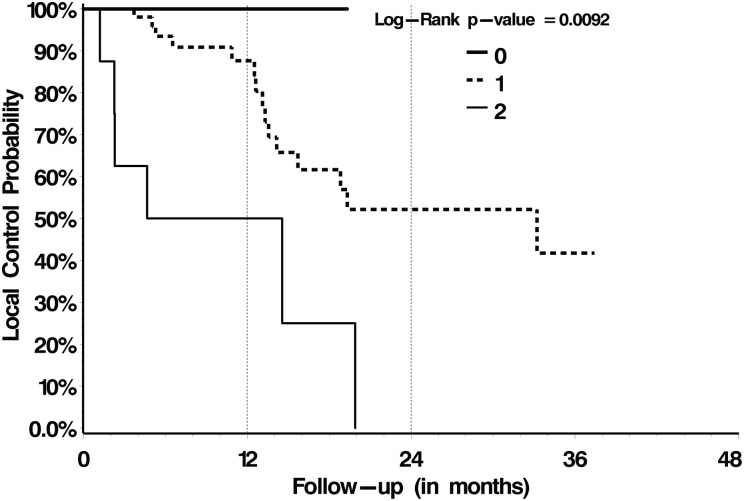
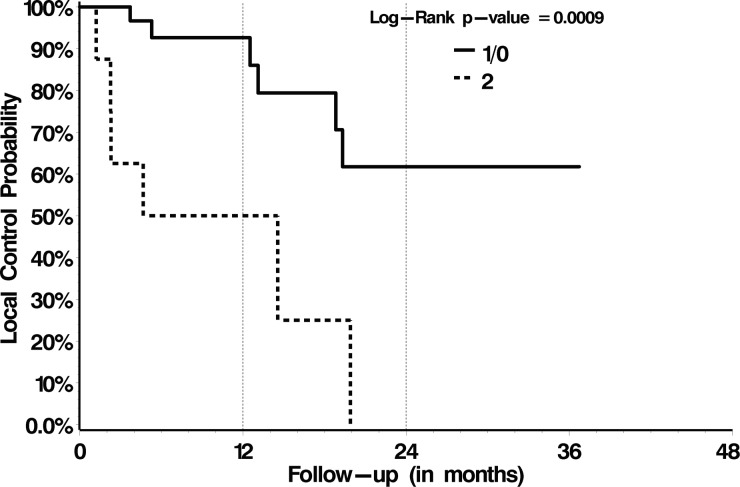
With respect to OS, treatment with post-SBRT systemic therapy versus no post-SBRT systemic therapy was the only significant prognostic factor identified on multivariate analysis, with 1-year OS rates of 78% versus 56% (P = .02), respectively (Table 4). Those patient and treatment characteristics summarized in Table 2 failed to be prognostic. With respect to LC, we analyzed those patient and treatment factors summarized in Table 2 and dosimetric variables summarized in Table 3. Multivariate proportional hazards analysis identified treatment with 18–26 Gy in 1 or 2 fractions (P = .02) and a postoperative epidural disease grade of 0 or 1 (P = .003) as significant predictors of LC (Figs 2 and 3, respectively, and Table 4). Subgroup analysis for only those patients with preoperative high-grade epidural disease (grade 2 or 3, n = 48/80) indicated significantly greater LC rates when downgraded to 0/1 versus 2 (P = .0009; Fig. 4).
Table 4.
Significant predictors of local control and overall survival on univariate and multivariate analyses
| Overall Survival |
Local Control |
||||
|---|---|---|---|---|---|
| Factor | Univariate | Multivariate | Factor | Univariate | Multivariate |
| Systemic therapy post-SBRT | P = .0209 | P = .0246/HR = 2.338 (95% CI, 1.115–4.903) | 18–26 Gy/1–2 fractions vs 18–40 Gy/3–5 fractions | P = .0292 | P = .0224;/HR = 0.322 (95% CI, 0.122–0.852) |
| Preoperative Bilsky grade | P = .0956 | NS | Postoperative Bilsky grade 0/1 vs 2/3 | P = .0092 | P = .003;/HR = 0.225 (95% CI, 0.084–0.604) |
| 18–26 Gy in 1–2 fractions vs 18–40 Gy in 3–5 fractions | P = .09 | NS | CTV V80 | P = .0914 | NS |
| Presence of lung and liver metastases | P = .0715 | NS | Systemic therapy post-SBRT | P = .1012 | NS |
Abbreviations: NS, not significant; CTV V80, volume of the clinical target volume encompassed by 80% of the prescribed dose.
Only those factors with P≤ .1 are shown for the univariate analysis and only those significant at P≤ .05 are shown for the multivariate analysis.
Fig. 2.
LC according to SBRT dose.
Fig. 3.
LC for the entire cohort according to postoperative epidural disease Bilsky grade.
Fig. 4.
LC probability for those 48 patients presenting with preoperative Bilsky grade 2 or 3 epidural disease according to their postoperative epidural grade.
Toxicity
According to the Common Toxicity Criteria for Adverse Events v. 4.0, 3 patients experienced grade 1/2 gastrointestinal toxicities, 3 had grade 1/2 genito-urinary toxicities, and 7 were observed to have worsening pain, likely a pain flare reaction because it was transient post-SBRT and has been previously described.14 There were no grade 3 or 4 acute toxicities. We observed 9 vertebral compression fractures (VCFs). Five were de novo and 4 were progressions of preexisting baseline fractures. The median time to VCF was 6.7 months. One patient had a hardware failure and required reoperation. There were no cases of radiation-induced myelopathy, radiculopathy, or wound breakdown.
Discussion
We report high rates of LC following spine SBRT in the postoperative patient, a first report that clearly describes the predictive value of epidural disease grade in this population and a therapeutic benefit to epidural disease resection with respect to LC. In addition, we confirm that high dose and low fraction number SBRT results in superior outcomes compared with more fractionated regimens.
The use of spine SBRT in the postoperative patient is an emerging indication. The aim is to improve upon existing rates of imaging-based LC compared with the current standard of adjuvant CRT.2–4 The postoperative spine SBRT literature has been limited to preliminary experiences involving small cohorts5,15; however, in 2013, the Memorial Sloan Kettering Cancer Center (MSKCC) reported its experience following postoperative SBRT in 186 patients.16 The researchers observed failures in 34 patients (18.3%) and reported 1-year LC of 83.6%. The surgical approach was described as separation surgery such that epidural disease was resected circumferentially, and SBRT practice varied from low-dose, fractionated SBRT (58% treated with 18–36 Gy in 5–6 fractions median total dose 30 Gy) to high dose fractionated SBRT (19.9% treated with 24–30 Gy in 3 fractions, median total dose 24 Gy) to single fraction SBRT (21.5% with 24 Gy). Preoperatively, 136 patients (74%) had spinal cord compression (grade 2/3), 40 patients (23%) had dural compression (grades 1b, 1c), and 6 patients (3%) had no compression. Postoperatively, 21 patients (11%) had residual cord compression (grade 2/3), while 98 patients (53%) and 67 patients (36%) had either dural compression (grades 1b, 1c) or no compression (grades 0, 1a), respectively. With respect to predictors of LC, those treated with low dose hypofractionated SBRT were at greater risk for failure compared with recipients of high dose hypofractionated SBRT (P = .04). However, no significant advantage to single fraction SBRT was observed compared with the high dose hypofractionated SBRT cohort.
Our series represents the second largest to be reported, at 80 patients. We report 21 local failures (26%) and 1-year LC and OS rates of 84% and 64%, respectively. Unlike the researchers in the MSKCC series,16 we did not control the surgical intent and procedure, which was left to the discretion of the surgeon. As a result, we had a range of operative procedures (Table 2). To adjust for the surgical variability, we focused on the epidural disease grade (Table 1). Preoperatively, 48 patients (60%) had high-grade epidural disease (grade 2/3), 25 patients (31.3%) had grade 1b or 1c, and 7 patients (8.7%) had no compression (grades 0, 1a). Postoperatively, 8 patients (10%) had residual high-grade epidural disease (grade 2 only), while 41 (51%) and 31 patients (39%) had either dural compression (grades 1b, 1c) or no compression of the dura (1a, 0), respectively. Although these results are similar to the MSKCC experience,16 we observed a significant relationship between postoperative epidural grade (0 vs 1 vs 2, P = .003 confirmed on multivariate analysis) and LC, as described in Fig. 3 and Table 4. We further analyzed only those patients presenting with high-grade epidural disease (grade 2 or 3, n = 48), who were then downgraded surgically to a 0 or 1 versus a 2, and a significant relationship was observed with superior rates of LC for those surgically downgraded (Fig. 4). This implies a therapeutic benefit to maximal epidural disease resection followed by SBRT.
Epidural disease is one of the major factors limiting spine SBRT efficacy. Several reports have concluded that epidural disease progression is the most common pattern of failure,5 including the current series (15/21 [71%]). Fundamentally, this is explained by either not encompassing the epidural space appropriately within the target volume, underdosing the tumor and more specifically the epidural space, or simply inherent bad tumor biology. With respect to target volume coverage, 90% of cases were treated with a donut approach, with the entire circumference adjacent to the thecal sac taken as the CTV (Fig. 1), and no significant relationship was observed between donut and nondonut type of dose distributions and LC (data not shown). Therefore, if anything, we tended to overcontour the epidural space because we tried not to take any chances with missing potential microscopic postoperative residual epidural disease. With respect to dose and biology, although we did not observe any significant relationship with LC and thecal sac dose (thecal sac biologically effective doses were also calculated), or any other dosimetric parameter (eg, minimum target dose) that could be a reasonable surrogate for dose within the epidural space, we did observe that high total doses ranging from 18 to 26 Gy delivered in 1–2 fractions yielded superior LC rates compared with 18–40 Gy delivered over 3–5 fractionated regimens (Fig. 2, Table 4, P = .02 on multivariate analysis)). This observation is similar to the MSKCC experience reported for both the postoperative patient16 and the patient with no prior radiation or surgery and treated with SBRT alone.17 There is a biological basis to support these data, as it has been shown that more extreme dose-per-fraction regimens (>8–10 Gy) activate additional mechanisms of cell kill linked to the ceramide pathway that would otherwise not be activated.18 Therefore, even though we have to relatively underdose the epidural space to keep the thecal sac dose acceptable with respect to the risk for radiation myelopathy, the additional biological tumor effects from the more extreme high dose per fraction regimens provide the additional cell kill beyond that expected by the absolute dose to yield a tumor control benefit. This may also overcome the inherently bad biology of this cohort, as tumors showed the propensity to break through into the epidural space and compress the thecal sac prior to treatment. Regardless of the potential explanations, it is clear that epidural disease progression requires innovative strategies to overcome this limitation and consequently improve LC.
One such approach has been to combine outpatient minimal access spine surgery followed by SBRT in patients with focal high-grade epidural disease and without significant instability or deformity.15 The intent of our minimal access spine surgery technique is therapeutic by debulking the epidural disease, which in turn also improves the spine SBRT distribution because there is now space between the tumor and the spinal cord to be spared. Additional benefits include a 2-cm limited incision that minimizes complications associated with impaired wound healing and a day surgery such that patients are discharged the same day with a 6.5-day median time to SBRT planning postop.15 This type of thinking and development of innovative therapeutic strategies focused on epidural disease resection as the primary surgical intent, represents a shift in the surgical ideology such that surgery becomes an adjunct to SBRT as opposed to vice versa. Our data now provide evidence for the first time to support this rationale, as we have shown that LC is linked to epidural grade (Fig. 3); and, moreover, high-grade (2 or 3) epidural disease that was downgraded to a postoperative grade 0 or 1 had superior LC rates to those of postoperative grade 2 (Fig. 4).
With respect to OS, our analysis identified post-SBRT systemic therapy as a significant prognostic factor. This is likely a surrogate for selecting patients who are to be longer-term survivors and have responsive disease, as otherwise systemic therapy would typically be withheld. The lack of significance with other clinical factors on univariate analysis—including those from Table 2 and widespread metastatic versus oligometastatic (≤5 sites of metastatic disease at the time of SBRT) disease, number of spinal metastases, and time from primary diagnosis to development of metastatic disease (data not shown)—may reflect the limitations of the study because the sample size was limited and the study was retrospective in nature. Importantly, we also recognize that the primary tumor types were heterogeneous, as were the systemic therapies, which ranged among several different chemotherapy agents and targeted therapies such that we could not do any specific analysis. Therefore, this finding is suggestive of a potential prognostic factor that can aid in patient selection and needs further validation. Other limitations include lack of documented pain scores and quality of life outcomes. An ongoing study at our institution is currently evaluating outcomes for this population, with pain measured using the Brief Pain Inventory, and quality of life with the European Organisation for Research and Treatment of Cancer QLQ-BM22 assessment tool.
Although our toxicity profile was favorable, with no major late adverse events such as radiation myelopathy, we acknowledge that retrospective evaluation of toxicities is suboptimal. However, a single radiation oncologist treated all patients with meticulous clinical and imaging-based follow-up, and thus we are confident in our major toxicity reporting as opposed to that of low-grade acute events. In addition, we have a centralized electronic medical record system that allows for high-quality data collection if the toxicity is documented. We did observe VCFs in 11% of patients, which is expected given the current literature,19 as the doses associated with SBRT are likely causing osteoradionecrosis20 and an independent pathomechanism that surgery does not mitigate. One case of hardware failure was observed (1/51 instrumented patients, 2%) which was in a patient previously radiated, and a low rate of hardware failure is consistent with the literature.21
Conclusion
Postoperative spine SBRT is efficacious and safe. Although the optimal SBRT dosing is unknown, the current data support our current standard of 24 Gy in 2 fractions. Aggressive epidural resection improves local control post-SBRT, and as such, multidisciplinary care involving spine surgery, radiation oncology, and medical oncology can maximize the therapeutic plan in these patients.
Funding
None declared.
Conflict of interest statement. None declared.
References
- 1.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 2.Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien) 1998;140:957–967. doi: 10.1007/s007010050199. [DOI] [PubMed] [Google Scholar]
- 3.Missenard G, Lapresle P, Cote D. Local control after surgical treatment of spinal metastatic disease. Eur Spine J. 1996;5:45–50. doi: 10.1007/BF00307826. [DOI] [PubMed] [Google Scholar]
- 4.Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys. 2011;81:e861–e868. doi: 10.1016/j.ijrobp.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 5.Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine. 2011;14:151–166. doi: 10.3171/2010.9.SPINE091005. [DOI] [PubMed] [Google Scholar]
- 6.Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phy. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 7.Sahgal A, Roberge D, Schellenberg D, et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol). 2012;24:629–639. doi: 10.1016/j.clon.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Arrigo RT, Kalanithi P, Cheng I, et al. Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery. 2011;68:674–681. doi: 10.1227/NEU.0b013e318207780c. [DOI] [PubMed] [Google Scholar]
- 9.Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13:324–328. doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 10.Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyde D, Lochray F, Korol R, et al. Spine stereotactic body radiotherapy utilizing cone-beam CT image-guidance with a robotic couch: intrafraction motion analysis accounting for all six degrees of freedom. Int J Radiat Oncol Biol Phys. 2012;82:e555–e562. doi: 10.1016/j.ijrobp.2011.06.1980. [DOI] [PubMed] [Google Scholar]
- 12.Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phy. 2012;82:107–116. doi: 10.1016/j.ijrobp.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85:341–347. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Chiang A, Zeng L, Zhang L, et al. Pain flare is a common adverse event in steroid-naïve patients after spine stereotactic body radiation therapy: a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2013 doi: 10.1016/j.ijrobp.2013.03.022. May 9. doi:pii: S0360-3016(13)00333-7. 10.1016/j.ijrobp.2013.03.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Massicotte E, Foote M, Reddy R, Sahgal A. Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol Cancer Res Treat. 2012;11:15–25. doi: 10.7785/tcrt.2012.500230. [DOI] [PubMed] [Google Scholar]
- 16.Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. 2013;18(3):207–214. doi: 10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 19.Cunha MV, Al-Omair A, Atenafu EG, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys. 2012;84:e343–e349. doi: 10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Al-Omair A, Smith R, Kiehl T, et al. Radiation-induced vertebral compression fracture following spine stereotactic radiosurgery: clinical-pathologic correlation. J Neurosurg Spine. 2013;18:430–435. doi: 10.3171/2013.2.SPINE12739. [DOI] [PubMed] [Google Scholar]
- 21.Harel R, Chao S, Krishnaney A, Emch T, Benzel EC, Angelov L. Spine instrumentation failure after spine tumor resection and radiation: comparing conventional radiotherapy with stereotactic radiosurgery outcomes. World Neurosurg. 2010;74:517–522. doi: 10.1016/j.wneu.2010.06.037. [DOI] [PubMed] [Google Scholar]